Note: Descriptions are shown in the official language in which they were submitted.
f' 1340326
Pyrazolesulfonylurea derivative, preparation thereof,
herbicide containing said derivative as active
ingredient and herbicidal method by use thereof
This invention relates to a novel pyrazolesulfonylurea
derivative represented by the formula (I), preparation
thereof, a herbicide containing said derivative as an
active ingredient and a herbicidal method by use
thereof.
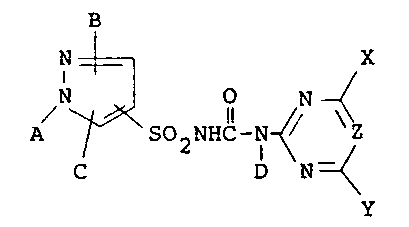
B
IN ~ X
A ~ SO2NHC~ Z (I)
C D N ~
y
wherein A represents a hydrogen atom, a Cl-C8 alkyl
group or a phenyl group which may be substituted with
Cl-C8 alkyl groups, halogen atoms or nitro groups; B
and C represent independently hydrogen atoms, halogen
atoms, nitro groups, Cl-C8 alkyl groups, arylalkyl
groups, Cl-C~ alkox~ groups, haloalkyl groups, -C02R
(where R is a hydrogen atom, a Cl-C8 alkyl group, an
allyl group or a propargyl group), -CONRlR~ lwhere R
is a hydrogen atom, a Cl-C8 alkyl group or a phenyl
group, R2 is a hydrogen atom or a Cl-C8 alkyl group, or
Rl and R2 taken together may represent -(CH2)m- (_ is
4, 5 or 6), -CH2CH2OCH2CH2- or -CH2CH2N(CH3)CH2CH2-],
-S(O)nR3( where R3 is a Cl-C8 alkyl group, a phenyl
1~ ~
, 13~032~
group or an arylalkyl group and n is 0, 1 or 2),
-SO2NR4R5 [where R4 is a Cl-C8 alkyl group, R5 is a
hydrogen atom or a Cl-C8 alkyl group, or R4 and R5
taken together may represent -(CH2)p- (p is 4, 5 or 6),
CH OCH C~2- or -cH2cH2N(cH3)cH2cH2
group which may be substituted with Cl-C8 alkyl groups,
halogen atoms or nitro groups; D represents a hydrogen
atom or a Cl-C8 ~lkyl group; X and Y represent
independently hydrogen atoms, halogen atoms, Cl-C8
alkyl groups, Cl-C8 alkoxy groups, Cl-C8 a~ko~xyalkyl
groups, -CF3 groups, Cl-C8 h'aloàlkoxy groups,
alkylamino groups, dialkylamino groups,
R6
-OCHC02R~ (where R6 and R7 each represent hydrogen
~ atoms or Cl-C8 alkyl groups) or either X or Y may form
a five-membered ring containing an oxygen atom together
with Z; and Z represents a nitrogen atom or C-R8 (where
R8 represents a hydrogen atom, a haloalkyl group or may
form a five-membered ring containing an oxygen atom
together with X or Y).
The compounds of this invention are novel compounds not
disclosed in literature and have excellent herbicidal
activities.
Japanese Unexamined Patent Publication Nos.102577/1980
and 139466/1981 disclose pyridinesulfonylurea
derivatives and Japanese Unexamined Patent Publication
No.169688/1981 discloses pyrolesulfonylurea
derivatives, respectively, as useful herbicides.
Heretofore, in using herbicides, it has been pointed
out that the economical cost involved in the use of a
herbicide depends on the application amount of the
active ingredient per unit area. For this reason,
studies have been continued for many years to obtain
~;r
1~40326
compounds which can exhibit high herbicidal effects at
lower application rate.
In the prior art, as pyrazole derivatives, there have
been known a large number of compounds, as disclosed in
Japanese Patent Publication No.36648/1979 and Japanese
Unexamined Patent Publication Nos.41872/1979,
2276/1982, 58670/1982 and 133265/1976.
The present inventors have made various investigations
for many years and consequently found that the
compounds of this invention have markedly high
herbicidal effects as compared with the above pyrazole
derivatives known in the art and are practically
- useful. On the other hand, as sulfonylurea derivatives
containing a nitrogen-containing heterocyclic ring, the
aforementioned pyrolesulfonylurea and pyridinesulfonyl-
urea are known. As compared with these known
compounds, the compounds of this invention have been
found to exhibit markedly higher herbicidal effects.
The present invention has been accomplished on the
basis of these findings.
That is, the compounds of this invention may be said to
be epoch-making herbicides capable of decreasing
markedly the amount of the active ingredient applied
per unit area as compared with these compounds known in
the art, having a very great economical effect as
compared with the herbicides of the prior art, and also
capable of alleviating markedly the environmental
pollution which may be caused by application of a large
amount of pesticides.
Typical examples of the derivatives represented by the
above formula (I) suitable for use as herbicides may
include those as shown in Table 1, Table 2 and Table 3
set forth below.
13gO326
The Compounds Nos. in these Tables are referred to in
the following description.
Table 1
O N
C
Comp. A B C ~ Y Z
No .
CH3 CH3 CH3 CH3 CH3 CH
2 CH3 CH3 CH3 CH 3 O CH3 CH
3 CH3 CH3 CH3 O CH3 O CH3 CH
4 CH3 CH3 CH3 CH3 O CH3 N
CH3 CH3 CH3 ~ CH3 O CH3 N
6 ~ CH3 CH3 CH3 H CH
7 (~ CH3 CH3 CH3 CH3 CH
8 ~ CH3 CH3 CH3 O CH3 CH
9 ~ CH3 CH, O CH3 OCH3 CH
1 0 ~ CH3 CH~ CHI OCH3 N
l340326
-- 5 --
-No. A B C X Y Z
1 1 ~ CH3 CH3 OCH3 OCH8 N
1 2 CH3 ~ CH3 CH3 CH3 ~H
13 CH3 ~ CTH3 CH3 o CH3 CH
1 4 CH3 ~ CH3 OCH3 0 CH3 CH
1 5 CH3 ~ CH3 CH3 OC~3 N
1 6 CH3 ~ CH3 ~CH3 OC-~3 N
1 7 CH3 ~ CH3 CH3 CHs N
18 CH3 ~ CH3 C ~3 C ~ CH
1 9 C .~ s ~) C L~ 3 C ~ 8 C ~ C (C~2C~
CH3 <~ C7~3 CH3 -OCH2C'~3 C
21 CH3 ~ CH3 C~ N (CH3)2! N
22 CH3 ~> C7L~3 ~CH3N (CH3)2 N
2 3 CH3 ~>- CH3 CHS CH, CH3 CH
2 4 CHt ~ C~3 Cr~3 . CH; O CH3 CH
CH3 ~CH3 CH3 CH3 ~5H3 N
13~0~26
-- 6
Comp. A B C ~r, y Z
2 6 CH3 ~C~Hs CH3 CH3 0C-~3 CH
2 7 5H3 ~C2Hs CH3 C 'd ~ O CH3 N
28 CH3 ~ CH3 CH3 CH3 CH
2 9 C-~3 _~ H3 5-;~ CH3 .O CH3 CH
3 0 CH3 ~ C-dt ~3 O CH3 -N
31 C'~3 ~) C-~3 CH3 C~9 CH
32 CH~ CH3 CH3 3C-~3 CH
C~3
3 3 CHs ~ CH~ CH3 ~ Cld3
3 4 CHt 4~C~ crdt C~d3 ~ C-dy C H
3 5 CH:~ ~Cf~ CH3 C~3 OCH3 N
3 6 CH C ~~ CH3 C-L~3 OCH3 CH
3 7 CH~ C ~ CH3 CE~3 O CH~ N
3 8 CH3 ~NO2 CH3 C-.~3 3 Cd3 CH
3 9 CH3 ~NO2 CH3 C~3 OC~I3
1340~26
Comp. A B C ~ Y Z
4 o CH3 ~ H CH3 CH3 CH
41 CH3 ~ ~ CH3 O CEI3 CTrl
4 2 CH3 ~) H CH3 O ''H~ N
4 3 CH3 CH3 (~ CH3 CH3 - C H
4 4 CH3 cr~3 ~ c-~3 O CH3 CH
4 5 CH3 CH3 ~ OCH3 OCH3 CH
4 6 CH3 CH3 ~ CH3 ~ CH3 N
4 7 CH~ CH3 ~ O CH3 O CH3 N
4 8 H ~> -CH3 CH3 CH3 CH
4 9 T~ CH3 C-~3 O ~H3 CH
5 0 H ~> CH3 CH3 OCH~, N
5 1 -CH3 CH3 ~ CH3 CHl 5H3 CH
5 2 -CH2 CH3 ~ CH3 CH3 O CH3 CH
53 -CH2 CH3 ~> CH3 CH3 OCH3 N
54 -CH,CH2CH3 ~ CH3 CH3 CH3 CE~
-CH~,CEI2CH3 ~> CH9 Cl~3 OCH3 CH
.
1340326
Comp. A B C ~: Y Z
No .
5 6- CH~ CH2 CH3 ~ CH3 c-~3 O CH3 N
5 7 <~ ~ CH3 CH9 ;CH~ CH
5 8 ~ ~ CH3 CH3 OCH3 CH
59 ~ ~ CH3 CH3 ~ CH3
6 0 CH3 ~ <~ CH3 CH3 ~ CH
61 CH5 ~ ~> CH3 ~ CTc~3 CH
6 2 CH3 ~ ~> CH8 ~ C;~3 N
6 3 H ~ ~ C~J9 CH3 CH
64 E~ O ~ . Ci~3 OCH3 C
H ~ ~ CH3 OCH3 N
6 6 CH5 ~ (~CH3 c~-~3 CH3 CH
6 7 CH3 ~ (3 CH3 CH3 ~ CH 3 CH
68 CH.J ~ OCH3 OCH3 OCH3 CH
- 6 9 CH3 (~ OCH3 OCH2CH3 OCH2CH3 CH
7 0 CH3 ~ O C~d3 CH3 O CH3
71 CH3 ~ OCTl-I3OCHa OCH3 N
134032fi
Comp.A B C X Y Z
7 2C H3 ~ O CHJ CH3 CH3 N
7 3CH5 ~> O CH3 CH3 C~ C (CH2)~
7 4CH3 ~ oCH3 CEI3 -OCH2 CEl3C -
75 CH3 <~) OCH3 OCH3 N(CH3)3 N
76 CH3 ~CH~ OCH~ CH3 CH3 CH
7 7CH3 _~CH3 ~ CH3 CH3 O CH3 CH
7 8C H5 ~CH3 ~ CH3 CH3 O CH3 N
7 9CH3 _~C~ o CH3 CH3 CH3 CH
8 oCH3 ~C~ OC-H3 CH3 OCH3 CH
81 CH3 ~C~ OCH3 CH3 OCH3 N
8 2CH3 _~N 3 O CH3 CEI3 CH3 CH
8 3C~3 _~5N~3 o CH3 CH3 ~ ~H3 CH
8 4CH3 _~NO2 OCH3 CH3 OCH~ ~
8 5CH3 CH3 C~ CH, CH3 CH
8 6CH3 CH3 'C~ CH~ o CH3 CH
134032~
-- 10 --
Comp. A B C X Y Z
8 7 CH3 CH3 C~ o CHa ~ CEI3 CH
88 CH3 CH3 C~ CH3 OCH5
8 9 CH3 CHg C~ O CH3 O CH3 N
9 o CH3 CH3 C~ CH3 C'~ID N
9 1 C~3 CH3 C~ CH3 C~C(CH2)~C~
. .
9 2 C-H3 CH3 c,e: c-~3-OCH 2 CHa C -
9 3 C~3 CH3 C~ OCH3 N(CH3)2 N
9 4 CE~3 CH3 .C~ CH2C~13 OCH3 CH
9 5 CE~3 CTn3 C~ CH3 CH2OCH3 CH
9 6 CH3 ~H3 C~ CH3OCH3CO.~CH3 CH
9 7 CH3 ~ C~ CH3 CH3 C'~
9 8 C~3 ~ C~ C H3 O CH3 CH
9 9 CH3 ~ O CH3 O CH3 CH
1 00 CH3 ~ C~ CH3 OCH3 N
__
1 01 CH3 (~ CC oCE~3 OCH3 N
10 2 C H3 ~H3l CH, CH3 CH
- ll 13~0326
Col~p.
No. A B C ~r~ y I Z
1 0 3 CH3 ~ CH3 C~ CH3 _ _
1 04 CH3 ~ CH3 C~ CH5 OCHs N
10 5 CT~3 CH3 Br CH3 CH3 CH
1 0 6 CH3 Cr~3 Br CE~3 ~ CH3 , CH
1 0 7 CH3 CH3 Br O CH3 O CH3 CH
10 8 cr~3 CH" Br c-~3 O CH3 N
10 9 CH3 CHy Br OCH3 OCH3 N
1 1 ~ CH3 H C4~ CH3 CH3 I CH
1 1 1 CH3 H C~ cr-~3 OCH3 CH
1 1 2 CH3 H C~ O CH3 OCHg CH
1 1 3 CH3 H C~ crd3 OCH3 N
1 1 4 CH3 H C~ OCH~ OCH~ N
. _ _
1 1 5 CH~CH~ CH3 C~ CH3 ICH3 Crd
1 1 6 cr-~CH 2 CH3 C~ c-n3 OCH3 lCH
1 1 7 CHsCH2 CH3 C~ CHs OCH3 N
1 18 CH3CrlaCH2CHg C~ CH3 CH3 CH
-
. . _
134032~
-- 12 --
NoP A B C X Y Z
1 1 9CH3 CH2CH3CH3 CC CHg OCH3 CH
1 2 ~cr~3 CH2 CH2cH3 C~ CH3 ~ CH3 CH
12 1 CE3 CH (CH3)2 C~ CH3 CE~3 CH
1 2 2CT.~,3CH (CH3) 2 C~ CH3 O CH3 CII
1 23 CH3 CH (CH3)2 C~ C-~3 OCH3 CH
12 4 H CH3 C~ CH3 CH3 CH
1 2 5 ~ CH3 C~ C~3 O CEI3 CH
12 6 H CH3 C~ C-~3 OCH3 N
12 7 ~3 CE33 , C~ CH3 CE~3 5H
1 28 ~) CH3 i-~ CH3 OCH8 CH
129 ~ CH3 C~ CH3 OCH3 N
1 3 0~-~3 CH3 -~> CH3 CH3 I CH3 CH
131 c-~3 CHz ~ CH3 CH3 OCH3 CE~
1 32 CHl CHz ~ CH3 OCH3 oc~3 cTd
1 3 3C~3 CH ~ ~ CH3 CH3 1OCld8 N
13 4 C-~3 CH2 -~ CH3 OCH3 OC.-~I" N
134032fi
-- 13 --
Comp .
No A B C X Y Z
135 CH3 CH3 CH2~ CH3 CH3 CH
136 CH5 CH3 CH ~ CH3 OCH3 CH
137 CH3 CH3 CH2-O CH3 O CHS N
138 CH3 CF3 CH3 CH3 CH3 CH
139 C~3 CF3 C~d3 CH3 OCH3 CH
140 Cld3CF3 CH3 OCH3 OCH3 CH
141 CH3 CF3 CH3 CH3 OCH3 N
142 CH3 CF3 CH3 OCH3 OCH3 N
143 CH3 CF3 CH 3 CH3 CH3
144 CH3 CF" CH3 CH3 C~ N
145 C.~3CF3 C H3 CH3 C~ C(CH2)2C~
146 CH3 CF3 CH3 CH3 -OCH2CH2C-
147 CH3 CF3 C~d3 C~ N(CH3)2 N
148 CH3 CF3 CH3 OCH3 N(CH3)2 N
149 C'd3CF3 CH3 OCH~CHa NHCH3
150 CH3 CF3 ' CH3 CH3 , C~ CH
._
1340326
,_ ,
-- 14 --
Comp. A B C I ~ Y
1 5 1 CH8 CF3 CHs CC C~ CH
1 52 H CF3 CH3 CH8 C~-d3 CH
153 H CF3 CH3 CHt OCH3 CH
1 5 4 H C~3 C-~3 C~3 OCH3 N
1 55 O CF3 CTd3 Cf~3 CH3 CH
15 6 ~ CF3 CH3 CTL~3 OCH3 CH
1 5 7 ~ CF~ crd3 c--~3 OCH3 N
. 1 58 CH3C02 CH~ CH3 CH3 CH3 CH
1 59 CLI3CO2 C~IJ crd3 CH3 OCH3 CH
1 6 0 CH3CO2 CH3 CHa OCH3 OCH3 CH
1 61 CH3CO~ CH" CH3 CH3 OCH3 N
1 62 CH3C02 CH3 CH,sOC-~3 OCH3 N
1 63 CH3CO2 CH3 CH3 CH3 CH3 N
1 6 4 CH3CO2 C~3 C~3 CH3 C~ N
16 5 CH3CO~ CH3 CT.~3CH3 C~ C(CH2)2C~
16 6 CH3CO~ CH3 CH3 CH3 -OCHy C H2C-
,
1340326
-- 15 --
No. A B C X Y ' Z
1 6 7 CH3 CO~ CH3 CH3 C~ N(CTd3)~ ~J
1 6 8 CH3 C~2 CH3 CH3 0 CH3 N(CH3) 2 N
1 6 9 CH3 CO2 CH3 CH3 C~ CH3 5H
1 70 CH3 CO2 CH, CH3 CH3 OCH2 CH3 CH
1 71 CH3 C~2 CH3 CH3 lCH3 OCH2CH3 N
1 72 CH3 C02 CE~3 CH3 Cd3 NHCH3 N
17 3 CH3 CO, CH 3 CE~3 CH3 H CH
1 74 CH3 CO2 CH 3 CH3 H H ~ CH
1 75 CE~ C02 CEI~ CH3 C~ C~ . CH
1 76 CH3 C;32CH2CH3 CH3 CH3 CHd ' CH
1 77 CH3 CO25E32CTd3 CT~3 CH3 OCH3 CH
1 78 CH3 CO2C-d2CH3 CH3 OCEI3 OCE~3 : CH
17 ~ CH3 CO2C-d2CH3 ! CH3 CH3 ,O CH3
180 1CH3 CO2CH2CH3 CH3 OCH3 OCH3 N
1 81 CH3 CO2CH2CH3 CH, CH3 CH3 ~, N
182 CH3 CO2C~H, -~ CH3 I CH3 CH, CH
13~0326
._ .
-- 16 --
Comp. A B I C ~ Y Z
No.
183 c-~3 c03C3Hr~~ CH3 CHa OCH3 CH
184 CTd~ C02 C3H7-ll CH3 OCH3 O CH3 CH
185 CH3 CO~ C3H7-n CH3 CH~ OCH3 N
186 CH3 CO, C3H7 -n CH8 o r~-~3 OC--~3 N
187 CH3 CO2 C3H7-i CH8 C-~3 CH3 CH
188 CH3 CO, C3H8-i CH3 c-~3 OCH3 CH
189 CH3 CO3 C3H7-i CH8 OC;~3 OCH3 CH
190 CH3 CO2 C3H7-i CH3 CH3 OCH3 N
191 C-d3 CO2 C3H7-i CH3 O''H3 OCH3 N
192 CH3 COiCH2CH=CH2 CH8 CH3 CH3 CH
193 CH8 CO2 CH2 CH=CH3 CH3 CH3 O CH3 C H
194 CE~ CO.~CH2CE~CH2 CH3 O CHl OCH3 CH
195 ~ CH3 CO2CH2CH=CH2 C-d3 CH3 OCH3 N
196 CH3 C4CHaCH=CH2 CH3 OCH3 OCH3 N
197 CH, CO,CH2 C--CH C-d3 ! CH
198 CH" CO2CH2C-- CH CH3 CH3 CH3 CH
- 17 - 1340~26
Comp. A ~ C g Y~;
No .
1 9 9 CH3 CO;~CH2C--CH CH3 CH3 OCH3 N
2 00 c-~3 C32H CH3 CH3 CH3 CH
20 1 CH3 CO2H CH3 CH3 OCH3 CH
2 0 2 CH3 CO2H CH3 O CH3 .O CH3 CH
2 03 CH3 CO2H CH3 CH3 OCH3 '~-
2 0 4 CH3 CO2H CH3 O CH3 O CH3 N
20 5 H CO2 CH3 C-~3 CH3 CH3 CH
2 0 6 H CO2 CH3 CH3 CT.~3 OCH3 CH
20 7 H CO2 CH3 CH3 OCH3 OCH3 CH
2 0 8 H CO2 CH3 CH" CH3 ~ CH3 N
20 9 H CO~ CH3 CHl OCH3 OCH3 N
2 10CHa CH3 C~2 CH3 C-H, c-~3 CH3 CH
21 1CH2 CH3 CO2 CH3 C-.I3 CH3 OCH3 CH
212 CH2 CH3 CO~ CH3 CH3 CH3 OCH3 N
213CH2CH2CH3 C~2 CH3 C~3 CH3 CH3 CH
214CH2CH2CH3 CO2 CH3 I CH~s CH3 OCH3 CH
1340~2fi
- 18 -
Comp. A ~ C ~ Y Z
215 CH2CH~ CH3 CO1CH3 CH3 CH3 OC~3 N
216 CH(CH3 )aC02C-'~3 C~3 CH3 C~3 C~
217 CH(CH8 )~CO2CH3 CH3 C.I3 OCHS CT
218 CH(CH3 )2 CO~5H3 CH3 CH3 OCH3 N
219 ~CO2CH3 Cr~3 ''CH3 CH3 C~
220 ~i CO2CH3 CH3 ' CE3 OCH3 CH
221 ~ C32CH3 CH3 , OCH3 OCH3 CE
222 ~, CO2CH3 CH3 CH3 OCH3 N
223 ~CO,CH3 CE3 OCE3 OCH3 N
224 CH3CO2CH3 H I CH3 CH3 CH
225 CH3CO2CE3 H CE~ OCH3 CH
226 CH3CO2 CH3 H . CH3 O CH3 N
227 CH3C ~2 CH3 CH,CH3 CH3 I CH3 CH
228 CH3C O2 CH3 CH2 CH3 CH3 O CH3 CH
229 CH3CO2CH3 CH2CH3 CH3 OCH3 N
230 ~ CH3I CO~CH3 CH(CH3)1 CH3 CH3 CH
-
- 1340326
-- 19 --
No. A B C ~ y z
231 CH3 Coa CH3 CH(CH3)2 CH3OCH3 CH
232 C~3 C02 CH3 CH(CH3)2 OCH3OCH3 CH
233 c~3 C~8 CH3 CH(CH3)2 CH3OCH3 N
234 CH3 COq CH3 CH(CH3)2 OCH3OCH3 N
235 CH,~ C02 CH3 C(CH3)3 CH3 CH8 CH
236 CH3 Coa CH3 C(CH3)3 C~3OCH3 CH
237 CH3 CO, CH3 C(CH3)3 CH3,OCH3 N
238 CH3 CoN(cH3)2 c-~3 CH3; CH3 CH
239 CH3 CoN(cH3)2 CH3 C-~3OCH3 "Çl
240 CH3 CON(CH3)2 CH3 OCH3OCH3 CH
241C1-~3 CON(CH3)2 CE~3 5T,~3OCH3 N
242C .I3 CO N( CH~)2CT~3 O CH3O CH3 N
243C-.I3 CON(C2H5)2 c-~3 CT,~3CH3 CH
244 CH3 CO~(C3H6)2 CH3 CH3OCH2 CH
245 CH~ CON(C2r'~5), CH3OCH3 OCH3 CH
246 CH~ CON(C.Hl)2CH,, CH3OCH3 N
- 134û326
-- 20 --
Comp. A ' B C ~ y Z
No.
247 CH3 CON(C2H6)a C33OCEI3 OCEI3 N
248 CH3 CON ~CH~ CH3 CH3 CH3 CH
C 2 H 5
249 CH3 CON ~ CH3 CH3 C-d3 OCH3 CH
C3 H6
250 CH3 CON C~3 CH3 CH3 OCH3 N
C2 H 5
251 CH3 CONHCH3 C~3 C~3 CH3 CH
252 CH3 CONE~CH3 CH3 CEI3 OCH3 CH
253 CH3 CONHCH3 5H3 CH3 OCH3 N
254 CH3 CONHC2H,, ~ CH~ CH9 .CH3 CH
255 CHg CONHC2H5 CH3 CH3 OCH3 CH
256 C~3 CONH C2 H5 C-~3 5rd3 OCH3 N
257 CH3 CON~ 3 CH3 CH3 CH3 CH
CH3
258 CH3 CON~ CHi3 CH3 OCH3 ~ CH
CH3
25 ~ CH3 CON~ CH3 CH3 OCH3, N
260 I CH3 C O~ CH3 ! CH3 CH3 CH
134032~
-- 21 --
_No . _ _ __ _ _ ._ __
261 CH3 C02 CH3 ~ CH3 OCH3 C~
262 CH3 COy CH3 ~ OCHa OCH3 CH
263 CH3 COy CH3 ~ CHg OCH3 N
264 cr~3 CO~ CH3 ~ OCH3 OCH3
265 CHI COz CH3 ~CH3 CH3 OCH3 CH
266 CH3 CO, CH3 ~CH3 5H3 OCH3 N
267 C-da COz CH3 ~-C2H6, CH3 OCH3 CH
268 CH3 C02 CH3 ~CzH6 CH3 OCH3 N
CH3
269 CH3 C~2 CH3 ~) CH3 OCH3 CH
270 C-13 CO, CH3 C~ CH3 OCEI3 :~
271 "H3 C03 C~3 ~ C~ Cr~3 C H3 CH
272 CH3 C~3 CH~ ~ C~ CH3 OCH3 CH
273 CX3 C~a CH3 ~-c,e,CEI3 ,05H3 N
. _....................... C~
274 CH3 C02 CH3 _~ CX3 ,C H3 CH
- 22 - 1340326
Comp- A B C X Y Z
_No . -
C~.~
2 75 CH3 COaCH3 C~ CH3 OCH3 CH
2 7 6 CH3 CO~ C~,~) CH3 0 C~3 N
NOa
2 7 7 CE3 CO3 CH3 ~ CH3 CH3 "H
2 7 8 CH3 CO2 CH3~5N(~ CH3 ~ CH3 CH
NO.~
27 9 CH3 CO2CH3 ~ CH3 OCH3
280 H CO2CH3 ~ CH3 CH3 CH
2 81 H CO2CH3 ~ CH3 OCE~3 CH
282 H CO2CH3 <~) CH3 O_E~3 N
283 CH3 CO2CH3OCH3 CH3 CH3 CH
28 4 CH3 CO2CH3OCE33 CT~3 OCH3 CH
-285 CH3 CO2CH3OCH3 05H3 OCH3 CH
286 C~L~3 CO2CH3OC;I3 CH3 OCH3
287 CH3 CO2CH3OCH~ OCH3 OCH3 N
288 C~3 CO2CH3OCHaCH3 CH, CH3 CE
289 C~3 CO,CH3OCH2CH3 CH3 OCH3 CH
- 23 - 1340~26
Comp. A B C ~ Y
No . __
2 9 0 CH3CO2CH3OCHa CH3 CH3 o c-~3 N
2 9 1 CH3 CH3 OCH3 CH3 CH3 CH
292 CH8 CH3 OCH3 CH3 OCH3 CH
2 93 C'H3 CH3 OCE~3 OCH3 OCH3 CH
29 4 CH3 CH3 OCH3 CH3 05H3
295 c~3 CH3 OCr~3 OC-~3 05H3 N
29 6 Cr.~3CH3 OCH3 CH3 CH3 N
297 CH3 cr~i3 OCH3 OCH3 N(CH3)2 N
29 8 Cr~3 CH3 ~C-~3 CH3 c~3 CH
29 9 CH3 CH3 OCH3 OCHCO3CH3
CH3
30 0 CH3 Cr~3 OCH3 OCH305H2 C~3 N
30 1 CH3 CH3 OCH2 CH3 CH3 CH3 CH
302 CH3 CH3 OCH2 CH3 CH3 o5H3 CE~
303 C~3 CH3 O''H2CH3 CH3 OCH3 N
3 0 4 CH3 CH3 H CH3 C'~3 CH
3 0 5 CH3 CH3 ! H CH3 OCrc~3 CH
-
- 24 - 1340326
Comp. A B C ~ Y Z
306 CH3 CH3 H OCH3OCH3 CH
307 CH3 CH3 H CH3 ~CH3
308 CH3 CH3 H O CH OO CH3 N
309 CH3 CH3 H Crd3CH a N
310 CH3CH2CH3 H CH3 CH3 CH
311 CH3CH~CH3 H CH3OCH~ Cd
312 CH3CH2CH3 H C~3OCH3
313 C~3C~d ( CH3)2 H CH3C r~3 CH
314 C~3CH( Cr~I3)2 H C~3O~H3 C~
315 CH3CH(CHa)2 H CHa ~CH3
316 , E~ CH3 CH3 C~d3CH 3 Cd
317 H CH3 CH3 CH3O CH3 CT~
318 rd CH3 CH3 ~CH3 ~~H3 CH
319 H C ~d3 C1d3 CH3OCH3 N
320 H CH3 CH3 ~CH5 ~CH3
321 i H CH2 CH3 CH2 CH3 CH3CH 3 CH
- 25 - 1340~26
No. A B C X Y l Z
322 H CH2 CH3 CH~ CH3 CH3 OCH3 CH
323 H CH2 CH3 CH2 CH3 CH3 OCH3 N
324 CH3 H CH3 CH3 CH3 CH
325 CH3 H CH3 CH3 O CH3 CH
326 CH3 H CH3 O CH3 O CH3 CH
327 CH3 H CH3 CH3 OCH3 N
328 CH3 H CH3 O CH3 O CH3 N
329 CH3 H CH3 CH3 CH3 N
330 CH3 H CH2 CH3 CH3 CH3 CH
331 CH3 H CH2 CH3 CH3 OCH3 CH
332 CH3 H CH~ CH3 CH3 OCH3 N
333 CH3 H CH(CH3)2 ', CH3 CH3 CH
334 CH3 H CH( CH3)2 , CH3 O CH3 CH
335 CH3 H CH( CH3)2 CH3 OCH3 N
336 CH3 H C ( CH3)3 CH3 CH3 CH
337 CH3 H IC ( CH3)3 ,CH3 O CH3 CH '
_
- 26 - 1340326
Comp. A B C X Y Z
No .
338 CH3 H C (CHa)3 CH3 ~ CH3 N
339 H CH~ CH3 O CH3 CH3 CHJ CE~
340 H CH2 CH3 OCH3 CH3 OCH3 CH
341 H CH~ CH3 OCH3 CH3 OCH3 N
342 HCH(CH3)2 O CH3 CH3 CH3 CH
343 HCH(CH3)2 OCH3 CH3 OCH3 CH
344 HCH(CH3)2 OCH3 CH3 OCH3 N
345 H ~ O CH3 CH3 CH3 CH
346 ~ O CH3 CH3 1 O CH3 CH
347 H ~ OCH3 CH3 OCH3 N
348 CH3CH3 CH 3 O CH3 CH3 CH3 CH
349 CH3CH2 CH3 O CH3 CH3 ~ CH3 CH
350 CH3CH2 CH3 OCH3 CH3 OCH3 CH
351 CH3CH2 CH3 o CH3 CH3 ~ CH3 N
352 CH3CH2 CH3 OCH3 OCH3 OCH3 ! N
353 CH3! CH2CH2 CH3 OCH, , CH3 ' CH3 CH
-
- 27 - 1340326
Comp. A B C X Y Z
354 CH3CH2 CH2 CH3O CH3 CH3 O CH8 CH
355 CH3CH2 CH3 CH3OCH9 CH3 O CH3 N
356 CH3CH (CH3)2 O CH3 CH3 CH3 CH
357 CH3CH (CH3)~ O CH3 CH3 O CH3 CH
358 CH3CH(CH3)2 OCH3 OCH3 OCH3 CH
359 CH3CH(CH3)2 OCH3 CEl3 OCH3 N
360 CH3CH(CH3)2 OCH3 OCH3 OCH3 N
361 CH3CH(CHs)~ OCH3 CH3 -Cf~- CH
362 CH3CH(CH3)2 OCH3 OCH3CH2C.~20CH9 CH
363 CH3CH(CH3)2 OCH3 CH3CH!2(t3-~H3 N
364 CH3C ( CH3) 3 O CH3 CH3 CH2 CH
365 CH3C ( CH3)3 O CH3 CHa O CH2 CH
366 CH3C (CH3)~ OCH3 Cd3 OCH:! N
367 CH3S CH3 CH3 CTd3 CH3 CH
368 CH3S CH3 CH3 CH3 O CH3 CH
369 CH3S CH3 CH3 !CH3 O CH3 ' N
-
~3~0326
-- 28 --
Comp. A B C X Y Z
370 CH3S (O) CH~ CH3 CH3 CH3 CH
371 CH3S (O)CH3 CH3 CH3 OCH3 CH
372 CH3S (O) CH3 CH3 CH3 O CH3 N
373 CH3SO2 CH3 CH3 CH3 CH3 CH
374 CH3S ~2 CH3 CH3 CH3 ~ CH3 CH
375 CH3S03 CH3 CH3 CH3 OCH3 N
376 CH3SO2CH2 CH3 CH3 CH3 CH3 ~ CH
377 CH3SO2 CH2 CH3 CH3 CH3 OCH3 CH
378 CH3SO2 CH2 CH3 CH~ CH3 ~ CH3 N
379 CH3SO2 C~ H,-n CH3 CH3 CH 3 CH
380 CH3SO2C3 H,-~ CH3 CH3 OCH3 CH
381 CH3SO2C~H,-n CH3 OCH3 OCH3 CH
382 CH3SO2 C3 H,-n CH3 CH~ o CH3 N
383 CH3SO2C3 H,-n CH3 OCH3 OCH3 N
384 CH3 CH3 CO2 CH3CH3 CH3 CH
385 ; CH, CH8 ~cO2 CH3CH~ IO CH3 CH
- 1340~2fi
-- 29 --
Comp. A B C X y z
No.
3 8 6 CH3 CH8 CO2CH3 O CH3 O CH3 CH
3 8 7 CH3 CH3 C 02 CH3 CH 3 O CH 3 N
3 88 CH3 CH3 CO2CH3 OCH3 OCH3 N
3 8 9 CH3 CH3 C 02 CH3 C~3 CH3 N
3 9 0 CH3 CH3 CO2CH3 CP.3 C~ C(CH2)
3 91 CH3 CH3CO2CH3 CHt ~ OCH2 CH2C -
3 9 2 CH3 CH3CO2CH3 CH3 C~ CH
393 CH3 CH3CO2CH3 c~3 OCH2CH3 . CH
3 9 4 CP,3CH3CO3 CH3 CH3 H CH
3 9 5 CH3 CH3CO2 CH3 CH3CH2 O CH3 CH
3 9 6 CH3 CH3C 02 CH3 CH3 CF3 CH
39 7 CH3 CH3CO2CH3 CH3OCH2 CF3 CH
3 9 8 CH3 CH3CO2 CH3 C'r.3OCH2C02CH3 CH
3 9 9 CH3 CH3CO2CH3 C~ c~ CH
4 0 0 CH3 CH3CO2 CH3 Br Br CH
40 1 CH3 CH3CO,CH3 CH3 C~ N
-
~ 30 - 134032~
Comp. A B C X Y Z
No .
402 CH~ CH3 CO~ CH3 C~N(CH3)2 N
403 CH3 CH3 CO2 CH3 OCH3N(CH3)2 N
404 CH3 CH3 CO2 CH3 CH~OCH2CH3 N
40 5 CH3 CH3 CO2 CH3 CH,,NHCH2CH3 N
406 CH3 CH3 CO2 CH3 CH3CH2OCH3 N
407 CH3 CH3 CO2 CH3 CH3OCI CO2H N
408 CH3 ~ CH3 CO2CH2 CH3 CH~ CH3 CH
409 CH3 CH8 CO2CH2 CH3 CH3o CH3 CH
410 CH3 CH3 CO2CEI2CH3 OCH3OCH3 CH
411 CH3 CH3 CO2CH2CH3 CH3OCH3 N
412 CH3 CH3 CO2CH2CH3 OCH3OCH3 N
413 CH3 CH3 I CO2CH2CH3 CH3C~ C(CH2)2C~
414 CH3 CH3 CO2CH2CH3 CH3- OCH2CH2C -
415 CH3 CH3 CO2C3Hr-n CH3CH3 I CH
416 CH3 CH3 CO2C8H7-n CH3OCH3 CH
417 CH3 CH3 C02 C3H7-n ~ OCH3 OCH3 CH
- 31- 1340~2~
Comp. A B C ! X I Y Z
4 1 8 CH3 CH9 COO CgH~ -~1 CH3 O CH3 N
41 9 CH3 CHt COoC3H7 ~1 OCH3 OCH3 N
4 2 0 CH3 CH3 CO2 C3 H~ -n CH3 CH 3 CH
421 CH3 CH3 COaC3H, -n CH3 OCH3 CH
4 2 2 CH3 CH3 CO2 C3H, ~1 OC H 9 0 CH3 CH
4 2 3 CH3 CH3 CO2 C5 H~ ~1CH3 O CH3 N
4 2 4 CH3 CH~ CO2 C3 H7 -~1OCH 3 O CH3 N
4 2 5 CH3 CH3 Coa CH~ CHCHa CH3 CH3 CH
42 6 CH3 CH3 CO2CH2C~-CE~ CH3 OCH3 CH
427 CH3 CH3 CO2CH2CH--CE~OCH3 OCH3 CH
428 CH3 CH3 CO2 CY2CH--CHaCH3 . OCH3 N
42 9 CHt CH3 CO2CE~aCH=CHa~ OCH3 OCH3 N
4 3 0 CH3 CH3 CO2 CE~2C-CEICH3 CH3 CH
4 31 CH~ CH3 CO2CH C --CH CH3 OCH3 CH
4 32 CH3 CH3 CO2CHC _CH OCH3 OCH3 CH
433 I CH3 CH3 CO2CHC_CH CH3 OCHt N
_ 1340~26
-- 32 --
Comp. A B C X Y . Z
434 CH3 CH3CCLCHCsCH O CH3 0 CH3 N
435 CH3 CH3CO2H CH3 CH3 CH
436 CH3 CH~C O2H CH3 0 CH, CH
437 CH3 CH3C 02H OCH3 0 CH3 CH
438 CH3 CH3CO2H CH3 0 CH3 N
439 CH3 CH8C 02H O CH 3 0 CH3 N
440 CH3 CH3CO2H CH3 CH3 N
441 CHgCH3 CH~CO2 CH3 CH3 , CH3 CH
442 CH2CH3 CH,CO2 CH3 CH3 OCH3 CH
443 CH2CH3 CH3COg CH3 OCH3 OCH3 ~ CH
444 CH,CH3 CH3CO2 CH3 CH3 OCH3 N
445 CH2CH3 CH3C~a CH3 OCH3 OCH3 N
446CH2 CH2CH3 CH3CO2 CH3 CH3 CH3 I CH
447CH2CH,CH3 CH3CO2 CH3 CH3 OCH3 CH
448CHaCH2CH, CH3CO2 CH3 CH3 OCH3 N
449CH(CE~)2 CH~C 4 CH, CH3 CH3 CH
- 33 - 13 4032 6
Comp..... A B C X Y I Z
No .
450 CH (CH3)2 CH3 CO2CH3 CH3 OCH3 CH
451 CH(CH3)2 CHt - CO2CH3 CH3 OCH3 N
452 ~ CH3 CO3CH3 CH3 CH3 CH
453 ~ CH3 CO2CH3 CH3 OCH3 CH
454 ~ CH3 C O2 CH3CCH3 O CH3 CH
455 (~ CH3 CO2CH3 CH3 OCH3 N
456 ~ CH3 C O, CH3OCH3 O CH5 N
457 CH3 H 'C03 CH3CH3 CH3 CH
458 CH3 H CO2CH~ CH3 OCH3 CH
459 CH3 H C ~2 CH3 CH3 O CH3 CH
460 CH3 H CO~CH3 CH3 OCH3 N
461 CH3 H CO3CH3 OCH3 OCH3 N
462 CH3 CH2CH3 CO~CH3 CH3 CH3 CH
463 CHt CH2CH3 CO2CH3 CH3 OCH3 CH
464 CH3 CH2CHt CO2 CH3OCH3 O CH3 CH
465 CH3 CH2 CH3 CO2 CH3 CH3 O CH3 N
1340326
-- 34 --
Comp. A B C ~ Y
466 CH3 CH2CH3 CO2CH3 OCH3 OCH3 N
467 CH3 CH2 CH.lCH3 C02CH9 CH3 CH3 CH
468 CH3 Cd2CH2CH3 CO,CH3 CH3 OCH3 CH
469 CH3 CH2 CH2CH3 COaCH3 O CH3O CH3 CH
470 CH3 CH2CH,CH3 C02CH3 CH3 OCH3 N
471 CH3 CH2 CH,CH3 CO, CH3 O CH3O CH3 N
472 CH3 CH(CH3)~ CO2CH3 c~3 CH3 CH
473 CH3 CH(CH3)2 C02CH3 cH3 OCH3 CH
474 CH3 CH(CH3)2 CO"CH~ OCH3 OCH8 CH
475 CH3 CH(CH3)2 CO,CH3 CH3 OCH3 N
476 CH3 CH(CH3)2 C02CH3 OCH3 OCH3
477 CH3 iC (CH3 )3 CC2CH3 CH3 CH3 CH
478 CH3 C (CH3)3 CO2CH3 CH3 OCH3 CH
479 CH3 C (CH3)3 co2CH3 CH3I OCH, N
480 CH3 CH9 CON(CE~ )2 CH3 CH3 CH
CH8 CH3 ! CON(CH3)2 l CH3 o CH3 I CH
13~0326
-
~ 35 --
No. B C ~ Y
482 CH3 CH3CON(CH3)2 OCH3 OCH3 CH
483 CH3 CH3CON(CH3)2 CH3 OCH3 N
484 CH3 CH3CON(CH~)a OCH3 OCH3 N
485 CH3 CH3CON(C2H5)2 CH3 CH3 .CE~,
486 CH3 CH~cON(C2H5)2 CH3 OCH3 ,CH
487 CH3 CH3CON(C2H5)2 OCH3 OCH3 CH
488 CH3 CH3CON(C2H5)2 CH3 OCH3 , N
489 CH3.CH3CON(C8H5)2 OCH3 OCH3 N
490 CH3 CH3 'C2H5 CH~ CH3 CH
491 CH3 CH CoN~CH3 CH3 O CH3 CH
492 CH3 CH3CON~c H CH3 OCH3 N
493 CH3 CH3CONH CH3 CH3 CH3 CH
494 CH3 CH3CO NH CH3 CH3 ~ CH3 CH
495 CH" CH3CONHCH3 CH3 OCH3 N
496 CHl CH5CONH C2H5 CH3 CH3 CH
-
. .
- 36 - 1340~25
! Comp. A B C ~ Y Z
49 7 CH3 CH3 CONHC2H5 CH3 OCH3 CH
498 CH3 c~3 CONHC2H5 CH3 OCH3 N
4 9 9 CH3 CH CoN,CH3 CH CH3 CH
5 0 0 CH3 CH3 CON ~ CH3 O CH3 CH
CH3
5 01 CH3 CH3 CON~ CH3 OCH3 N
5 0 2 CH3 j~ CO2 CH3 CH 3 CH3 CH
503 CH3 ~ CO3 CH3 CH3 OCH3 CH
5 o 4 CH3 ~ CO2 CH3 OCH3 OCH3 CH
50 5 CH3 ~ CO2 CH2 c~3 OCH3 N
5 0 6 CH3 ~ C~a CH3 OCH3 OCH3 N
5 o 7 CH3 O CO2 CH3 CH3 CH3 N
508 C~3 ~ CO2 CH3 CH3-OCH2CH2C-
509 CH3 (~ CO2 CH3 CH3C~ C(CH2)
51 0 CH3 ~ C~2 CH3 OCH3N(CH3)3 N
51 1 CH3 ~CH3 C02 CH3 CH3 CH3 CH
1340~26
-- 37 --
NoP A B C ~ Y Z
51 2 CH3~CH3 C03 CH3 CH3OCH3 CH
51 3 CH3~CH3 C~2C~3 CH3OCH3 N
514 CH~ ~ C O~ CH3 CH3 CH3 CH
5 1 5 CH3 ~ CO,CH3 CH3OCH3 CH
~CH3
516 CH3 C CO,CP.3 CH3OCH3 N
51 7 CH3 H~ CO2CE;3 CH3 CH3 CH
518 CH3 CH~ CO2C~3 CH3OCH3 CH
CH3
51 9 CH3 ~ CO,CH3 CH9OCH3 N
5 2 0 CH3 ~C~ CO2CH3 CH3 CH3 CH
5 2 1 CH3~- C~ C O~ CEl3 CH3O CH3 CH
5 2 2 CH3~ C~ CO, CH3 CH3O CH3 N
52 3 CH3 ~> CO2CH3 CH3 CH3 CH
5 2 4 CH" C~ C02 CH3 CH3~ CH3 CH
525 CH~ ~ CO2CH, CH3OCH3 N
13~0326
- 38 -
Comp. A I B C ' X y
No .
526 CH3 ~ CO2 CH3 CH8 CH3 CH
527 CH3 ~ CO~ CH3 CH8 O CH3 CH
528 CH3 ~ CO3 CH3 CH3 OCH3 N
529 CH3OCH3 CO2 CH3 CH3 CH3 CH
530 CH 3OCH3 CO3 CH3 CH3 OCH3 CH
531 CH3oCH3 C02 CH3 O CH3 . OCH3 : CH
532 CH3OCH3 CO, CH3 CH3 OCH3 N
533 CH3OCH3 CO2 CH3 OCH3 OCH3 N
534 CH3OCH3 CO2 CH3 CH3 CH3 N
535 CH3OCH2CH3 CO2 CH3 CH3 . CH3 CH
536 CH3OCH2CH~CO2 CH3 . CH3 O CH3 CH
537 CH3OCH2CH3CO2 CH3 CH3 OCH3 - N
538 CH3CH3S CH3 CH3 CH3 CH
539 C H~ CH3 S CH3 CH3 OC H3 CH
540 ~ CH3 S CH3 O CH3 OGH3 CH -
1340326
- 39 -
omp. A B C X Y Z
No .
541 CH3 CH9S CH9 CH9 OCH3 N
542 CH9 CH9S CH9 O CH9 O CH9 N
543 CH9 CH9SO~CH9 CH9 CH9 CH
544 CH9 CH9S ~2 CH9 CH9 O CH9CH
545 CH9 CH9SO~CH9 OCH9 OCH3 CH
546 CH3 CH9S O, CH 9 CH9 O CH3 N
547 CH3 CH3S 02 CH3 O CH9 O CH9 N
548 CH9 CH9SO2CH9 CH3 CH9 N
549 CH9 CH9SO2 CH2 CH9CH 9 CH3 CH
550 CH9 CH9SO2 CH2 CH9CH9 O CH9CH
551 CH3 CHtSO2CH2CH3OCH9 OCH9 CH
552 CH9 CH9SO2CH2CH9CH9 O CH9 N
553 CH9 CH9SO2CH2CH9OCH9 OCH9 N
554 CH9 CH9SC9H7-n CH9 CH9 CH
555 CH~ CH9SC9H7-n CH9 OCH9 CH
556 CHt CH9 S C~ H, -n I O CH9 O CH9 CH
_ 40 _ 1340~2fi
Comp. A B C X Y Z
No .
557 CH3 CH8 SC3H7-n CH3 OCH3 N
5 5 8 CH3 CH3 S C3H, -n O CH3 O CH3 N
55 9 CH3 CH3 SC3H7 -n CH3 CH3 N
5 6 0 CH3 CH3 ,S(O)C3H7-n CH3 CH3 CH
561 CH3 CH~ S(O)C3H7-n CH3 OCH3 CH
56 2 CH3 CHS S(O)C3H7~n OCH3 O CH3 CH
5 6 3 CH3 CH8 S(O)C3H7 -n CH3 OCH3 N
56 4 CH3 CH3 S(O)C3H7-n OCH3 OCY3 N
565 CH3 CH3 S03 CSHT-~ CH3 CH3 CH
56 6 CH3 CH3 S~a C3HT -n CH3 OCH3 CH
567 CH3 CH~ SO2 C3HT -n OCH3 OCH3 CH
568 CH3 CH3 SO2 C3 Hr -n CH3 OCH3 N
569 CH3 CH3 SO, C3H7 -n OCH3 OCH3 N
570 CH3 CH3 SO, C3H7 -n CH3 CH3 N
571 CH3 CH, S~2 C3H7 -n CH3 CH3 C(CH3)~
572 CH3 CH3SO, C8H, -n CH3 -OCH 2 CH25-
- ' 134032fi
-- 41 --
Comp. A B C X Y Z
No .
5 73 CHs CH3SO"C~H7-n CH3 C~ CH
5 7 4 CH3 CH3SO3C3H7-n CH3OCH2CH3 CH
5 7 5 CH3 CH3SOaC3Hr-n CH3 H CH
5 7 6 CH8 CH3SO2 C3H7-n H H CH
5 7 7 C~3 CH3S O2 C3H, -n CH3CH2OCH3 CH
578 CH3 CH3SO3C3H7-n CH3OCH2CO2CH3 CH
5 7 9 CH3 CH3SO2C3H7 -n CH3 . CQ N
580 CH3 CH3SO2C3H7-n C~ N(CH3)2 N
581 CH3 CH3SO2C~H7-n OCH3 N(CH3)2 N
582 CH3 CH3SO2C3H7-n CH3 OCH2CH3 N
5 8 3 CH3 CH3S O~ C3H7 - nCH 3 NHCH3 N
5 8 4 CH~ CH3S O2 C3H7 -n CH3 OCH2CF3 N
OCH-CO2H
5 8 5 CH3 CH3S O2 C3Hr -n CH3 CH3 N
5 8 6 CH3 CH3SO2c3H7-i CH3 CH3 CH
587 CH3 CH3So2c~H7-l CH3 CH3_ CH
588 CH3 CH,So2c3H7-i OCH" CH3 ! CH
- 42 - 1 3~0 3 2 6
NoP A B C X , Y , Z
5 8 9CH3 CH3SO2C3H, -i CH3 ~ CH3 N
59a CH3 CH3SO2C3H7-i OCH3 OCH3 N
5 91 CH3 CH3SO~C3H7 -i CH3 CH3 N
5 9 2CH3 CH3SO2C3H7 -i CH3 - OCH3CH2C -
5 93 CH3 CH3SO8C3HT -1 CH3 C~ C(CH2)2
59 4 CH~ CH3S03C3H7 -i OCH3 N(CH~)2 N
595 CH3 CH3SO2N(CH3)l CH3 CH3 CH
596 CH3 CX3SO2N(CH3)2 CH3: OCH3 ' CH
5 9 7CH3 CH3SOs,N( CH3)2OCH3, O CH3 CH
5 9 8CH3 CH3So2N( CH,), CH3 o CH3 N
599 CH3 CH3SO~N(CH3)2 OCH3 OCH3 N
6 0 0CH3 CH3SO2N( CH3)2 CH3 .? CH3 N
60 1 CH3 CH3SO2N(CH3)2 CH3 -aCH2CH2C-
6 0 ZCH3 CH3SO2N(CHa)2 JCH3 C~ 1 C(CH2)2C~
603 CH3 CHsSO~N(CH3)2 OCH8 N(CH,)2 N
60 4 CH3 ¦ CH3SO3N(C2H5), CH3 CH3 CH
1340~2fi
-- 43 --
Comp. A B C X Y , Z
No .
6 0 5 CH3 CH3 SO2N (C2H5)2CH3 OCH3 CH
606 CH3 CH3 SO2N(C2H5)2OCH3 OCH3 CH
6 ~ 7 CH3 CH3 SO2N (C2H5) 2 CH3 o CH3 N
6 0 8 CH3 CH~ SO2N (C2H5)2O CH3 O CHt N
6 ~ 9 CH8 CH3 SO2N(C2H5)2 CH8 CH3 N
610 CH3 CHt SO2N~ CH3 CH3 CH
C2H5
6 1 ~ CH3 CH3 . SO2N~C H CH3 OCH3 CH
61 2 CH3 CH SO N,CH3 OCH3 OCH3 CH
613 CH3 CH3 SO2'N~C H CH3 OCH3 N
6 1 4 CH3 CH3 SO2N OCH, OCH3 N
C2H5
615 C~3 CH3 SO3N~C H CH3 CH3 N
61 6 . CH3 CEI.3 SO2NHCH3 CH3 CH3 CH
61 7 CH3 CH3 SO2NHCE~3 CH3 OCH3 CH
6 1 8 CH3 CH3 SOaNHCH3 CHt OCH3 N
61 9 ¦ CH3 CH3 SO~NHC2H~ CH3 ICH3 CH
~- 134q~2~
-- 44 --
Comp. A B C X Y Z
6 2 0CH3 CH3S 02NHC3H6 CH, O CH3 C
6 21 CH3 CH3So2NHc2H6 CH3 OCH3 N
6 2 2CH3 CH3SO2NHC3H7-n CH3 CH3 CH
6 23 CH3 CH3SO2NHC3H7-n CH3 OC H3 CH
624 CH3 CH3SO2NHC 3H7-n CH3 OCH3 N
6 2 5CH3 H S CH3 CH3 OCH3 CH
6 26 CH3 H SCH3 CH3 OCH3 N
6 2 7CH3 HS(O)CH3 CH3 C~3 CH
628 CH,~ HS(O)CH3 CH3 OCH~ CH
6 2 9CHt HS (O) CH3 CH3 ~ CH3 N
6 3 0CH3 HSO2 CH3 CH3 CH3 CH
63 1 CH3 HSO2CH3 CH3 OCHa ¦CH
6 3 2CH3 HS 02 CH3 O CH3 O CH3 CH
6 3 3CH3 HS O~ CH3 CH3 ~ CH3 N
6 3 4CH3 HS02CH3 O CH3 O CH~ N
6 3 5CH" HS 02 C2H6 CH3 CH3 CH
-
- 45 - 1340-32~
Comp. A B C ~ Y Z
No .
636 C H3 H SO2 C2 H5 CH3 OCH8 CH
637 CH 3 H S ~2 Ca H~ CH3 O CH3 N
638 CH 8 H S ~2 C3H,-~ CH3 CH3 CH
639 CH 3 H SO2C3H7-~ CH3 O CH3 CH
640 CH3 H SO~C3H7-~ OCH3 OCH3 CH
641 CH8 H SO2C3H7-~ CH3 OCH3
642 CH3 H S O, C3HT-~O CH3 o CH3 N
643 CH3 H S O ~ C3H7-~CH3 CH3 N
644 CH3 H SO2C3Hr-~ CH3 -OCH 2CH2C-
645 CH3 H SO2C3H,-n CH3 C~ C(CH2)2C~
646 CH3 H S ~2 C3H7-~ O CH3N (CH3) 2 N
647 CH3 H SO2C3H,-i CH3 CH3 CH
648 CH3 H S O2 C3H, -iCH3 O CH3 CH
649 CH3 H SO2C3H,-i OCH3 OCH3 CH
650 CH~ H SO3 C3H7 -iCH3 ~ CH3 N
651 C~3 H SO2C3H7-i OCH~ OCH3 N
1340~2fi
-- 46 --
Comp. A B C X Y Z
652 CH3 HS02C3H7-l CH3 CH8 N
6 53 CH8 HSO2C H9-n CH3 CH3 N
654 CH3 HSO2C~H9-n CH3 OCH3 CH
6 55 CH3 H SOaC H~, -n CH3 OCH3 N
6 5 6 CH3 HSO,N (CH3) ~ CH3 CH3 CH
657 CH5 HSO2N(CH~)2 CH~ OCH3 CH
658 CH3 H SO~N(CH3)s OCH3 OCH~ CH
6 5 9 CH~ HS O,N (CH3)2 CH3 ~ CH3 N
6 6 0 CH3 HS 03N (CH3), 0 CH3 0 CH, N
6 61 CHt HSO2N (CH3)2 CH3 CH3 N
6 6 2 CH~ HSO2N(C3H5)2 CH3 CH3 CH
6 6 3 CH~ HS 02N (C~Hs)2 CH3 0 CH3 CH
6 6 4 CH3 HS O,N (CqH5)2 0 CH3 0 CH3 CH
6 6 5 CH3 HS 02N (C2H5)2 CH3 0 CH3 N
6 6 6 CH3 H SO,N(CqH5), OCH3 OCH~ N
6 6 7 CH3 HSO2 NH CH 3 CH3 CH3 CH
- 47 - 13~0~2
Comp. A B C X Y Z
No.
668 CH3 HSO2NHCH3 CH3OCHS CH
669 CH3 HSO2NHCH3 CH3OCH3 N
670 CH3 HSO3NHC2H6 CH3CH8 CH
671 CH3 HS02NHC2H5 CH3OCH3 CH
672 CH3 HS02NHCoH5 CH3OCH3 N
673 H CH3 SCH3 CHt CH3 CH
674 H CH3SCH3 CH3OCH3 CH
675 H CH3SCH3 CH3OCH3 N
676 H CH3SO2CH3 CH3CH3 CH
677 H CH3SO2CH3 CH3OCH3 CH
678 H CH3SO3CH3 CH3OCH3 N
679 H CH3So2c2H5 CH3CH3 CH
680 H CH~S03 C2Hs CH3OCH3 CH
681 H CH,SO2C2H5 CH~OCH3 N
682 H CH~SOaC3H7-~ CH3CH3 CH
683 H CH3 SO3C3H,-~ CH~OCH3 CH
- 48 - 13~0~2~
Comp.- A B C X Y Z
No.
684 H CH3 S02 C3H~ -nOCH3 .OCH3 CH
685 H CH8 SOaC3H7 -n CH3 OCH3 N
686 H CHt SO2C3H7 -nOCH3 OCH3 N
687 H CH3 S02N(CH3)2 CH3 CH3 CH
688 H CH3 S02N (CH3)2CH3 O CEI3 CH
689 H CH3 S02N(CH3)2 CH3 OCH3 N
690 H CH3 S02N(C2Hs)2CH3 - CH3 - CH
691 H CH3 S03N(C2H5)2CH3 OCH3 CH
692 H CH3 S~2N(C2~s)~ CH3 OCH3 N
693 H H S 02 CH3 CH3 CH3 CH
694 H H SO2CH3 CH3 .OCH3 CH
695 H H SO,CH3 CH3 OCH3 N
696 H H S ~2 C3H7 -n CH~ CH3 CH
697 H H S02C3Hr~ll CH3 OCH3 CH
698 H H SO2C3H~-n CH3 OCH3 N
699 H H SO~N(CH3)2CH3 CH3 CH
-
-
~ 49 ~ 1340326
Comp. A B C
700 H H S02N(CH3)a CH3OCH3 CH
7 0 1 H H S02N(CH3 )2 CH3 OCH3 N
7 0 2 H H So2N(c2Hs)3 CH~ CH3 CH
703 H H S02N(C2Hs)2 CH3 OCH3 CH
7 0 4 H H S02N(C2H~)2 CH3 o CH3 N
705 ~ CH3 SO2CH3 CH3 CH3 CH
7 0 6 (~ CH3 S 02 CH3CH3 ~ CH3 CH
707 (~ CH3 SO2CH3 CH~OCH3 N
7 0 8 ~ CH3 SO2C~H7 ~~ CH3 CH3 CH
7 0 9 ~) CH3 SO2C3H7 ~~ CH3 OCHs CH
710 ~ CH3 SO2C3H7-n CH3OCH3 N
71~ ~ CH3 SO2N(CH3)2 CH~ CH3 CH
712 ~ CH3 SO2N(CH3)2 CH3 OCH3 CH
71 3 ~ CH3 SO2N(CH3)2 CH3 OCH3 N
71 4 ~ CH3 SO2N(C2H~)2 CH3 CH3 CH
71 5 ~ CH3 SO2N(C,H~)2 CH~ OCH3 CH
~ 50 - 1340326
C omp ~ A ~ C X I Y Z
71 6 ~ CH3SO~N(C2H5)3 CH3 OCH3 N
71 7 CH3 H H CH3 CH3 CH
7 18 CH3 H H CH3 OCH3 CH
71 9 CH3 H H OCH3 OCH3 CH
7 2 0 CH3 H H CH3 OCH3 N
7 2 1 C~3 H H O CH3 O CH3 N
7 2 2 H H H CH3 CH3 CH
723 H H H CH3 OCH3 CH
7 2 4 H H H O CH3 O CH3 CH
7 2 5 H H H CH3 OC~I3 N
7 2 6 H H H O CH3 O CH3 N
-
~ - 51 - 1340~2~
Comp.
N A B C X Y Z
727CH2CH3 C~2CH3 CH3 3 3 CH
728CH2CH3 C~2CH3 CH3 3H3 N
729CH3 CO2CH2CH3 ~ ~ CH3CH3 CH
730CH3 CO2CH2CH3 ~ CH3OCH3 CH
731CH3 CO2CH2CH3 ~ OCH3OCH3 CH
732CH3 -~2CH2cH3 ~ CH3OCH3 N
733CH3 -~2CH2cH3 ~ OCH3OCH3 N
734CH3 CH3 02C3 7 i CH3CH3 CH
735CH3 CH3 ~2C3H7 i CH3OCH3 CH
736CH3 CH3 CO2C3H7-i ~ 3C 3 CH
737CH3 CH3 CO2C3 7 i CH3OCH3 N
738CH3 CH3 C 2C3 7 O 3 3 N
739 CH3 H H CH3 -OCH2CH2C-
740 CH3 H H CH3 CQ C(CH2)2
. 3 H H 3 3 N
742 CH3 CH3 SC2H5 3 3 CH
-
- 52 - 1 3q n ~2
Comp
~ A B C X Y Z
743 CH3 CH3SC2H5 CH3 OCH3 CH
744 CH3 CH3SC2H5 3 3 CH
745 CH3 CH3SC2H5 CH3 OCH3 N
746 CH3 CH3SC2HS C 3 3 N
747 CH3 CH3 S ~ 3 3 CH
748 CH3 CH3 S ~ CH3OCH3 CH
749 CH3 CH3 S ~ CH3OCH3 N
750 CH3 CH3SCH2 ~ CH3 C 3 CH
751 CH3 CH3SCH2 ~ CH3OCH3 CH
752 CH3 CH3C 2 ~ C 3 3 CH
753 CH3 CH3SCH2 ~ CH3OCH3
754 CH3 CH3SCH2 ~ OC 3 3 N
755 CH3 CO ~ CH3 CH3OCH3 CH
756 CH3 CO ~ CH3 CH3OCH3 N
3 CON ~ CH3 CH3 OCH3 CH
758 CH3 CON ~ CH3 CH3OCH3 N
~ _ 53 _ 1340~26
Comp
~ A B CX Y Z
759 CH3CON ) CH3 CH3 OCH3 CH
760 CH3CON~ CH3 CH3 OCH3 N
761 CH3CON~_~NCH3 CH3 CH3 OCH3 CH
762 CH3CH3 CON~ 3 3 CH
763 CH3CH3 CON~CH3 OCH3 CH
764 CH3CH3 CON~ 3 OCH3 CH
765 CH3CH3 CO ~CH3 OCH3 N
766 CH3CH3 CO~ ~ C 3 OCH3 N
767 CH3CH3 CO ~ 3 C 3 N
768 CH3CH3 CO ~ CQ CQ N
769 CH3CH3 CON ~ CH3 OCH3 CH
770 CH3CH3 CO ~ CH3 OCH3 N
771 CH3CH3 CON ~ CH3 OCH3 CH
772 CH3CH3 CON ~ CH3 OCH3 N
3CH3 CO ~ CH3 CH3 OCH3 CH
774 CH3CH3 ~_~ 3 3 3 N
1340~2fi
Table 2
~ O ~
~N ~ S02NHCNH~i ~Z
I N=<
A Y
Comp. A B C X Y Z
775 CH3 CH3 H CH3 CH3 CH
776 CH3 CH3 H CH3 OCH3 CH
777 CH3 CH3 H OCH3 OCH3 CH
778 CH3 CH3 H CH3 OCH3 N
779 CH3 CH3 H OCH3 OCH3 N
780 CH3 CH3 CH3 CH3 CH3 CH
~ 781 CH3 CH3 CH3 CH3 OCH3 CH
782 CH3 CH3 CH3 CH3 OCH3 N
783 ~ CH3 H CH3 OCH3 CH
784 ~ CH3 H CH OCH N
785 ~ H H CH3 OCH3 CH
786 ~ H H CH3 OCH3 N
787 CH3 3 ~ CH3 OCH3 CH
788 CH3 3 ~ CH3 OCH3 N
C~3 CQ C 3 C 3 CH
790 CH3 CH3 CQ CH3 OCH3 CH
-
_ 1340326
Co~;p. A B C X Y Z
791 CH3 CH3 CQ C 3 OCH3 CH
792 CH3 CH3 CQ CH3 OCH3 N
793 CH3 CH3 CQ 3 3 N
794 CH H CQ 3 C 3 CH
795 CH3 H CQCH3 OCH3 CH
796 CH3 H . CQOC 3 ~ 3 CH
797 CH3 H CQCH3 OCH3 N
798 CH3 H CQOC 3 ~ 3 N
799 CH3 CH3 NO2 3 3 CH
800 CH3 CH3 NO2 CH3 OCH3 CH
801 CH3 CH3 NO2 C 3 O 3 CH
802 CH3 CH3 NO2 CH3 OCH3 N
803 CH3 CH3 NO2 C 3 3 N
804 CH3 H NO2 CH3 OCH3 CH
3 H N~2 C 3 O 3 CH
806 CH3 H NO2 CH3 OCH3 N
- 1~4032fi
- 56 -
C~o~.p. A B C X Y Z
807 CH3 CH3 Br CH3 OCH3 CH
808 CH3 CH3 Br CH3 OCH3 N
809 CH3 CH3 Br OCH3 OCH3 N
810 CH3 CH3 C 2C 3 C 3 C 3 CH
811 CH3 CH3 C 2C 3 CH3 OCH3 CH
812 CH3 CH3 C~2C 3 OC 3 OC 3 CH
813 ~H3 CH3 C~2C 3 CH3 OCH3 N
814 CH3 CH3 C 2 3 3 3 N
815 CH3 H CO2C 3 CH3 C 3 CH
816 CH3 H CO2C 3 CH3 OCH3 CH
817 CH3 H CO2C 3 OC 3 ~ 3 CH
818 CH3 H CO2C 3 CH3 OCH3 N
819 CH3 . H CO2C 3 OC 3 3 N
820 CH3 H C~2CH3 CH3 CH3 N
821 CH3 H CO2C 3 CH3 C(CH2)2C
822 C 3 C~2CH3 CH3 C CH
_ 57 _ 1340326
Comp
B C ~ y z
823 CH3 H C~2CH3 CH3~C2H5 CH
824 CH3 C 2 3 CH3OCH2CO2CH5 CH
825 CH3 H CO2C 3 CH3 CQ N
826 CH3 H C~2CH3 OCH3N(CH3)2 N
827 C 3 H C~2CH3 CHOjCHCO2H N
828 CH3 CH3 CO2C2H5 CH3 CH3 CH
829 CH3 CH3 CO2C2H5 CH3 OCH3 CH
830 CH3 CH3 CO2C2H5 OCH3 OCH3CH
831 CH3 CH3 CO2C2H5 CH3 OCH3 N
832 CH3 CH3 CO2C2H5 oCH3 OCH3 N
833 CH3 H C~2C2H5 CH3 CH3 CH
834 CH3 H C~2C2H5 CH3 OCH3 CH
835 CH3 CH3 CO2C2H5 oCH3 OCH3CH
836 CH3 H C~2C2H5 CH3 OCH3 N
837 CH3 H C~2C2H5 OCH3 OCH3 N
838 H CH3 2 3 CH3 CH3 CH
-
- ~8 - 1340326
CN~om.P A B C X y z
839 H CH3 CO2 3 CH3 OCH3 CH
840 H CH3 C~2CH3 CH3 OCH3 N
841 H H C~2CH3 CH3 OCH3 CH
842 H H 2 3 OCH3 OCH3 CH
843 H H C 2C 3 CH3 OCH3 N
844 H CH3CO2C2H5 CH3 CH3 CH
845 H CH3CO2C2H5 CH3 OCH3 CH
846 H CH3CO2C2H5 CH3 OCH3 N
847 H HCO2C2H5 CH3 CH3 CH
848 H HCO2C2H5 CH3 OCH3 CH
849 H HCO2C2H5 OCH3 OCH3 CH
850 H HCO2C2H5 CH3 OCH3 N
851 H HCO2C2H5 OCH3 OCH3 N
852 ~ C 2C 3 CH3 OCH3 CH
853 ~ 2C 3 CH3 OCH3 N
854 CH2 H CO2C3H7-n CH3 OCH3 CH
139û326
- 59 -
Comp.
A B C X Y Z
855 CH3 H CO2C3H7-n OCH3 OCH3 CH
856 CH3 H CO2C3H7-n CH3 OCH3 N
857 CH3 H CO2C3H7-n CH3 OCH3 CH
858 CH3 H CO2C3H7-n OCH3 OCH3 CH
859 CH3 H CO2C3H7-n CH3 OCH3 N
86~ CH3 H CO2CH2CH=CH2 CH3 CH3 CH
861 CH3 H CO2CH2CH=CH2 CH3 OCH3 CH
862 CH3 H CO2CH2CH=CH2 CH3 OCH3 N
863 CH3 H CO2CH2C--CH CH3 CH3 CH
864 CH3 H CO2CH2C_CH CH3 OCH3 CH
865 CH3 H CO2C4Hg CH3 OCH3 CH
866 CH3 H C~2C4 9 CH3 OCH3 N
867 C2H5 H 2 3 CH3 CH3 CH
868 C2H5 H C~2CH3 CH3 OCH3 CH
869 C2H~ H C~2CH3 CH3 OCH3 N
870 CH3 H CO2H CH3 CH3 CH
.
~ - 60 - 134D32~
Comp. A B C X y z
871 CH3 H CO2H CH3 OCH3 CH
872 CH3 H CO2H CH3 OCH3 N
873 CH3 C2H5 C~2C 3 CH3 OCH3 CH
874 CH3 C2H5 C~2C 3 CH3 OCH3 N
875 CH3 CH3 CON(CH3)2 CH3 CH3 CH
876 CH3 CH3 CON(CH3)2 CH3 OCH3 CH
877 CH3 CH3 CON(CH3)2 CH3 OCH3 N
878 CH3 CH3 CON(CH3)2 OCH3 OCH3 N
879 CH3 CH3 SO2N(CH3)2 CH3 CH3 CH
880 CH3 CH3 SO2N(CH3)2 CH3 OCH3 CH
881 CH3 CH3 S~2N(CH3)2 OCH3 OCH3 CH
882 CH3 CH3 SO2N(CH3)2 CH3 OCH3 N
883 CH3 CH3 SO2N(CH3)2 OCH3 OCH3 N
884 CH3 CH3 SO_N(CH3)_ CH3 CH3 N
885 CH3 H SO2N(CH3)2 CH3 CH3 CH
886 CH3 H SO2N(CH3)2 CH3 OCH3 CH
- 61- 1~40326
CNoOm,p. A B C X Y Z
887 CH3 H SO2N(CH3)2 CH3 OCH3 N
888 CH3 CH3SO2N(C2H5)2 CH3 OCH3 CH
889 CH3 CH3SO2N(C2H5)2 CH3 OCH3 N
890 CH3 ~ CH3 OCH3 CH
891 CH3 CH3 2 ~--~ CH3 OCH3 N
892 CH3 CH3 S~2NO CH3 OCH3 CH
893 CH3 CH3 02N~ CH3 OCH3 N
894 CH3 CH3 S~2N ~ CH3 OCH3 CH
895 CH3 CH3 S02N NCH~ CH3 OCH3 CH
896 H CH3 H CH3 CH3 CH
897 H CH3 H CH3 OCH3 CH
898 H CH3 H CH3 OCH3 N
899 3 OE~ H CH3 OCH3 CH
900 CH3 ~ H OCH3 OCH3 CH
901 C 3 ~ H CP.3 OCH3 N
902 CH3 H ~ CH3 OCH3 CH
- 62 - 1340326
Co~.~
~o. R, C X Y Z
903 C~3 H ~ 3 3 ~H
S04 3 ~C~i3 OCH3 N
905 ~ H CQ CH3OCH3 CH
906 ~ H CQ ~ 3 C 3 CH
907 ~ H CQ CH3OCH3 N
908 ~CQ H C~2CH3 CH3OCH3 CH
909 ~ H C~2CH3 CH3OCH3 N
910 ~ H3 H C~2C2H5 CH3OCH3 CH
911 ~ H3 H C~2C2H5 CH3OCH3 N
91 C~3 ~ 3 C~2C 3 C~I3OCH3 CH
91 CH3 ~ 3 co2CIi3 3 3 CH
914 CH3 ~ CH3 C~2C 3 CH3OCH3 N
NO2 ~ 2 3 C~I3OCH3 CH
NO2 ~ 2 3 CH3OCH3 N
~ - 63 - 134032fi
.
Comp. A B C X Y Z
917NO2 ~ C~3 C~2CH3 CH3OC 3 CH
918NO2 ~ CH3 C~2CH3 CH3OCH3 N
919CH3 ~ H NO2 CH3OCH3 CH
CH
9203~ H NO2 CH3OCH3 N
921 ~ H CO2H CH3 OCH3 CH
922 ~ H CO2H CH3 OCH3 N
923 ~ H N~2 CH3 OCH3 CH
924 ~ H NO2 CH3 OCH3 N
925CH3 CH3 CQ CH3 H CH
926CH3 CH3 CQ CH3 CQ CH
CH3 CQ CH3 -OCH2CH2C-
928CH3 CH3 CQ CH3 CH3 N
929CH3 CH3 NO2 CH3 CQ CH
930CH3 CH3 NO2 CH3 -OCH2CH2C-
3 CH3 N~2 CH3 CH3 N
932C~3 CH3 SO2N(CH3)2 CH3 CQ CH
13~0~2~
-- 64 --
CN~om.P A B C X y z
933 CH3 CH3S02N (CH3) 2 CH3 -OCH2CH2C-
934 CH3 CH3S02N(CH3)2 CQ N(CH3)2 N
~ - 65 - 134032~ '
Table 3
C~S02NHCNH~
B
Conl~
;-' A B C ~ yz
935 CH3 H H CH
936 CH3 H H CH3 OCH3 CH
937 CH3 H H OCH3 OCH3 CH
938 CH3 H H CH3 OCH3 N
939 CH3 H H OCH3 OC~3 N
940 CH3 H H N
941 CH3 H H CH3 CQ CH
942 CH3 H H CH3 -OCH2CH2C-
943 CH3 H H ( H3)2 N
944 CH3 H H CH3 O~HCO2H N
945 CH3 CH3 H CH3 CH3 CH
946 CH3 CH3 H OCH3 OCH3 CH
- 66 - 134032~
Comp
' A B C X Y Z
947 CH3 CH3 3OCH3 CH
948 CH3 CH3 H CH3 OCY.3 N
949 CH3 CH3 3OC 3 N
950 CH3 H CH3 CH3OCH3 - CH
951 CH3 H CH3 3C 3 CH
952 CH3 H CH3 CH3OCH3 N
953 ~ H H CH3 OCH3 CH
954 @ H 3C 3 CH
955 @ H H CH3 OCH3 N
956 CH3 CQ H CH CH3 CH
957 CH3 CQ H CH3 OCH3 CH
958 CH3 CQ 33 CH
959 CH3 CQ H CH3 OCH3 N
960 CH3 CQ 33 N
961 CH3 Br H CH3 OCH3 CH
962 CH3 Br O 3 3 CH
~ - 67 - 1340326
Comp
' A B C X Y Z
963 CH3 Br H CH3OCH3 N
964 CH3 NO2 H CH3 CH3 CH
965 CH3 NO2 H CH3OCH3 CH
966 CH3 N~2 3 3 CH
967 CH3 NO2 H CH3OCH3 N
968 CH3 N~2 3 3 N
969 CH3 02 3 H CH3 CH3 CH
970 CH3C~2C 3 H CH3 OCH3 CH
971 CH32 3 3 3 CH
972 CH3C02C 3 H CH3 OCH3 N
973 OEI32 3 O 3 3 N
974 CH3CO2C2H5 H CH3 CH3 CH
975 CH3CO2C2H5 H CH3OCH3 CH
976 CH3CO2C2H5 H OCH3OCH3 CH
977 CH3CO2C2H5 H CH3OCH3 N
978 CH3C~2C2H5 3 3 N
- 68 - 13~032~
Comp.
A B C X Y Z
979 CH3 2 3 7 H CH3 OCH3 CH
980 CH3 2 3 7 3OCH3 CH
981 CH3 2 3 7 H CH3 OCH3 N
982 CH3 a~2CH2CH=CH2 H CH3OCH3 CH
983 CH3 (!~2CH2CH=CH2 H C 3OCH3 CH
984 CH3 C~2CH2CH=CH2 H CH3OCH3 N
985 CE~3 C~2CH3 CH3 CH3OCH3 CH
986 CH3 C~2CH3 CH3 C 3OCH3 CH
987 CH3 C 2CH3 CH3 CH3OCH3 N
989 CH3 CON(CH3)2 H CH3OCH3 CH
990 CH3 CON(CH3)2 3 3 CH
991 CH3 CON(CH3)2 H CH3OCH3 N
992 CH3 SO2N(CH3)2 H CH3OCH3 CH
993 CH3 SO2N(CH3)2 3 3 CH
994 CH3 SO2N(CH3)2 H CH3OCH3 N
995 CH3 SO2N(C2H5)2 H CH3OCH3 CH
- 69 - 13~0326
Comp. A B C X Y Z
996 CH3 S~2N(C2H5)2 3 3 CH
997 CH3 SO2N(C2H5)2 CH3 OCH3 N
998 CH3 S~2CH3 H CH3 OCH3 CH
999 CH3 SO2CH3 3 3 CH
.1000 CH3 S~2CH3 H CH3 OCH3 N
1001 H H H CH3 OCH3 CH
1002 H H 3 3 CH
1003 H H H CH3 OCH3 N
1004 C2H5 H H CH3 CH3 CH
1005 C2H5 H H CH3 OCH3 CH
1006 C2H5 H H C 3 3 CH
1007 C2H5 H H CH3OCH3 N
-
13~032~
- 70 -
The compounds of this invention represented by the
above formula (I) can readily be produced by selecting
suitably the following reaction scheme 1, 2 or 3.
Reaction scheme 1
N ~ S02NCO + HN
(IV) (V)
/N ~ S02NHC-N ~ z
A C D N
(I)
wherein A, B, C, D, X, Y and Z have the same
meanings as defined above.
That is, a pyrazolesulfonylisocyanate derivative (IV)
is dissolved in an inert solvent such as sufficiently
dried dioxane, acetonitrile, etc., and to the resultant
solution is added pyrimidine or a triazine derivative
(V). By stirring the mixture, the reaction proceeds
generally rapidly to give the compound (I) of this
invention. In case when the reaction proceeds
difficultly, a minute amount of a suitable base such as
lS triethylamine, triethylenediamine, pyridine, sodium
ethoxide, sodium hydride, etc. may be added to the
reaction mixture, whereby the reaction can proceed
easily.
13~0326
Reaction scheme 2:
X
D NJ
B NH~/ Z
I=L NC(
Cl CO 2 R g N ~ O ( V ) Y
N~S02NH2 ~ A \ S02NHCORg
A C C
(VI) (VII)
B X
N I O N~
~ . il~/ \\
~N~ 'S02NHC-N--~ Z
A C D N=~
Y
(I)
wherein A, B, C, D, X, Y and Z have the same
meanings as defined above, and Rg represents an
alkyl group or a phenyl group.
That is, a pyrazolesulfonamide derivative (VI) is
allowed to react with a chlorocarbonate in a solvent
such as acetone or methyl ethyl ketone in the presence
of a base such as potassium carbonate, followed by acid
precipitation with hydrochloric acid, etc., to give a
compound (VII). This compound is then heated in a
solvent such as toluene with a compound (V) to give the
compound (I) of this inventions.
Reaction scheme 3
13~032~
G
N¦
O=C=N~ Z
B N ~\ B G
N ~ (X) E N ~ O N ~
N ~ S02NH2 ~ / ~ S02NHCONH ~ Z
(VI) (VIII)
B G
R10~Na N ~ ~ I N
S02NHC-NH~
ORlo
(IX)
wherein A, B, C and Z have the same meanings as
defined above, E represents a halogen atom, G
represents H, Cl, Br, Cl-C4 alkyl, CF3,
methoxymethyl or methoxy ethyl, and Rlo represents
a lower alkyl group.
By referring to Japanese Une~Arined Patent Publication
No.154471/1981, a pyrazolesulfonamide derivative (VI)
can be allowed to react with pyrimidine or a triazine
isocyanate (X) to synthesize a compound ~VIII), which
is a part of the compound of this invention, followed
by reaction with a sodium alcoholate, to further
synthesize a compound (IX), which is also a part of the
compounds of this invention.
The other reactant aminopyrimidine can be synthesized
by referring to ~ The Chemistry of HeterocycliC
Compounds (Interscience Publishers Inc., New York) Vol.
16, The pyrimidines n.
- 72 -
13~0326
- 73 -
Aminotriazine can be synthesized according to the
method disclosed in " Journal of Organic Chemistry,
Vol. 28, pp.l812-1821 (1963) ".
2-Amino-4-methyl-5,6-dihydrofura[2,3-d]pyrimidine and
2-amino-4-chloro-5 -chloroethyl-6-methylpyrimidine can
be synthesized accordng to the method disclosed in "
Journal of Organic Chemistry, Vol.16, pp.ll53 (1951).
Most of the pyrazolesulfonamide (VI) or the
pyrazolesulfonyl isocyanate derivative (IV) used as the
starting material in the reaction scheme 1, 2 or 3 are
novel compounds, and they can be synthesized by
selecting suitably the following reaction scheme 4, 5 or
6 .
Reaction scheme 4
CQ5~3H A- ~ ~ 1) 9~CQ2
A- ~ S~2NH2 n-B~CO~
(VI)
B O B
N=h ll COCQ2 N~
A-~ S02NHCl!l~u-n C
,.
3~0326
-- 74 --
Reaction scheme 5
N=h
\ OH
A-N~
C \p2S5
B B B
C Q H2S,Na A-~-SH ~ 40H, A-N =~--SNH2
NaSCH2~) CQ2/CH3C02H ~. n-BuNCO
\3. COCQ2
B CQ2/cH3cooH ~ 2. n-BV a~ A_N~S02NC~
2 (IV)
Reaction scheme 6
B B
Nl\ 1) NaN02 ~ H IN~\ 1. NEI40H
A-N ~NH2 ~A-N SO CQ
C>~ 2) S02-Cu salts C . 2 2 nBuNCO
N~
A~'~ S02NCO
(IV)
.
1340326
Most of the pyrazolesulfonyl isocyanate derivatives
obtained hereinabove ar~ unstable in the air, and
should preferably be stored in a cold and dark place
under a nitrogen atmosphere when they are to be stored,
but alternatively they can be used as such without
purification in many cases in the subsequent reaction
step.
Most of the intermediates pyrazolesulfonamides (VI) to
be used in this invention are novel compounds, and
synthetic examples thereof are shown below as Reference
examples.
Reference example 1
Synthesis of 1,3-dimethyl-5-methoxypyrazole-4-sulfon-
amide:
- 75 -
13~032~
- 76 -
Into 70 ml of chlorosulfonic acid was added dropwise
under cooling at 5 ~C or lower 12.6 g (0.1 mol) of
1,3-dimethyl-5-methoxypyrazole. After the addition,
the mixture was stirred at 100 ~C for 8 hours. Then,
the reaction mixture was cooled to 80 ~C, followed by
dropwise addition of 11.9 g (o.l mol) of thionyl
chloride over 30 minutes. After the dropwise addition,
the mixture was stirred at 100 ~C for 2 hours. The
reaction mixture was cooled and poured carefully into
ice-water, whereby crude 1,3-dimethyl-5-methoxy-
pyrazole-4-sulfonylchloride was formed as crystals.
The crystals were separated by filtration, dissolved in
20 ml of tetrahydrofuran and the resultant solution was
added dropwise into 100 ml of aqueous ammonia (28%) at
10 ~C or lower. After the addition, the mixture was
stirred at room temperature for 30 minutes. The
reaction mixture was evaporated under reduced pressure
to remove low boiling components, whereby crystals were
- precipitated. The crystals were filtered, washed with
water and dried to give 11.5 g of 1,3-dimethyl-
5-methoxypyrazole-4-sulfonamide melting at 144 - 146
~C .
Reference example 2
Synthesis of 1,5-dimethyl-3-trifluoromethylpyrazole-
4-sulfonamide:
Into 100 ml of an ethanolic solution of 25 g (0.16 mol)
of trifluoroacetylacetone, there was added dropwise 7.4
g (0.16 mol) of methylhydrazine. After stirring at
room temperature for 3 hours, the mixture was left to
stand overnight and the solvent was evaporated to give
19.2 g of 1,5-dimethyl-3-trifluoromethylpyrazole as an
oil. Following then the procedures as Reference
example 1, this product was allowed to react with
chlorosulfonic acid and then with thionyl chloride to
1340326
obtain 29 g of 1,5-dimethyl- 3-trifluoromethylpyrazole-
4-sulfonyl-chloride, which was in turn reacted with
aqueous ammonia (28%) in tetrahydrofuran to give 21.5 g
of 1,5-dimethyl-
3-trifluoromethylpyrazole-4-sulfonamide melting at 204
- 206 ~C.
Reference example 3
Synthesis of 1,3-dimehyl-5-methoxycarbonylpyrazole-4-
sulfonamide:
Into 200 ml of chlorosulfonic acid, there was added
dropwise 50.4 g (0.3 mol) of Ethyl 1,3-dimethyl-5-
pyrazole carboxylate under cooling at 5 ~C or lower.
After heating at 120 ~C under stirring for 10 hours,
the reaction mixture was poured into ice-water and
extracted with ether. After washing with water and
drying, evaporation of the solvent gave 49 g of crude
1,3-dimethyl-4-chlorosulfonyl-5-pyrazole carboxylic
acid as a solid.
23.9 g (0.1 mol) of this product was dissolved in 60 ml
of tetrahydrofuran and the resultant solution was added
dropwise into 150 ml of aqueous ammonia(28%) at 10 ~C
or lower. The mixture was stirred at room temperature
for 4 hours and concentrated to dryness under reduced
pressure. Then, 300 ml of dry methanol was added to
the mixture, and the mixture was refluxed for 3 hours
while passing gaseous hydrogen chloride thereinto.
After the reaction, methanol was evaporated and
ice-water was added to the residue, followed by
extraction with ethyl acetate. After washing with
water and drying, the solvent was evaporated and the
crude crystals obtained were recrystallized from
methanol to give 9.0 g of 1,3-dimethyl-
5-methoxycarbonylpyrazole-4-sulfonamide melting at 174
oC .
- 176
1340326
- 78 -
Reference example 4
Synthesis of 1,3-dimethyl-5-n-propylsulfonylpyrazole-4-
sulfonamide:
With the use of 1,3-dimethyl-5-chloropyrazole as the
starting material, following the procedure in the above
Reference examples, there was obtained 5-chloro-1,3-
dimethylpyrazole-4-sulfonamide melting at 173 ~C. Into
a mixture of 14.1 g (0.067 mol) of the above
sulfonamide, 10.7 g (0.268 mol) of sodium hydroxide and
120 ml of DMF, 6.6 g (0.087 mol) of n-propylmercaptan
was added dropwise at room temperature. After the
addition, the mixture was heated at 80 to 90 ~C for 3
hours. After completion of the reaction, DMF was
evaporated under reduced pressure, and the residue was
diluted with water, adjusted to pH 4 with conc.
hydrochloric acid and the precipitated crystals were
collected by filtration. After drying, there was
obtained 14.3 g of 1,3-dimethyl-5-n- propylthio-
pyrazole-4-sulfonamide melting at 105 - 106~C.
As the next step, this intermediate was dissolved in 80
ml of acetic acid and 23 g of 30 % aqueous hydrogen
peroxide was added dropwise into the resultant solution
under ice-cooling. After stirring at room temperature
for 4 hours, the reaction mixture was poured into
water. The precipitated crystals were collected by
filtration to obtain 14 g of 1,3-dimethyl-5-n-
propylsulfonylpyrazole-4-sulfonamide melting at 127 -
129 ~C.
Following the procedures as described in Reference
examples 1 to 4, there were synthesized the substituted
pyrazolesulfoamides (IV) shown below~
-
~ 79 ~ 134032~
Reference example 5:
Synthesis of 1,5-dimethyl-3-phenylpyrazole-4-sulfon-
amide:
Obtained, following the above Reference examples, from
1,5-dimethyl-3-phenylpyrazole. m.p. 144-148 ~C
Reference example 6:
Synthesis of 5-chloro-1-methylpyrazole-4-sulfonamide:
Obtained from 5-chloro-1-methylpyrazole. m.p.159-152 ~C
Reference example 7:
Synthesis of 1,5-dimethyl-3-methoxycarbonylpyrazole-4-
sulfonamide:
Obtained from 1,5-dimethyl-3-methoxycarbonylpyrazole.
m.p. 183-185 ~C
Reference example 8:
Synthesis of 1,5-dimethyl-3-ethoxycarbonylpyrazole-4-
sulfonamide:
Obtained from 1,5-dimethyl-3-ethoxycarbonylpyrazole.
m.p. 159-161 ~C
Reference example 9:
Synthesis of 1,3-di-methylpyrazole-4-sulfonamide:
Obtained from 1,3-dimethylpyrazole. m.p.114-115 ~C
', 1340~26
- 80 -
Reference example 10:
Synthesis of 3,5-dimethylpyrazole-4-sulfonamide:
Obtained from 3,5-dimethylpyrazole. m.p.215-223 ~C
Reference example ll:
Synthesis of 1,5-dimethylpyrazole-4-sulfonamide:
Obtained from 1,5-dimethylpyrazole. m.p.l65-166 ~C
Reference example 12:
Synthesis of 5-methoxy-1-methyl-3-i-propylpyrazole-4-
sulfonamide:
Obtained from 5-methoxy-1-methyl-3-i-propylpyrazole.
m.p.114-115 ~C
Reference example 13:
Synthesis of 1,3-dimethyl-5-ethoxycarbonylpyrazole-4-
sulfonamide:
Obtained from 1,3-dimethyl-5-ethoxycarbonylpyrazole.
m.p.150-154 ~C
Reference example 14:
Synthesis of 3,5-dimethyl-1-phenylpyrazole-4-sulfon-
amide:
Obtained from 3,5-dimethyl-1-phenylpyrazole.
m.p.178-180 ~C
134032~
- 81 -
Reference example 15:
Synthesis of 3-ethoxycarbonyl-1-methyl-5-phenyl-
pyrazole-4-sulfonamide:
A solution of 7.5 ml of chlorosulfonic acid and 15 ml
of dry chloroform was cooled to 0 ~C, and a solution of
11.5 g (0.05 mol) of 3-ethoxycarbonyl-1-methyl-
5-phenylpyrazole in 25 ml of dry chloroform was added
dropwise thereinto. Then, the mixture was stirred at
room temperature for one hour and under reflux for 3
hours. After the reaction, by evaporation of
chloroform, there was obtained oily 3-ethoxycarbonyl-
l-methyl-5-phenylpyrazole-4-sulfonic acid. As the next
step, at room temperature 20.9 g of phosphorus
pentachloride was-added portionwise, followed by
stirring at 90 to 100 ~C for one hour. The reaction
mixture was added into ice-water and extracted with
ether to obtain 14.7 g of 3-ethoxycarbonyl-1-methyl-
5-phenylpyrazole-4-sulfonylchloride. Then, the
resultant sulfonylchloride was dissolved in 20 ml of
acetone and 4.48 g of KHCO3 was added to the solution.
After a solution of 2.7 g of aqueous ammonia (28 %) and
6.5 ml of acetone was added dropwise thereinto at room
temperature, the mixture was heated at 50 to 60 ~C for
30 minutes. After evaporation of acetone, water was
added to the residue, whereby 12.9 g of the title
compound was precipitated as a solid.
Recrystallization from benzene gave 8.4 g of the
product. m.p. 141-144 ~C
Following the procedures as described above, there were
synthesized the substituted pyrazolesulfon-
amides shown below.
Reference example 16:
Synthesis of 5-chloro-1,3-dimethylpyrazole-4-sulfon-
amide:
- 82 - 134032~
Obtained from 5-chloro-1-3-dimethylpyrazole. m.p. 173~C
Reference example 17:
Synthesis of l-ethyl-5-methyl-3-methoxycarbonyl-
pyrazole-4-sulfonamide:
Obtained from l-ethyl-5-methyl-3-methoxycarbonyl-
pyrazole. m.p. 165-168 ~C
Reference example 18:
Synthesis of 5-ethyl-1-methyl-3-methoxycarbonyl-
pyrazole-4-sulfonamide:
Obtained from 5-ethyl-1-methyl-3-methoxycarbonyl-
pyrazole. m.p. 188 - 191 ~C
Reference example 19:
Synthesis of l-ethyl-3-methyl-5-methoxycarbonyl-
pyrazole-4-sulfonamide:
Obtained from l-ethyl-3-methyl-5-methoxycarbonyl-
pyrazole. m.p. 130-133 ~C
Reference example 20
Synthesis of 3-ethyl-1-methyl-5-methoxycarbonyl-
pyrazole-4-sulfonamide:
Obtained from 3-ethyl-1-methyl-5-methoxycarbonyl-
pyrazole. m.p. 162 - 165 ~C
Reference example 21:
Synthesis of 1,3-dimethyl-5-dimethylcarbamoylpyrazole-
4-sulfonamide:
Obtained from 1,3-dimethyl-5-dimethylcarbamoylpyrazole.
m.p. 150 - 152 ~C
- 134032~
- 83 -
Reference example 22:
Synthesis of l-methylpyrazole-4-sulfonamide:
Obtained from l-methylpyrazole. m.p. 122-123 ~C
Reference example 23:
Synthesis of 1,3-dimethyl-5-i-propoxycarbonylpyrazole-
4-sulfonamide:
Obtained by heating 1,3-dimethyl-5-carboxylpyrazole-
4-sulfonamide btained in Reference example 3 in
i-propyl alcohol in the co-presence of hydrogen
chloride. m.p. 105-108 ~C
Reference example 24:
Synthesis of 1,3-dimethyl-5-(N,N-tetramethylene-
carbamoyl)-4-sulfonamide:
Obtained from 1,3-dimethyl-5-(N,N-tetramethylene-
carbamoyl)pyrazole. m.p. 92-95 ~C.
Reference example 25:
Synthesis of 4-ethoxycarbonyl-1-methylpyrazole-5-
sulfonamide:
(1) Synthesis of ethyl-5-hydroxy-1-methyl-4-
pyrazolecarboxylate
To a solution of 216 g (1 mol) of diethylethoxy-
methylenemalonate in 216 g of ethanol, there was added
46 g (1 mol) of methylhydrazine at 10 ~C. Then, at
room temperature, the mixture was stirred and further
refluxed for one hour, and thereafter the reaction
mixture was left to stand. The precipitated crystals
were filtered and dried to obtain 148 g of the title
compound melting at 134-135 ~C.
-
1340~2~
- 84 -
(2) Synthesis of ethyl 5-chloro-1-methyl-4-pyrazole
carboxylate:
A mixture of 10 g of ethyl 5-hydroxy-1-methyl-4-
pyrazole carboxylate and 50 ml of phosphorus
oxychloride was stirred at 90 to 100 ~C for 65 hours.
Excessive phosphorus oxychloride was evaporated under
reduced pressure, and the residue was poured into
ice-water. The precipitated crystals were filtered and
dried to obtain 4.5 g of 5-chloro-1-methyl-4-pyrazole
carboxylic acid. The filtrate was neutralized with
aqueous ammonia (28%) and extracted with ether. After
drying of the extract, evaporation of the solvent gave
4.0 g of ethyl 5-chloro-1-methyl-4-pyrazole
carboxylate. To 5-chloro-1-methyl-4-pyrazole
carboxylic acid were added 30 ml of thionyl chloride
and 0.2 ml of dimethylformamide, and the mixture was
refluxed for 5 hours. Then, excessive thionyl chloride
was evaporated and the residue was added to dry
ethanol. After stirring at room temperature for 3
hours, the solvent was evaporated and ether was added
to the residue. After washing with water, drying and
evaporation of the solvent, there was obtained 4.5 g of
ethyl 5-chloro-1-methyl-4-pyrazole carboxylate as an
oil. Total of the title compound: 8.5 g.
(3) Synthesis of ethyl 5-mercapto-1-methyl-4-pyrazole
carboxylate:
After 2.2 g (0.094 mol) of sodium was dissolved in 35
ml of ethanol, 50 ml of dimethylformamide was added to
the resultant solution, and most of ethanol was
removed. Then, under cooling, hydrogen sulfide was
passed into the mixture to saturation, followed by
addition of 7.4 g (0.039 ml) of ethyl 5-chloro-
l-methyl-4-pyrazole carboxylate. After stirring at 70
- 13~032fi
- 85 -
to 80 ~C for 3.5 hours, the reaction mixture was
concentrated under reduced pressure. Ice-water was
added to the reside, and insolubles were filtered off.
The filtrate was made acidic, extracted with chloroform
and dried, followed by evaporation of the solvent, to
give 6.8 g of the title compound as an oil.
(4) Synthesis of 4-ethoxycarbonyl-1-methylpyrazole-5-
sulfonamide:
To 100 ml of a 28 % aqueous ammonia, there was added 20
ml of aqueous solution containing 7.1 g of
ethyl-5-mercapto-1-ethyl-4-pyrazole carboxylate and 1.6
g of sodium hydroxide. To this reaction mixtur was
added 61 g of 6 % aqueous NaOCl solution at 5 - 10 ~C.
The precipitated crystals were filtered and washed with
water. The resultant moist sulfenamide (5.6g) was
suspended in water and an aqueous saturated solution of
5.5 g of potassium permanganate was added to the
suspension at room temperature. After stirring
vigorously at room temperature, the mixture was
filtered. The filtrate was made acidic and extracted
with ethyl acetate. After drying, the solvent was
evaporated to obtain 1.8 g of the title compound. m.p.
102 - 104 ~C
(5) Synthesis of 4-ethoxycarbonyl-1-methylpyrazole-
5-sulfonamide (alternative method):
A solution of 3.0 g of ethyl 5-mercapto-1-methyl-4-
pyrazole carboxylate in 50 ml acetic acid was stirred
while passing chlorine at 15 to 20 ~C. Then, after
nitrogen was passed into the mixture, the reaction
mixture was poured into ice-water and the crystals
precipitated were separated by filtration.
134032~
- 86 -
The sulfonyl chloride as prepared above was dissolved
in 20 ml of tetrahydrofuran, and added under
ice-cooling to 50 ml of 28 % aqueous ammonia. After
stirring at room temperature for 2 hours, the reaction
mixture was concentrated under reduced presure. The
precipitated crystals were filtered, washed with water
and further washed with n-hexane. After drying, 1.3 g
of the title compound was obtained. m.p. 102-104 ~C
Reference example 26
Synthesis of 4-methoxycarbonyl-1-methylpyrazole-5-
sulfonamide:
Synthesized similarly as in Reference example 25.
m.p. 127-128 ~C
The respective intermediates have the following
physical properties.
Methyl 5-hydroxy-1-methyl-4-pyrazole carboxylate:
m.p. 111-113 ~C
Methyl 5-chloro-1-methyl-4-pyrazole carboxylate:
m.p. 70-71 ~C
Methyl 5-mercapto-1-methyl-4-pyrazole carboxylate:
m.p. 64-66 ~C
Reference example 27:
Synthesis of 1,3-dimethylpyrazole-5-sulfonamide and
4-chloro-1,3-dimethylpyrazole-5-sulfonamide
(1) Synthesis of 1,3-dimethyl-5-mercaptopyrazole
To a solution of 84 g (0.75 mol) of 1,3-dimethyl-
- 5-hydroxypyrazole in 630 ml of xylene was added
1340326
- 87 -
portionwise 65.3 g (0.294 mol) of phosphorus
penlasulfide at 110 to 120~C. After heating under
reflux for 1.5 hours, the hot reaction mixture was
filtered. The filtrate was concentrated to give 21.4 g
of the title compound. m.p. 130 - 132~C.
(2) Synthesis of 4-chloro-1.3-dimethylpyrazole-
5-sulfonamide
Into a solution of 12 g (0.094 ml) of the
mercaptopyrazole obtained above in a mixture of acetic
acid (100 ml) and water (15 ml), chlorine was passed at
10~C for 2 hours. After completion of the reaction,
the mixture was poured into ice-water and extracted
with ether. The organic extracts were washed with
water, dried and evaporated to give 19.5 g of crude
4-chloro-1,3-dimethylpyrazole-5-sulfonyl chloride as an
oll .
Then, into 130 ml of aqueous ammonia (28 %) was added
dropwise the sulfonyl chloride obtained above in 50 ml
of THF at 10~C or lower. After the addition, the
reaction mixture was stirred at room temperature for 3
hours and then concentrated under reduced pressure to
form precipitates.
The preipitated solids were filtered, washed with water
and dried to give 10.8 g of the title compound. m.p.
135 - 138~C.
Further, the filtrate obtained above was extracted with
benzene several times and the aqueous layer was
concentrated under reduced pressure to dryness. The
residual product was extracted with 100 ml of ethyl
acetate. After evaporation of the solvent there was
also obtained 4.0 g of 1,3-dimethylpyrazole-5-
sulfonamide. m.p. 63 - 66~C.
-
,
~ - 88 - 134032~
Reference example 28:
Synthesis of 1,3-dimethyl-4-nitropyrazole-5-sulfonamide
(1) Synthesis of 5-chloro-1,3-dimethyl-4-nitropyrazole
Concentrated H2S04 (35 g) was added to 10 ml of 90%
HNO3 and at 75 - 85~C, 13 g (0.1 mol) of
5-chloro-1,3-dimethylpyrazole was added dropwise with
stirring to maintain the temperature at 85~C. After
completion of the addition, the mixture was heated at
75 - 85~C for 1.5 hr. The nitration mixture was cooled
and poured into ice. The suspention was filtered and
the solid was washed with water and dried to give 14.5
g of the title compound. m.p. 70~C.
(2) Synthesis of 5-benzylthio-1,3-dimethyl-
4-nitropyrazole
Benzylmercaptan (50.8 g) (0.41 mol) was added to 21 g
of sodium methoxide in 480 ml of DMF under ice-cooling
and then 65.2 g (0.37 ml) of 5-chloro-2,3-dimethyl-
4-nitropyrazole was added. After stirring at room
temperature for 3 hr., DMF was evaporated under reduced
pressure, and the residue ws diluted with ice-water.
The precipitated solids were filtered, washed with
water and further with n-hexane and dried to give 82.4
g of the title compound. m.p. 82 - 83~C.
(3) Synthesis of 1,3-dimethyl-4-nitropyrazole-
5-sulfonamide
Into a solution of 40 g (0.152 mol) of
5-benzylthio-1,3-dimethyl-4-nitropyrazole in 300 ml of
acetic aid and 30 ml of water chlorine was passed at 3
to 8~C for 1.5 hr. After completion of the reaction,
the mixture was poured into ice-water and extracted
with ether to give crude l,3-dimethyl-4-nitropyrazole-
5-sulfonylchloride as an oil.
134~32~
- 89 -
The sulfonylchloride obtained above in 50 ml of THF was
added into 150 ml of aqueous ammonia (28~) under
ice-cooling. After stirrig at room temperature for 5
hrs, the reaction mixture was evaporated under reduced
pressure. The precipitated solids were filtered,
washed with water and further with n-hexane and dried
to give 19.6 g of the title compound. m.p. 138 -
140~C.
Reference example 29:
Synthesis of 1,3-dimethyl-4-dimethylsulfamoylpyrazole-
5-sulfonamide:
(1) Syntheisis of N,N-dimethyl-(5-chloro-1,3-dimethyl-
pyrazole)-4-sulfonamide
Into 150 ml of chlorosulfonic acid was added dropwise
under cooling at 5~C or lower, 30 g (0.23 ml) of
5-chloro-1,3-dimethylpyrazole. After the addtion, the
mixture was stirred at 100~C for 8 hours. Then, the
reaction mixture was cooled to 80~C, followed by
dropwise addition of 36 g of thionyl chloride over 30
minutes. After the addition, the mixture was stirred
at 100~C for an additional 2 hours. The rection
mixture was cooled on ice and poured carefully ino
ice-water, whereby crude 5-chlro-1,3-dimethylpyrazole-
4-sulfonylchloride (50 g) was formed as crystals.
Subsequently, into a solution of 40 g of dimethylamine
in 250 ml of THF, the above compound was added under
cooling and the reaction mixture was stirred at room
temperature for 3 hours. After the reaction, THF was
evaporated under reduced pressure and into the residue
was added ether and washed with water. After drying
and evaporating the etherial solution, there was
obtained 47 g of the title compound. m.p. 53 - 55~C.
134~32fi
-- 90 --
(2) Synthesis of N,N-dimethyl-(5-benzylthio-1,3-
dimethylpyrazole)-4-sulfonamid
Obtained, following the above Reference example 28-(2),
from N,N-dimethyl-(5-chlro-1,3-dimethylpyrazole)-
4-sulfonamide. m.p. 108 - 109~C.
(3) Synthesis of 1,3-dimethyl4-dimethylsulfamoyl-
pyrazole-5-sulfonamide
Obtained, following the above Reference example 28-(3),
from N,N-dimethyl-(5-benzylthio-1,3-dimethylpyrazole)-
4-sulfoamide. m.p. 209 - 210~C.
Reference example 30:
Synthesis of l-methylpyrazole-3-sulfonamide:
To a solution of 32 g (0.33 mol) of
l-methyl-3-aminopyrazole in a mixture of conc.
hydrochloric acid (120 ml) and acetic acid (40 ml) was
added a solution of 34.1 g (0.494 mol) of sodium
nitrite in water (80 ml at -10~ to 0~C. The solution
was stirred at -5~C for 30 minutes and then added, in
several portions, into a sulfur dioxide saturated
solution of acetic aid (440 ml) containig 6.7 g of
cuprous chlride at -10~ to -5~C. The resultig solution
was strred at 0~ to 5~C for 4 hours and then poured
into ice-water and extracted with ether. The extracts
were washed with water and saturated aqueous sodium
bicarbonate, dried and evaporated to give 20.8 g of
crude sulfonylchloride as an oil. The sulfonyl-
chloride obtained bove was dissolved in 50 ml of THF
and added to 150 ml of aqueous ammonia (28%) at 10~C or
- 134032~
lower. After stirring at room temperature for 3 hours,
the reaction mixture was concentrated under reduced
pressure to form precipitates. The precipitated solids
were filtered, washed with water and further with
n-hexane and dried to give 24. g of the title compound.
m.p. 156 - 158~C.
Specific synthetic examples of the compounds according
to this invention are illustrated below by using the
substituted pyrazolesulfonamides (VI) obtained in -~
Reference examples, but this invention is not limited
thereto.
Example 1
Synthesis of N-[(4-methoxy-6-methylpyrimidin-2-yl)-
aminocarbonyll-1,3-dimethyl-5-methoxypyrazole-4-sulfon-
amide (Compound No. 292):
-- 91 --
.~
1340'32~
-
- 92 -
To a mixture of 10.25 g (0.05 mol) of the sulfonamide
obtained in Reference example 1, 8.29 g (0.06 mol) of
dry potassium carbonate and 50 ml of dry acetone was
added 4.95 g (0.05 mol) of n-butyl isocyanate at room
temperature, and the mixture was stirred for 4 hours.
Then, the reaction mixture was refluxed for one hour.
After the reaction, acetone was evaporated under
reduced pressure and the residue was dissolved in 200
ml of water. After separation of a trace of water
insolubles, the filtrate was made acidic with
hydrochloric acid and the crystals formed were
filtered, washed with water and dried to give 9.2 g of
N-(butylcarbamoyl)-1,3-dimethyl-5-methoxypyrazole-4-
sulfonamide melting at 135 - 142 ~C.
Into a mixture of 100 ml of dry benzene and 9.12 g
(0.03 mol) of N-(n-butylcarbamoyl)-1,3-dimethyl-5-
methoxypyrazole-4-sulfonamide, under reflux, 8.9 g
(0.09 mol) of phosgene was passed over 1.5 hours.
Then, the reaction mixture was further refluxed for 30
minutes. After completion of the reaction, evaporation
of benzene under reduced pressure gave crude
1,3-dimethyl-5-methoxypyrazole-4-sulfonyl isocyanate as
an oil.
The crude isocyanate was taken out in an amount of 1.39
g (0.006 mol), dissolved in 20 ml of dry acetonitrile
and 0.695 g (0.005 mol) of 4-methoxy-6-methyl-2-amino-
pyrimidine was added to the resultant solution. The
mixture was stirred at room temperature for one hour,
then refluxed for 30 minutes, followed by cooling.
The crystals formed were filtered, washed and dried to
obtain 1.09 g of N-[(4-methoxy-6-methylpyrimidin-2-yl)-
aminocarbonyl]-1,3-dimethyl-5-methoxypyrazole-4-
sulfonamide melting at 183-184 ~C.
13~032~
- 93 -
Example 2
Synthesis of N-[(4-methoxy-6-methyl-1,3,5-triazin-
2-yl)amino-carbonyl]-1,3-dimethyl-5-methoxycarbonyl-
pyrazole-4-sulfonamide (Compound No. 387):
With the use of 6.8 g of the sulfonamide obtained in
Reference example 3, following the procedure as in
Example 1, there was obtained 8.6 g (0.026 mol) of
N-(n-butylcarbamoyl)-1,3-dimethyl-5-methoxy-
carbonylpyrazole-4-sulfonamide. The compound obtained
was mixed into 200 ml of xylene and under reflux 7.7 g
(0.078 mol) of phosgene was passed into the mixture
over 1.5 hours. The reaction mixture was refluxed for
30 minutes, followed by evaporation of xylene, to
obtain crude 1,3-dimethyl-5-methoxycarbonylpyrazole-
4-sulfonyl isocyanate as an oil.
The crude isocyanate was taken out in an amount of 0.90
g (0.0035 mol) and dissolved in 20 ml of dry
acetonitrile and 0.35 g of (0.0025 mol) of 2-amino-
4-methoxy-6-methyl-5-triazine was added to the
solution. After the mixture was stirred at room
temperature for 2 hours, the reaction mixture was
refluxed for 30 minutes and cooled. The crystals
formed were filtered, washed and dried to obtain
N-[(4-methoxy-6-methyl-1,3,5-triazin-2-
yl)aminocarbonyl]-1,3-dimethyl-5-methoxycarbonyl-
pyrazole-4-sulfonamide melting at 195-198 ~C.
Example 3
Synthesis of N-[(4,6-dimethylpyrimidin-2-yl)amino-
carbonyl]-1,3-dimethyl-5-propenyloxycarbonylpyrazole-4-
sulfonamide (Compound No. 425):
134032~
- 94 -
Into a mixture of 17.4 ml of methanol and 8.7 g of 10 %
aqeous sodium hydroxide was added 3.3 g (0.00864 mol)
of N-[(4,6-dimethylpyrimidin-2-yl)aminocarbonyl]-
1,3-dimethyl-5-methoxycarbonylpyrazole-4-sulfonamide
(Compound No. 384), and the resultant mixture was
refluxed for 45 minutes. After the reaction, the
reaction mixture was made acidic with conc.
hydrochloric acid to obtain 2.3 g of a hydrolyzed
compound (Compound No. 435). The above compound was
taken out in an amount of 1.5 g (0.00408 mol),
dissolved in 8 ml of THF and 0.416 g of triethylamine
was added to the resultant solution. After stirring
for 10 minutes, a solution of 0.493 g of allyl bromide
in 8 ml of THF was added and the mixture was refluxed
for 3 hours. The precipitated salt was filtered off
and the filtrate was evaporated to obtain 0.5 g of the
crystals of the title comound. m.p. 180-183 ~C
Example 4
Synthesis of N-[(4.6-dimethoxy-1,3,5-triazin-2-yl)-
aminocarbonyl]-1,3-dimethyl-5-dimethylcarbamoylpyrazole-
4-sulfonamide (Compound No. 484):
In 15 ml of dry acetonitrile, there were added 1.97 g
(0.008 mol) of the sulfonamide obtained in Reference
example 21 and 1.68 g (0.0088 mol) of dichloro-S-
triazinyl isocyanate, and the mixture was refluxed for
one hour. The reaction mixture was then left to stand
at room temperature to give 2.2 g of crystals of N-[(
4,6-dichloro-1,3,5-triazin-2-yl)aminocarbonyl]-1,3-
dimethyl-5-dimethylcarbamoylpyrazole-4-sulfonamide.
m.p.182-185 ~C
Then, the above compound was taken out in an amount of
1.3 g (0.00297 mol) and added into 12 ml of methanol.
Into this mixture, there were added portonwise sodium
134032~
.~
- 95 -
methoxide prepared from 0.205 g of sodium and 5 ml of
methanol. After the reaction, the mixture was filtered
and methanol was evaporated. The residue was dissolved
in ice-water and made acidic with hydrochlorice acid,
whereby 0.8 g of the crystals of the title compound was
obtained. m.p. 170-175 ~C
Example 5
Synthesis of N-[(4-methoxy-6-methylpyrimidin-2-yl)-
aminocarbonyl]-1,3-dimethyl-5-dimethylsulfamoyl-
pyrazole-4-sulfonamide (Compound No. 596):
With the use of 39 g (0.186 mol) of the sulfonamide
obtained in Reference example 16, following the
procedure as in Example 1, there was prepared 57.6 g
N-(n-butylcarbamoyl)-1,3-dimethyl-5-chloropyrazole-4-
sulfonamide. m.p. 188 ~C
As the next step, 4.7 g of 55 % sodium hydride was
added to 100 ml of dimethylformamide and 9.1 g (0.074
mol) of benzylmercaptan was added to the mixture,
followed by portionwise addition of 15 g (0.049 g) of
the sulfonamide as prepared above. After stirring at
room temperature for several hours, the reaction
mixture was further stirred at 70 to 80 ~C for 5 hours.
After evaporation of the solvent, the residue was
added into ice-water and the insolubles were filtered
off. The filtrate was made acidic with hydro- chloric
acid to precipitate 19 g of N-(n-butyl-carbamoyl)-
1,3-dimethyl-5-benzylthiopyrazole-4-sulfonamide. Then,
into a solution of 19 g (0.0479 mol) of the above
compound dissolved in 100 ml of acetic acid, chlorine
was passed at 10 to 20 ~C for 1.5 hours. After the
reaction, the reaction mixture was poured into
ice-water to precipitate N-(n-butylcarbamoyl)-1,3-
~ 134032~
- 96 -
dimethyl-5-chlorosulfonyl-4-sulfonamide. Subsequently,
into a solution of 8.6 g of dimethylamine in 50 ml of
THF, the above compound was added and the mixture was
stirred at room temperature for 3 hours. After the
reaction, THF was evaporated, and the residue was
dissolved in an aqueous potassium carbonate solution.
After removal of the insolubles, the residual solution
was made acidic with hydrochoric acid to precipitate
10.0 g (0.026 mol) of N-(n-butylcarbamoyl)-
1,3-dimethyl-5-dimethylsulfamoyl-4-sulfonamide. m.p.
163-164 ~C
From the compound as prepared above, following the
procedures of Examples 1 and 2, there was synthesized
1,3-dimethyl-5-dimethylsulfamoylpyrazole-4-sulfonyl
isocyanate, which was in turn allowed to react with
2-amino-4-methoxy-6-methylpyrimidine to obtain the
title compound. m.p. 215-217 ~C
Example 6
Synthesis of N-[(4,6-dimethylpyrimidin-2-yl)amino-
carbonyl]-1,3-dimethyl-5-ethylthiopyrazole-4-sulfon-
amide: (Compound No.742)
Into 10 ml of dimethylformamide, there were added 1.5 g
(0.0042 mol) of N-[(4,6-dimethylpyrimidin-2-yl)amino-
carbonyl]-1,3-dimethyl-5-chloropyrazole-4-sulfonamide
(Compound No. 85), 0.4 g of ethylmercaptan and 0.67 g
of sodium hydroxide, and the resultant mixture was
stirred at 60 to 70 ~C for 4 hours. After the
reaction, the solvent was evaported, and the residue
was added into ice-water, followed by acidification
with hydrochloric acid, to precipitate 1.2 g of the
crystals of the title compound. m.p. 189-190 ~C
-
13~03~fi
- 97 -
Example 7
Synthesis of N-[(4,6-dimethoxypyrimidin-2-yl)amino-
carbonyl]-4-ethoxycarbonyl-1-methylpyrazole-5-sulfon-
amide (Compound No. 835):
To a mixture of 5.0 g of4-ethoxycarbonyl-
l-methylpyrazole-5-sulfonamide(Reference example 25),
4.45 g of dry potassium carbonate and 50 ml of acetone
was added 2.13 g of n-butyl isocyanate at room
temperature, and the mixture was stirred under reflux
for 3 hours. After the reaction, acetone was
evaporated under reduced pressure, and ice-water was
added to the residue, followed by filtration of the
insolubles. The filtrate was made acidic with
hydrochloric acid and the crystals precipitated were
filtered, washed with water and dried to give 5.1 g of
N-(n-butylcarbamoyl)-4-ethoxycarbonyl-1-methyl-
pyrazole-5-sulfonamide melting at 117-119 ~C. Then,
this product was added into 120 ml of dry toluene, and
9.1 g of phosgene was passed into the mixture under
reflux, followed further by refluxing for 1.5 hours.
After completion of the reaction, the reaction mixture
was concentated under reduced pressure to obtain crude
sulfonyl isocyanate.
The above crude sulfonyl isocyanate (0.98 g) was added
to a solution of 0.4 g of 2-amino-4,6-dimethoxy
pyrimidine in 20 ml of dry acetonitrile and the mixture
was stirred at room temperature. The crystals formed
were filtered, washed and dried to obtain 0.8 g of the
title compound melting at 170-172 ~C.
1340326
- 98 -
Example 8
Synthesis of N-[(4-methoxy-6-methylpyrimidin-2-yl)-
aminocarbonyl]-4-methoxycarbonyl-1-methylpyrazole-5-
sulfonamide(Compound No. 816):
With the use of the sulfonamide obtained in Reference
example 26, following the procedure of Example 7, there
was obtained N-(n-butylcarbamoyl)-4-methoxycarbonyl-
l-methylpyrazole-5-sulfonamide. m.p. 88-90 ~C
Further, following the procedure of Example 7, there
was synthesized a sulfonyl isocyanate, which was
further allowed to react with 2-amino-4-methoxy-
6-methylpyrimidine to obtain the title compound melting
at 183-184 ~C.
Example 9
Synthesis of N-[(4,6-dimethylpyrimdine-2-yl)-
aminocarbonyl]-4-chloro-1,3-dimethylpyrazole-
5-sulfonamide (Compound No. 789):
(1) Synthesis of N-(ethoxycarbonyl)-4-chloro-1,3-
dimethylpyrazole-5-sulfonamide
A mixture of 2.1 g (0.01 mol) of the sulfonamide
obtained in Reference example 27, 1.41 g (0.013 mol) of
ethyl chloroformate and 1.73 g (0.0125 mol) of
potassium carbonate in 30 ml of dry acetone was stirred
at room temperature for 18 hours. After the reaction,
acetone was evaporated under reduced pressure and the
residue was dissolved in water. The resultant solution
was made acidic with conc. hydrochloric acid and
extracted with ether. After evaporation of ether, the
precipitated solids were recrystallized from a mixture
134û32~
99
of benzene and n-hexane to obtain 2.0 g of the title
compound. m.p. 106 - 109~C.
(2) Synthesis of N-t(4,6-dimethylpyrimidine-2-yl)-
aminocarbonyl]-4-chloro-1,3-dimethylpyrazole-
5-sulfonamide
Into 1.41 g (0.005 ml) of the compound obtained above
in 10 ml of xylene was added 0.74 g (0.006 ml) of
2-amino-4,6-dimethylpyrimidine and the reaction mixture
was refluxed for 2 hours. The resultant mixture was
left to stand at room temperature to give 1.2 g of the
title compound. m.p. 191-194~C.
Example 10
Synthesis of t(4-methoxy-6-methylpyrimidine-2-
yl)aminocarbonyl]-l-methylpyrazole-3-sulfonamide
(Compound No. 936)
With the use of the sulfonamide obtained in Reference
example 30, following the procedure of Example 7, there
was obtained N-(n-butylcarbamoyl)-l-methylpyrazole-
3-sulfonamide. m.p. 168 - 169~C.
Further, following the procedure of Example 7, there
was synthesized a sulfonyl isocyanate, which was
further allowed to react with 2-amino-4-methoxy-
6-methylpyrimidin to btain the title compound. m.p.
185 - 186~C.
Next, similarly as in the above Examples, specific
compounds were prepared and they are shown together
with the compounds synthesized in Examples 1 to 10 in
Table 4.
134032~
-
-- 100 --
Table 4
Comp. No. m. p. ( ~C) Status
2 1 1 ~ 2 1 4 ~ite crystal
2 2 0 1 ~ 2 0 3 /~
3 1 7 1 ~ 1 7 2 /'
4 1 8 0 ~ 1 8 1 //
1 7 3 ~ 1 7 4 a
6 . 1 1 5 ~ 1 2 0 /'
7 1 7 1 ~ 1 7 5 //
8 1 7 7 ~ 1 7 9 /r
9 1 7 4 ~ 1 7 6 /~
1 0 1 6 3 ~ 1 6 6 /'
1 1 1 8 7 ~ 1 9 0 r'
1 2 1 83 ~ 1 84
1 3 2 1 9 ~ 2 2 0 //
1 4 2 1 0 ~ 2 1 1 /'
1 5 1 8 1 ~ 1 8 3
1 6 2 0 2 ~ 2 0 3 ~7
1 7 1 8 0 ~ 1 82
1 8 1 8 0 ~ 1 8 2 ,r
9 2 0 2 ~ 2 0 3
2 0 2 1 3 ~ 2 1 4 D t
134032fi
-- 101 --
Comp. No. m. p. ( ~C) Status
2 1 2 2 2 --- 2 2 4 ~ite crystal
22 226
8 5 2 1 2 ~ 2 1 7
8 6 1 9 2 ~ 1 9 6
8 7 1 8 4 ~ 1 88
8 8 1 4 8 ~ 1 5 2
8 9 1 53 ~ 1 5 7 a
1 1 a 1 9 9 ~ 2 0 2 /7
1 1 1 1 78 ~ 1 8 1
1 1 2 1 8 3 ~ 1 8 6 /~
3 2 2 0 ~ 2 2 7 r
1 1 4 1 6 4 ~ 1 6 S
1 3 8 2 4 2 ~ 2 4 4
1 3 9 2 3 2 ~ 2 3 4
4 0 2 1 0 ~ 2 1 4
4 1 1 9 0 ~ 1 9 3
1 4 2 1 9 a ~ 1 9 5 /~
1 4 6 2 4 2 ~ 2 4 4
4 7 2 1, 6 ~ 2 1 8
4 8 2 2 1 ~ 2 2 3
1 5 0 2 4 2 ~ 2 4 5
1 5 9 1 6 3 ~ 1 6 6 /7
1 6 0 1 9 8 ~ 2 0 0 /~
1 6 1 2 0 1 ~ 203 n
6 2 1 2 0 8 ~ 2 1 0
~ , .
..
1340326
- 102 -
Comp. No. m. p. ( ~C) Status
165 212 ~ 215~ite c~stal
166 219 ~ 220r/
177 197 ~ 199//
178 207 ~ 209//
179 191 ~ 193//
291 189 ~ 192//
292 183 ~ 184//
293 148 ~ 149 o
294- 158 ~ 161/7
295 174 ~ 175 a
304 230 ~ 232 a
305 206 ~ 208/~
306 202 ~ 204 a
307 185 ~ 195 a
308 185 ~ 188//
316 203 ~ 205
317 220 ~ 225
320 194 ~ 196 a
324 202 ~ 203//
325 187 ~ 188 a
327 191 ~ 194//
328 184 ~ 186
356 182 ~ 183
357 163 ~ 164
358 ! 176 ~ 178//
,
13~032~
- 103 -
Comp. No. m. p. ( ~C) Status
359 158 ~ 165 ~ite c~stal
360 167 ~ 169 //
361 169 ~ 171
384 195 ~ 198 /'
385 187 ~ 190
386 182 ~ 183 //
387 195 ~ 198 /'
388 173 ~ 176
391 209 ~ 211
403 185 ~ 188 "
409 186 "
410 180 ~ 183 /'
411 175 ~ 180
565 179 ~ 180 D
566 178 ~ 179
567 182 ~ 183 /7
568 145 ~ 147 - R
569 132 ~ 133 /'
570 170 ~ 171
571 2a4 ~ 206
572 183 ~ 184
573 163 ~ 165
580 185 ~ 186
581 178 ~ 179
1340326
- 104 -
Comp . No . m . p . ( ~ C )Status
210 182 ~ 185, White crystal
211 177 ~ 178 "
228 195 ~ 199 "
389 194 ~ 195 "
390 192 ~ 193 "
392 198 ~ 201 "
394 205 ~ 207 "
396 142 ~ 146 "
399 178 ~ 181 "
425 180 ~ 183 "
435 189 ~ 192 "
441 196 ~ 199
442 182 ~ 184 "
443 178 ~ 180 "
444 183 ~ 185 "
445 175 ~ 178 "
463 162 ~ 164 "
465 166 ~ 169 "
484 170 ~ 175 "
545 185 ~ 187 "
13~0~26
- 105 -
Comp. No. m.p. (~C) Status
546 166 ~ 169 White crystal
595 227 ~ 230 "
596 215 ~ 217 "
- 597 203 ~ 205 "
598 209 ~ 211 "
599 189 ~ 191 "
600 221 ~ 223 "
601 229 ~ 230 "
602 252 ~ 254 "
717 216 ~ 219 "
718 205 ~ 208 "
719 202 ~ 205 "
720 188 ~ 190 "
721 192 ~ 194 "
727 174 ~ 177 "
728 194 ~ 197 "
729 199 ~ 201 "
730 174 ~ 177 "
731 192 ~ 195 "
733 180 ~ 183 "
734 179 ~ 181
735 198 ~ 200
737 140 ~ 142 "
3~32fi
- 106 -
Comp . No . m. p . ( ~C) Status
739 195 ~ 198 White crystal
740 216 ~ 219 "
7 ~1 199 ~ 203 "
742 189 ~ 190 "
750 173 ~ 176 "
766 190 ~ 193 "
768 167 ~ 170 "
775 184 ~ 187 ll
776 160 ~ 162 "
778 162 ~ 165 "
789 191 ~ 194 "
790 165 ~ 168 . "
791 176 ~ 178 . "
792 158 ~ 160 . "
793 180 ~ 183 "
799 194 ~ 195 "
800 212 ~ 213 "
801 166 ~ 168 "
802 170 ~ 171 "
803 198 ~ 200 "
815 196 ~ 198 "
~ 816 183 ~ 184
i 817 1 110 ~ 113
i
13~0326
- 107 -
Comp. No . m. p . ( ~C) Status
818 171 ~ 172 White crystal
819 163 ~ 165 "
833 163 ~ 165 "
834 152 ~ 154 "
835 170 ~ 172 "
836 138 ~ 139 "
837 142 ~ 144 "
879 212 ~ 215 "
880 , 206 ~ 209 "
881 . 169 ~ 172 "
882 175 ~ 177 "
883 190 ~ 193 "
884 193 ~ 195 ; "
925 178 ~ 180 "
926 . 173 ~ 176 "
927 200 ~ 201 "
928 156 ~ 158
~ 929 218 ~ 220 ~ "
930 ~ 220 ~ 222 "
931 189 ~ 191 "
932 - 205 ~ 207 "
933 218 ~ 220 "
934 213 ~ 216 "
.
- 108 - 1340326
Comp. No. m.p. (~C) Status
935 212 ~ 215 White crystal
936 185 ~ 186 "
937 182 ~ 183 "
938 176 ~ 180 "
939 181 ~ 184 "
940 182 ~ 184 "
941 -195 ~ 197 "
942 -211 ~ 213 "
943 197 ~ 200 "
~ 134032~
-- 109 --
In application of the compounds of this invention as
herbicides, they can be applied by mixing with solid
carriers, including for example clay, talc, bentonite,
diatomaceous earth and others or liquid carriers,
including for example water, alcohols (methanol,
ethanol and the like), aromatic hydrocarbons (benzene,
toluene, xylene and the like), chlorinated
hydrocarbons, ethers, ketones, esters (ethyl acetate,
etc.), acid amides and others. They can be provided
for practical use with addition of any desired additive
selected from a emulsifier,a dispersing agent,a
suspending agent, a wetting agent, a spreader and a
stablizer and in any desired form such as a soluble
concentrate, an emulsifiable concentrate, a wettable
powder, a dust, a granule, a suspension concentrate,
etc.
In the following, there are shown examples of
formulations of herbicides containing the compounds of
this invention as active ingredients, but they are not
limitaive of this invention. In the exemplary
formulations shown below, "parts" mean "parts by
weight".
Exemplary formulation 1: Wettable powder
Compound No.2 of this invention 50 parts
Ziegleit A 46 parts
(kaolin type clay:produced by Ziegleit Kogyo Co.,)
Ltd.)
Solpol 5039 2 parts
(mixture of nonionic surfactant and anionic
surfactant; trade name; produced by Toho Kagaku
Co., Ltd.)
Carplex (anticaking agent) 2 parts
(white carbon; trade name, produced by Shionogi
Seiyaku Co., Ltd.)
- 13~0326
-- 110 --
All of the above components are mixed and pulverized
homogeneously to prepare a wettable powder. In use,
the above wettable powder is diluted with water to 50
to 50,000 times, and sprayed in an amount of the active
ingredient of 0.0005 kg to 10 kg per hectare.
Exemplary formulation 2: Wettable powder
Compound No.86 of this invention 75 parts
Ziegleit A 19 parts
(kaolin type clay:produced by Ziegleit Kogyo Co.,)
Ltd.)
Solpol 5039 2 parts
(mixture of nonionic surfactant and anionic
surfactant; trade name; produced by Toho Kagaku
Co., Ltd.)
Carplex (anticaking agent) 4 parts
(white carbon; trade name, produced by Shionogi
Seiyaku Co., Ltd.)
The above components are mixed and pulverized
homogeneously to prepare a wettable powder.
Exemplary formulation 3: Wettable powder
Compound No.140 of this invention 50 parts
Ziegleit A 46 parts
(kaolin type clay:produced by Ziegleit Kogyo Co.,)
Ltd.)
Solpol 5039 2 parts
(mixture of nonionic surfactant and anionic
surfactant; trade name; produced by Toho Kagaku
Co., Ltd.)
Carplex (anticaking agnet) 2 parts
(white carbon; trade name, produced by Shionogi
Seiyaku Co., Ltd.)
~ 134032~
111 --
The above components are mixed and pulverized
homogeneously to prepare a wettable powder.
Exemplary formulation 4: Wettable powder
Compound No.159 of this invention 50 parts
Ziegleit A 46 parts
(kaolin type clay:produced by Ziegleit Kogyo Co.,)
Ltd.)
Solpol 5039 2 parts
(mixture of nonionic surfactant and anionic
surfactant; trade name; produced by Toho Kagaku
Co., Ltd.)
Carplex (anticaking agent) 2 parts
(white carbon; trade name, produced by Shionogi
Seiyaku Co., Ltd.)
The above components are mixed and pulverized
homogeneously to prepare a wettable powder.
Exemplary formulation 5: Wettable powder
Compound No.292 of this invention 25 parts
Ziegleit A 71 parts
(kaolin type clay:produced by Ziegleit Rogyo Co.,)
Ltd.)
Solpol 5039 2 parts
(mixture of nonionic surfactant and anionic
surfactant; trade name; produced by Toho Kagaku
Co., Ltd.)
Carplex (anticaking agent) 2 parts
(white carbon; trade name, produced by Shionogi
Seiyaku Co., Ltd.)
The above components are mixed and pulverized
homogeneously to prepare a wettable powder.
1340326
.
-
-- 112 --
Exemplary formulation 6: Wettable powder
Compound No.384 of this invention 50 parts
Ziegleit A 44 parts
(kaolin type clay:produced by Ziegleit Kogyo Co.,)
Ltd.)
Solpol 5039 4 parts
(mixture of nonionic surfactant and anionic
surfactant; trade name; produced by Toho Kagaku
Co., Ltd.)
Carplex (anticaking agnet) 2 parts
~white carbon; trade name, produced by Shionogi
Seiyaku Co., Ltd.)
The above components are mixed and pulverizedhomogeneously to prepare a wettable powder.
Exemplary formulation 7: Wettable powder
Compound No.387 of this invention 45 parts
ziegleit A 51 parts
(kaolin type clay:produced by Ziegleit Kogyo Co.,)
Ltd.)
Solpol 5039 2 parts
(mixture of nonionic surfactant and anionic
surfactant; trade name; produced by Toho Kagaku
Co., Ltd.)
Carplex (anticaking agent) 2 parts
(white carbon; trade name, produced by Shionogi
Seiyaku Co., Ltd.)
The above components are mixed and pulverized
homogeneously to prepare a wettable powder.
Exemplary formulation 8: Emulsifiable concentrate
134032~
- 113 -
Compound No.817 of this invention2 parts
Xylene 78 parts
Dimethylformamide 15 parts
Solpol 2680 5 parts
(mixture of nonionic surfactant and anionic
surfactant; trade name; produced by Toho Kagaku
Co., Ltd.)
The above components are homogeneously mixed to prepare
an emulsifiable concentrate. In use, the above
emulsifiable concentrate is diluted to 10 to 10,000
times and sprayed in an amount of the active ingredient
of 0.0005 to 10 kg per hectare.
Exemplary formulation 9: Suspension concentrate
Compound No.387 of this invention 25 parts
Agrisol S-710 10 parts
(nonionic surfactant;trade name; produced by Kao-
Atlas Co., Ltd.)
Runox 1000 C 0.5 part
(anionic surfactant; trade name; produced by Toho
Kagaku Co., Ltd.)
1 % Rodopol water 20 parts
(thickener; trade name; produced by Rohne
Poulainc)
The above components are mixed to provide a suspnsion
concentrate preparation.
Exemplary formulation 10: Granule
Compound No. 816 of this inention0.1 prts
Bentonite 55 parts
Talc 44.9 parts
All of the above components are mixed and pulverized
homogeneously, then a little amount of water is added
134032~
- 114 -
and the whole is stirred, kneaded and granulated by
excluding granulator, then dried to prepare a granule.
Exemplary formulation 11: Granule
Compound No. 817 of this invention 0.25 parts
Bentonite 55 parts
Talc 44.57 parts
All of the above components are mixed and pulverized
homogenerously, then a little amount of water is added
and the whole is stirred, kneaded and granulated by
excluding granulator, then dried to prepare a granule.
Exemplary formulation 12: Granule
Compound No. 834 of this invention 0.5 parts
Bentonite 55 parts
Talc 44.5 parts
All of the above components are mixed and pulverized
homogenerously, hen a little amount of water is added
and the whole is stirred, kneaded and granulated by
excluding granulator, then dried to prepre a granule.
Exemplary formulation 13: Granule
Compound No. 835 of this invention 1 part
Bentonite 55 parts
Talc 44 parts
All of the above components are mixed and pulverized
homogeneously, then a little amount of water is added
and the whole is stirred, kneaded and granulated by
excluding granulator, then dried to prepare a granule.
-
134~32~
- 115 -
If desired, the compound of this invention can be
applied as a mixture with other kinds of herbicides,
various insecticides, sterilizers or adjuvants during
preparation or spraying.
As the other kinds of herbicides as mentioned above,
there may be included those as described in Farm
Chemicals Handbook, 69 th edition (1983).
The compounds of this invention can also be applied, in
addition to the agricultural and horticultural fields
such as farm fields, paddy fields, fruit gardens and
the like, to athletic grounds, vacant lands, belts
along the railroads and others. The amounts of the
pesticide to be applied, which may differ depending on
the scenes to be applied, the time of application, the
application method, the kinds of the objective grasses
and the crops harvested, may generally range suitably
from 0.005 to 10 kg per hectare.
The following test examples are set forth for
illustration of the utility of the compounds of this
invention as herbicides.
Test example 1: Herbicidal effect test by soil treat-
ment
In a plastic box of 15 cm length, 22 cm width and 6 cm
depth, there was placed a deluvium soil, seeds of (A)
rice (Oryza sativa), ~B) barnyardgrass (Echinochloa
crusgalli), (C) large crabgrass (Digitaria adscendens),
,
,_ 13~032~
- 116 -
(D) annual sedge (Cyperus microiria), (E) lambsquarters
(Chenopodium ficifolium), (F) common purslane
(Portulaca oleracea), (G) hairly galinosoga (Galinosoga
ciliata), and (H) yellow cress (Rorippa atrovirens)
were sown mixedly. After covering soil to about 1.5 cm
over the seeds, herbicides were sprayed evenly on the
soil surface to predetermined proportions of the active
ingredient. In spraying, the wettable powder as shown
in the foregoing exemplary formulations was diluted
with water and sprayed over the entire surface by means
of a small sprayer. Four weeks after spraying, the
herbicidal effect on rice and the various weeds were
examined according to the judgement criteria shown
below. The results are shown in Table 5.
Judgement criteria:
5... Growth control rates of more than 90% (almost
completely withered)
4Growth control rates of 70 to 90%
3............. .. 40 to 70 %
2............. n 20 to 40 %
1............. " 5 to 20 %
0............. " less than 5 %
(substantially no effect)
The above growth control rates are determined by
measuring the top fresh weights of the treated plants
and those of the non-treated plants, and calculated
from the following formula:
Growth Top fresh weight of the
controle = 1 - treated plants x 100
rate(%) Top fresh weight of the
non-treated plants
~ 134032~
- 117 -
Table 5
Amount or
Comp. active
ingredient(A) (B) (C) (D) (.E) (F) (G) (H)
No. (~g/ha)
0.1 6 5 5 5 5 5 5 5 5
0.08 5 5 5 5 5 5 5 5
0.1 6 5 5 5 5 5 5 5 5
0.08 5 5 5 5 5 5 5 4
0.1 6 5 5 5 5 5 5 5 5
0.08 5 5 5 5 5 5 5 4
0.16 5 5 5 5 5 5 5 5
0.08 5 5 5 5 5 5 5 5
0.1 6 5 5 5 5 5 1 5 5 5
86
0.08 5 5 5 5 5 5 5 5
0.1 6 5 5 5 5 5 5 5 5
87
0.08 5 5 5 5 5 5 5 5
88 al6 5 1 5 5 5 5 5 5 5
0.08 5 5 5 5 5 5 5 5
0.1 6 5 5 5 5 5 5 5 5
1 1 1
0.08 5 5 5 5 5 5 5 5
0.1 6 5 5 5 5 5 5 5 5
139 0.08 5 5 5 5 5 5 5 5
ao4 5 5 5 5 5 5 5 5
- 118 - ~ 134032~
- Amount of
Comp. active
ingredient ~A) (B) (C) (D) (E) (F) (G) (H)
No. applied
0.1 6 5 5 5 5 5 5 5 5
1 400.0 8 5 5 5 5 5 5 5 5
0.04 5 5 5 5 5 5 5 5
0.16 5 5 ~ 5 5 5 5 5 5
141 0.08 5 5 5 5 5 5 5 5
0.04 5 5 5 5 5 5 5 5
0.08 5 5 5 5 5 5 5 5
1 5 9~-~ 4 5 5 5 5 5 5 5 5
0.02 5 5 5 5 5 5 5 5
0.16 5 5 5 5 5 5 5 5
292
0.08 S 5 5 5 5 5 5 5
0.1 6 5 5 5 5 5 5 5 5
2 9 3
0.08 5 5 5 5 5 5 5 3
0.1 6 5 5 5 5 5 5 5 2
2 9 4
0.08 5 5 5 5 5 5 5
0.08 5 , 5 5 5 5 5 5 5
384 0-04 5 ' 5 5 5 5 5 5 5
0.02 5 i 5 5 5 5 5 5 5
0.08 5 ~ 5 5 5 5 5 5 5
385 ~-~ 4 5 5 5 5 5 5 5 5
0.02 5 5 5 5 5 5 5 5
0.08 5 5 5 5 5 5 5 5
386 0-04 - 5 5 5 5 5 1 5 5 5
0.02 5 5 5 5 5 5 5 5
0.08 5 5 5 5 5 5 5 5
' 387 0-04 5 5 5 5 5 5 5 5
0.02 5 5 5 5 5 5 5 5
- 119 - - 13~0326
r
. Amount of
CQ~ 1ngredient(A? (B) (C) (D) (E) (F) (G) (H)
No. appl ed
0.08 5 5 5 5 5 5 5 5
388 0.04 5 5 5 5 5 5 5 5
0.02 5 5 5 5 5 5 5 5
0.08 5 ~t 5 5 5 5 5 5 5
4 09 0.04 5 5 5 5 5 5 5 5
0.02 5 5 5 5 5 5 5 5
0.08 5 5 5 5 5 5 5 5
41 0 ~-~ 4 5 5 5 5 5 5 5 5
0.02 5 5 5 5 5 5 5 5
0.08 5 5 5 5 5 5 5 5
41 1
0.04 5 5 5 5 S 5 5 5
392 0.16 5 5 5 5 5 5 5 5
~ 0.08 S 5 5 5 5 5 5 5
394 0.16 5 5 5 5 5 5 5 5
0.16 5 5 5 5 5 5 5 5
-396
0.08 5 5 5 5 5 5 5 5
0.165 5 5 55 5 5 5
425
0.08 4 5 5 5 5 5 5 5
442 0.16 5 5 5 5 5 5 5 5
0.08 4 5 5 5 5 5 5 5
443 0.16 5 5 5 5 5 5 5 5
0.08 5 5 5 5 5 5 5 5
444 0.16 5 5 555555
0.08 5 5 5 5 5 5 5 5
445 0.16 5 5 5 5 5 2 5 5
0.08 5 5 5 5 5 4 5 5
~
- 120 - 13~0326
AIT~unt of
Comp. active
mgredient (A) (B) (C) (D) (E) (F) (G) (H)
No . ~I pl i etl
(kg/ha)
0.16 5 5 5 5 5 5 5 5
463 0.08 5 5 5 5 5 5 5 5
0.04 5 5 5 5 5 5 5 5
0.16 5 5 5 5 5 5 5 5
465 0.08 5 5 5 5 5 5 5 5
0.04 5 5 5 5 5 5 5 5
546 0.16 5 5 5 5 5 5 5 5
595 0.16 5 5 5 5 5 5 5 5
596 0.16 5 5 5 5 5 5 5 5
597 0.16 5 5 5 5 5 5 5 5
598 0.16 5 5 5 5 5 5 5 5
599 0.16 5 5 5 5 5 5 5 5
600 0.16 5 5 5 5 5 5 5 5
601 0.16 5 5 5 5 5 5 5 5
0.l6 5 5 5 5 5 5 5 5
734 0.08 5 5 5 5 5 5 5 5
0.0~ 5 5 5 5 5 5 5 5
0.16 5 5 5 5 5 5 5 5
735 0.08 5 5 5 5 5 5 5 5
0.04 5 5 5 5 5 5 5 5
0.16 5 5 5 5 5 5 5 5
737 0.08 5 5 5 5 5 5 5 5
0.04 5 5 5 5 5 5 5 5
13~0326
- 121 - -
Amount of
Comp. active
ingredient (A) (B) (C) (D) (E) (F) (G)
No. applied
(kg/ha)
815 0.16 5 5 5 5 5 5 5 5
0.08 4 4 4 5 4 4 4 4
816 0.16 5 5 5 5 5 5 5 5
0.08 5 5 5 5 5 5 5 5
817 0.16 5 5 5 5 5 5 5 5
0.08 5 5 5 5 5 5 5 5
818 0.16 5 5 5 1 5 5 5 5 5
0.08 5 5 4 5 5 5 5 5
819 0.16 5 5 5 5 5 4 5 4
0.08 5 4 5 5 4 4 5 4
833 0.16 5 5 5 5 5 5 5 5
0.08 4 4 4 5 4 4 4 4
834 0.16 5 5 5 5 5 5 5 5
0.08 5 5 5 5 5 5 5 5
835 0.16 5 5 5 5 5 5 5 5
0.08 5 5 5 5 5 5 5 5
836 0.16 5 5 5 5 5 5 5 5
0.08 5 4 4 5 4 5 5 5
0.16 5 5 5 5 5 4 5 5
837
0.08 5 4 5 5 4 3 4 4
Control 0.16 3 2 2 4 3 2 2 3
Compound
A 0.08 2 0 1 4 1 1 1 3
Control 0.16 4 4 3 3 4 3 4 4
Compound
B 0.08 3 3 3 1 3 2 3 3
Control 0.16 3 2 2 4 3 2 2 3
Compound
C 0.08 2 1 1 2 1 1 1 2
Control 0.16 5 4 4 4 3 3 3 4
Compound
D 0.08 4 3 4 3
1340326
- 122 -
Control Compounds in the above Table 5 are as follows:
Control Comp. A (disclosed in Japanese Unexamined
Publn. No. 169688/1981):
~ S 03 NHCNH
- H
OCH3
Control Comp. B (disclosed in Japanese Unexamined
Publn. No. 169688/1981):
~ SO~NHC~H ~ ~
Control Comp. C (disclosed in Japanese Unexamined
Publn. No. 169688/1981):
CH3O2C ~ SO NHCNH ~/ ~
CH3
Control Comp. D (disclosed in Japanese Unexamined
Publn. No. 102577/1980):
8 N~ocH3
~ SO2~HCNH ~ ~
13~032~
- 123 -
Test example 2: Herbicidal effect test by stem-leaf
treatment
In a plastic box of 15 cm length, 22 cm width and 6 cm
depth, there was placed a deluvium soil, seeds of ~A)
rice (Oryza sativa), (C) large crabgrass (Digitaria
adscendens), (D) annual sedge (Cyperus microiria), (E)
lambsquarters (Chenopodium ficifolium), (G) hairly
galinosoga (Galinosoga ciliata), (H) yellow cress
(Rorippa atrovirens), (I) corn (zea mays), (J) soybean
(Glysine max), (K) wheat (Triticum vulgare), and (L)
tomato (Lycopersicum esculentum) were sown in shapes of
spots, respectively, followed by covering of soil to
about 1.5 cm over the seeds. After respective plants
have grown to the second and the third leaf stage,
herbicides were sprayed evenly onto the stem-leaf
portion at predetermined proportions of the active
ingredient.
In spraying, the wettable powder as shown in the
foregoing exemplary formulations was diluted with water
and sprayed over the entire surface of the stem-leaf
portions of various weeds by means of a small sprayer.
Four weeks after spraying, the herbicidal effect on
rice and the various plants were examined according to
the judgement criteria as shown in Test example 1. The
results are shown in Table 6.
Some of the compounds of this invention have
selectivities on some kinds of crops.
In the following Table 6, the Control Compounds A, B
and D are the same as those in the foregoing Test
example 1.
134032~
-- 1~4 --
Table 6
Amount of
Camp ingredient ~A) (C ) (D ) ~E ) (G ) (;~ ) (I ) (J ) (K ) (L )
(~g/ha)
0.16 5 5 5 5 5 5 5 3 4 2
0.0 8 5 5 5 5 5 5 5 2 3
0.1 6 4 5 5 5 4 5 5 5 5 5
0.08 4 5 5 5 4 5 4 4 4 5
0.1 6 5 5 5 5 5 5 5 5 5 4
0.08 5 5 5 5 5 5 5 5 4 4
0.1 6 5 5 5 5 5 5 5 5 5 5
o.n8 5 4 4 5 4 5 5 5 5 3
Q16 5 5 5 5 5 5 5 5 5
86 0.08 5 5 5 5 5 5 5 5 5 0
0.1 6 5 5 5 5 5 5 5 5 5 5
87 0.08 5 5 5 5 5 5 5 5 5 5
0.1 6 5 5 5 5 5 5 5 5 5 5
88
0.08 5 5 5 5 5 4 5 5 $ 5
- 0.16 5 5 5 5 5 5 5 5 5 4
1 1 1
' 0.08 5 5 5 4 4 5 4 4 4 3
0.1 6 5 5 5 5 5 5 5 5 5 5
1 59 0.~8 5 5 5 5 5 5 5 5 4 4
0.04 5 5 5 5 5 5 5 5 4 3
1340326
.
-- 125 --
Ccmp ~nont of
No ingredient (A) (C) (D) (E) (G) (H) ~ J) (K) (L)
(kg/ha)
01 6 5 5 5 5 5 5 5 5 5 5
2 9 3
0.08 5 5 5 5 5 5 5 5 4 5
0.1 6 5 5 5 5 5 5 5 5 5 4
2 9 4
0.08 5 5 5 5 5 4 4 4 4 3
0.1 6 5 5 5 5 5 5 5 5 5 - 5
384 0.08 5 5 5 5 5 5 5 5 5 5
0.04 5 5 5 5 ~5 5 1 5 5 5 5
0.1 6 5 5 5 5 5 5 5 5 5
385 0.08 5 5 5 5 5 5 5 5 5 o
0.04 5 5 5 5 5 5 5 5 5 0
0.1 6 5 ~ 5 5 5 5 5 5 5 5 5
386 008 5 5 5 5 5 5 5 5 5 5
034 5 5 5 5 5 5 5 5 5 5
0.1 6 5 5 5 5 5 5 5 5 5 5
387 0.08 5 5 5 5 5 5 5 5 5 5
0.04 5 5 5 5 5 5 5 5 5 5
0.1 6 5 5 5 5 5 5 5 5 5 5
388 0.08 5 5 5 5 5 5 5 5 5 5
0.04 5 5 5 5 5 ' 5 5 5 5 5
0.1 6 5 5 5 5 5 5 5 5 5
409 0.08 5 5 5 5 5 5 5 5 5 o
0.04 5 5 5 5 5 5 5 5 5 0
0.16 5 5 5 5 5 5 5 5 5 z
41 0 0.08 5 5 5 5 5 5 5 5 5
0.04 5 5 5 5 5 5 5 5 5 0
0.1 6 5 5 5 5 5 5 5 5 5 ~ 5
41 1 0.08 5 5 5 5 5 5 5 5 5 5
0.04 5, 5 5 5 5 5, 5 5 5 5
1340~2~
-- 126 --
P~nount of
C~p. active
No. ingredient (A) (C) (D) (E) (G) (H) (I) (J) (K) (L)
applied
(kg/ha)
392 0.16 4 5 5 5 5 3 4 o 4 5
394 0.16 5 4 5 5 5 5 5 5 5 5
396 0.16 5 5 5 5 5 5 5 5 5 5
425 0.16 5 4 5 5 5 5555 5
442 0.16 5 5 555 5 5 5 5 o
0.08 4 5 5 5 5 4 55 4 ~
443 0.16 4 5 5 55 5 5 5 5 5
0.08 4 4 5 5 55 5 5 5 5
444 0.16 5 5 5 5 5 5 5 5 5 5
0.08 5 5 5 5 4 5 5 5 5 5
445 0.16 5 5 5 5 5 5 5 5 5 5
0.08 5 5 5 5 5 4 5 5 5 5
0.16 5 5 5 5 5 5 5 5 5 5
463 0.08 5 5 5 5 5 5 5 5 5 5
0.04 5 5 5 5 5 5 5 5 5 5
0.16 5 5 5 5 5 5 5 5 5 5
465 0.08 5 5 5 5 5 5 5 5 5 5
0.04 5 5 5 5 5 5 5 5 5 5
546 0.16 5 5 5 5 5 5 5 5 5 5
595 0.16 5 5 5 5 5 5 5 5 5 5
596 0.16 5 5 5 5 5 5 5 5 5 3
597 0.16 5 5 5 5 5 5 5 5 5 5
598 0.16 5 5 5 5 5 5 5 5 5 5
599 0.16 55 5 5 555 5 55
600 0.16 5 4 5 55 5 5 5 5 5
~ - 134032~
.
-- 127 --
a'lp actlve
ingr~dient (A) (C) (D) (E) (G) (H) (I) (J) (K) (L)
~ ~pl; e~l
(kg/ha)
601 0.16 5 5 4 5 5 5 5 5 5 5
0.16 5 5 5 5 5 5 5 5 5 5
734 0 . 08 5 4 5 4 5 4 4 5 5 5
0.16 5 5 5 5 5 5 5 5 5
735 0. 08 - 4 5 5 4 5 4 5 5 5 0
0.16 5 5 5 5 5 5 5 5 5 ~
737 0 . 08 5 5 5 4 5 4 5 5 5 0
0.16 5 5 5 5 5 5 5 5 2 5
815 0 . 08 5 4 5 5 5 5 5 5 1 4
0.16 5 5 5 5 5 5 5 5 4 5
816 0. 08 5 4 5 5 5 5 5 5 3 5
0.16 5 5 5 5 5 5 5 5 2 5
817 0. 08 4 4 5 5 5 5 5 5 1 4
0.16 5 4 5 5 5 5 5 5 2 3
818 0 . 08 4 3 5 5 5 5 5 5 0
0.16 5 5 5 5 5 5 5 5 3 5
819 0.08 4 4 5 5 5 4 4 5 2 4
0.16 5 4 5 5 5 5 5 5 2 5
833 0. 08 5 3 5 5 5 5 5 5 1 4
0.16 5 5 5 5 5 5 5 5 3 5
834 0.08 5 4 5 5 5 5 5 5 2 3
~_ 13~032~
-- 128 --
~nount of
No ingredient (A) (C) (D~ (E) (G) (H) (I) (J) (K) (L)
~ ~3~?1 i ~
(kg/ha)
0.16 5 4 5 5 5 5 5 5 2 4
835 0.08 4 3 5 5 5 5 5 5 1 2
0.16 4 3 5 5 5 5 5 5 2 3
836 0.08 3 2 5 4 5 4 4 4 0
0.16 5 3 5 5 5 5 5 5 2 5
837 0.08 4 3 5 4 5 4 4 4 1 4
Control 0.16 4 4 4 4 4 4 4 4 4 2
0.08 3 4 4 4 4 4 4 4 3 2
Control 0.16 3 3 3 3 3 3 3 4 2 2
~olm(l O.08 3 2 2 3 3 2 3 3 1 2
Control 0.16 3 4 3 3 4 3 3 3 2 2
~DP 0.08 3 3 3 3 3 3 3 2 2 2
_ 1340326
-
- 129 -
Test example 3: Herbicidal effect test under paddy
condtion
In a Wagner pot of 1/50 m2, there was placed an
alluvial soil, water was put thereinto, soil and water
were mingled and a paddy condition of 2 cm depth of the
water was made. Seeds of (B) barnyardgrass
(Echinochloa crus-galli), (M) ducksalad (Monochoria
vaginalis), (N) false pimpernel (Lindernia procumbens),
(O) toothcup (Rotala indica) and (P) bulrush (Scirpus
hotarui) were sown mixedly therein and tuberns of (Q)
arrowhead (Sagittaria pygmaea) and (R) perennial flat
sedge (Cyperus serotinus) were placed thereon. Then,
young rice plants of the 2.5 leaf stage were
transplanted.
On the next day, the diluted solution containing the
compound of this invention was dropped on the water
surface in predetermined proportions of the active
ingradient.
Three weeks after application, the herbicidal effect on
rice and the various weeds were examined according to
the judgement criteria as shown in Test Example 1. The
results are shown in Table 7.
.
1340326
-- 130 _
Table 7
Amount of
Compoundactive
No.ingredient (A) (B) (M) (N) (O) (P) (Q) (R)
applied
(kg/ha)
0.04 0 5555555
816 0.02 0 5555555
0.01 0 4 5 55555
0.04 0 5555555
817 0.02 0 555 5 5 55
0.01 0 4 55555 5
0.04 0 5 5 5 5 5 5 5
834 0.02 0 5 5 5 5 5 5 5
0.01 0 4 5 5 5 5 5 5
0.04 0 5 5 5 5 5 5 5
835 0.02 0 5 5 5 5 5 5 5
0.01 0 4 5 5 5 5 5 5