Note: Descriptions are shown in the official language in which they were submitted.
1~398~
Optically active cyclopropanecarboxamides
The present inventlon relates to novel optically
active cyclopropanecarboxamides, to pairs of enantiomers
thereof, to processes for their preparation, and to their use
as agricultural fungicides.
It has already been disclosed that certain N-
benzyl-cyclopropanecarboxamides have agricultural fungicidal
activities. (see Japanese patent Laid-open No. 15,867tl986)
There have now been found novel optically active
cyclopropanecarboxamides and pairs of enantiomers consisting
of said active compounds and enantiomers corresponding
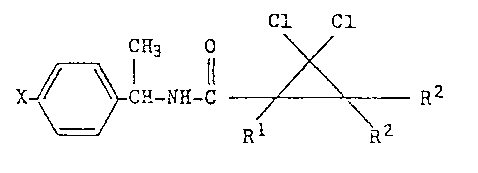
thereto of the formula (I)
X ~ CH-NH-C ~ R2
wherein X represents halogen, and
R1 and R2 each represent hydrogen or C1_6-alkyl.
The compounds of the formula (I) are obtained by a
process in which
*
23189-6577
13~9862
a) in the case where the formula (I) is optically active:
compounds of the formula (II)
X ~ IH-NH2 (II)
(~herein X has the meanins stated above)
are reacted with compounds of the formula (III)
Cl Cl
O \/
M-9 ~ \ R2 (III)
Rl R2
~vherein Rl and R2 have the meanings stated above, and M represents
hydroxyl or hP lo~Dn ) ~
Yith the proviso that at least one of the compounds (II) and (III) must
be opticPlly active,
in the presence of an inert solvent, if appropriate
in the presence of an acid binder,
or
b) the racemic modification having the same formula as
the above formula ~)~ subjected to a physical treatment, or
optically active compounds of the formula(I),either ~ acid
moiety or amine moiety of which is optically active, are
subjected to a physical treatment.
~it- 213 -U S
13398~
The compounds of the formula (I) ac~ording to the invention ha~e
a strong fungicidal action.
Surprisingly, the present compounds o~ formula (I) shou a
fungicidal action substantially far hi8her than that of the known
compounds disclosed in the above-me~tioned Japanese Patent Laid-
open No. 15867/1986, the known compounds havins the sa~e formul~ as
the formula (I), and having a structure wherein both t~e Pcid moiety
and the amine moiety are o~ the racemic configuration. The compounds
according to the in~ention exhibit a great fungicidal action against
pathogenic fung~, particularly a&a~nst rice blast caused by Pyricularia.oryzae.
The other objects and the various advantages of the invention
will be more apparent from the following descr,ptions.
In tne formula (I), X represents halogen, namely fluorine, chlorine,
bromine or iodine;
- Rl a~d.R2 each represent hydrogen or an ~lkyl radical ~ith 1 to 6
carbon atoms, such as methyl, ethyl, n- or iso-propyl, n-, iso-, sec-
or tert-butyl, n-pentyl or n-hexyl.
Preferably, X in the formula (I) represents chlorine or bromine;
Rl represents an alkyl radic~l with 1 to 4 carbon atoms; and
R2 represents hydrogen or an Plkyl radical with 1 to ~ carbon
atoms.
Nit ~ U S
1339862
More preferably, X in the formula (I) represents chlorine or
bromine;
Rl represents methyl, ethyl or isopropyl; and
R2 represents hydrogen or methyl.
In the case of the optically active compounds of the formula (I),
it is preferred that the compounds should have a structure ~herein the
a~ine moiety is of the R(+)-configuration and the acid ~ iety is of the
racemic configuration. More preferably, the amine moiety has a
structure of a mirror-image isomer having an R(+)-configuration.
Example of the compounds of the formula (I) according to the
invention are shown belov.
~it 2~3 . U S
- - 13~9862
Cl Cl
Br~C~ C /X C~3
Compound (a) Epimer , wherein the ~ine moiety is of
the R(+)-configuration;
[~Dl+61.2~ (c=1.02g/lOOml Or ethanol)
Compound (b) Enantiomer, wherein the amine
moiety is of the R(+)-configuration;
[~]~I+6~.2~ (c=1.13g/lOOml of ethanol)
Compound (c) A pair of the optically active isomer
[~]Dl+6~.2~ (c=1.13g/lOOml of ethanol) and
the corresponding er~ntiomer
it 21~ ~ ~ S
- 1339862
Cl Cl
CH3 0 ~
Cl ~ CH-NH-C / ~ ~ CH3
CH3 H3
Compound (d) Epimerst wherein the amine moiety is of
the Rl+)-configuration
Compound (e) Enantiomerj- wherein the amine
moiety is of the R(+)-co~figuration
Compound (f) Pair of the enantiomers
If the starting msterials, emoloyed in the process (a), are
R(+)-4-bromo-~-phenethylamineand l,3,3-trimethyl-2,2-dichloro-cyclo-
- propane carboxylic scid chloride, the reaction may be illustrated by
the rollo~ing scheme.
Cl Cl
; Br ~ CS-~H2 + Cl-C / X CH3
CH3 C~3
Cl Cl
-~Cl , Br ~ Ca-~H-C ~ \CH 3
(amine moiety: R(+); acid ~ iety: +)
~it 21~ - U S
-- 6 --
133g~62
In the formula (II), X has the meaning stated above, and preferabl~J
represents the same radicals as those preferred in the formula (I).
Some of the starting compounds of the formula (II) are already
known in the field of organic chemistry. Examples Or the Compounds
(II) are:
R~+)-4-bromo-~-phenethylamine, and
R(~ chloro-~-phenethylamine.
Next, with regard to the starting compounds of the
formula (III), wherein Rl, R2 and M have the meanings stated above
Rl and R2 of the formula (III) preferably have the same meanings as
those preferred in the formula (I). M preferably represents hydroxyl
or chlorine.
Some of the compounas OI the formula (III) are '~o~ a~d described
in Japanese Pate~t Lal~-open~No. 15867/1986. Examples of
these compounds are 1,3,3-trimethyl-2,2-dichloro-cyclopropane carboxylic
acid and its chloride~
In carrying out the process (a), use may be made of any of inert
organic solvents as the diluent. Examples of the diluents ~re aliphatic,
cycloaliphatic and sromatic, optionslly chlorinated, hydrocarbor.s, such
a~ heYane, cyclohe~ane, petroleum ether, ligroin, benzene, toluene,
lene, methylene chloride, chloroform, carbon tetrachloride,
chlorobenzene, ethylene chloride, and trichloroethylene; ethers, for
example diethyl ether, methyl ethyl ether, diisopropyl ether, dibutyl
~it 213 - ~ S
-- 7 --
1339862
ether, propylene oxide, dioxane and tetrahydrofuran; ketones, for example
acetone, methyl ethyl ketone, methyl isopropyl ketone and methyl
isobutyl ketone; nitriles, such as acetonitrile, propionitrile and
acrylonitrile; alcohols, such as methanol, ethanol, isopropanol, butanol
and ethylene glycol; esters, such as ethyl acetate and amyl acetate;
acid amides such as dimethylform2mide and dimethylacetamide; sulfones
and sulfoxides, s.uch as dimethyl sulfoxide and sulfolane; and bases,
such as pyridine.
When the starting msterials of the formula (III) are the carboxylic
acids, the process (a) may be conducted in the presence of a dehydration-
condensation promoter, for instance, N,N'-dicyclohexyl-carbodiimide.
When the starting materials of the formula (III) are the acid
halides, the process (a) can be carried out in the presence of an acid
binder.
Examples of acid binders are hydr~xides, carbonates,
bicarbonates and alcoholates of ~lkali metals; and tertiary amines such
as triethylamine, diethyl~ ine, pyridine and the like;
In the process (a), the reaction temperature may ~ary within a wide
temperature range. For instance, the process (a) may be carried out at
a temperature of about -20~C to the boiling point of the reaction
mixture, preferably a temperature of about 0 to 100~C. Although the
process (a) may preferably be conducted under normal pressure, it is
al30 po~sible to employ a higher or lo~er pressure.
~I~ 2~ U S
-- 8 --
1~39862
In the process (a), it is possible to employ about 1 mole or a
slightly excess amount of the compound of the formula (III) per 1 mole
of the compound of the formula (II). These starting compounds may ~e
reacted with each other in an inert solvent to obtain a desired optically
active compound of the formula (I).
In the process (b), it is possible to obtain a desired enantiomer
or a pair of the enantiomers by means of oolumn chromatography.
A preferred pair Or enantiomers..of the formLla (I) c~n
easily be obtained by subjecting a corresponding racemic compound to a
column chromatographic operation.
A preferred e~antiome~ of the formLla (I) can easily be
obtained by sub~ecting a corresponding epimer, ~herein the amine moiety
is of the R(+)-configuration, to a column chromato&raphic operation.
The active compounds according to the invention exhibit a po~erful
fungicidal and bactericidal action, so that the compounds can be used
for combating undesirable pathogenic fungi and bacteria.
~ it 2 1 ~ - U S
The active compounds according to the invenl ~9 862
exhibit a powerful microbicidal action and can be employed
in practice for combating undesired microorganisms. The
active compounds are suitable for use as plant protection
agents.
Fungicidal agents in plant protection are employed
for combating Plasmodiophoromycetes, Oomycetes, Chytridiomy-
cetes, Zygomycetes, Ascomycetes, Basidiomycetes and Deutero-
mycetes.
Bactericidal agents are employed in plant
protection for combating Pseudomonoadaceae, Rhizobiaceae,
Enterobacteriaceae, Corynebacteriaceae and Streptomyceta-
ceae.
Some causative organisms of fungal and bacterial
diseases included under the abovementioned main headings,
are mentioned below as non-limiting examples:
Xanthomonas species, such as, for example, Xanthomonas
campestris pv. oryzae;
Pseudomonas species, such as, for example, Pseudomonas
syringae pv. lachrymans;
Erwinia.species, such as, for example, Erwinia amylovora;
Pythium species, such as, for example, Pythium ultimum;
Phytophthora species, such as, for example, Phytophthora
infestans;
Pseudoperonospora species, such as, for example; Pseudopero-
nospora cubensis;
Plasmopara species, such as, for example, Plasmopara
-- 10 --
~it 211 -U S
1339862
viticola;
Peronospora species, such as, for example, Peronospora pisi
or P. brassicae;
Erysiphe species, such as, for example, Erysiphe graminis;
Sphaerotheca species, such as, for example, Sphaerotheca
fuliginea;
Podosphaera species, such as, for example, Podosphaera
leucotricha;
Venturia species, such as, for example, Venturia inaequalis;
Pyrenophora species, such as, for example, Pyrenophora teres
or P. graminea;
~Conidial form: Drechslera, Synonym: Helminthosporium);
Cochliobolus species, such as, for example, Cochliobolus
sativus;
(Conidial form: Drechslera, Synonym: Helminthosporium);
Uromyces species, such as, for example, Uromyces appendi-
culatus;
Puccinia species, such as, for example, Puccinia recondita;
Tilletia species, such as, for example, Tilletia caries;
Ustilago species, such as, for example, Ustilago nuda or
Ustilago avenae;
Pellicularia species, such as, for example, Pellicularia
sasakii;
Pyricularia species, such as, for example, Pyricularia
oryzae;
Fusarium species, such as, for example, Fusarium culmorum;
Botrytis species, such as, for example,.Botrytis cinerea;
~it 2~ -Us
1~39862
Septoria species, such as, for example, Septoria nodorum;
Leptosphaeria species, such as, for example, Leptosphaeria
nodorum;
Cercospora species, such as, for example, Cercospora
canescens;
Alternaria species, such as, for example, Alternaria
brassicae;
PseudocercosporeLla species, such as, for example, Pseudo-
cercosporella herpotrichoides.
Especially, the acti~e compounds according to the inve~tion e.~hibit
a ~e~f powerful fungic~dal actio~ a3ainst a ric~ plant blast-causing
fun~us (~yric~laria orlzae).
The good toleration, by plants, of the active
compounds, at the concentrations required for combating
plant diseases, permits treatment or -above-ground parts of
plants, of vegetative propagation stock and seeds, and of
the soil.
~ ~he acti~e compounds according to the invention h~~e ~ ~ery lov
le~el of toxity to ~arm-bloQded animals, so that the compounds can be
safely used.
~it 21~ . V S
1339862
The active compounds can be converted into the
customary formulations, such as solutions, emulsions,
suspensions, powders, foams, pastes, granules, aerosols,
natural and synthetic materials impregnated with active
compound, very fine capsules in polymeric substances,
coating compositions for use on seed, and formulatLons used
with burning equipment, such as fumigating cartridges,
fumigating cans and fumigating coils, as well as ULV cold
mist and warm mist formulations.
These formulations may be produced in known
manner, for example by mixing the active compounds with
extenders, that is to say liquid or liquefied gaseous or
solid diluents or carriers, optionally with the use of
surface-active agents, that is to say emulsifying agents
and/or dispersing agents and/or foam-forming agents. In the
case of the use of water as an extender, organic solvents
can, for example, also be used as auxiliary solvents.
- As liquid solvents diluents or carriers, there are
suitable in the main, aromatic hydrocarbons, such as-xylene,
toluene or alkyl napthalenes, chlorinated aromatic or
chlorinated aliphatic hydrocarbons, such as chlorobenzenes,
chloroethylenes or methylene chloride, aliphatic
hydrocarbons, such as cyclohexane or paraffins, for example
mineral oil fractions, alcohols, such as butanol or glycol
as well as their ethers and esters, ketones, such as
acetone, methyl ethyl ketone, methyl isobutyl ketone or
Nit 213 -U S
- 1339862
cyclohexanone, or strongly polar solvents, such as
dimethylformamide and dimethyl-sulphoxide, as well as water.
By liquefied gaseous diluents or carriers are
meant liquids which would be gaseous at normal temperature
and under normal pressure, for example aerosol propellants,
such as halogenated hydrocarbons as well as butane, propane,
nitrogen and carbon dioxide.
As solid carriers there may be used ground natural
minerals, such as kaolins, clays, talc, chalk, quartz,
attapulgite, montmorillonite or diatomaceous earth, and
ground synthetic minerals, such as highly-dispersed silicic
acid, alumina and silicates. As solid carriers for granules
there may be used crushed and fractionated natural rocks
such as calcite, marble, pumice, sepiolite and dolomite, as
well as synthetic granules of inorganic and organic meals,
and granules of organic material such as sawdust, coconut
shells, c~rn cobs and tobacco stalks.
As emulsifying and/or foam-forming agents there
may be used non-ionic and anionic emulsifiers, such as
polyoxyethylene-fatty acid esters, polyoxyethylene-fatty
alcohol ethers, for example alkylaryl polyglycol ethers,
alkyl sulphonates, alkyl sulphates, aryl sulphonates as well
as albumin hydrolysis products. Dispersing agents include,
for example, lignin sulphite waste liquors and
methylcellulose.
Adhesives such as carboxymethylcellulose and
natural and synthetic polymers in the form of powders,
Nit 21~ -U 8
- - 1339~62
granules or latices, such as gum arabic, polyvinyl alcohol
and polyvinyl acetate, can be used in the formulation.
It is possible to use colorants such as inorganic
pigments, for example iron oxide, titanium oxide and
Prussian Blue, and organic dyestuffs, such as alizarin
dyestuffs, azo dyestuffs or metal phthalocyanine dyestuffs,
and trace nutrients, such as salts of iron, manganese boron,
copper, cobalt, molybdenum and zinc.
The formulations in general contain from O.l to 95
per cent by weight of active compound, preferably from 0.5
to
~it 2~ S - lS -
-- - 1339862
The active compounds according to the invention
can be present in the formulations or in the various use
forms as a mixture with other known active compounds, such
as fungicides, bactericides, insecticides, acaricides,
nematicides, herbicides, bird repellents, growth factors,
plant nutrients and agents for improving soil structure.
The active compounds can be used as such or in the
form of their formulations or the use forms prepared there-
from by further dilution, such as ready-to-use solutions,
emulsions, suspensions, powders, pastes and granules. They
are used in the customary manner, for example by watering,
immersion, spraying, atomising, misting, vaporising, inject-
ing, forming a slurry, brushing on, dusting, scatterting,
dry dressing, moist dressing, wet dressing, slurry dressing
or encrusting.
In the treatment of parts of plants, the active
compound concentrations in the use forms can be varied
within a substantial range. They are, in general, from l to
0.0001% by weight, preferably from 0.5 and 0.001%~
For the treatment of seed, amounts of active
compound of 0.001 to 50 g, especially 0.01 to 10 g, are
generally employed per kilogram of seed.
For the treatment of soil, active compound
concentrations, at the point of action, of 0.00001 to 0.1%
by weight, especially of 0.0001 to 0.02%, are generally
employed.
The preparation and use of the active compounds
- 16 -
~it 2'~ -U 8
1339862
according to the invention can be seen from the following
examples.
~it 213 -U S - 17 -
1339~62
Preparative Examples:
Example 1
Cl Cl
Br ~ C -NH-C / / ~ \ C~3
CH3 CH3
(amine moiety: R(+) acid moiety: +)
(Compound 1)
4.0 g of R(+)-4-bromo-a-methyl-benzylamine and 2.2 g of triethyl-
amine ~ere dissolYed in 30 ml of tetranydrofuran, and the resulting
solution vas cooled vith ice. To this solution uere added portionwise
a solution of 4.28 g of 1,3,3-trimethyl-2,2-dichloro-cyclo~ropane
carbo~lic acid chloride in 20 ml of tetrahydrofuran under stlrring.
After this addition, the reaction mixture uas brought to room temperature,
stirred for 5 hours, he~ted to 60~C and then stirred for further 2 hours.
The tetrahydrofuran ~as distilled off, and the residue uas mixed with
50 ml Or chloroform. The reaction mixture ~as uashed with the follouing
liquids in this order: uater, a dilute hydrochloric acid, a dilute
aqueous sodium bicarbonate solution, and again ~ater. The chloroform
layer ~as dried o~er Pnhydrous sodium sulfate. The chloroform ~as
distilled off under a reduced pressure, and the residue uas recrystallized
from a sol~ent mixture of 7 parts of n-hexane and 3 parts of ether,
so that 8.38 g Or the epimer, i.e. R~ 4-bro ~ -a-methylbenzylamide
ot ~ 1,3,3-trimethyl-2,2-dichloro-cyclopropane carboxylic acid ~ere
obtained.
~it 213 - U S
- 18 -
1339862
mp. 148 - 159~C
[~]~1+61.2~ (c=1.02g/lOOml of ethanol)
~ e same operation as ExaIlple 1 may be carried out to react R(+)-4-
chloro c,~ lbenzylam~ne with 1,3,3-trin~thyl--2,2-dichloro cyclopr~pane
carboxylic acid chloride, so that the epimer, i.e. R(+)-~-chloro-
~-methylbenzylamide of '-1,3,3-trimethyl-2,2-dichloro-cyclopropane
carboxylic acid can be obtained.
ExP~ple 2
7.0 g of the compound obtained in Example 1 uere sub~ected to a
column chromatos-aphic operation, so that 3.48 g of the desired
e~ntiomer ~ere obtained from the first fraction. This isomer ~as
obtained as a colorless crfstalline material ha~ing a melting point of
1~7 - 148~C and a specific rotation of [Q)DI+64.2~ (c=1.13g/lOOml of
ethanol). This isomer is called "Compound 2".
C ~ - .
Rf= 0.22 IIatroscPn; silica gel; -
- chloroform : ethyl acetate : n-hexane = 4 : 1: 5)
Conditions of chromatogra~hic operation:
Internal diameter : 30 mm; Length ; 500 mm;
B Nitrogen gas preSSUre : 1,o kg/om2;
"Wako-gel" (c-3003 . i50 g
eluent:
n-he.~ane : ethyl acet~te : chloroform = 8: 1 : 1
~ Tr~le-~nar~
Nit 213 - U S
1,~ --
1339862
.
According to the same methc~ as that described above, R(+)-4-
chloro-a-methylbenzylamide of +-1,3,3-trimethyl-2,2-dichloro-cyclopropane
carboxylic acid can be treated to obtain the corresPonding enan-
tiometer.
Exam~le 3
A mixture of racemic compounds, consisting of four stereo-isomers
of 4-bromo-~-methylbenzylamide of 1,3,3-trimethyl-2,2-dichlorocyclopropane
carboxylic acid, waS subjected to a colum~ chromatographlc operation
under the same conditions as in Example 2, so that a pair of the
e~antiomers was obtained in an amount of 0.97 g from the first
fraction, mp. 159.5 - 160.5~C. This compound is called "Compound 3".
By a method cimil~r to that described above, a mixture of racemic
comDounds, which consisted of four stereo-isomers of 4-chloro-~-
methylbenzylamide o~ 1,3,3-trime~hyl-2,2-dichlorocyclopropane c rboxylic
acid was tre.~ted to obtain a pair of the e~ntiomers having a melting
point of 153 - 155~C. This product is r~lled "Compound 4".
The structures and the properties of Compounds 1 - 4 produced
~ according to the invention are sllmm~rized in Table 1.
~it 211 - U S - 20 ~
- 1339862
~ W ~ ,,
L.
Q ~ ~ ~ X
Q Q . Q t~ :~
X 5 5
Q Q Q ~ X
P C ID ~ ~ n ~ ~ I
3 1 ~ , 0 5
O O I' I
0 3 3 ~ -~ Q~O
3 0 0 0 ' C
Q
g
~ tD ~/
3 ~ o /\
~ ~ 3 Q
~ ~ + _ ~ /
~ O
~5 ~3 p, .
CO u U
P
O
o r~ S2 0 ~ R~$ rq
1~ 1--~~ ".
I 1~ + ~ O +:~ -
o o~ w a~
_- 3
O 1~ r~
' O o 1' 0 o
~
W ~o ~
~ I _
_ ~ , 1~ ~ o
~n O ~D ~O
~it 21~ -US
-- 21 --
- 1~39862
~iotest Example:
~no~n comparison compound
A-1:
Cl Cl
Cl ~ CH_NH_C X \ CH3
. CH3 CH3
(according to Japanese Patent Laid-open No. 15867/1986)
Examnle 4
Rice blast test/.foliar treatment
Formul~tion of active com~ounds
Acti~re compound: 50 parts by weight
Car-ier: 45 parts by weight of diatomaceous earth/kaolin mixture
(1: 5 mixture)
Emulsifier: 5 parts by ueight of polyo~ethylene-alkyl-phenyl-
- ether
To prepare a suitable preparation of active compound, the stated
amount of active compound was mixed ~ith the stated amDunt Or carrier
and the stated amount of emulsifier, and the resulting wettable powder
was diluted ~ith ~ater to the desired concentration.
Test procedures
Rice plants (~ariety Asahi~ ~ere gro~n in porcelain pots having
a diameter o~ 12 cm. The rice plants, at the 3 - 4 le2f stage, were sprayed
uith the preparation Or active compound in an amount Or 50 ml per 3 pots.
~I~~ r~ R
~it 21~ - U S
- 22 -
- 1339862
On the ne~t day, the plants ~ere sprayed two times ~ith spore
suspe~sion of r ce blast fungus, Pyricl1laria o~zae (which had been
artifici~lly cultured), and placed in a moisture cha~ber at 25~C and
100% of relative humidity for two days.
Seven days after the inoculation, the infection de~ree of the
rice plants was determined and recorded accordir~ to the following
~sPs.smPnt 9~1 e:
Area wherein the pustules
Infection de~ree ha~e occurred
O O
0.5 2 or less
l 3 - 5
2 6 - l0
3 ll - 20
4 21 - 40
- 5 41 or more,
The percent protection was calculated by e~ploying the formula:
Protection (%) = A x lOO
~herein A represents the infection degree ir. the control
(untreated) section; and
B represents the infection degree in the treated section.
In this test, each test section consisted of three pots.
~it 21~
- 23 -
' - 1339862
The results of this test are given in Table 2.
Table 2
Active compound
Compound concentration Protection
No. (p~m) (%)
~ 1 5 100
100
2 - 5 100
1~0
3 5 100
100
4 5 10
100
~Known)
A-l 5 60
1 13
_
It will be understood that the specification and
examples are illustrative but not limitative of the
present invention and that other embodiments within the
spirit and scope of the invention will suggest themselves
to those skilled in the art.
.
~it 213 - U S
- 24 -