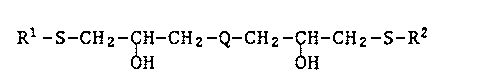Note: Descriptions are shown in the official language in which they were submitted.
o293
Additives for functional fluids
The present invention relates to novel compounds containing thioether
groups, to functional fluids of the series of lubricants, such as
lubricating oils and greases, hydraulic fluids and metalworking fluids
comprising compounds containing thioether groups, and to the use of these
compounds as additives.
Various additives are generally added to functional fluids to improve
their performance properties. Since, for example, lubricating oils must
possess a high load-bearing capacity for the transference of more
powerful forces, they are treated by the so-called extreme-pressure and
antiwear additives, which treatment markedly reduces the wear phenomena
which would otherwise occur. On the other hand, when oxygen and moisture,
for example, jointly act on a metal surface, corrosion may take place,
for which reason corrosion inhibitors are added. The oxidation reactions,
for example, which occur under the action of atmospheric oxygen and do so
to a much greater degree at elevated temperatures, can be inhibited by
the addition of antioxidants. It is known that some substances used as
additives for lubricating oils can combine several such characteristics;
they are referred to as multipurpose additives. Such substances are
naturally much sought after on economic and practical grounds.
US-PS 4,246,127, for example, discloses disulfides and polysulfides andtheir use as lubricant additives. EP-A 0,166,696 furthermore discloses
compounds containing thioether groups and compositions thereof with
lubricants and hydraulic fluids.
- 2 - 1 3 ~ 0293
Compound~ containing thioether groups have now been found which in
compositions with lubricants, hydraulic fluids or metalworking fluids
possess quite outstanding properties and which in combination, for
example, with lubricating oils distinctly enhance the performance of the
latter compared with compounds described earlier.
The present invention relates to compounds of the formula
R1-5-CHz-ÇH-CHz-Q-CH2-CIH-CH2-s-R2 (I)
H 11
in which R1 and R2 are identical or different and are tert-C4 to
tert-C7alkyl or C5 or C6cycloalkyl, and Q is -S~ S-S-,
--S-CH2-S- or -S-CH2--CH2--S--.
The compounds are expediently of the formula I, where R1 and R2 are
identical. In the formula I, R1 and R2 are preferably tert-butyl or
cyclohexyl.
Particularly preferred compounds are
tert.-Butyl-S-CHz-Ç}1-CH2-S-CH2-~H-CHz-S-tert.-Butyl (III),
H H
tert.-Butyl-S-CHz-ÇH-CHz-5-S-CHz-~H-CHz-S-tert.-Butyl (IV),
H H
tert.-Butyl-S-CHz-CI~-CH2-S-CI12-S-CI12-C~-CI12-S-tert.-Butyl (V),
0~ 0
tert.-Butyl-S-CHz-ÇH-CH2-S-CH2-C112-S-CH2-ÇH-CH2-S-tert.-Butyl (VI),
OH OH
S-CHz~ÇH~CH2~S-CHz~ÇH~CH2-S~-/ \- (VII),
._. ._.
~\ /--S-Cli2-~CH-CI12-S-S-CH2-ÇII-C112-S--~ /- (VIII),
~\ /--s-cH2-çH-cH2-s-cl~2-s-cl~2-~l~-cl~2-s--\ /- (IX),
~ 3 ~ 1 ~ 40 293
~ . . .
~/ \~--S--CH2--8H--CH2--S--CH2--CH2--S--CH2--8H--CH2--S--~/\. (X),
CH2-s-cH2-cH-cH2-s-cH2-8H-cH2-s-cH2
H3C\i\ i i /i~CH3 (XI),
H3C/ '!' '!' \CH
CH2-S-CHz-8H-CH2-S-S-CH2-8H-CH2-S-CH2
H3C\i\ i i /i~CH3 (XII),
H3C/ '!' '!' \CH
/CHz-S-CH2-8H-CH2-S-CH2-S-CH2-8H-CH2-S-CH2\
H3C\i\ i ~/ \i/CH3 (XIII)
H3C ~ \1/ CH3
and
CH2-S-CHz-CH-CH2-S-CH2-CH2-S-CH2-8H-CH2-S-CH2
H3C\i\ i i /i~CH3 (XIV).
H3C ~/ \1/ \CH
The present invention also relates to compositions which comprise
a) a functional fluid of the series of lubricants, hydraulic fluids and
metalworking fluids, and
b) at least one compound of the formula
Rl-S-CHz-8H-CH2-Q-CH2-8H-CH2-S-R2 (I)
H H
in which Rl and R2 are identical or different and are C3 to C7alkyl,
Cs to Clocycloalkyl or pinane-10-yl, and Q is -S-, -S-S-, -S-CHz-S- or
-S-CHz-CH2-S-.
1~4~293
-- 4 --
The present invention relates especially to compositions which comprise
a) a lubricating oil of the series of mineral oils, synthetic oils or
mixtures thereof, and
b) at least one compound of the formula I.
The compositions expediently comprise at least one compound of the
formula I, in which Rl and R2 are tert-C4 to tert-C7alkyl or Cs or
C6cycloalkyl. These compositions expediently comprise at least one
compound of the formula I in which Rl and R2 are identical.
The compositions preferably comprise at least one compound of the
formula I in which Rl and R2 are both tert-butyl or cyclohexyl.
Compositions which are particularly preferred comprise at least one of
the compounds of the formulae
tert.-Butyl-s-cH2-8H-cH2-s-cH2-8H-cH2-s-tert--Butyl (III),
H H
tert.-Butyl-s-cH2-8H-cH2-s-s-cH2-8H-cH2-s-tert--Butyl (IV),
H H
tert.-Butyl-S-CHz-C~-CH2-S-CH2-S-CH2-C~-CH2-S-tert.-Butyl (V),
O O
tert-~BUtYl~S~CH2~8H~CH2-S-CH2-CH2-S-CH2-8H-CH2-S-tert.-Butyl (VI),
H H
\ _ / S CHz 8H CH2-S-CH2-8H-CH2-S--~ \- (VII),
.\ /.-s-cH2-8H-cH2-s-s-cH2-8H-cH2-s-.\ /. (VIII),
~-- H H ~--
~/ \--s-cH2-8H-cH2-s-cH2-s CHz 8H CH2 S \ _ / (IX),
~/ \~--S--CH2--8H--CH2--S--CH2--CH2--S--CH2--8H--CH2--S--~/ \~ (X),
- 5 ~ 1340293
/CHz-S-CH2-8H-CH2-S-CH2-8H-CH2-S-CH2\
H3C\i\ i i /i~CH3 (XI),
H3C ~ ~ CH3
/CH2-S-CH2-8H-CH2-S-S-CH2-8H-CH2-S-CH2\
H3C\i\ i i /i~CH3 (XII),
H3C \-/ \1/ \CH3
/CHz-S-CH2-~H-CH2-S-CH2-S-CH2-8H-CH2-S-CH2~
H3C\i\ i i /i~CH3 (XIII)
H3C ~ ~ CH3
or
/CH2-S-CH2-8H-CH2-S-CH2-CH2-S-CH2-8H-CH2-S-CH2\
H3C\i\ i i /i~CH3 (XIV).
H3C ~ ~ CH3
Rl and R2 in the formula I independently of one another are an alkyl
group having 3 to 7 carbon atoms and can be, for example, a tert-butyl or
a pentyl, hexyl or heptyl group, it being possible also for the last
three groups to contain a tertiary substituted carbon atom.
Rl and R2 in the formula I independently of one another are a cycloalkyl
group having 5 to 10 carbon atoms and can be, for example, a cyclopentyl,
cyclohexyl, cycloheptyl, cyclooctyl, cyclononyl or cyclodecyl group.
Preferred is a cyclohexyl group. Rl and R2 in the formula I independently
of one another are for example pinane-10-yl having the formula
!cH2-
H 3 C~ i / \i
H3C
The preparation of the compounds according to the present invention is
carried out in a manner known per se.
~340293
-- 6 --
The preparation of the alkyl thiaglycidyl ethers employed as intermediate
for the compounds of the formula I, is performed in the following manner:
R-SH + Cl-CH2-C~ /CHz -NaCl/-H 0
R-S-CH2-C~--/CHz (II),
in which the substituent R is as defined above for R1 or R2. The use of a
phase transfer catalyst, for example tetrabutylammonium chloride, is
particularly advantageous for this reaction. The preparation of alkyl
thiaglycidyl ether is also described in US-A-2,965,652, US-A-2,731,437
and BE-A-609,375.
The alkyl thiaglycidyl ethers of the formula II may then be reacted, for
example, with sodium hydrogen sulfide to form compounds of the type of
the formula III, VII or XI. The mercaptans which are formed by the
addition of H2S to compounds of the formula II, may be converted to
compounds of the type of the formula IV, VIII or XII using, for example,
dimethylsulfoxide. These mercaptans furnish with formaldehyde compounds
of the type of the formula V, IX or XIII while the addition of compounds
of the type of the formula II to 1,2-dimercaptoethane gives rise to
compounds of the type of the formula VI, X or XIV.
The compounds of the formula I function already in very low amounts as
additives in functional fluids, such as lubricating oils, hydraulic
fluids and metalworking fluids. They are expediently added to the
functional fluids in an amount of 0.01 to 5 % by weight, preferably in an
amount of 0.05 to 3 % by weight, based on the functional fluid.
The lubricating oils in question are familiar to the person skilled in
the art and are described, for example, in "Schmierstoffe und verwandte
Produkte" ["Lubricants and Related Products"] by Dieter Klamann, (Verlag
Chemie, Weinheim, 1982) or in "Das Schmiermittel-Taschenbuch" ["Lubri-
cants Handbook"] by Schewe-Kobek, (Dr. Alfred Huthig-Verlag, Heidelberg,
1974). In addition to mineral oils, poly-~-olefins, ester-based lubri-
cants, phosphates, glycols, polyglycols and polyalkylene glycols, are
particularly suitable.
- 7 ~ 0 2 9 3
The compounds of the formula I are readily soluble in lubricants, are
accordingly particularly suitable as additives, for example, to lubri-
cating oils and greases and enhance the extreme-pressure and antiwear
properties. The oxidation-preventive and corrosion-preventive action of
these compounds even toward copper should be also pointed out. Further-
more, common sealant materials are not attacked. Finally and surprising-
ly, the preparation of so-called masterbatches is possible.
The presence of compounds of the formula I in lubricants allows at least
a partial replacement of ash-producing additives, i.e. especially zinc
compounds or phosphorus-containing additives, principally zinc dithio-
phosphates. It is desirable to limit the amounts of zinc and its com-
pounds and especially of phosphorus and its compounds, or avoid their use
altogether, as a component of an additive for a gasoline engine motor oil
in view of the possibility that the catalyst attached nowadays to the
exhaust of a gasoline engine may be deactivated by the zinc and phos-
phorus compounds discharged in the exhaust gases.
In contrast to the possible impairment of the catalyst of a gasoline
engine by the additives used hitherto, the compounds of the formula I
according to the present invention allow a continued reduction in
phosphorus and thereby enhance the operational reliability of the
catalyst.
Novel compounds and novel functional fluid compositions and especially
lubricating oil compositions containing these compounds having properties
further improved compared with products known hitherto, have also been
found. The products are distinguished by high resistance to oxidative
degradation and to solvolytic cleavability, and they impart, for example,
to lubricating oil compositions a surprisingly high wear resistance which
is particularly effective on the valve train, i.e. on the cams of the
camshafts and on the tappets of diesel engines and spark-ignition
engines. Furthermore, the compounds of the formula I and the compositions
comprising these compounds have a good seal compatibility and they do not
corrode copper.
- ~340293
The compositions according to the invention are suitable for use as
lubricating oil compositions for internal combustion engines in general,
they are particularly suitable for diesel engines and are very parti-
cularly suitable for spark-ignition engines. The term "spark-ignition
engines" refers to internal combustion engines operating on the piston
stroke principle. Internal combustion engines may be used for a variety
of purposes, for example in stationary engines or as drives in means of
transport such as ships, aircraft and especially in motor vehicles.
The invention can relate to compositions, for example, in which the
lubricating oil is an oil for diesel engines or spark-ignition engines.
The present invention accordingly also relates to the use of compounds of
the formula I as multipurpose additives for lubricants, hydraulic fluids
and metalworking fluids. Compounds of the formula I are expediently
employed in lubricating oils as multipurpose additives. Compounds of the
formula I are preferably used as extreme-pressure and antiwear additives
in lubricants and especially in lubricating oils.
The compositions according to the invention also relate expediently to
low-ash or ashless and phosphorus-free compositions which comprise (a) a
mineral oil or a synthetic oil or a mixture thereof or a hydraulic fluid
or a metalworking fluid, and (b) at least one compound of the
formula (I).
The present invention accordingly also relates to the use of compounds of
the formula I as phosphorus-free extreme-pressure and antiwear additives
for lubricants, hydraulic fluids or metalworking fluids.
The invention refers particularly to compositions of the specified type
according to API classification SF, SG, CD and/or CE or according to CCMC
categories G 1, G 2, G 3, D 1, D 2, D 3 and/or PD 1.
Accordingly, the compositions expediently represent motor oils for motor
vehicles, and essentially for passenger car engines and light commercial
vehicle engines, which correspond to at least one of the categories SF,
SG, CD or CE of the API (American Petroleum Institute) classification and
. ~
9 1340~93
to at least one of the categories G 1, G 2, G 3, D 1, D 2, D 3 or PD 1 of
the CCMC (Committee of Common Market Automobile Constructors) classifi-
cation.
The invention also comprises a method for reducing wear in diesel engines
and spark-ignition engines, and in particular on the cams of the cams-
hafts and on the tappets of the valve train, wherein the lubricating
system of the internal combustion engine is run on a composition of the
type described above.
The invention also comprises a method for avoiding the deactivation of
the catalyst attached to the exhaust of a gasoline engine, wherein the
lubricating system of the internal combustion engine is run on a compo-
sition of the type described above.
The lubricants may additionally contain other additives, added for the
purpose of further improving the basic properties of the lubricants;
these include: antioxidants, metal passivators, corrosion inhibitors,
viscosity index improvers, pour point depressants, dispersants,
detergents as well as further extreme-pressure additives and antiwear
additives. The above reservations concerning ash-producing compounds,
especially zinc compounds, and phosphorus compounds are accordingly also
valid for the additional additives, a certain content of zinc-containing
phosphorus/sulfur compounds being tolerated.
Examples of phenolic antioxidants
1. Alkylated monophenols
2,6-di-tert-butyl-4-methylphenol, 2,6-di-tert-butylphenol, 2-tert-butyl-
4,6-dimethylphenol, 2,6-di-tert-butyl-4-ethylphenol, 2,6-di-tert-butyl-
4-n-butylphenol, 2,6-di-tert-butyl-4-isobutylphenol, 2,6-dicyclopentyl-
4-methylphenol, 2-(~-methylcyclohexyl)-4,6-dimethylphenol, 2,6-diocta-
decyl-4-methylphenol, 2,4,6-tricyclohexylphenol, 2,6-di-tert-butyl-4-
methoxymethylphenol, o-tert-butylphenol.
134~293
2. Alkylated hydroquinones
2,6-di-tert-butyl-4-methoxyphenol, 2,5-di-tert-butylhydroquinone,
2,5-di-tert-amylhydroquinone, 2,6-diphenyl-4-octadecyloxyphenol.
3. Hydroxylated thiodiphenyl ethers
2,2'-thio-bis(6-tert-butyl-4-methylphenol), 2,2'-thio-bis(4-octylphenol),
4,4'-thio-bis(6-tert-butyl-3-methylphenol), 4,4'-thio-bis(6-tert-butyl-
2-methylphenol).
4. Alkylidene bisphenols
2,2'-methylene-bis(6-tert-butyl-4-methylphenol), 2,2'-methylene-bis-
(6-tert-butyl-4-ethylphenol), 2,2'-methylene-bis[4-methyl-6-(~-methyl-
cyclohexyl)phenol], 2,2'-methylene-bis(4-methyl-6-cyclohexylphenol),
2,2'-methylene-bis(6-nonyl-4-methylphenol), 2,2'-methylene-bis(4,6-di-
tert-butylphenol), 2,2'-ethylidene-bis(4,6-di-tert-butylphenol), 2,2'-
ethylidene-bis(6-tert-butyl-4- or -5-iso-butylphenol), 2,2'-methylene-
bis[6-(~-methylbenzyl)-4-nonylphenol], 2,2'-methylene-bis[6-(~,~-di-
methylbenzyl)-4-nonylphenol], 4,4'-methylene-bis(2,6-di-tert-butyl-
phenol), 4,4'-methylene-bis(6-tert-butyl-2-methylphenol), 1,1-bis(5-
tert-butyl-4-hydroxy-2-methylphenyl)butane, 2,6-di(3-tert-butyl-5-
methyl-2-hydroxybenzyl)-4-methylphenol, 1,1,3-tris(5-tert-butyl-4-
hydroxy-2-methylphenyl)-3-n-dodecylmercaptobutane, ethylene glycol
bis[3,3-bis(3'-tert-butyl-4'-hydroxyphenyl)butyrate], bis(3-tert-
butyl-4-hydroxy-5-methylphenyl)dicyclopentadiene, bis[2-(3'-tert-
butyl-2'-hydroxy-5'-methylbenzyl)-6-tert-butyl-4-methylphenyl]tere-
phthalate.
5. Benzyl compounds
1,3,5-tri(3,5-di-tert-butyl-4-hydroxybenzyl)-2,4,6-trimethylbenzene,
bis(3,5-di-tert-butyl-4-hydroxybenzyl)sulfide, isooctyl 3,5-di-tert-
butyl-4-hydroxybenzylmercaptoacetate, bis(4-tert-butyl-3-hydroxy-2,6-
dimethylbenzyl)dithiolterephthalate, 1,3,5-tris(3,5-di-tert-butyl-4-
hydroxybenzyl)isocyanurate, 1,3,5-tris(4-tert-butyl-3-hydroxy-2,6-di-
methylbenzyl)isocyanurate, dioctadecyl 3,5-di-tert-butyl-4-hydroxy-
benzylphosphonate, calcium salt of monoethyl 3,5-di-tert-butyl-4-
hydroxybenzylphosphonate.
10293
6. Acylaminophenols
4-hydroxylauranilide, 4-hydroxystearanilide, 2,4-bisoctylmercapto-6-
(3,5-di-tert-butyl-4-hydroxyanilino)-s-triazine, octyl N-(3,5-di-tert-
butyl-4-hydroxyphenyl)carbamate.
7. Esters of B-( 3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid
with monohydric or polyhydric alcohols, for example with methanol,
diethylene glycol, octadecanol, triethylene glycol, 1,6-hexanediol,
pentaerythritol, neopentyl glycol, trishydroxyethyl isocyanurate,
thiodiethylene glycol, bishydroxyethyloxaldiamide.
8. Esters of B-( 5-tert-butyl-4-hydroxy-3-methylphenyl)propionic acid
with monohydric or polyhydric alcohols, for example with methanol,
diethylene glycol, octadecanol, triethylene glycol, 1,6-hexanediol,
pentaerythritol, neopentyl glycol, trishydroxyethyl isocyanurate,
thiodiethylene glycol, dihydroxyethyloxaldiamide.
9. Amides of B-( 3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid
for example N,N'-bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)hexa-
methylenediamine, N,N'-bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)-
trimethylenediamine, N,N'-bis(3,5-di-tert-butyl-4-hydroxyphenylpro-
pionyl)hydrazine.
Examples of aminic antioxidants:
N,N'-di-isopropyl-p-phenylenediamine, N,N'-di-sec-butyl-p-phenylenedi-
amine, N,N'-bis(1,4-dimethylpentyl)-p-phenylenediamine, N,N'-bis(l-
ethyl-3-methylpentyl)-p-phenylenediamine, N,N'-bis(l-methylheptyl)-p-
phenylenediamine, N,N'-dicyclohexyl-p-phenylenediamine, N,N'-diphenyl-
p-phenylenediamine, N,N'-di(naphthyl-2)-p-phenylenediamine, N-isopropyl-
N'-phenyl-p-phenylenediamine, N-(1,3-dimethylbutyl)-N'-phenyl-p-
phenylenediamine, N-(l-methylheptyl)-N'-phenyl-p-phenylenediamine,
N-cyclohexyl-N'-phenyl-p-phenylenediamine, 4-(p-toluenesulfonamido)di-
phenylamine, N,N'-dimethyl-N,N'-di-sec-butyl-p-phenylenediamine, di-
phenylamine, N-allyldiphenylamine, 4-isopropoxydiphenylamine, N-phenyl-
l-naphthylamine, N-phenyl-2-naphthylamine, octylated diphenylamine, for
example p,p'-di-tert-octyldiphenylamine, 4-n-butylaminophenol, 4-butyryl-
aminophenol, 4-nonanoylaminophenol, 4-dodecanoylaminophenol, 4-octa-
- 12 - ~ 3~ 0293
decanoylaminophenol, di(4-methoxyphenyl)amine, 2,6-di-tert-butyl-4-di-
methylaminomethylphenol, 2,4'-di-aminodiphenylmethane, 4,4'-diaminodi-
phenylmethane, N,N,N',N'-tetramethyl-4,4'-diaminodiphenylmethane,
1,2-di[(2-methylphenyl)amino]ethane, 1,2-di(phenylamino)propane,
(o-tolyl)biguanide, di[4-(1',3'-dimethylbutyl)phenyl]amine, tert-
octylated N-phenyl-l-naphthylamine, mixture of mono- and dialkylated
tert-butyl-/tert-octyldiphenylamines, 2,3-dihydro-3,3-dimethyl-4H-1,4-
benzothiazine, phenothiazine, N-allylphenothiazine.
Examples of other antioxidants:
aliphatic or aromatic phosphites, esters of thiodipropionic acid or of
thiodiacetic acid, or salts of dithiocarbamic or dithiophosphoric acid.
Examples of metal deactivators, for example for copper:
triazoles, benzotriazoles and their derivatives, tolutriazoles and their
derivatives, 2-mercaptobenzothiazole, 2-mercaptobenzotriazole, 2,5-di-
mercaptobenzotriazole, 2,5-dimercaptobenzothiadiazole, 5,5'-methylenebis-
benzotriazole, 4,5,6,7-tetrahydrobenzotriazole, salicylidenepropylenedi-
amine, salicylaminoguanidine and their salts.
Examples of rust inhibitors:
a) Organic acids, their esters, metal salts and anhydrides, for example:
N-oleoylsarcosine, sorbitol monooleate, lead naphthenate, alkenylsuccinic
anhydride, for example dodecenylsuccinic anhydride, alkenylsuccinic
hemiester and hemiamide, 4-nonylphenoxyacetic acid.
b) Compounds containing nitrogen, for example:
I. Primary, secondary or tertiary aliphatic or cycloaliphatic amines and
amine salts of organic and inorganic acids, for example oil-soluble
alkylammonium carboxylates.
II. Heterocyclic compounds, for example:
substituted imidazolines and oxazolines.
c) Compounds containing phosphorus, for example:
amine salts of phosphoric acid partial esters or phosphonic acid partial
esters, zinc dialkyldithiophosphates.
- 13 - 13~0293
d) Compounds containing sulfur, for example:
barium dinonylnaphthalene sulfonates, calcium petroleum sulfonates.
Examples of viscosity index improvers:
polyacrylates, polymethacrylates, vinylpyrrolidone/methacrylate copoly-
mers, polyvinylpyrrolidones, polybutenes, olefin copolymers, styrene/
acrylate copolymer, polyethers.
Examples of pour point depressants:
polymethacrylate, alkylated naphthalene derivatives.
Examples of dispersants/surfactants:
polybutenylsuccinamide or polybutenylsuccinimide, polybutenylphosphonic
acid derivatives, basic magnesium, calcium and barium sulfonates and
phenolates.
Examples of antiwear additives:
compounds containing sulfur and/or phosphorus and/or halogens, such as
sulfurized vegetable oils, zinc dialkyldithiophosphates, tritolyl
phosphate, chlorinated paraffins, alkyl and aryl disulfides, alkyl and
aryl trisulfides, triphenylphosphorothionates, diethanolaminomethyltolyl-
triazole, di(2-ethylhexyl)aminomethyltolyltriazole.
The examples below elucidate the invention in greater detail. The partsand percentages are parts and percentages by weight, unless stated
otherwise.
Example 1: preparation of
tC4Hg-S-CHz-8H-CHz-S-CHz-8H-CHz-S- C4Hg tIII)
H H
~ 340293
- 14 -
Reactlon scheme
2tC~Hg-s-CH2~ ~ ~ CH2 + NaSH(~ % ~ H2O) + H~O
~
~ ~C ~ ~ H4--S--CH2--CH--CH2--)2S ~NaOH
H20
OH
73.1 g (0.5 mol) of tert-butyl glycldyl thloether are added
dropwlse wlth stlrrlng to 50 ml (0.268 mol) of technlcal 30
sodlum hydrogen sulflde solutlon, contalned ln a four-necked
flask and dlluted wlth 25 ml of water (duratlon about 1 hour).
The temperature of the reactlon mlxture whlch heats up ln the
reactlon, ls kept to 40-50~C by coollng. Stlrrlng ls then
contlnued at 50-60~C for 1 hour and the endpolnt of the
reactlon ls checked by the dlsappearance of the glycldyl
thloether spot ln the thln-layer chromatogram.
Worklng up ls carrled out vla phase separatlon.
The organlc phase ls the reactlon product. It ls
washed wlth about 20 ml of dllute sodlum blcarbonate solutlon
and ls flltered, after clear phase separatlon, through CELITE
and ls drled.
Trade-mark
i, ~
~ 3~029~
- 14a -
Yleld: 77.6 g~g5 % of theory; yellow vlscous llquld;
refractlve lndex n2~D:1.5261; bolllng polnt: 180~C/0.5 mbar
Example 2: preparatlon of
Herstellung von
OH
~ ~ Hg- S - CH2 - CH - CH2 - )2 Vl
Reactlon scheme
2~C4Hg - S-GH2- @ - CH2+ HS - CH2 - CH2 - SH CH~,
OH
~ ~ Hg- S - CH2 - CH - CH2 - S- CH2 -)2
Procedure
1=8.8 g (0.2 mol) of 1,2-dlmercaptoethane and 0.5 ml of sodlum
methylate solutlon (30 % ln methanol), contalned ln a two-
necked flask, are warmed to 60~C, the heatlng bath ls removed
and 61.4 g (0.42 mol) of tert-butyl glycldyl thloether are
added dropwlse ln the course of
~ ~ 10293
45 minutes at 60-70~C with stirring (exothermic reaction). When the
addition is completed, the mixture is further stirred for 30 minutes at
about 60~C and volatile substances are removed in vacuo in a rotary
evaporator. Working up is carried out as in Example 1.
Yield: 100 % of theory; colourless liquid; refractive index: n2D: 1. 5391
Example 3: preparation of
( C4Hg-S-CH2- H-CH2-S--)2 IV
Reaction scheme:
2 C4Hs-S-CH2- H-CH2-SH + (CH3)2S = 0
-HzO ~ (tc4Hg-s-cH2-cH-cH2-s-)2
QH
36 g (0. 5 mol) of the compound tert-C4Hg-S-CH2-CH-CH2-SH, 31.2 g
(0.4 mol) of dimethyl sulfoxide and 150 ml of toluene are combined in a
reaction vessel and 1.7 ml of the water of reaction is removed with
stirring by reflux. About 100 ml of toluene/dimethyl sulfoxide are then
distilled off at normal pressure, the organic phase is treated with 50 ml
of toluene, washed with aqueous sodium bicarbonate solution and then with
water, dried and concentrated.
Yield: 34.7 g~~6.7 % of theory, yellow liquid; refractive index
n2 D: 1.5450
Example 4:
Wear test:
Each of the compounds III, IV and VI, prepared as in Examples 1 to 3, is
mixed with an SAE 10 W-30 motor oil free from zinc dithiophosphates (the
base oil being an IS0 VG 32 mineral oil), designated AARG 45, at a
concentration of 1 % by weight, and the samples are subjected to the
so-called cam and tappet (C + T) test.
. . .
~ 5~=
- 16- ~-340~9
Brief description of the C + T test:
In an enclosed housing, a cam made of cast iron induction-hardened to
550-580 HV (Vickers hardness) rotates against a spring-loaded tappet made
of casehardened steel of 800-850 HV hardness. The housing is filled with
the test oil up to the middle of the cam axle. The oil is heated to 100~C
by means of built-in heating rods. When the required temperature is
reached, the force acting on the cam head is set to 1,000 N by adjusting
the spring pressure and the test is commenced at a speed of rotation of
the cam of 1,500 rpm. The wear is measured after one hour's running. If
the total wear of cam head and tappet is less than 0.25 mm, the force
acting on the cam head is increased by 100 N and the test is continued
for a further hour at the increased cam force until either the wear is
greater than 0.25 mm or a cam head force of 2,000 N is reached.
The result is reported as damage stage or, in the absence of any wear, is
recorded as greater than 2,000.
Table 1 lists the measured values, a higher numerical value (in N)
representing an improved antiwear performance.
Table 1
Base oil AARG 45 1000
+ 1 % compound of the formula III (Example 1) 1700
+ 1 % compound of the formula IV (Example 2) 1800
+ 1 % compound of the formula VI (Example 3) 1900
Example 5:
Oxidation stability test
The compounds prepared in Examples 1 and 2 (additives III and VI) are
subjected to the TFOU test.
- 17 - ~ ~40293
(TFOU test Thln-Fllm Oxygen Uptake Test, Natlonal Bureau of
Standards (NBS), Washlngton DC, USA)
Thls test ls a modlfled version of the "Rotary Bomb
Oxldatlon Test for Mlneral Olls" (RBOT) (ASTM D 2272). It ls
descrlbed ln full ln the paper by C.S. Ku and S.M. Hsu, A
Thln-Fllm Oxygen Uptake Test for the Evaluatlon of Automotlve
Crankcase Lubrlcants, Lubrlcatlon Englneerlnq, Vol. 40 (2),
75-83 (1984). In the TFOU test, the oll under test (1.5 g),
contalned ln a glass tube placed lnslde a steel pressure
contalner (bomb), ln the presence of 2 % of water, a llquld
oxldlzed and nltrated fractlon of a motor gasollne as catalyst
(4 % lnltlal concentratlon) and a llquld metal naphthenate as
a further catalyst (4 % lnltlal concentratlon) (the water and
the two llquld catalysts are avallable from the Natlonal
Bureau of Standards (NBS) under the Standard Reference
Materlal No. 1817 wlth certlflcate of analysls), ls sub~ected
to oxygen pressure of 6.3 bar (90 psl) (oxygen volume 65 ml).
The bomb rotates ln a sloplng posltlon at 100 rpm ln a llquld
bath at 160~C. The sloplng posltlon and the rotary movement
cause the oll to be dlstrlbuted as a fllm on the surface of
the glass contalner.
After a glven lnductlon tlme, the oll beglns to
oxldlze under these condltlons. The oxldatlon lnduces oxygen
uptake, manlfested by a dlstlnct pressure drop. The perlod to
the onset of oxldatlon, the so-called lnductlon tlme, ls taken
as a measure of oxldatlon stablllty.
", . , . , ,,, ~, ,
~ 340293
- 17a -
The test oll used is a motor oll based on an ISO VG
32 mlneral oll, comprlslng an addltlve package Oloa 4140 C
(Orogll) and a vlscoslty lndex lmprover (an olefln copolymer).
The vlscoslty corresponds to 15W-50 and the zlnc
dlthlophosphate content of the addltlve glves rlse to an
analytlcal value of 0.067 % of Zn and 0.06 % of P ln the oll.
The results glven ln Table 2 below represent the
tlme (ln mlnutes) to the break ln the pressure/tlme dlagram.
Long perlods correspond to effectlve stablllzlng
actlon. Concentratlon of the addltlves III and VI: 0.75 % by
welght based on the oll.
Trade-mark
r
,; ~
- 18 - ~ 3~0293
Table 2
TFOUT min
Base oil 68
+ 0,75 % by weight of the
compound III from Example 1 275
0,75 % by weight of the
compound VI from Example 2 272
Example 6:
Test for compatibility with sealant materials
The test employed is the Volkswagen method VW-Seal test, P-VW 3334 at
150~C over a test period of 96 hours. The evaluated parameters are the
change in the modulus 1/3 (at 1/3 elongation), the change in ultimate
tensile strength and the change in elongation.
The test of a test material of the type FKM (fluorine rubber) in a motor
oil containing either the compound of the formula III or a compound of
the formula VI brings about no significant changes in the condition of
the seals in respect of elongation and changes in tensile stress compared
with the motor oil without the additives of the formulae III and VI.
Example 7:
Test of corrosion behaviour towards copper
The Petter CEC L-02-A-78 test is used. The weight loss of copper bearings
is determined after 36 hours, both in a phosphorus-free oil and in an oil
containing phosphorus (P = 0.06 %) using a compound of the formula III
and a compound of the formula VI. The weight losses are less than 25 mg,
thus complying with the requirements of the CCMC categories G 1, G 2
and G 3.