Note: Descriptions are shown in the official language in which they were submitted.
1~3!~80~
- 1 -
OXIDATION OF WHITE LIQUOR IN THE PRESENCE OF LIME HUD
Background of the Invention
The present invention relates to the process of
recausticizing kraft liquor and more specifically to the
production of sodium polysulfide and sodium hydroxide from
the oxidation of white liquor.
In the conventional kraft process for the production
of wood pulp, wood chips are digested in an alkaline
cooking liquor called white liquor. The active components
of this liquor are sodium hydroxide and sodium sulfide.
Although sodium sulfide hydrolyses to sodium hydrosulfide
in white liquor, for the purposes of simplification only
sodium sulfide is referred to throughout the text of this
specification.
It is known that an increase in carbohydrate yield in a
kraft cook can be achieved by the addition of sodium
polysulfide to the conventional white liquor. Reference is
made to this process in an article published by Svenesk
Papperstidn. 49(9):191, 1946 by E. Haegglund. Sodium
polysulfide acts as a stabilizing agent of carbohydrates
towards alkaline peeling reactions. Thus polysulfide
cooking results in a significant pulp yield gain which then
provides increased pulp production permitting increased
liquor throughput in recovery at the same total solids or
thermal load.
One manner of producing polysulfide is to add
elemental sulfur to a white liquor which is disclosed in
Canadian Patent No. 444,274 to Fuller and Woodside. This
approach, however, leads to an imbalance of sodium and
sulfur in the kraft recovery process. The end result is a
progressive increase in the sulfidity of the white liquor
133~8~g
-- 2 --
and an eventual increase of sulfur gases emissions. The
extra sulfur which is lost in the environment represents a
pollution source which is no longer tolerable. An
alternative approach to adding sulfur to white liquor for
producing polysulfide, is to convert the sodium sulfide
already present in the white liquor to polysulfide by an
oxidation process. Based on this approach, several
processes for the continuous production and recovery of
polysulfide have been published. The different methods
have been reported by Green, R.P. in Chemical Recovery in
the Alkaline Pulping Process, Tappi Press, pp. 257 to 268,
1985 and by Smith, G.C. and Sanders F.W. in U.S. Patent
4,024,229. These procedures generally involve redox,
catalytic or electrochemical processes.
One process for converting sodium sulfide is
described by Barker in U.S. Patent 3,470,061 relating to
the redox formation of polysulfide by treatment of a liquor
containing sulfide with an insoluble manganese oxide with a
manganese valence greater than two. After oxidation of
sulfide to polysulfide by the manganese oxide, the spent
manganese oxidant, which is insoluble in the polysulfide
liquor, can be physically separated and reused after
partial regeneration by oxidation with air. As disclosed
by Barker and Ma in Tappi report 56 (5), 112, 1973, the
overall oxidation can be formulated as:
(1) xNa2S + (x-1)MnO2 + (x-1)H2O ----- Na2Sx + (x-1)MnO +(2x-2)NaOH
where x has typically a value of 2.
The regeneration of the manganese oxidant with air can be
expressed as:
(2) MnO + 1/2O2 ---- MnO2
The process for the regeneration of manganese oxide,
which includes separation, drying and oxidation, has been
1339~09
described by Barker and Ma in U.S. Patent 2,653,824.
Because the process requires a large amount of metallic
oxidant to produce enough polysulfide to obtain a
significant pulp yield increase, an alternative method to
regenerate the metallic oxidant has been proposed by
Barker and Becker in U.S. Patent No. 3,860,479. In this
last process a clear white liquor is fed in the bottom of
a packed tower which contains a catalyst selected from
one of the oxides of manganese, iron, cobalt, zinc,
aluminum, nickel and chromium or from one of the sulfides
of manganese, nickel, copper, iron and cobalt.
Simultaneously air or oxygen is also fed in the bottom of
the tower and the oxidized liquor is continuously removed
from the top of the tower. For a liquor with a sulfide
concentration of 30.2 grams of Na2S per litre (as Na20),
the polysulfide level of the white liquor exiting a
laboratory reactor tower ranged between 6.0 and 7.5 grams
of polysulfide sulfur per litre for a 17 hour period.
A further process for the production of polysulfide
from kraft white liquor oxidation is disclosed by Smith
and Sanders in U.S. Patent 4,024,229. The oxidation of
white liquor with air or oxygen occurs in the presence of
partially wet proofed activated carbon catalyst and is
based on the following reactions:
(3) 2Na2S + 02 + 2H20 ---- 2S + 4NaOH
(4) xS + Na2S ---- Na2S(X+1)
where x has a typical value of 1.
The sulfide and polysulfide may also react with
oxygen to produce thiosulfate:
(5) 2Na2S + 202 + H20 ---- Na2S203 + 2NaOH
(6) 2Na2S2 + 3~2 --~~ 2Na2S203
13~!~303
It is presently considered that oxidation of sulfide
in white liquor with air or oxygen is very slow without a
catalyst and leads mostly to the formation of thiosulfate
as shown in reactions 5 and 6, rather than polysulfide as
shown in reactions 3 and 4. However, it has been suggested
that the presence during oxidation of a granular grade of
activated carbon treated with polytetrafluoroethylene to
provide wet-proofing over a portion of the surface of the
carbon, results in a large increase in the rate of sulfide
oxidation and in a substantial formation of polysulfide.
The mechanism of action of the hydrophobic surface is not
well understood. It is believed, however, that the
polysulfide reaction takes place essentially at the solid
surface whereas the thiosulfate reaction occurs in the bulk
of the sulfide solution. This conception is disclosed by
an article in the Paper Trade Journal 159(13), 38-41, May 1
1975., Smith, Knowles and Green.
The process disclosed in U.S. Patent 4,024,229 has a
vertical or cylindrical vessel, referred to as a reactor
containing the wet-proofed activated carbon catalyst in a
fixed bed. The oxidation of white liquor in the reactor is
accomplished with compressed air. It has been reported
that the lime mud particles dispersed in white liquor, as a
result of incomplete separation of the mud from the white
liquor in the recausticizing plant of the kraft mill, tend
to contaminate the catalyst bed and reduce its activity.
Thus, to prevent deactivation of the bed with lime mud, the
white liquor pumped to the reactors needs to be extremely
clear which usually necessitates the passage of the
clarified white liquor through polishing filters placed
between the reactor and the white liquor separation unit.
The oxidized kraft white liquor from the process, which is
133~09
sometimes referred to as orange liquor, produces 4.6 g/L of
polysulfide sulfur for an initial sulfide concentration of
24 g/L (as Na20) and 9.8 g/L of polysulfide sulfur for
initial sulfide concentrations of 48 g/L (as Na20).
A process similar to that disclosed in U.S. Patent
4,024,229, but using a different catalyst is disclosed in
Japanese Patent No. 61,259,754. The catalyst in the
packed bed is shown to be made of active carbon granules
with 0.2 to 4 mm particle size and a macropore volume of
0.25 cc/g of which 35% consists of pores with a diameter
greater than 100 nm. However, unlike the catalyst used in
U.S. Patent 4,024,229, no wet-proofing treatment of the
carbon surface is apparently required to favor polysulfide
formation. It has been suggested that oxidation of sulfide
is controlled by internal diffusion (see Hara, S. and Ono,
T., Japan Tappi Journal 58(1): 46-51 (1988); Ohgushi, Y.
and Hara S-I, in "Preprints of the 1988 Spring Conference,
CPPA, May 19-21, 1988, Jasper, Alberta, Canada). Initial
results have indicated that with this activated carbon
catalyst, the polysulfide concentration in the oxidized
liquor is 5.7 g/L (as sulfur) for an initial sulfide
concentration of 24.4 g/L (as Na20).
For the production of polysulfide, it is apparent
that the formation of thiosulfate from reactions 5 and/or 6
is not desirable because thiosulfate is an inert species
during cooking. There are, however, other applications in
the kraft process where the full oxidation of sulfide to
thiosulfate may be desirable.
White liquor can represent an inexpensive source of
sodium hydroxide for the purification of flue gases, for
oxygen pulping and bleaching processes and for extraction
stages of bleaching sequences using chlorine or chlorine
1~39~30~
-- 6 --
dioxide. The major problem with the use of white liquor is
the formation of hydrogen sulfide when the pH of the stream
falls below 10. Moreover, even when the pH of the process
lies above 10, the sulfide in white liquor affects
adversely the brightness and the viscosity of the pulp in
pulping and bleaching processes using oxygen and in
bleaching sequences using chlorine or chlorine dioxide.
These problems can be eliminated, however, if the sulfide
is entirely converted to thiosulfate in an oxidation stage.
Smith and Sanders in U.S. Patent 4,162,187 describe
how the oxidation of white liquor with wet-proofed active
carbon catalyst of the process described in U.S. Patent
4,024,229 can be carried out to further oxidize the
polysulfide to thiosulfate as shown in reaction 6. A
procedure for oxidizing sodium sulfide in white liquor to
produce sodium thiosulfate has been disclosed by Hultman,
in U.S. Patent 4,053,352. In one embodiment of this
invention, the white liquor is oxidized at a temperature
within the range of about 50~C to 130~C and without the use
of catalyst by injecting air into the liquor while
maintaining an air flow within the range of about 50 to 150
m3/hr.m2 at standard conditions and with no catalyst.
Laboratory tests indicated that the rate of oxidation in
small scale equipment was low without a catalyst and that
the rate increased with increasing temperature and flow
rate. It was also shown that by conducting the test in
pilot plant scale equipment, the rate of oxidation
increased significantly due to the higher liquid height in
the pilot plant reactor.
Thus it has been shown that a number of processes are
available to produce polysulfide or thiosulfate from the
oxidation with air or oxygen of sulfide in white liquor.
1339'~03
However, of all the known processes for polysulfide
production, only the method disclosed in U.S. Patent
4,024,229 and a recent modification of this process has
reached commercial status. The main disadvantages of
this oxidation process are the high capital cost of the
equipment, the cost of the carbon catalyst, the
progressive deactivation of the catalyst and the frequent
acid washes required to reactivate, at least partly, the
contaminated catalyst. A simplified process to produce
sodium polysulfide or sodium thiosulfate from the
oxidation of sodium sulfide is, therefore, required.
SUMMARY OF THE lNv~l.LlON
It is an aim of the present invention to provide a
simplified process for the production of sodium
polysulfide or sodium thiosulfate from the oxidation of
sodium sulfide in a kraft white liquor.
It has surprisingly been found that when white
liquor is oxidized in the presence of lime mud particles
and with a sufficient degree of gas dispersion, the rate
of oxidation is much higher than when the white liquor is
oxidized without the lime mud particles. The
concentration of polysulfide in the white liquor oxidized
according to the present invention approaches that
obtained by the more expensive process disclosed in U.S.
Patent 4,024,229. One of the advantages of the present
process is that the catalyst is already an inherent part
of the conventional white liquor preparation process
produced during the causticizing of a kraft liquor with
lime. Furthermore, it is another advantage that the
equipment and conditions to carry out the present process
may be the same as that used for the causticizing of
green liquor with lime.
~3391309
-- 8 --
It has further been found that a typical white liquor
containing lime mud particles and having preferably a
sulfide concentration between 25 and 50 g/L (as Na20) is
oxidized with air or oxygen at temperatures ranging between
90 and 105~C in a stirred reactor, which may or may not be
under increased pressure. The catalyst for the oxidation
consists of lime mud particles which are produced in the
recausticizing plant of a kraft mill during the
causticizing of green liquor with lime. The catalyst is,
therefore, already part of the process of preparation of
the conventional white liquor whereas in the process
disclosed in U.S. Patent 4,024,229 the lime mud is
considered a contaminant of the carbon catalyst and is
generally removed. The chemical composition of the lime
mud particles is variable because the type and level of
impurities changed from mill to mill, but the major
component is always calcium carbonate.
The present invention provides in a process of
recausticizing kraft liquor containing sodium sulfide
wherein kraft green liquor is causticized with lime to
produce kraft white liquor and leave a residue of lime mud,
the improvement comprising the steps of oxidizing the kraft
white liquor in the presence of the lime mud to convert
sodium sulfide to sodium polysulfide and sodium hydroxide.
In another embodiment, oxidizing continues to produce
sodium thiosulfate.
There is also provided a process for production of
sodium polysulfide from kraft liquor containing sodium
sulfide comprising the steps of causticizing a kraft green
liquor with lime to produce a kraft white liquor
containing sodium hydroxide and a residue of lime mud, and
oxidizing the kraft white liquor in the presence of the
1~3~q~o-~
lime mud to convert sodium sulfide to sodium polysulfide
and sodium hydroxide.
The sodium polysulfide efficiency is calculated on a
sulfur basis from the formula:
sodium polysulfide formed by weight x 100
converted sodium sulfide by weight
If an efficiency of about 100 is achieved, then most of
the oxidized sodium sulfide is converted to sodium
polysulfide. If the efficiency is below 100 which is
found to generally be the case, then a portion of the
oxidized sodium sulfide has been converted to sodium
thiosulfate, and the remainder is sodium polysulfide. An
efficiency of 70% would indicate 70% sodium polysulfide
and the r~m~in~er sodium thiosulfate.
The process can be varied to suit specific
requirements, for instance, metal impurities particularly
manganese oxide increases the polysulfide efficiency.
Other variables include time of oxidizing, selection and
flow of oxygen or air through an efficient sparging
system and also the percentage of sulfide conversion that
occurs. If the oxidizing step is long, then the
oxidation step may form all sodium thiosulfate rather
than sodium polysulfide.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG 1 is a diagrammatic layout of a recausticizing
plant in a kraft process mill as known in the prior art;
FIG 2 is a diagrammatic layout of a pipe line
reactor for oxidizing kraft white liquor according to one
embodiment of the present invention;
FIG 3 is a sectional diagrammatic view showing a
test vessel used for carrying out the process of the
present invention;
-
._"
13~9~o~
-- 10 --
FIG 4 is a graph of white liquor oxidation showing
sulfide conversion against time for different catalysts;
FIG 5 is a graph showing different oxygen flow rates
comparing sulfide conversion to polysulfide on a time
base;
FIG 6 is a graph showing the variation in air flow
rate comparing conversion of sulfide to polysulfide on a
time base;
FIG 7 shows a graph of two different stirrer speeds
comparing sulfide conversion to polysulfide on a time
base;
FIG 8 shows a graph with gas dispension and no gas
dispersion comparing sulfide conversion to polysulfide on
a time base;
FIG 9 shows a graph of the variation in
concentrations of sulfide, polysulfide and thiosulfates
during the conversion process with a metallic impurity in
the lime mud.
DESCRIPTION OF THE PREFERRED EMBODIMENT
In a conventional kraft liquor cycle, the digestion
of wood chips takes place in an aqueous mixture of
sodium hydroxide and sodium sulfide called white liquor.
After the cooking operation, the waste liquor, sometimes
referred to as black liquor, is separated from the pulp
fibres, concentrated and burnt in a recovery furnace to
form a smelt. The smelt, which consists mainly of sodium
carbonate and sodium sulfide, is then dissolved in an
aqueous solution, usually a weak wash, to form the green
liquor. FIG 1 shows schematically a recausticizing plant
wherein the smelt and water are added to a dissolving
tank 10. The raw green liquor is passed through a buffer
tank 12 into a clarifier 14 where the dregs, comprising
mainly carbon particles and metallic compounds insoluble
in the green liquor are removed. The clarified
,. ~ ~
1 1 13~9809
green liquor is then mixed with lime in the slaker 16 to
convert the sodium carbonate into sodium hydroxide in
accordance with the formula:
(7) CaO + Na2CO3 + H2O ---- 2NaOH + CaCO3
During this reaction the lime and calcium carbonate are
insoluble and are, therefore, present in the liquid as
suspended solids. Several other impurities coming from the
lime or from the dregs are also insoluble and become part
of the suspended solids and the mixture of these insoluble
compounds forms the lime mud.
To complete the reaction, the slurry, which comprises
sodium hydroxide and calcium carbonate particles, is passed
through a series of agitated vessels called causticizers
18. The slurry is allowed to react at temperatures between
about 90 and 105~C for a period varying from 60 to 180
minutes. The reacted mixture is then passed to a clarifier
or white liquor filter 20 to separate the lime mud from the
white liquor. The white liquor goes to the white liquor
storage for use in the digestion of wood chips and
contains mostly an aqueous solution of sodium hydroxide and
sodium sulfide.
The thickened lime mud, in the embodiment shown, is
washed in a lime mud washing filter or sedimentation tank
24, passes through a storage tank 26 and a mud filter 28
where it is dewatered and then is calcined in a lime kiln
or in a fluidized bed calciner to yield reburned lime which
is then reused for causticizing green liquor in the slaker
16.
In plants which follow the process described in U.S.
Patent No. 4,024,229 the oxidation stage is carried out
after separation of the white liquor from the lime mud.
Thus the oxidation with the carbon catalyst takes place in
~3~9~09
- 12 -
special equipment located between the white liquor filter
20 and the white liquor storage tank 22.
In the present application the oxidizing step occurs
inside the recausticizing plant when the white liquor and
the lime mud are still mixed together and in one embodiment
in at least one of the causticizers 18.
In a commercial process the oxidizing may occur under
pressure, and in a pipe line reactor which may be connected
directly to a causticizer 18, or alternatively located in
the down flow from the white liquor filter 20.
If the oxidizing step occurs in one or more
causticizers, air or oxygen is introduced from one or
several spargers immersed deeply in the white liquor
containing the lime mud particles.
FIG 2 illustrates a pipe line reactor 30 from a
causticizer 18. The white liquor containing mud particles
is pressurized by a pump 32 and passed at high pressure
through a series of looped pipes 34 and back into the
causticizer 18. Oxygen or air is injected through nozzles
36 at loop bends. In one embodiment, pressure is in the
order of 1000 kilopascals, the length of the pipe line
reactor is about 45 meters and retention time in the
reactor is about 2 minutes. Whereas the pipe line reactor
30 is shown connected to a causticizer 18, in another
embodiment it is located in the down flow from the white
liquor filter 20 so that the oxidizing step occurs between
the white liquor filter 20 and the lime mud washing
filter 24.
For test purposes, the causticizing and oxidation
reactions are carried out substantially simultaneously
rather than consecutively because as soon as lime is added
to a green liquor, reaction commences. Once reaction has
1339809
- 13 -
commenced, lime mud is formed, thus the oxidizing step
can be started very shortly thereafter. Both steps may
be carried out in a single vessel although in a
production kraft mill separate locations for the two
steps may be preferable.
The concentration of lime mud particles in white
liquor during the causticizing step lies typically
between 90 and 500 g/L and the specific surface area
between 3 and 6 square metres per gram. Although the
specific surface area of the mud particles is relatively
low, the total surface area of solids per litre is high
between about 270 and 780 square metres.
Variations in process control occur when the
oxidation is carried out with fine bubbles of air or
oxygen sparged into causticizers where the temperature
is elevated and, the agitation intensity is varied.
Furthermore, the retention time may be varied and, the
liquid level controlled, so that the interfacial area and
number of contacts between the oxidant, be it air or
oxygen, the reductant (sodium sulfide) and the catalyst
(lime mud) is high thus favoring the oxidation of sulfide
in white liquor.
Another aspect of the present invention is the
role of metallic impurities in the lime mud. The
lime mud produced in the recausticizing plant is made
from reburned lime and is the solid reaction product of
the causticizing reaction as shown in equation 7. The
impurities in the lime mud are called non-process
elements because they are not among the active pulping
chemicals in the kraft process. The accumulation
of non-process elements in the lime mud cycle depends
on the input rate, the degree of closure of the
chemical cycle and the specific purges of
1339809
individual elements (Frederick, W.J., AIChE, Symp. Ser.
239 80:21, 1984). The impurities that may affect the
selectivity in the catalytic activity of lime mud during
the oxidation of white liquor are likely to be oxidizing
agents. Oxides of manganese, iron, cobalt, zinc,
aluminum, vanadium and copper, for example, are possible
oxidants in the lime mud. Of these substances, only the
oxides of iron, aluminum and manganese are at a
sufficiently high concentration in the lime mud to be
considered important. Wood is generally the main source
of these metal ions and accounts for nearly all input of
manganese. However, process water and makeup lime may
also be an important source of aluminum and iron. The
level of these impurities in the mud cycle depends on the
wood species, the chemical composition of the make-up
lime, the efficiency of the dregs removed from kraft
green liquor and the degree of closure of the mud cycle.
If these impurities in lime mud are found to play an
important role during the oxidation of white liquor, it
is possible to alter their level in the mud by, for
example, burning the dregs in the lime kiln. These
levels may also be changed by increasing the degree of
closure of the mud cycle and by selecting a proper makeup
lime. Furthermore, it is possible to enrich the mud in
metallic oxi~Ants by direct additions of these substances
to the lime mud cycle.
Although the concentration of these ox;~Ants in the
lime mud may be low, their effect on polysulfide and/or
thiosulfate generation is high because as these oxidants
get reduced during the oxidation reaction, they are
continuously reoxidized by the air or oxygen sparged in the
stirred tanks or pipe line reactor. Moreover, even if a
fraction of these metallic oxidants are reduced after the
. ~
- 15 - 1339~03
oxidation reaction, the oxidative calcination of lime mud
in the lime kiln tends to reactivate these oxidants in the
lime.
EXAMPLES
A number of oxidation experiments were carried out in
a laboratory scale. The tests were conducted in the
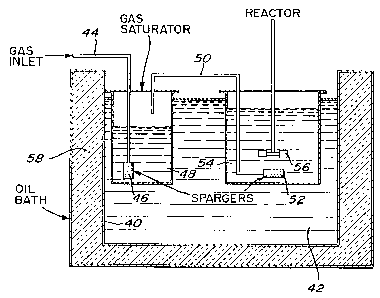
apparatus as shown in FIG 3 which comprises a one litre
vessel 40 immersed in a thermostatically controlled oil
bath 42. Gas, which was either air or oxygen, was first
passed through a gas line 44 and sparger 46 into a gas
washing bottle or saturator 48 from whence the saturated
gas is passed in a second gas line 50 to a sparger 52
within a reaction vessel 54. The gas saturator 48
minimizes evaporation losses from the reactor for test
purposes. A stirrer 56 provides agitation to the reactor
54 and the oil bath 42 has an insulating jacket 58
surrounding it.
Example 1
The catalytic activity of lime mud was tested during
the oxidation of white liquor with oxygen. FIG 4 gives the
percentage conversion of sulfide with and without suspended
catalytic solids in white liquor during oxidation. The
first series of tests were carried out wherein 0.75 litres
of white liquor with sulfide concentration of 28 grams per
litre (as Na20) were charged to the reactor. Oxygen at a
rate of 0.7 litres per minute was then sparged in the
liquors, kept at 100~C and agitated at a constant stirrer
speed of 1900 rpm for thirty minutes. Curve A represents
the oxidation of white liquors without a catalyst. By
adding activated carbon at concentrations of 4 and 53 grams
per litre, curves C and E respectively were produced. For
curve B an activated carbon aliquot coated with a
1339809
- 16 -
polyethylene film to produce a hydrophobic carbon catalyst,
similar to that used in the process described in U.S.
Patent 4,024,229, was added to the reactor to give a
concentration of 4 grams per litre. Finally to produce
curve D, (i.e. oxidation carried out in presence of lime
mud particles), a mill reburned lime with an available lime
content of 94% was added to 0.75 litres of green liquor
whose sulfide concentration was also 28 g/L (as Na20). The
lime-to-liquor ratio was 60 g/L of green liquor which
corresponds to a typical lime dosage in the recausticizing
plant of a kraft mill. After causticizing the green liquor
with the lime for two hours at 100~C, the concentration of
lime mud particles was approximately 105 g/L which is also
typical of a mill operation. The oxidation reaction was
then started at conditions identical to those used during
the oxidation of white liquor with and without carbon
particles added.
These results indicate that the rate of oxidation
increased drastically when carbon, wet-proof carbon or lime
mud was suspended in the liquor. As can be seen, the
catalytic activity of lime mud particles during the
oxidation of sulfide in white liquor with oxygen was
comparable with that of activated carbon, a known and
expensive catalyst. Because the polysulfide and
thiosulfate concentrations were not monitored during these
oxidation reactions, nothing can be concluded from these
tests on the selectivity of lime mud particles towards the
production of polysulfide.
The next series of tests illustrate the effectiveness
of variables on the rate of sulfide oxidation and on the
selectivity of lime mud under different operating
- 17 - 1~39~09
conditions towards polysulfide generation. The
polysulfide efficiency is defined here as follows:
sodium polysulfide efficiency =
sodium polysulfide formed by weight x 100
converted sodium sulfide by weight
where the polysulfide formed and the converted sulfide
during the oxidation are all expressed as g/L on a sulfur
basis. An efficiency of about 100% indicates that all
oxidized sulfide was converted to polysulfide sulfur
whereas an efficiency lower than 100% reveals the
simultaneous formation of thiosulfate.
For examples 2 to 6, each green liquor sample had an
initial temperature of 90~C and was causticized with
reburned lime at a constant stirrer speed of 1000 rpm.
This degree of agitation when translated to a mill scale
reactor represents relatively mild agitation conditions.
At the end of the causticizing, the white liquor
temperature had increased from 90 to 100~C as a result of
the exothermic reaction of lime hydration. After the 100
minutes causticizing time, a small sample of liquor was
removed for analysis. This sample was the zero time
oxidation sample. The gas valve for air or oxygen was
then opened to the reactor to permit the oxidation
reaction to proceed. Except for example 6, the oxidation
time was sixty minutes which is below the total retention
time available in mill causticizers. The temperature
during the oxidations ranged normally between about 100
and 104~C.
Prior to each oxidation test a fresh lime mud
suspension in white liquor was produced in the reactor
shown in FIG 3 by adding 45 grams of reburned lime to 0.75
litres of green liquor samples whose sulfide concentrations
ranged between 19 and 22 g/L (as S). The reburned lime
.
., ~ ,
~ 3393~3
- 18 -
used in all experiments was collected from the lime kiln of
a kraft mill and had the chemical composition given in
Table I.
CaO Na2O MgO MnO2 Fe2O3 Al2O3 Si 2 4 3
(%) (%) (%) (%) (%) (%) (%) (%) (%)
92.0 1.22 0.82 0.03 0.189 0.11 0.36 3.0 3.0
This lime can be considered as low in impurities and is
typically obtained in mills with an efficient dregs removal
operation and/or a low degree of closure in the lime mud
cycle.
Example 2
The effect on white liquor oxidation of increasing
the flow rate of oxygen from 0.12 to 0.4 L/min. is shown in
FIG 5. For these two oxidations, the initial sulfide
concentration was 19.3 g/L (as S) and the rotational speed
of the impeller was 1400 rpm. An increase of oxygen flow
rate increased both the rate of oxidation and the
polysulfide formation. The polysulfide efficiency was
similar at the two flow rates being approximately 30% at
the highest polysulfide concentrations. These results
indicate that when the oxidation was carried out with
oxygen in the presence of lime mud, polysulfide was formed
in significant quantities, but the major product of
oxidation was sodium thiosulfate. Under these conditions
the polysulfide efficiency was lower than that obtained in
the process according to U.S. Patent 4,024,229.
3o
13380~
-- 19 --
Example 3
For this series of oxidations, oxygen was
substituted with air, which is the usual oxidant in the
process disclosed in U.S. Patent 4,024,229. The effect
on oxidation of varying the air flow rate from .50 L/min.
to 1.4 L/min. is shown in FIG 6. These results show that
the rate of sulfide oxidation as well as the extent of
polysulfide formation increased with air flow rate.
Although the rates of sulfide oxidation with air were
much lower than with oxygen when compared on an equal
oxygen basis, the polysulfide efficiency was much higher
with air than with oxygen. Indeed the polysulfide
efficiencies with air were typically between 70 and 85~
which compares well with those obtained in the process of
U.S. Patent 4,024,229.
The tests indicate that the activity and selectivity
of the lime mud particles during the oxidation are
affected by the oxidant gas. Thus the resistance to gas-
liquid and liquid-solid mass transfer appear to be
influenced by the total flow rate and the presence or not
of nitrogen in the gas. (i.e. the partial pressure of
oxygen)
Example 4
Tests were then performed to assess the effect of
impeller rotational speed on white liquor oxidation. Air
was used in both tests as the oxidant at a flow rate of
0.5 L/min. The effect of increasing of the rotational
speed of the impeller from 1000 to 1920 rpm is shown in
FIG 7. With a 0.04 m diameter impeller used in the
experiments, the blade tip speeds range from 2.09 to 4.02
meters/second, which is within the range encountered
within commercial causticizers. With a more intense
agitation, both the sulfide conversion and the
polysulfide formation increased.
. .~
1339~
- 20 -
The polysulfide efficiencies at 60 minutes oxidation time
were approximately 75% for the two degrees of agitation.
The tests indicate that the higher the rotational speed
that can feasibly be obtained, the better.
Example 5
Tests were carried out to establish whether the
degree of dispersion of the gaseous oxidant in the
mixture of lime mud and white liquor influenced the rate
of oxidation. The gas sparger shown in FIG 3 was removed
and air was supplied in the reactor from a tubing (0.25
cm I.D.) placed under the impeller. The air flow rate
was 0.50 L/min. and the stirrer speed 1400 rpm for the
two oxidations with and without gas dispersion. As shown
in FIG 8, the rate of sulfide oxidation was reduced
markedly and no polysulfide was formed when air was not
dispersed with a sparger.
A low degree of dispersion of the oxidant gas in the
lime mud suspension not only reduced the rate of
oxidation, but also the polysulfide efficiency.
Example 6
The addition of metallic oxidants such as manganese
oxide to sulfide liquors is known to yield mostly
polysulfides. The reburned lime, which has a very low
level of impurity, was blended with MnO2 in the
proportions of 97 parts of lime and three parts of MnO2.
The weight percentage of MnO2 in the lime mud after the
causticizing reaction was 1.68%. During the two hours
oxidation, the oxygen flow rate was 0.10 L/min. and the
stirrer speed was 1950 rpm.
FIG 9 reveals that the presence of small quantities of
metallic oxidants such as manganese oxide in the lime mud
during the oxidation of white liquor with oxygen favors
.. .~,; .
1339~03
the formation of polysulfides up to approximately 50%
sulfide conversion. The polysulfide efficiency at the
maximum in polysulfide concentration was 80% which is much
higher than the value of 30% as shown in Example 2 where no
manganese oxide was added to the lime prior to the
oxidation.
Whereas the 1.6% MnO2 impurity figure of this test
may be somewhat higher than a commercial process,
comparable results were obtained with 0.5% MnO2 impurity
which is closer to a commercial figure.
The examples presented herewith should be considered
as illustrative only because they were obtained in a
laboratory reactor rather than a recausticizing plant of a
kraft mill. Higher heights of liquors occur in mill
reactors compared to a laboratory reactor and it is
expected that lower flow rates of oxygen or air will be
required at a mill scale to yield the same extent of
sulfide oxidation.
Various changes may be made to the different
embodiments of the process disclosed herein without
departing from the scope of the present invention which is
limited only by the following claims.