Note: Descriptions are shown in the official language in which they were submitted.
1 338907
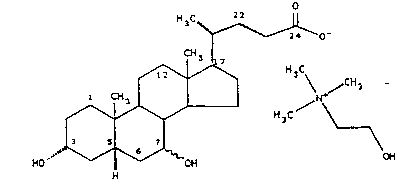
This invention provides 2-hy~lo~y-N,N,N-
trimethylethanaminium salts (= choline salts) of formula
\l O
~ C~I CNI ~ 3NCNzCNzO
/ .
~I),
HO ~ 1
wherein
R1 and R2, and R3 and R4, independently, together are
an oxo group, or are two hydrogen atoms or a hydrogen
atom and a hydroxy group, and
X is a C-O bond (i.e., a single bond directly
o
connecting -C- and -O-) or an -NH-CH2-CO- or
-NH-(CH2)2-S02- group,
X'
- 2 - l 338907
which are pharmacologically effective substances
which can be used, for example, as active ingredients of
cholagogue or biliary tract therapeutic agents, as well
as pharmaceutical and cosmetic preparations containing
them.
The production of 2-hydroxy-N,N,N-
trimethylethanaminium salts (= choline salts) of formula
I can be performed under conditions which are well known
to one skilled in the art.
lo Thus, for example, the free carboxylic acids of
formula II
~ ~ C
, \X-O-Y
/ ^\ ~ ~l~J,
Ho ~ ~ 2
wherein
R1 and R2, and R3 and R4, independently, together are
an oxo group, or are two hydrogen atoms or a hydrogen
atom and a hydroxy group, and
X is a C-O bond or an -NH-CH2-C0- or -NH-(CH2)2-S02-
group, and
Y represents a hydrogen or an alkali metal atom, is
reacted with a compound of general formula III
z
I
(CH3)3-N-HC2-CH2-OH
- 3 - l 3~89~7
wherein
Z represents a hydroxy group, if Y is a hydrogen
atom, or means the radical of an inorganic acid, if Y is
an alkali metal atom;
can be reacted in a polar inert solvent with
choline.
Suitable solvents include, for example, lower
alcohols (e.g., methanol, ethanol, propanol, isopropanol,
etc.) or lower ketones (e.g., acetone, methyl ethyl
ketone). On the other hand, dipolar aprotic solvents
(e.g., dimethylformamide, hexamethylphosphoric acid
triamide, etc.) are also suitable. But the latter
generally do not produce any advantages. If lower
alcohols and about 0.2 to 1 mol of acid per mol of
choline are used, the salts can be isolated in a simple
way, by the reaction mixture being concentrated by
evaporation and/or the reaction products being
precipitated by addition of nonpolar solvents, such as,
for example, acetone.
On the other hand, the alkali metal salts of
cholanic-24 acid derivatives of formula II (preferably
the sodium and potassium salts) can also be reacted with
choline salts of inorganic acids (preferably choline
chloride or choline sulfate). This reaction can also be
performed in inert polar solvents. If the reaction is
performed in one of the above-named lower alcohols by use
of approximately equimolar amounts of the two reactants,
then the reaction product, after filtering off of the
alkali metal salt of the inorganic acid, can be isolated
in a simple way, by the filtrate being concentrated by
evaporation and/or precipitated by addition of nonpolar
solvents such as, for example, acetone.
These reaction variants can of course also be
performed so that the free acids of formula II are
reacted with a choline salt or an inorganic acid in an
inert solvent, for example in the presence of alkali
- 4 - l 3389~7
metal bicarbonates (e.g., sodium bicarbonate or potassium
bicarbonate).
The 2-hydroxy-N,N,N-trimethylethanaminium salts of
general formula I have the same pharmacological effect as
the 5beta-cholanic-24 acid derivatives of general formula
II, wherein R1 and R2 are oxo, and R3, R4 and Y are H.
Further, they exhibit a bactericidal or virucidal
effectiveness.
Furthermore, the compounds according to the
invention surprisingly exhibit, among other properties,
good water solubility, good stability, relatively low
basicity, and relatively good resorbability.
For production of pharmaceutical agent specialties
the compounds according to the invention optionally are
processed with the usual additives and flavorings into
tablets, dragees and capsules, which normally contain 50
to 500 mg of active ingredient per dosage unit.
If the pharmaceutical agent specialty is to be used
for medicinal dissolution of cholesterol gallstones, in
animals, e.g., mammals, e.g., humans, as prophylaxis
against gallstone formation or as bactericidal or
virucidal agents, 5 to 20 mg of active ingredient per kg
of body weight per day is generally administered to the
patient, analogously to the known compound
- 1 3 3 8 9 0 7
-- 5 --
It will be appreciated that the actual preferred
amounts of active compound in a specific case will vary
according to the specific compound being utilized, the
particular compositions formulated, the mode of
application, and the particular situs and organism being
treated. Dosages for a given host can be determined
using conventional considerations, e.g., by customary
comparison of the differential activities of the subject
compounds and of a known agent, e.g., by means of an
appropriate, conventional pharmacological protocol.
The great water solubility of the substances
according to the invention further also make possible the
production of aqueous solutions, which, for example, are
suitable for infusion or injection, which can
advantageously be used for treatment of primary biliary
cirrhosis and primary sclerosing cholangitis as well as
also for treatment of organ-caused acute disturbances of
the bile flow or of intestinal absorption or which are
suitable for irrigation of the bile duct or of the
gallbladder by percutaneous transhepatic drainage.
Further, the substances according to the invention
are also suitable for making into water/oil and/or
oil/water emulsions. The lotions, creams or ointments
are suitable as dermatological preparations, for example,
for treatment of wounds or as cosmetics. Production of
these lotions, creams or ointments takes place according
to methods as they are well known to one skilled in the
art (Dr. J. Stephen Jellinek "Kosmetologie" [Cosmetology]
2nd edition, 1967, Dr. Alfred Huethig Verlag, Heidelberg
(West Germany) and European patent specification 0065
929).
- 6 - 1 33 8 9 0 7
The substances according to the invention are
further suitable as galenical auxiliary agents for
pharmaceutical preparations of active ingredients to
improve their transdermal resorption.
Without further elaboration, it is believed that one
~killed in the art can, using the preceding description,
utilize the present invention to its fullest extent. The
following preferred specific embodiments are, therefore,
to be construed as merely illustrative, and not
19 limitative of the remainder of the disclosure in any way
whatsoever.
In the foregoing and in the following examples, all
temperatures are set forth uncorrected in degrees Celsius
and unless otherwise indicated, all parts and percentages
are by weight.
1 338907
-- 7
Example 1
21.6 g of sodium salt of 3alpha,7beta-dihydroxy-
Sbeta-cholanic-24 acid (= 0.052 mol) is dissolved in 250
ml of ethanol with warming, mixed with a solution of 7.0
g of choline chloride in 50 ml of ethanol and reacted for
2 hours. Then the reaction mixture is allowed to cool,
the precipitated common salt is filtered out and the
filtrate is largely concentrated by evaporation and the
still flowable residue is instilled in 0.5 1 of acetone.
The mixture is stirred for two hours more, the product is
suctioned off and dried in a vacuum. Thus, 17.9 g of 2-
hydroxy-N,N,N-trimethylethanaminium salt of 3alpha,7beta-
dihydroxy-Sbeta-cholanic-24 acid with a melting point of
185 to 190C is obtained.
Example 2
6.05 g of choline (=0.05 mol) is dissolved in S0 ml
of ethanol, mixed with 19.6 g of 3alpha,7beta-dihydroxy-
Sbeta-cholanic-24 acid (= 0.05 mol) and after dissolution
is completed, is evaporated to dryness. The residue is
taken up in acetone, the separated crystallizate is
suctioned off and dried. 21.6 g of 2-hydroxy-N,N,N-
trimethyleth~n~rinium salt of 3alpha,7beta-dihydroxy-
Sbeta-cholanic-24 acid with a melting point of 186 to
190C is obtained.
- 1 3 3 8 9 07
-- 8
Example 3
6.05 g of choline (0.05 mol) is dissolved in 50 ml
of ethanol, mixed with 19.6 of 3alpha,7alpha-5beta-
cholanic-24 acid (= 0.05 mol) and, after dissolution is
completed, is instilled in 200 ml of acetone. It is
stirred for another hour, the separated crystallizate is
suctioned off and dried in a vacuum. Thus 20.0 g of 2-
hydroxy-N,N,N-trimethylethanaminium salt of
3alpha,7alpha-dihydroxy-5beta-cholanic-24 acid with a
melting point of 70 to 75C is obtained.
Example 4
11.7 g of 3alpha,12alpha-dihydroxy-5beta-cholanic-24
acid (= 0.030 mol) is dissolved in 30 ml of ethanol and
mixed with 14.3 ml (= 0.030 mol) of a 2.1 molar solution
of choline base in ethanol. Then the reaction mixture is
instilled in 250 ml of acetone, the precipitate is
suctioned off and dried in a vacuum. 12.8 g of 2-
hydroxy-N,N,N-trimethylethanaminium salt of
3alpha,12alpha-dihydroxy-5beta-cholanic-24 acid with a
melting point of 253 to 255C is obtained.
ExamPle 5
57 g of 3alpha,12beta-dihydroxy-5beta-cholanic-24
acid (= 0.145 mol) is dissolved in 100 ml of ethanol and
mixed with a solution of 17.5 g of choline base (145
mmol) in ethanol. Then the ethanol is distilled off, the
residue is mixed with 200 ml of acetone, the precipitate
is suctioned off and dried in a vacuum.
60 g of 2-hydroxy-N,N,N-trimethylethanaminium salt of
3alpha,12beta-dihydroxy-5beta-cholanic-24 acid with a
melting point of 170 to 174.5C is obtained.
Example 6
4.6 g of N-(3alpha,7alpha-dihydroxy-24-oxo-5beta-
cholan-24-yl) glycine (= 0.00832 mol) is dissolved in 10
9 _ 1 3 3 8 9 0 7
ml of ethanol and mixed with a solution of 1.0 g of
choline base (= 0.00832 mol) in 10 ml of ethanol. Then
the mixture is instilled in 200 ml of acetone, stirred
for some additional time, the crystals are suctioned off
s and dried in a vacuum.
3.6 g of 2-hydroxy-N,N,N-trimethylethanaminium salt
of N-(3alpha,7alpha-dihydroxy-24-oxo-5beta-cholan-24-yl)
glycine with a melting point of 80 to 83C is obtained.
ExamPle 7
8.04 g of 2-[(3alpha,7beta-dihydroxy-24-oxo-5beta-
24-yl)-amino]-ethane sulfonic acid (= 0.0133 mol) is
dissolved in 30 ml of ethanol and mixed with a solution
of 1.61 g of choline base (= 0.0133 mol) in 16 ml of
ethanol. Then the mixture is instilled in 200 ml of
acetone, stirred for some additional time, the crystals
are suctioned off and dried in a vacuum.
Thus, 6.9 g of 2-hydroxy-N,N,N-trimethylethanaminium
salt of 2-t(3alpha,7beta-dihydroxy-24-oxo-5beta-cholan-
24-yl)-amino]-ethane sulfonic acid with a melting point
of 80 to 85C is obtained.
- lo 1 338 90 7
Example 8
30 g of 3alpha-hydroxy-7-oxo-5beta-cholanic-24 acid
(0.1 mol) is suspended in 200 ml of ethanol and mixed
with a solution of 12.1 g of choline base (0.1 mol) in
100 ml of ethanol. Then the solvent is distilled off in
a vacuum, the residue is mixed with 500 ml of acetone,
the precipitate is filtered off and dried in a vacuum.
Thus 37.7 g of 2-hydroxy-N,N,N-trimethylethanaminium
salt of 3alpha-hydroxy-7-oxo-5beta-cholanic-24 acid with
an indefinite melting point is obtained.
t ~]20 = 11.2 (ethanol).
The preceding examples can be repeated with similar
success by substituting the generically or specifically
described reactants and/or operating conditions of this
invention for those used in the preceding examples.
From the foregoing description, one skilled in the
art can easily ascertain the essential characteristics of
this invention, and without departing from the spirit and
scope thereof, can make various changes and modifications
of the invention to adapt it to various usages and
conditions.