Note: Descriptions are shown in the official language in which they were submitted.
13~0230
DESCRIPTION
TITLE OF THE INVENTION Process for removing heavy metal
compounds from the filter dust from trash ;ncinerators,
flue gas dust precipitators and gas-cleaning units
GACKGROUND Of THE INVENTION
Field of the Invention
Processing of pulverulent residues laden ~ith
pollutants and originating in most cases from combustion
processes. Dust precipitation and gas cleaning have g?ined
importance in conjunction ~ith regulations for protection
of the environment. As a result, more stringent demands
have to be met also by the associated further process;ng
and storage of the pollutants and their carriers.
The invention relates to the further development,
improvement and simplification of the separation of pol-
lutants, vhich have to be treated separately, from a com-
lex particle mixture arising in combustion processes.
In particular, it relates to a process for removing
heavy metal compounds from the filter dust from trash in-
cinerators, flue gas dust precipitators and gas-cleaning
units by vaporisation, separation and subsequent conden-
sation and/or sublimation of the heavy metal compounds.
Discussion of background
Filter dust from industrial combustion installa-
tions, specifically electrostatic precipitator dust, hasa high content of harmful substances ~hich contaminate
the surroundings, pollute the environment and in some
- 2 - 1340230
cases are toxic. These include certain organic compounds
and above all heavy metal compounds, for example of the
elements Pb, Zn, Cd, Tl and Cu. Special regulations
therefore apply to such mixtures, and the filter dust
must be regarded and treated as special waste.
At present, the treatment is carried out in
accordance with 3 principles:
- Agglomeration and compaction of the dust to give
larger pieces by means of binders such as cement,
asphalt and the like. This is followed by final
deposition in a dump [cf."Behandlung und Verfest-
igung von Ruckstanden aus Kehrichtverbrennungs-
anlagen (Treatment and solidification of residues
from trash incinerators]" Schriftenreihe Umwelt-
schutz [Protection of the Environment Publica-
tions] No. 62, Bundesamt fur Umweltschutz [Federal
office of the environment], Berne 1987). In this
case, a product is formed, the mass of which is
greater than that of the loose starting material.
Final deposition is therefore expensive and raises
problems because the space available is in most
cases restricted.
- Chemical treatment with acids. The heavy metal
compounds are leached out with aqueous acids. The
heavy metal-free residue is deposited in a dump and
the acid solutions containing heavy metals are
processed further (cf. H. Vogg "Rauchgas-
reinigungsverfahren [Flue gas cleaning processes]",
paper read at the VDI seminar 43.32.02 "Abfallbe-
handlung und -verwertung durch Mullverbrennung
[Waste treatment and utilization by trash
incineration]" VDI, Dusseldorf 1986). These
leaching processes are not true solutions of the
waste problem, but merely shift it to a different
plane. The further treatment of the heavy metal-
containing solutions and their concentration
- 2a - 1~230
involves considerable consumption of energy. New
problems (effluent, pollution of the environment)
are created.
- Thermomechanical separation by heating of the dust
laden with heavy metals in a gas stream,
vaporization of the heavy metal compounds and
mechanical separation of the solid suspended
particles from the gas phase at
. .. . .
13~0230
high temperatures (hot-gas cyclone, hot-gas filter,
etc.). The heavy metal vapors are then condensed in
a cooler (cf. W. Weissweiler et al, "Anreicherung
von Thallium-und Bleihalogeniden [Concentrating of
thallium and lead halides]", Staub, Reinhaltung der
Luft, volume 46, No. 3, pages 120-124, March 1986).
The hot-gas separators (cyclones, electrostatic
precipitators, ceramic honeycomb filters, etc.) must
operate isothermally at high temperatures (1000~C
and higher), which makes severe demands on their
design and their operation. Moreover, separation of
the fine dust (less than lum particle diameter),
which is disproportionately important as a carrier
of the heavy metal compounds in percentage terms,
can hardly be accomplished by economical means.
SUMMARY OF THE INVENTION
Accordingly, one object of this invention
is to provide a novel process for removing heavy
metal compounds from the filter dust from combustion
plants of any type, which process, while utilizing
the principle of vaporization and subsequent conden-
sation, avoids the difficult and extensive mechani-
cal separation of pollutant vapor and dust particles
at high temperatures (hot-gas filtration, hot-gas
separation) and as far as possible suppresses a
later reattachment of the condensed pollutants to
the fine dust particles. The process should be sim-
ple and ensure the most economical individual
further processing of the various separated products
for final deposition.
In accordance with the present invention,
there is thus provided a process for removing heavy
metals and/or heavy metal compounds from the filter
dust of refuse incinerators, flue gas dust precipit-
ators and gas-cleaning units, in which the filter
. _ _ . . . . .. . -- . . . . .. . .
- 3a -
'~- 1340230
dust is heated in a reaction vessel at least up to
the vaporization temperature of the heavy metals
and/or heavy metal compounds which are to be
removed, the vaporized heavy metals and/or heavy
metal compounds are then quenched in a cooler and
converted to a liquid or solid state, and removed.
The process of the invention is characterized in
that before the vaporization of the heavy metals
and/or heavy metal compounds to be removed,
superfine dust particles with a particle size
smaller than 5~m are separated in a cold state from
the filter dust by mechanical, hydraulic or
pneumatic means or by filtration, and the remaining
filter dust fraction is fed to the reaction vessel.
BRIEF DESCRIPTION OF THE DRAWINGS
A more complete appreciation of the
invention and many of the attendant advantages
thereof will be
134023~
-- 4
readily obtained as the same becomes better understood
by reference to the following detailed description when
considered in connection with the accompanying drawings,
wherein:
Figure 1 shows a flow diagram of a discontinuous
process for removing heavy metal compounds by means of
a carrier gas;
Figure 2 shows a diagrammatic illustration of
equipment for discontinuous removal of heavy metal
compounds;
Figure 3 shows a flow diagram of a continuous
process for the removal of heavy metal compounds by
means of a carrier gas;
Figure 4 shows a flow diagram of a discontinuous
process for the removal of heavy metal compounds with
application of reduced pressure, and
Figure 5 shows a flow diagram of any desired
process for the removal of heavy metal compounds by
separating the fine dust off beforehand by means of a
carrier gas.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
Referring now to the drawings, wherein like
reference numerals designate identical or corresponding
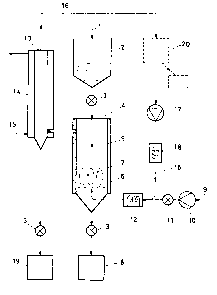
parts throughout the several views, Figure 1 shows the
flow diagram of a discontinuous process, operating
batchwise, for removing heavy metal compounds from
filter dusts by means of a fluidizing carrier gas. 1 is
the raw dust supply, indicated by an arrow, to the dust
silo 2 which is closed at the bottom by a periodically
operable gate valve 3 for solids. 4 is a reaction vessel
for high temperatures (about 1000~C, sometimes even
higher) which is provided with heat insulation 5 (if
appropriate, with additional heating 24, cf. Figure 2)
and which contains a sieve tray 6 ~displaceable) in its
interior at the lower end. In operation, a fluidized
layer 7 (fluidized bed, fluid bed) is generated above
~ 1~230
- 4a -
the sieve tray 6 (indicated by arrows). The lower,
conical end of the reaction vessel 4 is connected via
a gate valve 3 to a tank 8 which is to receive the
purified dust. 9 represents the fresh
-
13~0230
carrier gas (inlet indicated by an arro~). 10 is a fan
for conveying the carrier gas 9, and 11 is a corresponding
gas isolation valve. 12 represents a heater tup to
about 1200~C) for the gas stream partially laden ~ith
particles. This heater 1Z is advantageously provided ~ith
a contro~ system, since it largely determines the thermo-
dynamics in the reaction vessel 4. 13 is a cooler sur-
rounded by a cooling jacket 14 and having a greater length
as compared ~ith the cross-section. The coolant 15 (in
most cases water) enters at one end of the cooling jacket
14 and leaves at the other end (indicated by arro~s). 16
represents the circulating gas ~hich still contains cer-
tain fractions of dust particles held in suspension. 17
is the circulation pump (fan) and 18 is the preheater
(up to about 800~C) for the circulating gas 16. 19 is a
tank ~hich is to receive the heavy metal compounds and
~hich is connected via the discontinuously operable gate
valve 3 to the lo~er conical end of the cooler 13.
Figure 2 relates to a diagrammatic representation
of equipment for the discontinuous removal of heavy metal
compounds from filter dusts by processes according to ~
Figure 1. 3 are the conventional, discontinuously (batch-
~ise) operable gate valves for solids and 4 is the reac-
tion vessel for high temperatures, ~hich is provided with
a heater 24 and heat insulation 5 in the form of approp-
riate casings. 6 represents a sieve tray which is here
designed as a t~in tray ~ith a laterally disp~aceable
lover part (indicated by double arro~). The gas stream
can be regulated in this ~ay. After the batch has been
completed, the reaction vessel 4 can be partially opened
for discharging the purified dust. 7 is the fluidized bed
marked by arro~s. 25 is the ra~ dust feed uhich takes
place in the upper part of the reaction vessel 4 through
a central pipe. 21 is the carrier gas, 22 is its feed
line, 23 is the blast box of the reaction vessel 4, 16 is
the circulating gas taken from the cooler 13, and 14 is
the cooling jacket through ~hich the coolant 15 flo~s.
It must be ensured that the cooler 13 is connected to the
reaction vessel 4 by a line ~hich is as short as possible.
.. ~ . .. . .. , . . .. ... ., . ~
1340230
-- 6
in practice, the former immediately adjoins the latter,
so that there is no undesired condensation of the vapors
of the heavy metal compounds in the connecting line. For
the sake of clarity, 4 and 13 are shown separately in
the drawing.
Figure 3 shows a flow diagram of a continuous
process for removing heavy metal compounds from filter
dusts by means of a fluidizing carrier gas. The
reference numerals 1, 2, 3, 8 and 19 and their meanings
correspond exactly to those of Figure 1. 9 is the fresh
carrier gas which is delivered by the fan 10 and is
passed via the valve 27 and the heater 12 into the
reaction vessel 4 provided with the heat insulation 5.
The valve 27 can be designed as a non-return valve,
differential valve or a controlled or regulated valve.
The lower end of the reaction vessel 4 is directly
adjoined by the cooler 13 which is surrounded by the
cooling jacket 14 and is of very slender design. Water
is used in most cases as the coolant 15. 28 represents
the additives feed which may be necessary or desirable.
These can have a physical or chemical action. In most
cases, they are materials which lower the vaporization
temperature, such as metal halides, NH4Cl, etc. or
reducing agents such as C, CO, etc.
Figure 4 shows a flow diagram of a discontinuous
process for removing heavy metal compounds from filter
dusts by the application of reduced pressure ("vacuum").
The reference numerals 1, 2, 3, 8 and 19 correspond
exactly to those of Figure 1. The reaction vessel 4,
provided with a heater 24 (up to about 1200~C) and a
heat insulation 5, has at the lower end a conical part,
is designed to be vacuum-tight and can be sealed from
the surroundings by vacuum-tight gate valves 3. The
reaction vessel 4 is connected via a short duct directly
to the vacuum-tight cooler 13 which is provided with a
cooling jacket 14 (coolant 15) and which can be
1340230
- 6a -
connected from time to time via the likewise vacuum-
tight gate valve 3 to the heavy metal compounds tank 19.
At the upper outlet of the cooler 13, there is a vacuum-
tight three-way valve 29, whose normal operating
position (as drawn) is such that it opens
~ 13~0230
-- 7 --
the ~ay from the cooler 13 via a filter 30 (fine filter)
to a vacuum pump 3Z. The filter 30 is connected to a fine
dust ~filter dust) tank 31. From time to time, a temor-
ary air feed 33 (dashed arrow) for flooding the unit can
be effected via the three-~ay valve,29.
Figure S relates to a flow diagram of any desired
process for the removal of heavy metal compounds from
filter dusts by separating the fine dust off beforehand
by means of a carrier gas. 1 is the ra~ dust supply to
the dust silo 2 which is connected by a pipe to a m;xer
34. 9 is the carrier gas ~hich is delivered by the fan
10 into the mixer 34. In the latter, intense turbulence
and fluidization of the dust particles takes place. The
fluidized gas/dust mixture passes into the separator 35
~here the coarser fraction (in the present case of parti-
cle diameter > S ~m) is separated off as dust 36 and pas-
sed into a unit 37 (for example according to Figures 1 to
4) for removing heavy metal compounds. The separator
~ 35 can consist of a filter (for example an electro-
I Z0 static precipitator) or of a cyclone. Carrier gas ~
_ dust (finer fraction of a particle diameter of < S ~m)'38leave the separator 35 and are delivered to the unit 39
for further processing. This can in principle consist of
a trash incinerator, a fine filter or a scrubber.
Embodiment example 1:
See Figures 1 and 2.
In a small pilot unit for batch~ise operation,
filter dust from a trash incinerator ~as treated. The re-
action vessel 4, consisting of a ceramic material and pro-
vided vith a sieve tray 6, had a cylindrical shape. Itsinternal diameter ~as 50 mm and its height (internal,
cylindrical part) ~as 200 mm. The cooler 13 consisted
of a double-~alled pipe of stainless steel, having an inter-
nal diameter of 10 mm and a length of 1000 mm. ~ater
flo~ed as the coolant through the cooling jacket 14.
The filter dust contains inter alia, the following
metals:
~ , . . ..
- 8 - 13 40 230
Ca: 11.5 wt.%
Cu: 0.09 wt.%
Zn: 3.3 wt.%
Pb: 0.8 wt.%
Cd: 0.05 wt.%
Sn: 0.34 wt.%
Sb: 0.16 wt.%
Ba: 0.34 wt.%
The reaction vessel 4 was charged with
10 g of filter dust and heated to a temperature of
1000~C. At this temperature, the dust was fluidized
by means of a stream of nitrogen as the carrier gas
9 and held in suspension in the fluidized bed 7. The
major part of the heavy metal compounds vaporized in
the reaction vessel 4 and was continuously condensed
in the cooler 13. The precipitated metal salts were
here only slightly contaminated by superfine parti-
cles entrained by the gas stream. After 1 hour, the
process was complete and the reaction vessel 4 was
cooled. The filter dust was analyzed before and
after the thermal separation of the heavy metal com-
pounds. This gave the following picture:
Before After
separation separation
Total quantity 10.0 g 8.0 g
Zn content 3.3 wt.% 0.3 wt.%
Pb content 0.8 wt.% 0.4 wt.%
Cd content 0.05 wt.% 0.005 wt.
Moreover, samples of the filter dust
before and after thermal separation were leached
with CO2-saturated water and the solutions thus
obtained were examined for their heavy metal ion
content:
Heavy metal Before After
separation separation
(mg/l of solution) (mg/l of solution)
zn2+ 1600 63
1~023~
pb2+ 13 7.8
Cd2+ 37 2.4
Embodiment example 2:
See Figures 1 and 2.
In a pilot unit, filter dust from a trash
incinerator was treated similarly as in example 1.
The unit was of similar structure as in example 1,
but had greater dimensions.
The reaction vessel 4 was charged with
1000 g of filter dust and held at a temperature of
1000~C for 3 hours. Instead of nitrogen, the acid
off-gas of a trash incinerator was used as the
carrier gas 9. After the charge had been completed,
the reaction vessel was cooled and the residue was
examined. The result was as follows:
Before After
Analyzed separation separation
total quantity 10.0 g 8.2 g
Zn content 3.3 wt.% 0.02 wt.%
20 Pb content 0.8 wt.% 0.3 wt.%
Cd content 0.05 wt.% 0.003 wt.%
In addition, samples of the filter dust
before and thermal separation were leached with CO2-
saturated water, and the solutions thus obtained
were examined for their heavy metal ion content:
Heavy metal Before After
separation separation
(mg/l of solution) (mg/l of solution)
zn2+ 1600 1.3
Pb2+ 13 2
Cd2+ 37 < 1
This shows that it was possible, by using
a chemically active gas instead of the largely inert
nitrogen, to achieve a better yield of heavy metal
removal from the filter dust.
Embodiment example 3:
See Figure 3.
- lO- 13~02~0
In a pilot unit for continuous operation,
filter dust from a trash incinerator was processed.
The reaction vessel 4 consisted of refractory
ceramic material and had a casing-like heat insula-
tion 5 consisting likewise of ceramic material andglass wool. The cylindrical interior had a diameter
of 100 mm and a height of 500 mm. The cooler 13,
provided with a cooling jacket 14 carrying a water
flow, was of cylindrical shape and had an internal
diameter of 20 mm and a height of 1600 mm.
The filter dust was injected continuously
into the reaction vessel 4 and fluidized by carrier
gas 9 preheated to 1400~C (heater 12). Analogously
to example 2, the carrier gas consisted of acid off-
gas from a trash incinerator. In addition, 5 wt.% ofNH4Cl, relative to the quantity of the filter dust,
was injected as additive (25) into the reaction
vessel 4. The throughput of filter dust was about l
g/second, and that of carrier gas was about l
dm3/second, relative to the standard state. This
gave a flow velocity of about 0.55 m/second in the
reaction vessel 4 at a temperature of 1000~C, and a
flow velocity of about 12.5 m/second at the inlet
and about 5 m/second at the outlet of the cooler 13.
The downstream separator 26 was designed as a
cyclone and constructed and adjusted in such a way
that the coarser fraction was separated off down to
a particle size of 5um with a separation efficiency
of at least 95%.
Before and after the separation, the dust
was examined as in the preceding examples. The
result was:
Before After
Analyzed separation separation
total quantity 10.0 g 8.4 g
Zn content 3.3 wt.% 0.3 wt.%
Pb content 0.8 wt.% 0.1 wt.%
~.~
11 - 1~ 40 230
Cd content 0.05 wt.% 0.0005 wt.%
At the same time, samples of the filter
dust were leached according to example 1, and the
solutions thus obtained were examined for their
heavy metal ion content:
Heavy metal Before After
separation separation
(mg/l of solution) (mg/l of solution)
zn2+ 1600 43
Pb2+ 13 O.g
Cd2+ 37 0.2
Embodiment example 4:
See Figure 3.
In a unit for continuous operation, filter
dust from a trash incinerator was treated. The re-
action vessel 4 consisted of refractory ceramics and
had a heat insulation 5 in the form of a casing,
likewise consisting of ceramic material and slag
wool. The cylindrical interior had a diameter of
200 mm and a height of 800 mm. The cooler 13,
consisting of stainless steel, was built up from 3
parallel-connected cylindrical pipes each of 25 mm
internal diameter and 1800 mm length.
The carrier gas 9 used was nitrogen at a
rate of 4 dm3/second (relative to standard state)
which was heated in the heater 12 to a temperature
of 1300~C, in order to release an excess of sensible
heat to the charge and to cover all heat losses. The
filter dust, which was not preheated, was added to
the reaction vessel at a rate of about 1 g/second.
At the same time, a rate of about 0.05 g/second of
coke powder (85 wt.% C) was injected, which amounted
to about 4 wt.% C relative to the filter dust. The
separator 26 in the form of an electrostatic pre-
cipitator had a separation efficiency of 92% forparticles down to 3um diameter. The fine fraction
was recycled via the circulation pump 17 into the
- 12 - 13 40 23q
circulation. The dust was analyzed before and after
the separation:
Before After
Analyzed separation separation
5 total quantity 10.0 g 9.1 g
Zn content 3.3 wt.% 0.1 wt.%
Pb content 0.8 wt.% 0.005 wt.%
Cd content 0.05 wt.% not detectable
The samples of filter dust were leached
according to example 1, and the solutions obtained
were examined for their heavy metal ion content:
Heavy metal Before After
separation separation
(mg/l of solution) (mg/l of solution)
Zn2+ 1600 8
pb2+ 13 Not detectable
Cd2+ 37 Not detectable
Embodiment example 5:
See Figure 3.
Filter dust from a trash incinerator was
treated in the pilot unit according to example 3.
The filter dust was continuously injected
into the reaction vessel 4 and fluidized. The
carrier gas 9 used was nitrogen preheated to 1200~C
by means of the heater 12. At the same time, 3% by
volume of CO, relative to the volume of nitrogen,
were injected as additive 28 into the reaction
vessel 4. As a result, a reactive (reducing) mixture
was formed. The rate of filter dust per gas volume
was about 150 g/m3, and the throughput of filter
dust was about 0.15 g/second, and that of carrier
gas was about 1 dm3/second. The mean flow velocity
in the reaction vessel 4 at a temperature of 1000~C
was about 0.5 m/second, and that in the cooler 13
was about 12 m/second at the inlet and about
5 m/second at the outlet. A cyclone was used as the
separator 26.
- 13 - 134023~
The dust was examined before and after the
separation, analogously to the preceding examples.
The following values were obtained:
Before After
5 Analysed separation separation
total quantity 10.0 g 8.2 g
Zn content 3.3 wt.% 0.5 wt.%
Pb content 0.8 wt.% 0.4 wt.%
Cd content 0.05 wt.% 0.005 wt.%
In addition, samples of the filter dust
were leached with a CO2-saturated aqueous solution,
and the lyes were examined for their heavy metal ion
content:
Heavy metal Before After
separation separation
(mg/l of solution) (mg/l of solution)
Zn2+ 1600 122
pb2+ 13 4.2
Cd2+ 37 4.1
Embodiment example 6
In a pilot unit for continuous operation,
electrostatic precipitator ash from a trash
incinerator was treated. Unlike Figure 3 the filter
ash was first fluidized with nitrogen (throughput
about 0.14 dm3/second) in a mixer and then passed
into an electrically heated reaction furnace. In the
latter, the fluidized mixture was brought to a
temperature of 1100~C and held at this temperature
for a residence time of 3 seconds. On this occasion,
the major part of the heavy metal compounds was
vaporized and passed into the gas phase. The
gas/particle mixture then flowed through a hollow
cylindrical cooler of 10 mm internal diameter, on
the inner wall of which the heavy metal compounds
condensed. The cooled gas stream, laden with
particles and largely free of heavy metal compounds,
J
- 14 - 1340230
was passed into a dust filter, where the dust was
separated out.
The analysis gave the following picture:
Before After
5 Analyzed separation separation
total quantity 8.0 g 6.5 g
Zn content 3.3 wt.% 0.07 wt.%
Pb content 0.8 wt.% 0.02 wt.~
Cd content 0.05 wt.% 0.001 wt.%
Samples of the filter dust were leached
with CO2-saturated water. This gave the following
heavy metal content of the solution:
Heavy metal Before After
separation separation
(mg/l of solution) (mg/l of solution)
zn2+ 1600 45
pb2+ 13 0 9
Cd2+ 37 0.8
Embodiment example 7:
See Figure 4.
In a small pilot unit for discontinuous
batchwise operation, filter dust from a trash
incinerator was processed. The reaction vessel 4
consisting of ceramic material had a hollow cylin-
drical shape with a conical bottom. It was fittedwith heating 24 and a casing-shaped heat insulation
5. The internal diameter was 40 mm and the height
was 240 mm. The cooler 13 consisted of a double-
walled pipe of 6 mm internal diameter and 1500 mm
length. The cooling jacket 14 was charged with water
as the coolant 15.
The charge consisted of 100 g of filter
dust, which was heated in the reaction vessel 4 to
1000~C. At the same time, the entire unit was evacu-
ated slowly via the three-way valve 29 by means of
the vacuum pump 32 at a pressure drop of
10 kPa/minute down to a final pressure of l kPa
.. , .. . . ~, . ..
- 15 - 134~230
(about 0.01 bar). This step thus took about 10
minutes. The charge was kept in this state for about
1 hour. During this time, the heavy metal compounds
largely vaporized under the reduced pressure and
condensed or sublimed in the slim cooler 13, where
they were discharged via the vacuum-tight gate valve
3, after the batch has been completed. Air was then
admitted to the unit via the three-way valve 29, the
dust residue was discharged from the bottom of reac-
tion vessel 4 and the latter was charged with thenext batch.
The fine dust entrained by the vapor
stream was kept away from the vacuum pump 32 in the
filter 30 and discharged from time to time.
The dust was analyzed before and after the
separation, the following picture being obtained:
Before After
Analyzed separation separation
total quantity 10.0 g 8.4 g
20 Zn content 3.3 wt.% 0.4 wt.%
Pb content 0.8 wt.~ 0.4 wt.%
Cd content 0.05 wt.% 0.005 wt.%
Samples of the filter dust were leached
with CO2-saturated water. The heavy metal content of
the solutions was found to be as follows:
Heavy metal Before After
separation separation
(mg/l of solution) (mg/l of solution)
zn2+ 1600 87
Pb2+ 13 6.4
Cd2+ 87 1.7
Embodiment example 8:
See Figure 5.
Filter dust from a trash incinerator was,
before removal of the heavy metal compounds,
mechanically treated in such a way that it was
- 15a - 1340230
divided into a coarser fraction and a finer frac-
tion.
Fresh carrier gas 9 was injected into the
mixer 34 by means of a fan 10. The throughput was
about 0.55 dm3/second. Filter dust from the dust
silo 2 was fed to the mixer, so that a suspension of
about 100 g of dust/m3 was formed. The mixture
fluidized in this way was conveyed in the cold state
through a cyclone 35, where the coarser fraction of
more than 5um particle diameter was precipitated.
This fraction represented about 98% of the mass of
the total dust. It was fed directly to a unit 37 for
removing heavy metal compounds - in the present case
according to Figure 3. The dust 38 passing through
the cyclone 35 and conveyed by the carrier gas still
amounted to only about 2% by weight of the mass of
the original dust and was passed into a unit 39 for
further processing. This dust was precipitated in a
superfine filter and further treated separately.
The invention is not restricted to the
embodiment examples. The filter dust is heated in
the reaction vessel up to the vaporization tempera-
ture of the heavy metal compounds, which are to be
removed, at the prevailing partial pressure and the
resulting vapor is separated purely thermally from
the dust particles, by quenching the mixture and
converting the steam by condensation or
- 16 - 13~ 2~0
solidification or sublimation into the liquid or solid
state. In the process ~ith fluidization by means of a
gaseous medium, at least a part of the carrier gas is re-
cycled as circulating gas in the cyclic process to the
charging point of the filter dust. The carrier gas is
advantageous~y preheated in such a ~ay that the entire
heat requirement - apart from losses - is covered by its
sensible heat. In this ~ay, high heat transfer coeffic-
ients for heating of the charge are achieved. The proce-
dure can in principle be continuous or discontinuous,in the form of batches. In the latter case, it is also
possible to operate batch~ise ~ithout carrier gas under a
greatly reduced partial pressure of the heavy metal com-
pounds, ~hich are to be vaporized, or in vacuo. In this
~ay, the vaporization temperature can be substantially
lo~ered, the vaporization process can be accelerated and
the yield of heavy metal compounds in the condensate/sub-
limate can be improved. Solid, liquid or gaseous substances
can additionally be added in principle to the filter dust
in all processes in the reaction vessel, by admixing the
additives to the dust itself or to the carrier gas, if~
any, or introducing them directly into the reaction ves-
sel. These substances have a physical and/or chemical
action on the charge, by accelerating the vaporization of
ZS the heavy metal compounds as a result of reducing the va-
porization temperature, converting the heavy metals into
more volatile substances, and the like. In an advantageous
manner, metal halides, ammonium halides or reducing agents,
preferably carbon or carbon monoxide, are added. It is
of advantage to split the filter dust, before the remova~
of the heavy metal compounds, in the cold state purely
mechanically to a coarser fraction and a finer fraction
tfor example less than 5 ~m particle size) and separately
to treat each fraction further. The separation can be
carried out by mechanical, pneumatic or hydraulic means
with the aid of cyclones, electrostatic precipitators,
dry filters, scrubbers and the like. The coarser fraction
is taken to the heavy metal removal unit, and the finer
fraction is taken to the unit for concentration,
. .~ , ~,
1340230
~ - 17 -
agglomeration, briquetting and dumping (final deposition)
and the like.
Obviously, numerous modifications and variations
of the present invention are possible in the light of the
S above teachings. It is therefore to be understood that,
~ithin the scope of the appended claims, the invention
may be practiced other~ise than as specifically described
herein.