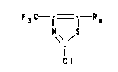Note: Descriptions are shown in the official language in which they were submitted.
-
- 1 1338418
Mlcroblcldal ComPosltlon
The present lnventlon relates to funglcldal and
mlcroblcldal composltlons whlch contaln as actlve lngredlent a
2-chloro-4-trlfluoromethyl-thlazol-5-carbonlc acld derlvatlve
of formula I, to the preparatlon of these derlvatlves and to
composltlons contalnlng them, as well as methods for
controlllng or preventlng lnfestatlon of plants by
phytopathogenous mlcroorganlsms.
The 2-chloro-4-trlfluoromethyl-thlazol-5-carbonlc
acld derlvatlves correspond to the formula I
N S (~
q'
whereln R 18 an organlc radlcal wlth up to 40 carbon atoms
whlch optlonally contalns nltrogen, oxygen or sulfur atoms and
whlch can be transformed by hydrolysls or oxydatlon dlrectly
lnto the thlazol bound carboxyl group.
Thlazol-5-carbonlc acld derlvatlves are known from
the llterature. 2,4-Dlmethylthlazol-5-carboxamldes are
dlsclosed as funglcldes ln US patent 3,725,427; 2-chloro-4-
trlfluoromethylthlazol-5-carbonlc acld derlvatlves are
dlsclosed as antldotes (safeners) to reduce the phytotoxlc
actlon of strong herblcldes on cultlvated plants ln US patents
4,199,506, 4,251,261, ln the Canadlan Patents Nos. 1,151,654,
1,161,441 and 1,174,867.
21489-7362
` ` 1338418
It has been found that the 2-chloro-4-
trifluoromethyl-thiazol-5-carbonic acid derivatives of formula
I have extraordinary good microbicidal activity and are able
to cure or prevent cultivated plants from infestation of
phytopathological microbes and fungi.
The compounds of formula I are stable at room
temperature. They can be used in the agricultural and in
related fields for controlling pests, especially for
controlling and preventing phytopathogenic microorganisms.
The derivatives of formula I are characterised by excellent
fungicidal activity over a wide application range and
unproblematic handling. These derivatives further possess
nematicidal properties which makes them suitable for
controlling nematodes, especially phytopathogenic nematodes.
Because of their pronounced microbicidal activity
the derivatives of formula I are preferred, wherein the group
R contains a maximum of 25 carbon atoms, among them the
derivatives falling under the formula Ia
F3C--j_i R,
N\/S
i,
wherein Ra is a radical selected from among cyano, -COXR1,
-CONR3R4 or -COD,
X is oxygen or sulfur,
1 ~ 1 18 alkyl or C3-C18 cycloalkyl which is
unsubstituted or substituted by halogen, a group -YR2, A,
nitro, -N(R3)COA, -[N(R3)]m-C(A)=NC1-C4alkyl, -lN(R3)]m-
C(A)=NH or lN(R3)]m-CO-N[(CO)mR3]-NR3, -[N(R3)]m-CON[(CO)m
-- 2
. ~
~ 21489-7362
~ 3 - l 33841 8
R3]-N(R3)m~~CO)mR4 ln whlch groups one of the lndlces m must
be zero, further Cl-Cl8alkyl or C3-C18cycloalkyl can be
substltuted by cyano, a group -C(X)mXRlo, XCXR -(X)m-cxA~
-(X)CXN (R3)N(R3)R4, -CHA-COORlo, -C(OR7)=(OR8)Rg,-PO(R5)R6,
C3-C8cycloalkyl or C5-C8cycloalkenyl; Rl further represents
C3-Cl8cycloalkenyl, whlch ls unsubstltuted or substltuted by
halogen, Cl-C4alkoxy, Cl-C4alkylthlo, Cl-C4haloakoxy,
Cl-C4haloalkyl or a group -CO(O)m-Rlo,-COA, -CON(A)R3 or
-PO(R)5R6~ Rl further represents -(E)mU or ~(E)mQ,
R2 ls Cl-C8alkyl or C3-C8cycloalkyl, whlch ls
unsubstltuted or substltuted by Cl-C8alkoxy, Cl-C8alkylthlo,
C2-C8alkoxyalkyl, halogen, cyano or a group -CX(X)mRlo, ~(X)m~
CXA, -(X)mCXRlo, -N(R3)COA, -[N(R3)]m-C(A)=NH, [N(R3)]m-C(A)-
NCl-C4alkyl, A, -X-U or XQ; R2 further represents
C3-C8alkenyl or C3-C8cycloalkenyl, whlch ls unsubstltuted or
substltuted by halogen or a group -(E)mU or ~(E)mQ;
m ls zero or one;
Y ls oxygen, sulfur, SO2 or SO2;
A ls a radlcal -N(R3)R4;
D ls a radlcal -N(R3)N(R4)(CO)mR3;
R3 and R4 lndependently of each other represent
hydrogen, Cl-C8alkyl or C3-C8cycloalkyl, whlch ls
unsubstltuted or substltuted by Cl-C8alkoxy, C2-C8
alkoxyalkoxy, Cl-C8alkylthlo, cyano, a group -COORlo,
Cl-C4alkylcarbamoyl, dl-Cl-C4alkylcarbamoyl, plperldlno-
carbonyl, pyrrolldlnocarbonyl, plperldlno or pyrrolldlno; R3
~-D 21489-7362
- 3a - 1338418
and R4 further represent C3-C8alkenyl or C3-C8cycloalkenyl
whlch ls unsubstltuted or substltuted by halogen, Cl-C8alkoxy,
lD
21489-7362
1 3384 1 8
C3-C8cycloalkyl or a group cyano, -COORlot C1-C4alkylcarbamoyl
or piperidinocarbamoyl group; R3 and R4 represent further
C3-C8alkynyl, which is unsubstituted or substituted by U, or
R3 and R4 represent a radical ~(E)m U or ~(E)m Q;
R3 and R4 together with the nitrogen atom, to which
they are bound form a saturated or unsaturated heterocycle
with 5 to 9 ring members which may include once or several
times -NH-, -N-Cl-C4alkyl,
-CO- or -C(OR7)0R8 and which may be substituted by
halogen, cyano, Cl-C8alkoxy, amino, C1-C4alkylamino
di-C1-C4alkylamino or a group -COORlo;
R5 and R6 are independently of each other hydrogen,
Cl-C4alkyl or C2-C4alkoxy;
R7 and R8 are independently of each other Cl-C4alkyl or
R7 and R8 form together an alkylene chain of 2 to 4 chain-
links,
Rg and Rlo are independently of each other hydrogen,
Cl-C8alkyl, C3-C8cycloalkyl, C3-C8alkenyl, C2-C8alkoxyalkyl,
C3-C8alkoxyalkoxyalkyl, Cl-C4haloalkyl, ~(Cl-C3alkylene)mU,
-(Cl-C3alkylene)mQ, C1-C4haloalkoxy;
U is phenyl or naphthyl, which is unsubstituted or
substituted once or several times by halogen, Cl-C4alkyl,
-Y-C1-C4alkyl, C1-C4haloalkyl, C1-C4haloalkoxy, cyano, nitro,
carboxyl, -COOR7, -CONH2, -CONHR7, -CON(R7), -SO2NHR7,
-SO2N(R7)2, pyrrolidino, piperidino, carbonyl or
piperidinocarbonyl;
E is a Cl-C8alkylene or C2-C8alkylene chain, which
is unsubstituted or substituted by halogen, Cl-C4alkoxy,
C
~ 21489-7362
`` ~ 1338418
C1-C4alkylthio, C1-C4haloalkoxy or by a group -CO(O)mR1o,
-(CO)mA, ~(CO)mQ and which may be interrupted by
-CO- or -C(OR7)OR8-;
Q is a saturated or unsaturated heterocycle with 5 to 12 ring
members which may contain 1 to 4 heteroatoms or 1 to 2
heteroatoms in combination with a sulfinyl or sulfonyl group
or which may be interrupted by 1 to 2 carbonyl groups and
which may be fused to the phenyl ring together with inert
carrier material and a surfactant for the control or
prevention of infestation of cultivated plants by pathogenic
micro-organisms; with the proviso that when Ra is -COXR1, if
X=S then R1 or R2 is not C1-C5alkyl; if X=S and R1 or
R2=-(E)mU and m=O or 1 then U is not phenyl; if X=O then R1 is
not hydrogen, C1-C1Oalkyl, C3-C5alkenyl, C3-C5alkinyl,
C1-C5alkyl substituted by halogen; if X=O and R1=-~E)mU and
m=O or 1 then U is not phenyl or phenyl substituted by
halogen, C1-C4alkyl, trifluoromethyl or nitro; or when
Ra is -CONR3R4 then R3 and R4 are not hydrogen or
Cl -C5alkyl .
Good microbicidal activity show especially the
derivatives of the formula Ib
o
F3C--j i--C-OR,
~ ~ ( I b )
wherein R1 has the meaning given above.
Also good activity is shown by the derivatives of the
formula Ic
- 4a -
21489-7362
o 1338418
F3C~ C-SR2
N~ ~S ( I c )
i,
wherein R2 has the meaning given above, as well as the
compounds of the formula Id
C - 4b -
- 21489-7362
-5- 1 3384 1 8
~73C I I C--N--R~
Nq~S ~
wherein R3 and R4 have the meaning given above.
Some of the 2-chloro-4-trifluoromethyl-thlazol-5-carbonic acid
derivatives are new, those corresponding to the formula Ia
P3C I I R~
Nq~S ~)
Gl
wherein
Ra is a radical cyano, -COXRi, -CORi, -CONR3R4 or -COD, in
which
D and X have the meaning given in claim 3,
Ri is unsubstituted Cll-C18alkyl or C3 C18cyc y
Cl-C18-alkyl or C3-C18cycloalkyl substituted by halogen, a
group -YR2, A, nitro, -N(R3)COA~ -[N(R3)]m--C(A)-NCl-C4alkYl,
-[N(R3)]m--C(A)5NH or -[N(R3)]m--CON[(COm)R3]-N(R3),
[ ( 3)]m CN[(C)mR3]~N(R3)m~~(C)mR4~ in which group one of
the indices m must be zero, Cl-C18-alkyl or C3-C18cycloalkyl
are further substituted by cyano, a group -C(X)m--XRlo, -
XCXRlo,
~`D
21489-7362
-6- 1338418
-(X)m--C, -(X)m--CXN(R3)N(R3)R4, -CHA-COOR1or -C(OR7)(OR8)Rg,
-PO(R5)R6, C3-C8cycloalkyl or C5-C8cycloalkenyl; with the
proviso that -XRi is not C1-C10haloalkoxy, C2-C10alkoxy-
alkoxy, phenylthlo or benzyloxy if these group~ are
unsubstituted or phenoxy substituted or unsubstituted;
Ri further is C3-C8alkenyl or C3-C18cycloalkenyl, which is
unsubstituted or substltuted by halogen, C1-C4alkoxy,
C1-C4alkylthio, C1-C4haloalkoxy, C1-C4haloalkyl or a group
-CO(O)mR1o, -COA, -CON(A)R3 or -PO(R5)R6 with the proviso that
-XRi iæ not unsubstituted C3-C5alkenyloxy;
Ri further is C3-C8alkinyl which is unsubstituted; if X is
sulfur, Ri is also a group -(E)m--U or ~(E)m~~Q;
R2 is C1-C3alkyl or C3-C8cycloalkyl, which is unsubstituted or
substituted by C1-C8alkoxy, C1-C8alkylthio, C3-C8alkoxyalkoxy,
halogen, cyano or a group -CX(X)mR1o, -(X)m-CX-A, -(X)mCXR
-NIR3)COA, -[N(R3)1m--C(A)-N, -[N(R3)1m--C(A)-NC1-C4alkyl, A,
-XU or -XQ;
R2 further is C3-C8alkenyl or C3-C8cycloalkyl which is
unsubstituted or halosubætituted or is a group -(E)mU or
~(E)mQ;
m is zero or 1;
Y i5 oxygen, sulfur, -SO- or -52-
~A is a group -N(R3)R4,
D is a group -N(R3)N(R4)(CO)mR3,
R3 and R4 independently of each other are hydrogen,
C1-C18alkyl or C3-C18cycloalkyl which is unsubstituted or
substituted by C1-C8alkoxy, C2-C8alkoxyalkoxy, cyano a group
L~D
2148g-7362
' -
-6a- l 33841 8
-COOR10, C1-C4alkylcarbamoyl, di-C1-C4alkylcarbamoyl,
C1-C4alkylamino, di-C1-C4alkylamino, piperidinocarbonyl,
pyrrolidinocarbonyl, piperidino or pyrrolidino;
R3 and Ri further represent C3-C8alkenyl or C3-C8cycloalkenyl,
which is un6bustituted or substituted by halogen, C1-C8alkoxy,
C3-C8cycloalkenyl, cyano, a group -COOR1o, C1-C4alkyl-
carbamoyl, di-C1-C4alkylcarbamoyl, U, pyrrolidinocarbonyl or
piperidinocarbonyl;
R3 and Ri further are a group -(E)m--U or ~(E)m~~Q with the
proviso that only one of R3 and Ri is hydrogen or C1-C4alkyl;
R3 and R4 together with the nitrogen atom, to which they are
bound alæo pre~ent an unsaturated or ~aturated heterocycle
with 5 to 9 ring-members which i8 interrupted once or several
time~ by oxygen, sulfur, nitrogen, imino, C1-C4alkylimino,
-CO-, or -C(OR7)OR8- and which may be substituted by halogen,
cyano, Cl-C8-alkoxyamino, C1-C4alkylamino, di-C1-C4alkylamino
or -COOR1o; R5 and R6 are independently or each other
hydroxyl, C1-C4alkyl or C2-C4alkoxy;
R7 and R8 are independently of each other C1-C4alkyl or R7 and
R8 form together a 2 to 4 membered alkylene chain,
`D 21489-7362
~ 7 ~ 1 3 3 8 4 1 a
Rg and Rlo are independently of each other hydrogen, C3-Cgcyclo-
alkyl, C3-Cgcycloalkenyl, C3-C8alkinyl, C2-Cgalkoxyalkyl, C4-Cs-
alkoxyalkoxyalkyl, Cl-C4haloalkyl, -(Cl-C3alkyl) - U,
-(Cl-C3alkyl)m--O" Cl-C4haloalkoxy, Cl-C4haloalkoxy-Cl-C4alkyl;
U is a phenyl or naphthyl ~Y~St, which is unsubstituted or substi-
tuted once or several times by halogen, Cl-C4alkyl, -Y-Cl-C4alkyl,
Cl-C4haloalkyl, Cl-C4haloalkoxy, cyano, nitro, -COOH, -COOR7,
-CONH 2, -CONHR7, -CON(R7), -SO 2 NHR7, -SO 2 N(R7) 2, pyrrolidino,
piperidino, pyrrolidinocarbonyl or piperidinocarbonyl;
E is a C1-Coalkylene or C2-Cgalkenylene chain, which is unsubsti-
tuted or substituted by halogen, Cl-C4alkoxy, Cl-C4alkylthio,
Cl-C4haloalkoxy or a rest -CO(O)mRlo~ ~(CO)m- A or ~(CO)mQ and/or
is interrupted by a chain - ~cr -CO- or C(OR7)0Rg,
Q is an unsaturated or saturated heterocycle with 5 to 12 chain-
members, which contains 1 - 4 heteroatoms or a sulfonyl or sulfinyl
group in combination with 1 or 2 heteroatoms and which can also
contain one or two carbonyl groups or be benzannelated.
New are also the 2-chloro-4-trifluoromethylthiazol derivatives of
formula Ib
F3C ~ -ORl
(Ib)
Cl
wherein R; is Clo-Clgalkyl or C3-Cgcycloalkyl unsubstituted or
substituted by halogen or Cl-Csalkyl or Rl is Cl-Clgalkyl or
c3-cl8cycloalkyl substituted by a ~ -XRz, A, nitro, -N(R3)COA,
-[N(R3)]m C(A), -lN(R3)]m C(A)~NH, -[N(R3)]m CoN[(Co)~R33-NR3,
-[N(R3)]m CON[(CO)m- R3]-N(R3)m (CO)mR4 in which~le~ one of
the indices must be zero, Cl-Clgalkyl or C3-Clgcycloalkyl are
further substituted by cyano, a ~e~ -C(X)m XRlo, -lX) - CXA,
-(X)CXN(R3)R4, -CHA-COORlo, -C(OR7)(0Rg)Rg, -PO(Rs)R6, C3-C~cyclo-
alkyl or C3-Cgcycloslkenyl,
3 3 ~
Ri further 18 C3-C8alkenyl or C3-C18cycloalkenyl whlch ls
unsubstltuted or substltuted by halogen, Cl-C4alkoxy,
Cl-C4alkylthlo, Cl-C4haloalkoxy, Cl-C4haloalkyl or by a group
-CO(O)mRlo, -COA, -CON(A)R3 or -PO(R5)R6,
Ri further ls C3-C8alkynyl, whlch ls unsubstltuted,
Ri ls also a group ~(E)m~ U or ~(E)m- Q wlth the
provlso, that -(E)m--U ls not unsubstltuted benzyl or
substltuted or unsubstltuted phenyl and
m X Y A D, R3, R4~ Rs~ R6~ R7~ R8' 9 10
and Q have the meanlng glven above under formula Ia.
Especlally good actlvlty showed those 2-chloro-4-
trlfluoromethyl-thlazol-5-carbonlc acld derlvatlves of the
formula Ib, whereln Rl ls Cl-C18alkyl whlch ls substltuted by
halogen, cyano, Cl-C4alkylthlo, Cl-C4alkoxycarbonyl,
Cl-C4alkylsulfonyl, C3-C8cycloalkyl or C3-C8cycloalkenyl or a
rest -(E)m--U or ~(E)m~~Q and E, m, Q and U have the meanlng
glven above under formula Ia, most actlve were those, whereln
Rl corresponded to a radlcal tetrahydropyran-2-ylmethyl, 2,2-
dlmethyl-1,3-dloxolan-4-ylmethyl, 1,2-dlhydrobenz-1,4-dloxan-
2-ylmethyl, thlophen-2-yl-3,4-methylenedloxybenzyl,
5-methylthlazol-2-ylethyl, para-tolylether-l-yl, bornyl,
norbornyl, fenchyl, menthyl, cyanoethyl, phenoxyethyl,
methylsulfonylethyl, phenylthloethyl, ethoxycarbonyl-
metaethoxycarbonyleth-l-yl, 5,5-dl-methyl-tetrahydrofuran-2-
on-3-yl, 2-oxopyrrolldlnomethyl, a-methoxy-carbonylbenzyl, a-
cyanobenzyl, a-benzoylbenzyl, a-methoxycarbonyl, a-
phenylbenzyl or morphollnomethyl.
l-D
~1489-7362
- 8a - 1338418
Good actlvlty showed also the derlvatlves of the
formula Ib, whereln Ri ls Cl-C12alkyl, substltuted by ~~X)m~
CXA and, whereln A, m and X have the meanlng glven above,
under formula Ib.
New are further the 2-chloro-4-trlfluoromethyl-
thlazol- 5-thlocarbonlc acld derlvatlves of the formula Ic
ID 21489-7362
- 9 - 1 3384 1 8
F3C - - C- SR2
(Ic)
Cl
wherein Rz has the meaning given above under formula Ia.
New are also the 2-chloro-4-trifluoromethylthiazol-5-carboxamides of
the formula Id
~ R
F3C - 4
(Id)
Cl
wherein R3 and R4 independently of each other are hydrogen, C1-Cg-
alkyl or C3-Cgcycloalkyl which iR unsubstituted or substituted by
Cl-Cgalkoxy, Cl-Cgalkoxyalkoxy, Cl-Cgalkylthio, cyano, a rest
-COORlo~ Cl-C4alkylcarbamoyl, di-Cl-C4alkylcarbamoyl, Cl-C4alkyl-
amino, di-Cl-C4alkylamino, piperidinocarbonyl, pyrrolidinocarbonyl,
piperidino or pyrrolidino;
R3 and R4 further represent C3-Cgalkenyl or C3-Cgcycloalkenyl which
is unsubstituted or substituted by halogen, Cl-Cgalkoxy, C3-Cgcyclo-
alkyl or a rest cyano, -COORlo~ Cl-C4alkylcarbamoyl or piperidino-
carbamoyl; R3 and R4 represent further c3-c8alkynyl~ which is
unsubstituted or substituted by U, or R3 and R4 represent 8 radical
~(E)m- U or ~(E)m Q; R3 and R4 together with the nitrogen atom,
to which they are bound form a saturated or unsaturated heterocycle
with 5 to 9 ring member~ which may include once or several times
-NH-, -N-Cl-C4alkyl, -CO- or -C(OR7)0Rg and which may be substituted
by halogen, cyano, Cl-Cgalkoxy, amino, Cl-C4alkylamino, di-Cl-C4-
alkylamino or a rest -COORlo and wherein E, m, Q, Rlo and U have the
meaning given above under formula Ib.
Of thexe amides, those wherein -N(R3)R4 corresponds to a rest
defined below, have e~pecially good sctivity.
-N-dichlorobenzyl-N-methylcarbamoyl,
-N-benzyl-N-isopropylcarbamoyl,
lo - 1 33841 8
-N-cyclohexyl-N-methoxycarbamoylethylcarbamoyl,
-N-2,6-dimethylphenyl-N-methoxycarbamoyleth-1-yl or
-N-(l-cyclopent-1-yl)-N-methylcarbamoyl.
New are further the 2-chloro-4-trifluoromethyl-thiazol-5-carbo-
hydrazides of the formula Ie
F3C~ -CO ~ ~ (CO) -R4 (Ie)
Cl
wherein m, each R3 independently of the other and R4 have the
meaning given above under formula Ia. Among those, the hydrazides of
the formula If showed especially good activity
F3C-- -CO ~ ~ (CO) -R'5
R'3' ~'4 m (If)
Cl
wherein R'3', R'4 and R'~ independently of each other represent
hydrogen, C1-Cgalkyl, C3-Cgcycloalkyl, phenyl or benzyl whereby
phenyl and benzyl are unsubstituted or substituted by halogen,
C1-C4alkyl, C1-C4haloalkyl, C1-C4alkoxy, C1-C4haloalkoxy, nitro,
carboxyl, C1-C4alkoxycarbonyl, carbamoxy or methylcarbamoyl,
R'3' and R'4 togethetr represent also a 4 - 5 membered alkylene
chain, which may be interrupted by oxygen, sulfur, a imino or
C1-C4alkylimino and which may be substituted once or several times
by C1-C4alkyl.
~,
In these definitions, the alkyl ~Yn~tr, where not otherwise
specified, are understood to have 1 to 18 carbon atoms. They can be
straight-chained or branched. The most usual ~c ~ are e.g. methyl,
ethyl, n ~ro~yl, isopropyl, n-butyl, isobutyl, sec.butyl,
tert.butyl, n-pentyl, isopentyl, n-hexyl and n-octyl. The alkenyl
and alkynyl ~ Pcan also be straight-chained or branched and
contain 3 to 18 carbon atoms. The most used ~ulei-Pare e.g. allyl,
methallyl, butene, butadiene, propynyl, methylpropynyl, 1-butynyl
~ 1 33841 8
and 2-butynyl. Cycloalkyl or cycloalkenyl groups have preferably 3 to 13 carbon
atoms and can also be benzannellated. Typical representatives are e.g.
cyclopropyl, cyclopentyl, cyclohexyl, cyclohexenyl, indan, tetrahydronaphthalin,decalin. Halogen stands for fluorine, chlorine, bromine and iodine atoms,
especially fluorine and chlorine. Haloalkyl and haloalkenyl groups are mono- or
poly-substituted with halogen atoms.
The above-mentioned group can be unsubstituted or substituted, typicalsubstituents of such groups are halogen or alkyl, alkenyl, alkynyl, cycloalkyl, aryl
or aralkyl groups which are bound via oxygen, sulfur or an imino group. The arylgroups can be substituted in turn. These groups can also be bound via a sulfinyl-,
sulfonyl-, carbonyl-, carbonyloxy-, carbamoyl-, sulfamoyl- or an amino-oxy-bridge
to the alicyclic hydrocarbon group.
The substituent Q, as well as the resets R3 and R4 with the nitrogen atom, to
which they are bound can represent unsaturated or saturated heterocycles with 5
to 12 ring members, which may include one two or three additional heteroatoms
or a sulfinyl- or sulfonyl group. They can further contain one or two carbonyl
groups and be benzannellated unsubstituted or substituted.
Possible heteroatoms are in this context one, two or three additional nitrogen
atoms, up to two oxygen or sulfur atoms, whereby two oxygen atoms cannot be
in vicinal position.
Examples for such heterocycles are listed below:
pyrroline, pyrrolidine, imidazoline, imidazolidine, pyrazoline, pyrazolidine, isazoline,
isazolidine, oxazoline, oxazolidine, isothiazolidine, thiazoline, thiazolidine,
diathiazolidine, oxa-diazolidine, piperidine, piperazine, tetrahydropyrimidine and
pyrazine, morpholine, thiomorpholine, thiazine, hexahydrotriazine,
tetrahydropyrazine, oxadiazine, oxatriazine, hexahydroazepine,
hexahydrodiazepine, diazepine, hexahydrodiazepine, azacyclooctan, indoline,
isoindoline, benzimidazoline, benzindazoline, benzoxa-
~ - 12 - 1338418
zoline, benzthiazoline, benzisooxazoline, benzthiazole, tetra-
hydrochinoline, tetrahydroisochinoline, tetrahydrochinazoline,
tetrahydrochinoxaline, tetrahydrophthalazine, benzomorpholine,
benzothiomorpholine, tetrahydrobenzazepine, tetrahydrobenzdiazepine,
tetrahydrobenzoxazepine, 1,5-diabicyclol4.3.0]nonane, dihydro-
benzoxazepine, 1,6-diabicyclol5.3.0]decane, 1,4-diabicyclo[3.3.0]-
octane, 1,5-diazablcyclol4.4.0]decane.
The above heterocycles can also be substituents. Further examples of
heterocyclic systems which may occur as substituents are e.g.
pyrrole, imidazole, pyrazole, isoxazole, thiazole, triazole,
oxadiazole, thiadiazole, tetrazole, oxatriazole, thiatriazole,
furan, tetrahydrofuran, dioxole, dioxolane, oxathiole, oxathiolane,
thiophen, tetrahydrothiophen, dithiolan, dithiazole, pyridine,
pyran, thiopyran, pyridazine, pyrimidine, pyrazine, tetrahydro-
pyran, tetrahydrothiopyran, dioxin, dioxan, dithiin, dithian,
oxazine, thiazine, oxathiine, oxathiane, triazine, oxadiazine,
th~ zine, oxathiazine, dioxazine, azepine, oxepin, thiepin,
diazepine, oxazepine, indole, benzofuran, benzothiophen, indazole,
benzimidazole, benzdioxol, benzdithiol, benzisoxazole, benz-
thiazole, benzoxazole, benzoxathiole, benztriazole, benzoxadiazole,
benzofurazane, benzothiadiazole, quinolin, isoquinolin, chromene,
chromane, isochromene, isochromane, thiochromene, isothiochromene,
thiochromane, isothiochromane, cinnoline, chinazoline, chinoxaline,
phtalazine, benzdioxin, benzdithiin, benzoxazine, benzdioxan,
benzoxathiane, benzotriazine, benzazepine, benzdilazepine, benz-
oxazepine, purine, pteridine, phenoxazine, phenothiazine.
g ro~pS
The heterocyclic Fest~rcan be substituted as mentioned above.
Some derivatives of the formula I are known from the literature and
can be produced by known methods.
~ - 13 - 1 3384 1 8
The thiazol derivatives of formula I are produced e.g. according to
US patent 4,199,506, by condensing an acrylic acid ester of the
formula II with chlorocarbonyl-sulfonylchloride of the formula III,
according to the reaction scheme
F 3 C ~ CN ~ -OR' + Cl~ -S-Cl ~ F 3 C ~ OR'
(II) (III) II----~\ /S
The 2-oxo-4-trifluoromethyl-thiazol-5-carbonic acid derivative
otained is heated with phosphoroxychloride whereby according to the
reaction conditions and the amount of phoshoroxychloride used, a
2-chloro-4-trifluoromethyl-thiazol-5-carbonic acid derivative of the
formula Ia or 2-chloro-4-trifluoromethyl-thiazol-carbochloride of
the formula IIa
cl
(Ia) (IIa)
Acrylic acid derivatives of the formula III can be prepared
according to J. Het. Chem. 9 (1972) S13 by condensing an acetoacetic
ester with trifluoromethylnitril in a boiling solvent in the
presence of sodium acetate, according to the reaction scheme
F 3 C-CN + CH 3 ~-cH2-coR- Na2O~CH 3 ~ F
H2II
Starting from 2-chloro-4-trifluoromethyl-thiazol-5-carbochloride of
formula IIa, the following active derivatives of formula I can be
prepared according to known methods:
~_ - 14 - 1338418
,
F3C_ CCl F3C ~ XR
+ R1-XH
Cl Cl
(IIa) (Ib)
F 3 - ~-A
+ amine HA(D) ~ /~ + amine HA (D)
~1
(Id)
F 3 C - ~ - ~ ~ XH F 3 C - ~ - ~ ~ XR
~1 Cl
(IIa) (Ic)
In these formulae A and R1 have the ~ning given above under
4l~s~a~b /c
formula Ia and G is an ~Astabl~ nucleofuge rest, such as a halogen
atom or a lower-alkyl sulfoxy rest.
In these reactions there are used inert solvents and diluents are
used to suit the particular reaction conditions. There may be
mentioned as examples:
halohydrocarbons, especially chlorohydrocarbons, such as tetra-
chloroethylene, tetrachloroethane, dichloropropane, methylene
chloridè, dichlorobutane, chloroform, chloronaphthalene, dichloro-
naphthalene, carbon tetrachloride, trichloroethane, trichloro-
ethylene, pentachloroethane, difluorobenzene, 1,2-dichloroethane,
l,l-dichloroethane, 1,2-cis-dichloroethylene, chlorobenzene, fluoro-
benzene, bromobenzene, iodobenzene, dichlorobenzene, dibromobenzene,
chlorotoluene and trichlorobenzene; ethers, such as ethyl propyl
ether, methyl tert.-butyl ether, n-butyl ethyl ether, di-n-butyl
ether, diisobutyl ether, diisoamyl ether, diisopropyl ether,
anisole, phenetole, cyclohexyl methyl ether, diethyl ether, ethylene
glycol dimethyl ether, tetrahydrofuran, dioxan, thioanisole and
~ - 15 - 1338418
dichlorodiethyl ether; nitrohydrocarbons, such as nitromethane,
nitroethane, nitrobenzene, chloronitrobenzene and o-nitrotoluene;
nitriles, such as acetonitrile, butyronitrile, isobutyronitrile,
benzonitrile and m-chlorobenzonitrile; aliphatic or cycloaliphatic
hydrocarbons, such as heptane, pinane, nonane, cymol, petroleum
fractions within a boiling range of from 70 to 190C, cyclohexane,
methylcyclohexane, Decalin, petroleum ether, hexane, ligroin, tri-
methylpentane, 2,3,3-trimethylpentane and octane; esters, such as
ethyl acetate, ethyl acetoacetate and isobutyl acetate; amides, for
example formamide, methylformamide and dimethylformamide.
Surprisingly, it has been found that the compounds of formula I of
this invention have, for practical field application purposes, a
very advantageous microbicidal spectrum against phytopathogenic
fungi and bacteria. Compounds of formula I have very advantageous
curative, systemic and, in particular, preventive properties, and
can be used for protecting numerous cultivated plants. With the
compounds of formula I it is possible to inhibit or destroy the
microorganisms which occur in plants or in parts of plants (fruit,
blossoms, leaves, stem~, tubers, roots) in different crops of useful
plants, while at the same time the parts of plants which grow later
are also protected from attack by such microorganisms.
The compounds of formula I are effective against the phytopathogenic
fungi belonging to the following classes: Fungi imperfecti (e.g.
Botrytis, Helminthosporium, Fusarium, Septoria, Cercospora,
Pyricularia, Alternaria); Basidiomycetes (e.g. the genera Hemileia,
Rhizocotonia, Puccinia); and, in particular, against the class of
the Ascomycetes (e.g. Yenturia, Podosphaera, Erysiphe, Monilinia,
Uncinula). In addition, the compounds of formula I have a systemic
action. They can also be used as dressing agents for protecting
seeds (fruit, tubers, grains) and plant cuttings aginst fungus
infections as well as against phytopathogenic fungi which occur in
the soil.
~ - 16 - 1338418
Apart from their microbicidal activity, the compounds of formula I
have nematocidal properties which make them especially suitable for
controlling plant nematodes. For this utility, the compositions of
the invention can be used curatively, preventively or systemically.
They exhibit a broad range of activity against the various species
of nematode and therefore satisfy the requirements of practice.
In the rates of application indicated below, the co ounds of the
invention, are especially well tolerated by plants.
Accordingly, the invention also relates to microbicidal compositions
as well as to the use of the compounds of formula I for controlling
phytopathogenic microorganisms, in particular phytopathogenic fungi,
or for protecting plants from attack by said microorganisms.
The invention further embraces the preparation of agrochemical
compositions, which comprises homogeneously mixing the active
ingredient with one or more compounds or groups of compounds
described herein. The invention furthermore relates to a method of
treating plants, which comprises applying thereto the compounds of
formula I or the novel compositions.
Target crops to be protected within the scope of the present
invention comprise e.g. the following species of plants:
cereals (wheat, barley, rye, oats, rice, sorghum and related crops),
beet (sugar beet and fodder beet), pomes, drupes and soft fruit
(apples, pears, plums, peaches, almonds, cherries, strawberries,
raspberries and blackberries), leguminous plants (beans, lentils,
peas, soybeans), oil plants (rape, mustard, poppy, olives, sun-
flowers, coconut, castor oil plants, cocoa beans, groundnuts),
cucumber plants (cucumber, marrows, melons), fibre plants (cotton,
flax, hemp, jute), citrus fruit (oranges, lemons, grapefruit,
mandarins), vegetables (spinach, lettuce, asparagus, cabbages,
carrots, onions, tomatoes, potatoes, paprika), lauraceae (avocados,
c~nn~ In, camphor), or plants such as maize, tobacco, nuts, coffee,
- 17 _ 13384i8
sugar cane, tea, vines, hops, bananas and natural rubber plants, as
well as ornamentals (flowers, shrubs, deciduous trees and conifers).
This recitation constitutes no limitation.
The compounds of formula I are normally applied in the form of
compositions and can be applied to the crop area or plant to be
treated, simultaneously or in succession, with further compounds.
These compounds can be both fertilisers or micronutrient donors or
other preparations that influence plant growth. They can also be
selective herbicides, insecticides, fungiGides, bactericides,
nematicides, mollusicides or mixtures of several of these prepara-
tions, if desired together with further carriers, surfactants or
application promoting adjuvants customarily employed in the art of
formulation.
Suitable carriers and adjuvants can bè solid or liquid and corres-
pond to the substances ordinarily employed in formulation tech-
nology, e.g. natural or regenerated mineral substances, solvents,
dispersants, wetting agents, tackifiers, thickeners, binders or
fertilisers.
A preferred method of applying a compound of formula I, or an
agrochemical composition which contains at least one of said
compounds, is foliar application. The number of applications and the
rate of application depend on the risk of infestation by the
corresponding pathogen (species of fungus). However, the compound of
formula I can also penetrate the plant through the roots via the
soil (systemic action) by impregnating the locus of the plant with a
liquid formulation, or by applying the compounds in solid form to
the soil, e.g. in granular form (soil application). The compounds of
formula I may also be applied to seeds (coating) by impregnating the
~eeds either with a liquid formulation containing a compound of
formula I, or coating them with a solid formulation. In special
cases, further types of application are also possible, e.g. selec-
tive treatment of the plant stems or buds.
~ - 18 - 1 3384 1 8
The compounds of formula I are used in unmodified form or, preferab-
ly, together with the adjuvants conventionally employed in the art
of formulation, and are therefore formulated in known manner to
emulsifiable concentrates, coatable pastes, directly sprayable or
dilutable solutions, dilute emulsions, wettable powders, soluble
powders, dusts, granulates, and also encapsulations in e.g. polymer
substances. As with the nature of the compositions, the methods of
application, such as spraying, atomising, dusting, scattering,
coating or pouring, are chosen in accordance with the intended
objectives and the prevailing circumstances. Advantageous rates of
application are normally from 50 g to 5 kg of active ingredient
(a.i.) per hectare, preferably from 100 g to 2 kg a.i./ha, most
preferably from 200 g to 600 g a.i./ha.
The formulations, i.e. the compositions, preparations or mixtures
containing the compound (active lngredient) of formula I and, where
appropriate, a solid or liquid adjuvant, are prepared in known
manner, e.g. by homogeneously mixing and/or grinding the active
ingredients with extenders, e.g. solvents, solid carriers and, where
appropriate, surface-active compounds (surfactants).
Suitable solvents are: aromatic hydrocarbons, preferably the
fractions containing 8 to 12 carbon atoms, e.g. xylene mixtures or
substituted naphthalenes, phthalates such as dibutyl phthalate or
dioctyl phthalate, aliphatic hydrocarbons such as cyclohexane or
paraffins, alcohols and glycols and their ethers and esters, such as
ethanol, ethylene glycol, ethylene glycol monomethyl or monoethyl
ether, ketones such as cyclohexanone, strongly polar solvents such
-as N-methyl-2-pyrrolidone, dimethyl sulfoxide or dimethylformamide,
as well as vegetable oils or epoxidised vegetable oils such as
epoxidised coconut oil, sunflower oil or soybean oil; or water.
The solid carriers used e.g. for dusts and dispersible powders, are
normally natural mineral fillers such as calcite, talcum, kaolin,
montmorillonite or attapulgite. In order to improve the physical
properties it is also possible to add highly dispersed silicic acid
- 19 1 33841 8
or highly dispersed absorbent polymers. Suitable granulated adsorp-
tive carriers are porous types, for example pumice, broken brick,
sepiolite or bentonite; and suitable nonsorbent carriers are
materials such as calcite or sand. In addition, a great number of
pregranulated materials of inorganic or organic nature can be used,
e.g. especially dolomite or pulverised plant residues. Particularly
advantageous application promoting adjuvants which are able to
reduce substantially the rate of application are also natural
(animal or vegetable) or synthetic phospholipids of the series of
the cephalins and lecithins, which can be obtained e.g. from animal
or plant cells, in particular from soybeans. Examples of useful
physical forms are phosphatidyl choline mixtures. Examples of
synthetic phospholipids are dioctanoylphosphatidyl choline and
dipalmitoylphosphatidyl choline.
Depending on the nature of the compound of formula I to be formula-
ted, suitable surface-active compounds are non-ionic, cationic
and~or anionic surfactants having good emulsifying, dispersing and
wetting properties. The term "surfactants" will also be understood
as comprising mixtures of surfactants.
Suitable anionic surfactants can be both water-soluble soaps and
water-soluble synthetic surface-active compounds.
Suitable soaps are the alkali metal salts, ~lk~line earth metal
salts or unsubstituted or substituted ammonium salts of higher fatty
acids (Clo-C22), e.g. the sodium or potassium salts of oleic or
stearic acid, or of natural fatty acid mixtures which can be
obtained e.g. from coconut oil or tallow oil. Mention may also be
made of fatty acid methyllaurin salts.
More frequently, however, so-called synthetic surfactants are used,
especially fatty sulfonates, fatty sulfates, sulfonated benzimid-
azole derivatives or alkylsulfonates.
- 20 ~ 1 3384 1 8
The fatty sulfonates or sulfates are usually in the form of alkali
metal salts, alkaline earth metal salts or unsubstituted or sub-
stituted ammonium salts and contain a C8-C22alkyl radical which also
includes the alkyl moiety of acyl radicals, e.g. the sodium or
calcium salt of lignosulfonic acid, of dodecylsulfate or of a
mixture of fatty alcohol sulfates obtained from natural fatty acids.
These compounds also comprise the salts of sulfated and sulfonated
fatty alcohol/ethylene oxide adducts. The sulfonated benzimidazole
derivatives preferably contain 2 sulfonic acid groups and one fatty
acid radical containing 8 to 22 carbon atoms. Examples of alkylaryl-
sulfonates are the sodium, calcium or triethanolamine salts of
dodecylbenzenesulfonic acid, dibutylnaphthalenesulfonic acid, or of
a condensate of naphthalenesulfonic acid and formaldehyde. Also
suitable are corresponding phosphates, e.g. salts of the phosphoric
acid ester of an adduct of p-nonylphenol with 4 to 14 moles of
ethylene oxide.
Non-ionic surfactants are preferably polyglycol ether derivatives of
aliphatic or cycloaliphatic alcohols, or saturated or unsaturated
fatty acids and alkylphenols, said derivatives containing 3 to
30 glycol ether groups and 8 to 20 carbon atoms in the (aliphatic)
hydrocarbon moiety and 6 to 18 carbon atoms in the alkyl moiety of
the alkylphenols.
Further suitable non-ionic surfactants are the water-soluble sdducts
of polyethylene oxide with polypropylene glycol, ethylenediamino-
propylene glycol and alkylpolypropylene glycol containing 1 to
10 carbon atoms in the alkyl chain, which adducts contain 20 to
250 ethylene glycol ether groups and 10 to 100 propylene glycol
ether groups. These compounds usually contain 1 to 5 ethylene glycol
units per propylene glycol unit.
Representative examples of non-ionic surfactants are nonylphenol-
polyethoxyethanols, castor oil polyglycol ethers, polypropylene/
polyethylene oxide adducts, tributylphenoxypolyethyleneethanol,
~ - 21 - ~ 3384 1 8
polyethylene glycol and octylphenoxypolyethoxyethanol. Fatty acid
esters of polyoxyethylene sorbitan, e.g. polyoxyethylene sorbitan
trioleate, are also suitable non-ionic surfactants.
Cationic surfactants are preferably quaternary ammonium salts which
contain, as N-substituent, at least one C8-C22alkyl radical and, as
further substituents, unsubstituted or halogenated alkyl, benzyl or
hydroxy-lower alkyl radicals. The salts are preferably in the form
of halides, methylsulfates or ethylsulfates, e.g. stearyltrimethyl-
ammonium chloride or benzyldi(2-chloroethyl)ethylammonium bromide.
The agrochemical compositions usually contain 0.1 to 99 % by weight,
preferably 0.1 to 95 % by weight, of a compound of formula I, 99.9
to 1 % by weight, preferably 99.8 to 5 % by weight, of a solid or
liquid adjuvant, and O to 25 % by weight, preferably 0.1 to 25 % by
weight, of a surfactant.
Whereas commercial products will preferably be formulated as concen-
trates, the end user will normally employ dilute formulations.
The compositions may also contain further auxiliaries such as
stabilisers, antifoams, viscosity regulators, binders, tackifiers as
well as fertilisers or other active ingredients for obtaining
special effects.
Such agrochemical compositions constitute an object of the present
invention.
The following non-limitative Examples serve to illustrate the
invention in more detail. Temperatures are given in Centigrades in
the following examples and tables, pressures are given in millibar
(mbar), percentages and parts are by weight.
~ - 22 - 1 3384 1 8
Example 1.1: Preparation of 2-chloro-5-(2-phenoxyethoxycarbonyl)-4-
trifluoromethyl-thiazole
CF3 ~ COOCH2CHzO-~
Cl
6.3 g (0.045 mol) of 2-phenoxyethanol are added slowly while
stirring to a solution of 11 g (0.44 mol) of 2-chloro-5-chlor-
carbonyl-4-trifluoromethyl-thiazole in 100 ml of absolute toluene.
The solution is then cooled to 0 - 5 and 3.6 g (0.045 mol) of
triethylamine are added dropwise while stirring. Triethylamine-
hydrochloride precipitates from the reaction mixture. After every-
thing is added, the suspension is stirred for 20 hours at room
temperature and then poured onto ice-water. The organic phase is
separated, dried over sodium sulfate and concentrated in a rotatory
evaporator. The residue cristallizes. In order to purify it, the
crystals are suspended in petrol-ether, filtered and dried. In this
manner 8.7 g (57 %) of the title compound are obtained, m.p. 70-72.
Example 1.2: Preparation of 2-chloro-5-(N-benzyl-N-isopropylamido)-
4-trifluoromethyl-thiazole
CF3 - - C0 -CHz~
TH( CH3)2
C1
A solution of 2.8 g (0.027 mol) of triethylamine and 4.1 g
(0.027 mol) of N-isopropylbenzylamine in 50 ml of ethylacetate are
added dropwise at O to 5 into a stirred solution of 6.5 g
(0.025 mol) of 2-chloro-3-chlorocarbonyl-4-trifluoromethyl-thiazole.
After the addition is complete, the reaction mixture, which has
turned into a yellow solution is stirred for 15 hour~ at room
temperature and then poured onto ice-water. The organic phase is
separated, dried over sodium sulfate, purified with active charcoal,
filtered and concentrated on a rotatory evaporator. The residue, a
red-brown oil is purified by chromatography in ethyl acetate/hexan
~ - 23 - ~ 3384 1 8
over a silica-gel column. After evaporation of the eluant, there
,e s~ns 6.5 g (73 %) of a colourless oil with correct chemical
analysis.
Example 1.3: Preparation of 2-chloro-5-(1-methoxycarbonyl-eth-1-
yloxy-carbonyl)-4-trifluoromethyl-thiazole
CF 3-- b ~--COOCHCOOCH 3
./
~1
A mixture consisting of 6.94 g of 2-chloro-5-carboxyl-4-trifluoro-
methyl-thiazole, 5 g of 2-bromopropionic acid-methyl ester and 4.5 g
of potassium carbonate, suspended in 50 ml of anhydrous dimethyl-
formamide is stirred under nitrogen atmosphere at room temperature
during 3 hours. The reaction-mixture is then filtered and the
filtrate is poured into ice-water/ethyl-acetate 1:1. The organic
phase is separated, washed 3 times with ice-water, dried and
concentrated in a rotatory evaporator. The residue, a colourless
oil is distilled for purification. One obtains 8.5 g (75 %) of title
product as colourless oil b.p. 75-80/0.015 mbar.
~ - 24 - 1 3384 1 8
In snalogy to these examples the following compounds are prepared:
b
~.,
Cl
Table 1
No. R1 phys. constant
1.001 1-dodecyl
1.002 1-octadecyl
1.003 cyclopropylmethyl
1.004 cyclopentylmethyl
1.005 cyclohexylmethyl
1.006 cyclohexylethyl
1.007 tetrahydrofuran-2-yl-methyl
1.008 pentahydLo~yLan 2-yl-methyl m.p. 60 - 63
1.009 2,2-dimethyl-1,3-dioxolan-4-yl-methyl oil
1.010 1,2-dihydrobenz-1,4-dioxan-2-ylmethyl resin
1.011 furan-2-ylmethyl
1.012 thiophen-2-ylmethyl oil
1.013 3,4-methylendioxybenzyl m.p. 96 - 98
1.014 thiophen-2-ethyl
1.015 5-methyl-thiazol-4-ylathyl m.p. 64 - 67
1.016 phenylethyl
1.017 para tolyl-eth-1-yl oil
1.018 3,45-trimethoxybenzyl
1.019 geranyl
1.020 2-hexen-1-yl
1.021 1-hexen-6-yl
1.022 1-hexen-3-yl
1.023 2-chloro-2-propen-1-yl
1.024 3-chloro-2-propen-1-yl
1.025 3-phenyl-2-propen-1-yl
1.026 cyclopentyl
1.027 cyclohexyl
1.028 2-methyl-cyclohexyl
1.029 3-methyl-cyclohexyl
1.030 4-methyl-cyclohexyl
1.031 cyclododecyl
1.032 2,3-dimethyl-cyclohexyl
1.033 2,4-dimethyl-cyclohexyl
1.034 2,6-dimethyl-cyclohexyl
1.035 3,5-dimethyl-cyclohexyl
1.036 4-tert.butyl-cyclohexyl
1.037 bornyl m.p. 65 - 67
-1.038 norbornyl oil
1.039 fenchyl oil
1.040 menthyl oil
1.041 2,2-dichlor-cyclopropylmethyl
1.042 -CH2-CN
1.043 -CH2-CH2-CN oil
~ - 25 ~ 1 3384 1 8
No. Rl phys. con~tant
1.044 -CH2-PO(OC2Hs)2
1.045 2-nltro-ethyl
1.046 2-allyloxy-ethyl
1.047 2-benzyloxy-ethyl
1.048 para-chlorbenzyloxyethyl
1.049 ortho-chlorbenzyloxyethyl
1.050 cyclopropyloxyethyl
1.051 cyclohexyloxyethyl
1.052 2-phenoxyethyl m.p. 70 - 72
1.053 para-chlorphenoxyethyl
1.054 -CHz-CHz-SCH3
1.055 -CH2-CH2-SO-CH3
1.056 -CH2-CH2-SO2-CH3 m.p. 86 - 88
1.057 -CHz-CH2-CH2-SCH3
1.058 -CH2-CH2-CH2-SO-CH3
1.059 -CH2-CH2-CH2-SO2-CH3
1.060 -CH2-CH2-S-C4Hg(")
1.061 -CH2-CHz-S-CH2-CH'CH2
1.062 cyclohexylthioethyl
1.063 benzylthioethyl
1.064 para-chlorbenzylthioethyl
1.065 phenylthioethyl m.p. 47 - 49
1.066 phenylsulfonylethyl
1.067 para-tolylthioethyl
1.068 para-chlorphenylthioethyl
1.069 phenylthiopropyl
1.070 ~-naphthylthioethyl
1.071 2-phenylthio-1-methyl-ethyl
1.072 2-phenylthio-1-chloromethyl-ethyl
1.073 -CH2-CH2-S-CH2-COOC2Hs
1.074 -CHzCH2SCH(CH3)COOC2Hs
1.075 -CH2-CH2-S-CO-N(CH3)2
1.076 piperidinoylthioethyl
1.077 -CH2-CH2-S-CS-N(CH3)2
1.078 piperidinothiocarbonylthioethyl
1.079 -CH2-COOCH3
1.080 -CH2-COOC2Hs b.p. 80 - 85/
0.025 mbar
1.081 -CH2-COOC4Hg(n)
1.082 -CH(CH3)COOCH3 b.p. 25 - 80/
0.015 mbar
1.083 -C(CH3)2COOC2Hs
1.084 -CH2-CH2-COOC2Hs
1.085 5,5-dimethyl-tetrahydrofuran-2-on-3-yl m.p. 100 - 102
1.086 -CH2-CO-N(C2Hs)2
1.087 -CH2CONlCH(CH3)2~2
1.088 -CH2CON[CH2(CH3)C2Hs]2
1.089 -CH2CON(CH2CH-CHz)2
1.090 2-methylpiperidinoylmethyl
1.091 azepinoylmethyl oil
~_ - 26 - 13384i8
No. Rl phys. constant
1.092 anllidomethyl
1.093 N-methyl-anilidomethyl
1.094 N-(2,6-dimethylphenyl)-N-(methoxy-
carbonyl-eth-1-yl)-carbamoylmethyl m.p. 95 - 97
1.095 1-(piperidinocsrbonyl)-eth-1-yl m.p. 62 - 66
1.096 -CH2-CHz-NH-CO-CH3
1.097 cyclopropancarbamoyl-ethyl
1.098 -CHz-CH2-NH-CO-CH2-Cl
1.099 -CH2-CH2-NH-CO-CHC12
1.100 benzamoyl-ethyl
-1.101 thienyl-2-carbamoylethyl
1.102 furylcarbamoylethyl
1.103 -CH2-CH2-NH-CO-NH-CH
1.104 C(CH3)3
1.105 phenylureylene-ethyl
1.106 -CHzCH2N(CH3)COCH3
1.107 -CH2CHzN(CH3)COCHC12
1.108 -CH2CH2N(CH 3 ) CONHCH 3
1.109 -CH2CH2N(CH2)SO2CH3
1.110 N-methyl-phenylsulfamoyl-ethyl
1.111 -CH2CH2N(C3H7-i)COCHClz
1.112 -CHzCH2N(CH2CH~OH2)CONHC12
1.113 2-oxo pyLLolidino-ethyl m.p. 58 - 62
1.114 dicyclohexylmethyl
1.115 a-phenylbenzyl oil
1.116 a-methylbenzyl
1.117 a-carboxylbenzyl
1.118 a-carboxyl-para-chlorbenzyl
1.119 a-methoxycarbonyl-benzyl m.p. 62 - 64
1.120 a-ethoxycarbonyl-benzyl
1.121 a-cyanobenzyl m.p. 78 - 81
1.122 a-benzoyl-benzyl m.p. 105 - 109
-1.123 a-methoxycarbonyl-a-phenylbenzyl m.p. 106 - 110
1.124 -cH2-cH2-N(cH3)2
1.125 pyrrolidinoethyl
1.126 piperidinoethyl
1.127 morpholinoethyl m.p. 185 - 187
(hydrochloride)
1.128 anilinoethyl
1.129 para (1-methoxycarbonyl)ethoxyphenyl
1.130 para(3-methyl-1,3-oxazolidin-2-yl)phenyl
1.131 para(N'N'-(dimethyl-ureylene)-phenyl
1.132 meta(N',N'-dimethyl-ureylene)phenyl
1.133 ~-cyano-~-methoxycarbonyl-styryl-4-yl
1.134 B,~-di(methoxycarbonyl)-styryl-4-yl
1.135 ~,~-dicyano-styryl-4-yl
- 27 - 1 3384 1 8
Nr. Rl phys. constant
1.136 -COOC2Hs m.p. 59 - 60
1.137 -COOH m.p. 122 - 125
1.138 -C00 benzyl m.p. 56 - 58
1.139 -COO(4,4-dimethyl-tetrahydro-fur-3~
yl-2-on) m.p. 100 - 102
1.140 -CO0(4-methyl-thiazol-5-ylethyl) m.p. 64 - 67
1.141 -COO(2,3,5,6-diepoxy-cyclo-
hexan-1-yl) syn. isomer m.p. 144 - 175
1.142 -COO(2,3,5,6-diepoxy-cyclo-
hexan-l-yl) anti isomer m.p. 144 - 120
1.143 a-(4-chlorphenyl)benzyl
1.144 a-(2-chlorphenyl)benzyl
1.145 a-(4-chlorphenyl)-4-chlorbenzyl
1.146 a-(2-chlorphenyl)-2-chlorbenzyl
1.147 a-(2-chlorphenyl)-4-chlorbenzyl
1.148 a-(4-fluorphenyl)-benzyl
1.149 a-(2-fluorphenyl)-benzyl
1.150 a-(4-fluorphenyl)-4-fluorbenzyl
1.151 a-(2-fluorphenyl)-2-fluorbenzyl
1.152 a-(2-fluorphenyl)-4-fluorbenzyl
1.153 a-(4-tolyl)-benzyl
1.154 a-(4-anisyl)-benzyl
1.155 a-(4-methoxyphenyl)-benzyl
1.156 a-(3-trifluorphenyl)-benzyl
Table 2
- CF3 - b ~ - co s R2
~.,
Cl
Nr. R2 phys. constant
2.001 -CHz-COOH
2.002 -CH2-COOCH3
2.003 -CH2-COOC2Hs
2.004 -CH(CH3)COOH
2.005 CH(CH3)COOCZHs
2.006 -CH2-CHz-COOH
2.007 -CHz-CO-N(C2Hs)2
2.008 piperi~n~ -ylmethyl
2.009 azepinamoylmethyl
2.010 anilidomethyl
2.011 para chloranilidmethyl
2.012 2-carboxylphenylmethyl
2.013 benzyl m.p. 60 - 62
~ - 28 - 1338418
Table 3 3
CF3 - CO-N ~
(Id)
Cl
Nr. R3 R4 phys. constant
3.001 allyl allyl
3.002 allyl 2-methoxy-ethyl
3.003 allyl isopropyl
3.004 2-methyl-2-propen-1-yl isopropyl
3.005 2-methyl-2-propen-1-yl cyclohexyl
3.006 2-chlor-2-propen-1-yl 2-methoxy-ethyl
3.007 2-chlor-2-propen-1-yl i~opropyl
3.008 2-chlor-2 plo~en-l-yl cyclohexyl
3.009 2-chlor-2-propen-1-yl 2-chlor-2-propen-
l-yl
3.010 3-chlor-2-propen-1-yl propyl
3.011 propyl 2-methoxy-ethyl
3.012 methyl cyclohexyl
3.013 methyl benzyl
3.014 methyl 2,6-dichlor-benzyl m.p. 118 - 120
3.015 isopropyl benzyl oil
3.016 isopropyl 4-chlor-benzyl
3.017 H benzyl
3.018 H phenyl
3.019 methyl phenyl
3.020 methyl 4-chlor-phenyl
3.021 ethyl 2-chlor-4-brom-phenyl
3.022 ethyl 3-trifluormethyl-phenyl
3.023 H -CH2-COOC2Hs
3.024 methyl -CHz-COOCH3
3.025 isopropyl -CH2-CO-NH-C3H7(i)
3.026 cyclopropyl -CHz-CO-N(C2Hs)2
3.027 ethyl -CHz-CH2-CN
3.028 allyl -CH2-CH2-CN
3.029 cyclohexyl -CH2-CH2-CN
3.030 phenyl -CHz-CHz-CN
3.031 phenyl -CH2-CH2-COOH
3.032 cyclohexyl -CHz-CH2-COOCH3 m.p. 116 - 120
3.033 2,6-dimethyl-phenyl -CH(CH3)COOCH3 m.p. 120 - 123
3.034 phenyl -CHz-COOH
3.035 phenyl -CH2-CN
3.036 4-chlor-phenyl -CH2-CN
3.037 2,4-tichlor-phenyl -CH2-CN
3.038 3,4-dichlor-phenyl -CH2-CN
3.039 3-trifluormethyl-phenyl -CH2-CN
3.040 methyl l-cyanocyclopent-l-yl m.p. 130 - 133
3.041 H 3-trifluoromethyl-cyclohexyl
3.042 H -NHz
3.043 H -N(CH3)2
3.044 methyl -NH-CH3
- 29 - 1 3384 1 8
Nr. R3 R4 phys. constant
3.045 H anilino m.p. 137 - 138
3.046 H 2-benzoyl-hydrazo
3.Q47 H 2-phenylsulfonyl-hydrazo
3.048 pyrrolidino
3.049 piperidino
3.050 2-methylpiperidino
3.051 2-ethylpiperidino
3.052 hexahydroazepino
3.053 morpholino
3.054 2,2,5,5-tetramethyl 1,3-oxazolidin-3-yl
3.055 5,5-dimethyl-2,2-tetra-methylen-1,3-oxazolidin-3-yl
3.056 5,5-dimethyl-2,2-pentamethylen-1,3-oxazolidin-3-yl
3.057 2-phenyl-1,3-oxazolidin-3-yl
3.058 2,2-tetramethylen-benzthiazol-3-yl
3.059 2-oxo p~.,olidino
3.060 hexahydro-2-oxo-azepino
3.061 3-oxo-thiomorpholino
3.062 2-oxo-1,3-oxazolin-3-yl
3.063 2-trichloromethyl-1,3-oxazolidin-3-yl
3.064 H 2-chlorbenzyl
3.065 H 2-hexylbenzyl
3.066 C2Hs C2Hs m.p. 40 - 41
3.067 allyl H m.p. 56 - 58
3.068 phenylethyl H m.p. 88 - 89
3.069 C2Hs 2,6-dichlorbenzyl m.p. 89 - 91
3.070 2-chlorobenzyl H m.p. 115 - 116
3.071 allyl allyl m.p. 100 - 101
3.072 C4Hg-n 2,6-dichlorbenzyl nD 1.5491
3.073 H ethoxycroton-2-yl m.p. 72 - 74
3.074 CH(CH3)2 2-chlorallyl nD 1.5027
3.075 4-chlor-2-fluor-6-iso-
propoxyphenyl H m.p. 123 - 125
3.076 cyano-dimethylmethyl methoxyethyl m.p. 130 - 132
3.077 chlorphenyl H m.p. 125 - 128
3.078 cyano-dimethyl-methyl H m.p. 78 - 80
3.079 2,2-dimethylindanyl H m.p. 174 - 175
3.080 3,5-bistrifluormethyl-
phenyl H m.p. 125 - 127
3.081 diphenylmethyl H m.p. 177 - 179
3.082 2,6-difluorophenyl H m.p. 160 - 161
3.083 5-trifluoromethyl-
thiazol-2-yl H m.p. 136 - 138
3.084 2-carboxyl-4-chlorphenyl H m.p. 132 - 134
3.085 3-trifluormethylcyclo-
hexyl H m.p. 106 - 109
3.086 2,4,6-trichloranilino H m.p. 184 - 186
3.087 furfuryl H m.p. 100 - 102
3.088 3,4-methylendioxybenzyl H m.p. 129 - 131
3.089 4-amidosulfonylphenyl H m.p. 186 - 189
3.090 1,2-diphenyleth-1-yl H m.p. 146 - 148
~ 30 - 1338418
Nr. R3 R4 phys. constant
3.091 -methylbenzyl H m.p. 131 - 133
3.092 benzoylamido H m.p. 192 - 194
3.093 4-fluorbenzyl H m.p. 128 - 130
3.094 2,2-diphenyleth-1-yl H m.p. 127 - 129
3.095 l-cyano-cyclopent-l-yl methoxycarbonylmethyl m.p. 145 - 147
3.096 l-cyano-cyclohex-1-yl H m.p. 145 - 147
3.097 2-methoxycarbonyl-4-
chlorphenyl H
3.098 2-methylaminocarbonyl-
4-chlorphenyl H
3.099 2-dimethylamino H
3.100 anilino H
3.101 2-chloranilino H
3.102 4-chloranilino H
3.103 l-cyanocyclopent-l-yl CH3
2. Formulation Examples for active ingredients of the formula I
(% = per cent by weight)
2.1 Wettable powders a) b) c)
active ingredient from the Tables 1-3 25 %50 % 75 %
sodium lignosulphonate 5 % 5 %
sodium lauryl sulphate 3 % - 5 %
sodium diisobutylnaphthalene-
sulphonate - 6 % 10 %
octylphenolpolyethylene glycol ether
(7-8 mol ethylene oxide) - 2 %
highly dispersed silicic acid5 % 10 % 10 %
kaolin 62 % 27 %
The active ingredient is mixed well with the adjuvants and ground
well in a suitable mill. Wettable powders are obtained that can be
diluted with water to give suspensions of any desired concentration.
2.2 Emulsifiable concentrate
active ingredient from the Tables 1-3 10 %
octylphenolpolyethylene glycol ether
(4-5 mol ethylene oxide) 3 70
- 31 -l 338418
calcium dodecylbenzenesulphonate 3 %
castor oil polyglycol ether
(35 mol of ethylene oxide) 4 %
cyclohexanone 30 %
xylene mixture 50 %
Emulsions of any desired concentration can be prepared from this
concentrate by dilution with water.
2.3 Dusts a) b)
active ingredient from the Tables 1-3 5 % 8 %
talcum 95 %
kaolin - 92 %
Dusts that are ready for use are obtalned by mixing the active
ingredient with the carriers and grinding in a suitable mill.
2.4 Extruder granulate
active ingredient from the Tables 1-3 10 %
sodium lignosulphonate 2 %
carboxymethylcellulose l %
kaolin 87 %
The active ingredient is mixed with the adjuvants, ground and moistenedwith water. This mixture is extruded and then dried in a stream of air.
2.5 Coated granulate
active ingredient from the Tables 1-3 3 %
polyethylene glycol (MW 200) 3 %
kaolin 94 %
(MW S molecular weight)
The finely ground active ingredient is uniformly applied in a mixer to
the kaolin moistened with polyethylene glycol. A dust-free coated
granulate is obtained in this manner.
~ - 32 -1338418
2.6 Suspension concentrate
active ingredient from the Tables 1-3 40 %
ethylene glycol 10 %
nonylphenolpolyethylene glycol
ether (15 mol of ethylene oxide) 6 %
sodium lignosulphonate 10 %
carboxymethylcellulose 1 %
37 % aqueous formaldehyde
solution 0.2 %
silicone oil in the form of
a 75 % aqueous emulsion 0.8 %
water 32 %
The finely ground active ingredient is intimately mixed with the
adjuvants. In this manner, a suspension concentrate is obtained from
which suspensions of any desired concentration can be prepared by
dilution with water.
3. Biological Examples:
Example 3.1: Action against Puccinia graminis on wheat
a) Residual protective action
Wheat plants are treated 6 days after sowing with a spray mixture
(0.06 % active ingredient) prepared from a wettable powder formulation
of the test compound. After 24 hours the treated plants are infected
with a uredospore suspension of the fungus. The infected plants are
incubated for 48 hours at 95-100 % relative humidity and about 20C and
then stood in a greenhouse at about 22C. Evaluation of rust pustule
development is made 12 days after infection.
b) Systemic action
Wheat plants are treated 5 days after sowing with a spray mixture
(0.02 % active ingredient, based on the volume of the soil) prepared
from a wettable powder formulation of the test compound. After 48 hours
the treated plants are infected with a uredospore suspension of the
~ - 33 - l 3384 1 8
fungus. The plants are then incubated for 48 hours at 95-100 % relative
humidity and about 20C and then stood in a greenhouse at about 22C.
Evaluation or rust pustule development is made 12 days after infection.
Compounds of the Tables 1-3, especally 3.040 exhibit good activity
against Puccinia fungi. The Puccinia attack was inhibited almost
completely. Puccinia attack is 100 % on untreated and infected control
plants.
Example 3.2: Action against Cercospora arachidicola on groundnut
plants
Residual protective action
Groundnut plant~ 10-15 cm in height are sprayed with a spray mixture
(0.02 % of active ingredient) prepared from a wettable powder
formulation of the test compound, and infected 48 hours later with a
conidia suspension of the fungus. The infected plants are incubated for
72 hours at about 21C and high humidity and then stood in a greenhouse
until the typical leaf specks occur. Evaluation of the fungicidal
action is made 12 days after infection and is based on the number and
size of the specks.
Compared with untreated and infected control plants (number and size of
the specks - 100%), Cercospora attack on groundnut plants treated with
compounds of the tables 1-3 is substantially reduced. Thus
c~ ,ou..d 1.009 inhibits the occurence of specks almost completely (0 to
10 %).
Example 3.3: Action against Erysiphe graminis on barley
a~ Residual protective action
Barley plants about 8 cm in height are sprayed with a spray mixture
(0.006 % active ingredient) prepared from a wettable powder formula-
tion of the test compound. The treated plants are dusted with conidia
~ 34 ~ 1 3 3 8 4 1 8
of the fungus after 3 to 4 hours. The infected barley plants are stood
in a greenhouse at about 22C. The fungus attack is evaluated after
10 days.
Compounds of the Tables 1-3 exhibit good activity against Erysiphe
fungi. For example, the compounds 1.009, 1.012, 1.065, 1.115, 1.127,
1.137, 3.077, 3.084 and 3.086 inhibited Erysiphe attack almost
completely (attack - 0-10 %). On the other hand, Erysiphe attack is
100 % on untreated and infected control plants.
xample 3.4: Residual protective action a~ainst Venturia inaequalis on
apple shoots
Residual protective action
Apple cuttings with 10-20 cm long fresh shoots are sprayed with a spray
mixture (0.02 % a.i.) prepared from a wettable powder formulation of
the test compound. The plants are infected 24 hours later with a
conidia suspension of the fungus. The plants are then incubated for
5 days at 90-100 % relative humidity and stood in a greenhouse for a
further 10 days a 20-24C. Scab infestation is evaluated 15 days
after infection.
Compounds from the Tables 1-3 exhibit good activity against Venturia.
Attack is 100 % on untreated and infected shoots.
Example 3.5: Action against Botrytis cinerea on beans
Residual protective action
Bean plants about 10 cm in height are sprayed with a spray mixture
(0.02 %) prepared from the test compound formulated as wettable powder.
After 48 hours, the treated plants are infected with a conidia
suspension of the fungus. The infected plants are incubated for 3 days
at 95-100 % relative humidity and 21C, and evaluation of the fungus
attack is then made. Many compounds of Tables 1 - 3 very strongly
inhibit fungus attack. At a concentration of 0.02 % compounds 1.004,
~ _ 35 _ 1338418
1.012, 1.065, 1.115, 1.127, 1.137, 3.077, 3.084 and 3.086 are fully
effective (attack 0 to 5 %) Botrytis attack on untreated and infect
bean plants i8 100 %.
Example 3.6: Action against Pyricularia oryzae on rice-plants
a) Residual protective action
Two week old rice plants are sprayed with a spraying mixture containing
0.006 ~0 of active substance, that was prepared from a wettable powder
of the substance to be tested. After 48 hours the plants were infested
with a suspension containing conidia of the fungus. The infected plants
are kept at 24~ and 95-100 % relative humidity for S days before the
fungus infestation is evaluated.
b~ Systemic action
Two week old rice plants in flower pots are sprayed with a spraying
mixture containing 0.006 % of active substance, that was prepared by
diluting a wettable powder of the substance to be tested with the
required amount of water. The flower pots are then added with water so
that the stems of the rice plants stand in water. After 48 hours the
plants are infected with a suspension containing conidia of the fungus.
The fungus-infectation is evaluated after an incubation period of
5 days during which the rice plants were kept at about 24 and 95-100 %
relative humidity.
The compounds of tables 1 to 3 are very effective against Pyricularia
orizae. The Pyricularia infection is 100 % on infected but not treated
control plants. The tested compounds, especially 1.115 and 3.040
inhibited fungus attack on rice plants to 0-5 %.
Example 3.7: Action against Tilletia caries on wheat
Winter barley of the type Probus is infected with spores of Tilletia
caries, to the rate of 3 g of dry fungus-spore per kg of barley seed.
The infected seeds are dried and then macerated in a rolling-mixer with
an aqueous solution of the compound to be tested, so that 60 ppm of
active substance per weight of seed gets applied. The seeds are then
1~ - 36 - 1 3384 1 8
dried. The infected and treated barley is sown in October in a field by
means of a sowing machine. Lots of 2 m length containing 3 rows are
arranged in triple repetition.
The test is evaluated when the spicules are ripe by evaluating the
percentage of spicules infested with Tilletia caries.
The compounds of tables 1 to 3 are very effective against Tilletia
caries. While plants from infected but not treated seed showed a 100 %
Tilletia-infestation, the tested co .ounds reduced the infection to
O - 5 %.
Example 3.8: Action against Helminthosporium ~ramineum on barley
Winter barley of the type "Cl" which is naturally infected with
Helminthosporium gamineum is treated in a rolling-mixer with a solution
of the compound to be tested, so that 60 ppm per weight of seed get
applied.
The infected and treated barley is sown in October in a field by means
of a sowing machine, so that lots of 2 m containing 3 rows of plants
are arranged in triple repetition.
The test is evaluated when the spicules develop and the percentage of
stalks which are infected with Helminthosporium gramineum are counted.
The compounds of tables 1 - 3 are very effective against
Helminthosporium gramineum. While plants from infected but not treated
seed showed a 100 % Helminthosporium-infection, the tested compounds
reduced the infection to O to 5 %.
Example 3.9: Action a~ainst Phytophtora on tomato plants
a) Residual-protective action
Three week old tomato-plants are sprayed with a spray-solution
containing 0.006 % of active substance, which has been prepared from a
wettable powder of the substance to be tested. The treated plants are
infected with a suspension of sporangia of the fungus. The plants are
~ 37 ~ 1 3 3 8 4 1 8
then kept at 20 and 90 - 100 % relative humidity. The test is
evaluated after a 5 day incubation period by evaluation the degree of
Phytophthora-infection.
b) Residual-curative action
Three week old tomato-plants are infected with a suspension of
sporangia of Phytophthora. After an incubation period of 22 hours in a
humid chamber at 20 and 90 - 100 % relative humidity, the infected
plants are dried and sprayed with a spray-solution containing 0.006 %
of active substance.
The tested compounds of tables 1-3 showed in these test remarkable
activity against Phytophtora.
xample 3.10: Action against Rhizoctonia solani (soil fungus) on
rice plants
a) Soil application, local protective action
12 days old rice-plants are watered with a spray-solution, made by
dilution of a formulation, containing 0.006 % of active substance, in
such a manner that none of the plant-parts above ground are
contamined. In order to infect the treated plant~, a suspension of
mycelium and sclerotia of Rhizoctonia solani is poured onto the soil
around the plant. After 6 days incubation period at 27 temperature
(day) and 23 (night) at 100 % relative humidity in a humid-box in the
clima-room, the fungus infection on the sheath, the leaves and the stem
is estimated.
b) Leaf application, local protective action
12 days old rice plants are sprayed with a spray-solution, which has
been prepared by diluting a formulation with water. After one day the
plants are infected by spraying them with a suspension of mycelium and
sclerotia of Rhizoctonia solani. After an incubation period of 6 days
at a temperature of 27 (day) and 23 (night) at 100 % relative
humidity in a humid box in the clima-room, the fungus infection on the
sheath, the leaves and the stem is evaluated.
~ - 38 - ~ 33841 8
The tested compounds of tables 1, 2 and 3 showed in this test good
activity against Rhizoctoria solani. Best protection was achieved with
c~ .oulld 3.040.
Example 3.11: Action against Xanthomonas oryzae on rice ~Oryza sativa)
a) Residual protective action
3 week old rice plants of the type "Calora" or "S6" are sprayed in the
green-house with a spray-solution containing 0.02 % of active
substance. After one day when the coating from the spray has dried, the
plants are put into a clima chamber of 24 temperature and 75 - 85 %
relative humidity, where the are infected. Infection is carried out by
cutting the points of the leaves with a scissors, which had been dipped
into a suspension of Xanthomonas oryzae. After 10 days incubation
period, the best is evaluated. Infected leaves will curl up and become
necrotic. The extent of the pathologic symptoms serves to determine the
extent of the residual activity of the substance tested.
b) Systemic action
3 week old rice plants of the type "Calora" or "S6" are sprayed in the
green-house with a spray-solution containing 0.006 % of active
substance. Three days later the plants are put into a clima-chamber of
24 temperature and 75 - 85 % relative humidity and infected. Infection
is carried out by cutting the points of the leaves with a scissors that
has been dipped into a suspension of Xanthomonas oryzae. After 10 days
incubation period, the test is evaluated. Infected leaves will curl up
and become necrotic. The extent of the pathologic symptoms serves to
determine the degree of the systemic activity of the substance to be
tested.
The tested compounds of tables l, 2 and 3 showed good activity against
Xanthonomas oryzae. The compounds 1.127 and 1.137 reduced the fungies-
infection to 0 - 20 % while infected not treated control plants were
100 % infected.