Note: Descriptions are shown in the official language in which they were submitted.
' ~ 134q3~
1 BLEACHING PROCESS FOR THE PRODUCTION OF HIGH BRIGHT PULPS
This invention relates to a multistage bleaching
process in which reducing agents and oxidizing agents
are used sequentially to bleach mechanical and
chemimechanical pulps to high brightness levels and
partially remove their yellow shade.
It is well recognized by those skilled in the art
of mechanical and chemimechanical pulping that the
quality of mechanical and chemimechanical pulps need
to be improved in termC of brightness levels, color or
yellowness and rapid reversion characteristics which
occur with yellowing. Therefore various processes are
currently employed in the pulp and paper industry to
bleach these pulps for their use in a wide variety of
paper products. The oldest process uses a reducing
agent such as dithionite or sodium and zinc
hydrosulfite (H) to brighten or bleach the pulps. With
this bleaching chemical moderate gains of 4 to 10
points are obtained. Maximum brightness levels of 68
to 70% iso can be reached with the addition of
approximately 1% to 1.25% on o.d. pulp of sodium
hydrosulfite. The process is usually carried out in an
aqueous phase at 3 to 5% consistency, a pH of 4.5 to
6.0, a temperature of about 60~C and a retention time
of up to one hour. The use of a chelating or
sequestering agent such as sodium tripolyphosphate
(STPP) to remove naturally occuring trace metals is
recommended. This agent is being added to the pulp
prior to the addition of the reducing agent or is
incorporated in the bleaching solution.
Today, peroxide (P) is the most commonly employed
oxidizing agent for bleaching mechanical and
chemimechanical pulps. This alkaline process is
normally carried out in a single stage or in a double
stage. In both cases, the bleaching is done at a pulp
consistency of 15 to 35%, moderate temperatures of 50
to 70~C, and retention times of 2 to 3 hours for each
C ~
2 1340348
l stage. In peroxide bleaching, stabilizers such as
sodium silicate and magnesium sulfate are added to the
bleach liquor to prevent decomposition of the
oxidizing agent. Sodium hydroxide is also used to
maintain an alkaline pH of 9.5 to 11 so as to increase
the concentration of the perhydroxyl ion OOH- which is
believed to be the active bleaching agent.
Furthermore, pulps are normally pretreated at low
consistency with organic chelating agents such as
sodium diethylenetriamine penta-acetate (DTPA) to
remove naturally occuring trace metals. Additional
quantities are added in the bleach liquor to complex
trace metals that are desorbed from the pulp as a
result of the reaction of the bleaching agents with
the chromophores of the pulp. In the bleaching of
commercial pulps, iso brightness of 74-76% are
conventionally achieved using this process with 3%
hydrogen peroxide on o.d. pulp in a single stage while
values of 76-78% iso-brightness are achieved in two
stages in which greater retention times and higher
peroxide charge are applied, i.e. 5% hydrogen peroxide
on o.d. pulp.
Two stage bleaching of groundwood pulp using
peroxide in the first stage and hydrosulfite
(dithionite) in the second stage is well known and
applied commercially (PH). An ISO-brightness level of
75-77% is achieved. However, much lower brightness
levels are achieved when this two stage sequence is
reversed (HP) (Schroter, H., Wbl. Papriefabr. 97, No.
23/24 (1969) p. 1023 and Joyce, P. and Mackie, M.,
CPPA, TAPPI International Pulp Bleaching Conference,
Toronto, Canada, June 11-14, 1979, Preprint Page 116).
Other multistage bleaching processes have been
disclosed in the literature but have not found
commercial application. For instance, Loras, V. and
Soteland, N. have published results for a three stage
/~
., . . . . ., . ... _._ ................................. .
13~03~
1 bleaching sequence utilizing borohydride, peroxide and
dithionite sequentially (BPH). This sequence was
reported to yield a brightness of 88% from an initial
level of 67%, an increase of 21 points. (High
Brightness Bleaching of Mechanical Pulp, Norsk
Skogindustri, 10/72 p. 255). It is also known from
U.S. Patent 3,100,732 to Smedberg to use a combined
and simultaneous action of an oxidizing agent and a
reducing agent; the patentee also discloses that when
using a double stage sequence, one uses the oxidizing
agent first and subsequently the reducing agent.
Liebergott, N., and Heitner, C. disclosed a multistage
process for bleaching high yield and ultra-high yield
pulps in which the pulp is treated sequentially with a
peroxygen compound (P), a reducing compound (R) and a
final peroxygen compound (P) to achieve higher
brightness levels (Eur. Pat. Appl. EP 187,477).
Tibbling, P. also disclosed a multiperoxide stage
mechanical pulp bleaching process in which the pulp is
treated sequentially with hydrogen peroxide in a first
stage (P) and a second stages (P) and sodium
hydrosulfite in a third stage (H) (Eur. Pat. Appl. EP
191,756). It is claimed that higher brightness levels
are obtained than for those obtained for the bleaching
sequence involving hydrogen peroxide (P) followed by
sodium hydrosulfite (H).
It is an object of the present invention to
provide a multistage bleaching process for mechanical
and chemimechanical pulps which gives high brightness
levels to such pulps and partially remove their yellow
shade.
According to the present invention, there is
provided a method for the bleaching of high yield or
ultra high yield pulp which comprises the steps of
sequentially treating the lignocellulosic fibres with
_~.. . . .. , ~ ,, ,
13403~8
1 a reducing compound and subsequently treating the same
fibres with peroxide in two successive stages.
In greater detail, the method or process includes
three stages where the wood pulp is subjected to
bleaching operations. The wood pulp which may be
utilized is any high yield or ultra yield pulp such as
mechanical, chemimechanical, chemithermomechanical,
groundwood, refiner mechanical pulp, thermomechanical
pulp, high yield and ultra high yield sulfite pulps.
In the first stage, the wood pulp is treated with
a reducing compound which may be chosen from many such
reducing compounds known to those skilled in the art.
During this first stage, preferred reaction conditions
include:
(1) a reducing compound charge of about 0.01 to
about 1.5% by weight of oven dried pulp;
(2) the presence of a chelating agent such as
DTPA or STPP;
(3) a reaction temperature of from about 60~C to
100~C;
(4) a reaction time of from about 4 to about 120
minutes;
(5) a pulp consistency of from 3% to about 35%
and
(6) a reaction terminating pH of about 3.5 to
about 11Ø
In the second stage, the pulp is bleached with a
peroxygen compound. Preferred conditions of bleaching
include:
(1) a peroxygen compound charge of about 0.1% to
about 5% by weight of oven dried pulp in the
presence of sodium hydroxide, sodium
silicate, magnesium sulfate and DTPA;
(2) a reaction temperature of between about 60~C
to about 100~C;
13~0 3~8
1 (3) a reaction time of from about 4 minutes to
about 180 minutes;
(4) a pulp consistency of from about 4% to about
40%; and
(5) a reaction terminating pH of from about 8.5
to about 10.5.
In the third and final bleaching stage a peroxygen
compound is utilized which is similar to the one used
in the second stage. The preferred reaction conditions
include:
(1) a peroxygen compound charge of about 0.1% to
about 12.0% in the presence of sodium
hydroxide, sodium silicate, magnesium sulfate
and DTPA;
(2) a reaction temperature of from about 60~C to
about 100~C;
(3) a reaction time of about 4 minutes to about
240 minutes;
(4) a pulp consistency of from about 4% to about
40%; and
(5) a reaction terminating pH from about 7.5 to
about 10Ø
The compounds utilized in the process of the
present invention may be selected from among these
well known to those skilled in the art. Thus, the
reducing compounds may be chosen from commercially
inorganic reducing agents such as sodium or zinc
3' hydrosulfite (dithionite), sodium or magnesium
bisulfite, sodium borohydride, sodium and potassium
borosulfate (borol),thiurea
dioxide, ammonium borohydride, hydrazine and organic
reducing agents such as amine-boranes and phosphine-
boranes. It will be noted that some of these reducingagents are sold commercially with a chelating agent
mixed therewith.
C
. _ . .... . . . . ..
6 13403~
1 Examples of the peroxide compound utilized in the
second and third stages may include conventional
inorganic peroxides such as hydrogen and sodium
peroxide and also organic peroxides such as benzyl
peroxide, ditertiary-butyl peroxide and peracetic
acid.
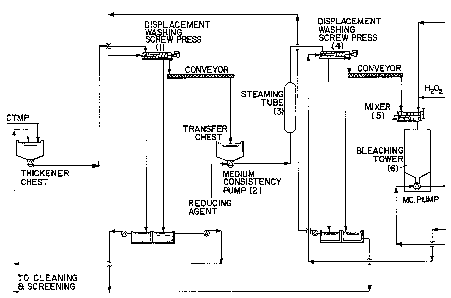
The process flow diagram of figure 1 illustrates
the various steps of a continuous operation in which
pulps are bleached in multistage according to the
present invention. In the process the pulp is first
washed with a dewatering device (1) such as, but not
exclusively, a standard screw press, a displacement
washing screw press, a twin wire press, a disc filter
or a twin roll press. These devices allow for water
removal from the pulp slurry as well as for washing of
contaminants such as sodium sulfite, metal ions,
organic extractives, dissolved solids, etc., which are
known to impair on the bleaching reactions between the
bleaching agents and lignocellulosic fibres. Following
this washing stage the pulp is mixed with the
bleaching liquor containing the reducing agent. Mixing
devices (2) such as single or double shaft mixers,
refiner type mixers, high shear mixers and medium or
low consistency pumps can be used. It is important in
this stage of the process to disperse the bleaching
liquor uniformly onto the fibre surface so that
bleaching reactions can prevail over darkening
reactions that also occur when lignocellulosic fibres
are submitted to high temperature. After this pulp
mixing stage the reducing agent reacts with the pulp
in an upflow tower or steaming tube (3). A chemical
charge of 0.75 to 1.25% sodium hydrosulfite and of 0.3
to 0.5% of sodium borohydride by weight of oven dried
pulp are the preferred charges. A temperature between
65 to 85~C; a consistency between 3.5 to 5% for sodium
hydrosulfite and of 10 to 12% for sodium borohydride;
a reaction time of between 1 to 40 minutes is
~J
. ~ . . . . . _.
1340348
1 preferred at this stage to favor a more effective use
of the reducing agent as it cannot be reused in the
system. A reaction terminating pH of about 4.5 to 5.0
for sodium hydrosulfite and of about 10.0 to 10.5 for
sodium borohydride is also recommended. Following this
first stage bleaching with a reducing agent the pulp
is dewatered and washed with a dewatering device (4)
such as those described previously and used in
position (1). The purpose is to wash the unreacted
reducing agents or byproducts produced from the
bleaching reactions so as to minimize its carry over
to the next bleaching stage. After this pressing and
washing stage the pulp is mixed with the peroxide
bleaching liquor in a mixer (5). Other devices such as
those described previously and used in position (2)
can also be used. The efficiency of the mixer is
important at this stage of the process to disperse the
bleaching liquor uniformly onto the fibre surface so
that oxidizing bleaching reactions of the chromophoric
groups on the lignocellulosic fibres occur and prevail
over darkening reactions that also occur when pulps
are submitted to high temperatures. We show in figure
1 a mixer (5) which allows for the addition of steam
and the peroxide bleaching liquor simultaneously.
Following this mixing stage the pulp is transferred to
a bleaching tower (6). The most preferred charge of
the peroxygen compound in this second stage bleaching
is in amount equal to the charge of the last bleaching
tower or to one third of the charge of the last
bleaching tower. Sodium hydroxide, sodium silicate and
magnesium sulfate are preferably added in charge
ranges of 0.5-3.0%, 0.0 to 3.0% and 0.01-0.05%
respectively. It is also preferable to add small
amounts of DTPA between 0.1-0.4%. All these components
stabilize the peroxygen compound, in the form of the
perhydroxyl ion, initiate and maintain a stable
bleaching reaction. In a commercial operation the
peroxide bleaching liquor mixed with the pulp at this
r~~
~,
.
. . . ~ .
134034~
l stage can be either prepared from fresh commercial
components dissolved in water in separate tanks or it
can be a residual bleaching liquor solution from the
last stage bleaching tower (9). We have found from
mass balance calculations that this latter is
preferable to minimize the operating and bleaching
cost of the process disclosed in this application. The
size of the second bleaching tower (6) is to be
determined considering the production rate, reaction
time and pulp consistency selected or desired. We have
found that a consistency in the 10-12% range, a
retention time of 60 to 90 minutes and a temperature
of 65 to 70~C are preferable to minimize the bleaching
cost of the process disclosed. In figure 1 we show a
schematic of a tower which is discharged with a medium
consistency pump so as to have an operation with an
efficient control over the bleaching conditions.
Following this second stage bleaching with an
oxidizing agent the pulp is dewatered and washed with
a dewatering device (7) such as those described
previously and used in position (1) and (4). The
purpose is to wash the byproducts produced from the
bleaching reactions which occured in the second stage
bleaching tower, avoid their carry over to the next
bleaching stage and eliminate these from the bleach
plant with an adequate white water recirculation
strategy. After this pressing and washing stage the
pulp is mixed with the peroxide bleaching liquor in a
mixer (8). The mixer used and its efficiency are
important at this stage for the same reasons as those
elaborated previously above. Following this mixing
stage the pulp is transferred to a bleaching tower
(9). The preferred charge of the peroxygen compound in
this third stage bleaching is 3 to 10% by weight of
oven dried pulps. Sodium hydroxide, sodium silicate
and magnesium sulfate are preferably added in charge
ranges of 0.25-0.3%, 0.01% to 3.0% and 0.01-0.05%
, . .. . . . , .. __
1340~4~
l respectively. It is also preferable to add small
amounts of DTPA between 0.2-0.4%. In a commercial
operation it is preferable in this last bleaching
stage that fresh peroxide bleaching liquor be used and
mixed with the pulp. This bleaching liquor is normally
prepared from fresh commercial components which are
dissolved in water in separate tanks. In addition to
this fresh liquor, a small quantity of the residual
bleaching liquor solution from the same tower (9) can
also be used. The residual bleaching liquor is
recovered with the last pair of dewatering presses (11
and 12). We have found that high charges of peroxide
are required in this last bleaching tower to achieve
high brightness levels. The size of the third
bleaching tower (9) is to be determined considering
the production rate, reaction time and pulp
consistency selected or desired. We have found that a
consistency in the 20-35% range is preferable to have
high effective concentrations of the oxidizing agents
so as to minimize the bleaching cost of the process
disclosed.
In figure 1 we show a schematic of a tower which
is discharged in a transfer chest (10) with a screw
conveyer. This device allows for a positive
displacement out of the tower so as to provide an
efficient control over the operating and bleaching
conditions of the pulp in the tower.
Following this third stage bleaching with an
oxidizing agent the pulp is washed and dewatered with
dewatering devices, (11) and (12), such as those
described previously. At this stage it is important to
add fresh water in the transfer chest (10) to wash the
pulp by dilution and minimize brightness reversion
subsequently. After the final stage of the bleaching
process the pulp is pressed so as to recover the
unreacted peroxide bleaching liquor and to reuse it in
C''
1341)~48
1 the process as shown in figure 1. This white-water
recirculation strategy and counter current washing
lowers the operating cost of the bleaching process
disclosed. The addition of sulfuric acid in the
transfer chest (10) or the addition of SO2 in the
fluffer (13) is also desirable to lower the aqueous
solution pH to about 6 to minimize brightness
reversion subsequently.
Having thus generally described the invention,
reference will be made to the following examples;
EXAMPLE 1.
A commercial spruce balsam chemithermomechanical
pulp from an Eastern Canadian mill was washed with
0.5% diethylenetriaminepentaacetate (DTPA) for 30
minutes at 60~C and 3% consistency to eliminate metal
ions which impair the bleaching reactions. Following
this treatment, the pulp was pressed to 25%
consistency and bleached. The experimental conditions
and chemical charges are given in Table 1.
The bleaching chemicals were mixed by hand with a
20-g pulp sample, while the pulp consistency was
simultaneously adjusted with demineralized water.
Subsequently, the bags were sealed and immersed in a
thermostatically controlled bath for the bleaching
reactions to occur. After bleaching, the pulps were
neutralized to destroy the bleaching agents and to
adjust the pulp pH to m; nimize brightness reversion.
For hydrogen peroxide bleaching, sodium metabisulfide
was used, while sulfuric acid was used for the other
bleaching agents.
The pulps were neutralized by diluting the pulp to
3% consistency with the neutralizing agent, mixing the
slurry for 5 minutes, and pressing the pulp to 18%
consistency. After neutralization, two samples of 3.5g
C ~!
_ ................ .. .
11 13~034~
1 each were used to make the handsheets. The pulps were
disintegrated for 2 minutes at a consistency of
approximately 0.3%. The sheets were made with
demineralized water on a British handsheet machine
following the procedures prescribed by the Canadian
Pulp and Paper Association. The sheets were pressed
for 2 minutes at 50 psig and dried for 24 h at 23~C
and 50% RH. The brightness was measured with an
Elrepho spectrophotometer. Reflectance measurements
with Filter Nos. 8, 9, 10, and 11 were made and used
to calculate the color coordinates (CIE LAB) reference
system. ISO brightness reported are the reflectance
values at 457 nm using filter No. 8.
15In the multistage bleaching experiments, each
stage was similar to the single stage. However, the
two-stage experiments were carried out with 30-g pulp
samples, and 40-g samples were used for three stages.
In all cases, a 7-g sample was taken at the end of
each stage and was processed to obtain brightness
values.
The results in Table I show the superiority of the
- multistage bleaching process disclosed in the present
invention compared to the bleaching processes which
constitute the prior art. High brightness values are
achieved (ISO-brightness and L*) and a great deal of
the yellow shade of the pulps is removed (B* values)
while the pulps have less greenish shade than those
bleached with peroxide only (P). These benefits remain
after reversion. It can also be observed that for the
bleaching process disclosed less peroxide is consumed
to achieve higher brightness levels.
~_J
. .
... . . . . . . .
12 134034~
1 EXAMPLE 2.
A commercial spruce balsam chemithermomechanical
pulp was pretreated and bleached following the
experimental procedures described in example 1 and
under the chemical charges and bleaching conditions
given in Table 2. The results in Table 2 show the
superiority of the multistage bleaching process
disclosed in the present invention compared to other
multistage bleaching sequences; peroxide-reducing
agent-peroxide (PRP) and peroxide-peroxide-sodium
hydrosulfite (PPH). With the sequences sodium
hydrosulfite-peroxide-peroxide (HPP) and sodium
borohydride-peroxide-peroxide (BPP) higher ISO-
brightness values are obtained for a given totalperoxide addition level while less peroxide is
consumed in the process. Inversely at a constant
peroxide consumption level lower ISO-brightness values
are obtained with the bleaching procedures of the
prior art compared with the process disclosed in this
application. In addition to higher brightness values
it can be seen from Table 2 that low B* values are
obtained which indicate that the pulp bleached
following the process disclosed is less yellow than
the control pulp, as well as the pulp bleached with
hydrogen peroxide only or upon bleaching with the
procedures described in the prior art.
EXAMPLE 3.
A commercial spruce balsam chemithermomechanical
pulp was -pretreated and bleached following the
experimental procedures described in example 1. In
this series of experiments the pulp was bleached under
different charges of the reducing agent in the first
stage. The charges used were from 0.01% to 0.5% while
the total peroxide charge was kept constant at 5%. The
r~ ~
13 ~ 0 3 4 ~
13
l results in Table 3 show that higher brightness values
are obtained with increasing charges of the reducing
agent. It can be observed that an optimum charge
between 0.1 to 0.3% is desirable. Progressively lower
B* values are obtained with the addition of the
reducing agent therefore eliminating a great deal of
the yellowness of the pulp.
EXAMPLE 4.
A commercial spruce balsam chemithermomechanical
pulp was pretreated and bleached following the
experimental procedures described in example 1. In
this series of experiments the pulp was bleached under
a given charge of 0.3% of the reducing agent, sodium
borohydride, in the first stage while increasing
charges of peroxide up to 5% by weight on oven dried
pulp were added in the second and third stages. The
results in Table 4 show that higher brightness values
are obtained with increasing charges of peroxide in
the second and third stages. It can be observed that
small brightness gains are realized with charge levels
slightly in excess of 3% so that preferred conditions
would be for an addition level of 4 to 5% in peroxide
o.d. weight when sodium borohydride is used.
Progressively lower B* values are obtained with the
addition of peroxide therefore eliminating a great
deal of the yellowness of the pulp.
EXAMPLE 5.
A commercial spruce balsam chemithermomechanical
pulp was pretreated and bleached following the
experimental procedures described in example 1. In
this series of experiments the pulp was bleached using
a constant charge of 0.5% sodium hydrosulfite as the
Cl
, . .
.. . ......
134034~
14
1 reducing agent in the first stage. In the second and
third stages progressively increasing peroxide charges
were added from 1% to 8% o.d. weight o.d. pulp. The
results in Table 5 show that higher brightness values
are obtained with increasing charges of peroxide in
the second and third stages. It can be observed that
smooth increases are obtained up to 8% added peroxide
allowing for high brightness levels and appreciable
pulp yellowness removal.
EXAMPLE 6.
A commercial spruce balsam chemithermomechanical
pulp was pretreated and bleached following the
experimental procedures described in example 1. In
this series of experiments the pulp was bleached using
a constant charge of 1.0% sodium hydrosulfite as the
reducing agent in the first stage. In the second and
third stages progressively increasing charges of
peroxide were added from 1% to 8% o.d. weight on pulp.
The results in Table 5 show that higher brightness
values are obtained with increasing charges of
peroxide in the second and third stages. It can be
observed that smooth increases are obtained up to 8%
added peroxide allowing for high brightness levels and
appreciable pulp yellowness removal. It can be
appreciated that higher brightness levels are achieved
compared to example 5 so that preferred reducing agent
charges are 1.0 to 1.25% o.d. weight on pulp.
It will be understood that the above described
embodiments are for purposes of illustration only and
that changes and modifications may be made thereto
without departing from the spirit and scope of the
invention.
f~ '
V
.. _ . . . .. . . . . _
134034~
_. 15
TABLE 1: BLEACHIK6 CHE~ICAES AND SEOUENCE5
CHEIIICAL CHAR6ES PER07tDE-
~ND 8L~ACHINS uNa~. HyDRa~ P~ROXIOE HY3RUSllEFlT~ 9lEACHIN6 FRocEss slscLosED
CCNDITIONS FULP SUEFITE PEROXIDE
IH) u~ (PH)IPH)IPP) (HPP)IHPPI~sPp1taPp)
RIRST STAGE
N~2slo3 7l - 3,00 3.883.0il3.a~
~l9SO~ I - 0.05 o.asa.oso~os - - - -
N~OH Z - 3.00 1~6al.ùa l~sa
O.T.P.A~ 7. a.~o o~o e.4aa.~oe.~a o~a 0.40 a.~aa.~a
H202 ADDED I - 8.00 2.s24.0a2.sa
~IBH~ - - - -a. 3ao ~ 30
Na2s2o4 ~ a - - - l~O~ I.aa - -
10 CONSISTE~CY t s.a ls.o lo.ola~al~.a s.a 5.2 la.alO.a
tE~lPERArURE C 70 70 7a 70 70 7o 7a 7a 70
RETERTION TIPE ~in. 3a 9o 9o 9o sa 3a 3a 2a 2a
pH IINITIAL1 - 5.5 11.011.0Il.a ll.a 5.4 5,311.7 II.S
pH (FIN~L) - 5.~ 9.6 6.~8.5 9.5 4.9 s.a 12.~10.4
SECOHD STA8E
N~2SiO3 X - - 3.aa ~.ao 3.aa 3.ao3.0D
15 ~IgSO~ 7 ~ - a.as o.as o.os o~oso~os
N2DH t - - 1.5~ 1.7~ 2.26 1.391.39
o.r.P~ x 0.40o.~a a~o o.~a o.~a a.4aa.40
H202 AODED 7 - - 2.s2 2.s8 4.aa 1.582.s0
N~2~204 ~ I.oal.oo
CONSlStENCY 2 5.0s.e 20.a lO.O Ia.a Ia.ala.o
TEI~PERATURE C 7~ 70 70 7a 7a 70 70
RETENTION II~Enin. 3B 30 90 ga 9~ 90 90
pH ~I~IITIAL) - 5.~ 5.~ I~.a 10.9111.8il.l 11.0
20 pH (ftNAL) - 4.2~.~ 10.7 a.1 8.0 la.llo~s
rHlRD StA6E
N~25103 x 3.aa 3.aa a.aa3.00
llgSa~ I a.os a as a.oso~os
N~OH t 1.75 2.eo l.aal oo
D T P A. t o.~a o~o a.4a o~o
H202 ADDED I 2.sa ~.aa l.sa2.50
25 CGN61STENC~ t 28.0 20.0 2a.022.0
lEtlFERATURE C 70 70 70 70
RETE11TION tl~~nln. 90 90 90 90
pH (IIIITIAL)
pH (FIIIAL) - lo~s la.6 10.519.5
tot~L
- - - - -
H202 ~AODED~ t 3.ea2.sa4.aa s.oa s~oo a.oo3.0a s oa
30 N202 (RESIOUAL) ~ a.7s1.322.46 2.1a 2.79 5.s61.66 2.gs
H202 (CO~SUI~ED) ~ 2.2l l.l8 1.51 2.82 2.21 2.~ '.3y 2.ls
O~TICAL PRCPERTIES
8EFORE REVERS1011 ~
SR16HT~ESS llso-~s7n~) 7. 52.s 68.s 71.1 J6.~ 78.s 19~osa.s 77.a 78.2
L- - as.l 91.~95.1 94.59~.895.3 95.9 9S.3 95.195.0
Al - -0.87 -0.98-2.l6 -1.99-l.95-1.66 -1.97 -2 18 -2.67-2.20
35 9- - S.25 9.689.16 e.61s.~Be.33 7.~9 6.6e 3.3S7.98
AfTER REVERSION ~
DR16HTNESS (1so-4s7n~) ~ 61~9 67.57s.77~.5 75.0 76.6 77.77e.~ 75.5 l7.B
L~ - s6.a 9l~l 9~.59~.29~.2 94.~ 94.6 95.09~.8 94.1
8 37 ~ -3.ao -l.94-l.75-3.Ja -1.80 ~2.0l -2.72-2.7~
- 9.6~ 19.a99.1119.~0~.997.a3 9.03 7.se s.eo7.~9
c
16 134~4~
CHEIlICAL CHAR6~S l-E 2 3'.EACliING CliEMICALS AND SEQUENCE
AND 9lEACHIN6 uNel~ - 3lEACHIN6 PROCESS DlSClO~ED
COHO l T l ONS PULP - ..... .
(pp) lpp) (PBP) (P3P) (PHP) (~HP) (PPHI (PPH) (9PP) (9PP) (HPP) (HPR)
.. . . ...... . .. . . .....
~IRST STASE
- - ~ - - - - - - - -
Na2giO3 1 3,LC 3.C0 3,CC 3.'0 3.C0 3.CC 3.C0 3.GD
~9so4 l o.as 0.05 Q.05 o~os 0.05 2.0~ 0.05 0.05 - 0.0~ - -
NaO~ .aCu1.~30 l.60 1.60 1.60 1.60 l.aQ l.90 1.03 I,DC
D.T.P.A. l O.~G C.~O ~lo G.49 C.40 û.40 C.4~ 0.40 0.4G 0.40 o~c 0.40
H202 ADDED I 2.53 1-52 2.53 - 2.s0 2.50
lla3H4 2 - - - - - - - - 0.30 0.30
Nd2620~ Z - - - - - - - - - - O. 50 C. 50
CONSISTENCY Z 10.3 lO.D lC.O IO,G 10.0 10.0 lC.O ID.O lo~o lO.O S.O 5.C
TERPERATueE C in 10 7C 7Q 70 7Q 10 1o iG 70 70 70
RETENTION II~IE ~in. 9Q 90 90 9~ 9C 90 90 90 20 tO 39 3C
pH IINITIAL) - ll~o ll~o ll.O il~o ll.D ll.O ll.O ll.Q ll,S 11.5 5.4 5.2
pH (EIHAL) - 9~s 9~s 3.6 6.6 8.4 3.4 a.1 3.1 l0.4 lC,4 s.2 S.4
SECOND ST~GE
Na2SiO3 2 3.003.00 - - - - 3.D~ I,GO 3,3C 3,C~ 3.~0 3,0C
119504 l o~ csc~ os - - - -G . CSO . iS0. C50 . 050 . C5 O. G5
NaOH 2 (.54l.5~ l.CO l.OO - - 1.70 1.70 l.39 l.39 1.14 1.14
D.T.P.~. I O.~CD.40 G.~O 0.40 o~o o~c c~o 0.40 0.~0 0.40 O.~C 0.40
H202 ADDED l 2.5C 1-53 1.72 - 1.72 2.5~ 1.85 2.50 2.27 2.5G 2.25
Na8~ . 3
N~2s2o4 1 - - - -o~ sn~ jo
CONSISTEHC~ I 20.02D.0 Io~a lo~o s~o s.3 29.0 t6.Q IQ.C IC.Q 15.G IO.C
TERPERATURE C 7D 7D 7~ 7C 70 iû 70 IC 70 70 70 1~
RETENtlON TlllE ~in. 90 9C 2~ 20 30 30 9G 90 90 9D 9C qû
pH (INITIAL) -ll~o ll.G l2.2l2.2 s.4 5.4 Il.G l~ o ll~c~
pl~ (~îNA~) - l0.7l~.7 lt~o ll.3 ~.4 ~.t l0.3 l0.3 1~.5 I~.S 3.6 6.6
IHIRD STA6E
~ - - - - - - - -
Ha2SiO3 7 3.DC 3.C03.90 3.CO - - 3.GD 3.W 3.C0 3.80
RgS04 I 0.05 0.050.05 o.as - - o~os o~ûs O.i5 0.05
NaOH I l.25 1.25 l~ oo - - l~o l~oo 1.7S l.7s
D.T P.A. l 0,43 0.~00.40 0.40 0.40 0.4~ 0.40 0.40 0.40 a.43
H20i ADDED x 2.58 lJ32.50 1.73 - 1.85 2~sQ 2.s0 2~sQ 2.25Ha?920~ 7. 2.28
Cû'nii31ciiCï ~ 20.I7 2û.020.0 20.0 5 G 5.C 20.6 2Q.0 20.0 20.û
TEllPERArURE C 7c 79 10 l9 10 79 70 t~ 70 7RETENTIOH TIRE ~in, 90 90 93 90 30 30 9o 90 9o 9o
pH (I~TlAl) - ll~o ll~oll o ll ~ s 5 5 5 ll l ll I ll O ll OpH (~IN~L) - II.D 11.0 9.6 9.6 5.5 5.s lo~s l0.5 lO.~ 10.4
TOTAl
- - ~ - -
11202 (A90EO) 2 5.03 3.0s s~oo 3.~5 5.CC 3.~5 5.00 3.79 s~oc ~ss 5~0C ~so
H202 (R~SIDUAL) I 2.l8 ~.95 2.43 l.45 2.62 l.45 2.s7 I.7Q l.9S 2.ss 2.97 2.5Q
H202 (coHsuREo) 2 2.82 2.0~ 2.51 2,0D 2.38 2.~0 Z.43 2.CD2. 1. 2.DC 2.03 7.W
OPTICAL PROPERTIES
- - - - - -
BEPORE REvER5 l oll I
3R16~1TNE55 (ISO-~7n~)l62.~ 7a.5 7~.979.0 78.9 77.s 76.1 1a.Q 77.~ la.2 7B.2 79.9 78.7
L~ - 37.395.3 9~.s 94.995.0s~.a9~.7 9~.9 94.~ 95.Q 95.2 9S,2 95.1A~ - -C.74-2.66 -2.60 -1,86~l.90~2.02-l~9~~1.9l-l.75-2.20 -2.ao -2.08 -2.30
8~ - a.37a.33 9.l7 7.288.l~6.2sa.6~ 7~9g a.2s 7.98 7.20 9.02 9,~C
AETER RE~ERSION:
aRlBHTNESS (150-457no)2 61.9 76.674.677.2 - 7s.3 - 76.9 - 17.0 - 78.2
Ll - a7.4 9~ s3.a 93.t - 9~,4 - 94.7 - 9~l 95.~ -
A~ - -1.25-3.30 -3.18 -2.16 - -l.78 - -1.95 - -2.79 - -1.93
9. - 8.7~7.33 3.9~7 6.4~ - s.c4 - 8.~3 - 7.39 - ~a.c3
~ '
v
13403~
.
....
TABLE 3: OHErllCAL CHAR6ES AND eLEACHlN8 COHDITION3 FGR
TH~ 58Q~EHCE SODIUtl 80ROHYDRIDE-PERO~IDI-PEROX19E (8PP)
. .
FIRST STA9E
I~-OH X 0.03 D.10 0.17 0.~3 I.ea 1.67
D.~.P.~. ~ a.4a 0.4a o.~a o.~o a,~o ~.40
Na8H4 7. ~.el 0.03 0.05 ~.10 0.3a o,sa
CONSlSTEliCY L ll.a 10.0 lo~O lo.~ lO.o 1l.O
TE11PER~TURE ~ 7a 70 70 70 70 7a
RET9NT1011 TlllE ~in. 21 2a 20 20 20 20
p~ (INITIAL) - 9.3 9,7 10.3 10.9 11.5 11.8
pH (FINAL~ - 7.6 9,1 9.5 s.a 10.4 II.Q
10 SECCND ST16E
N~2s~D3 Z 3.2a 3.0a 3.00 3.00 3.00 3~Da
l~gSO~ % 0.05 0.05 a.os o.as o.os O.OS
Na9H T ~ 2.aq 1.56 I.Sg 1.39 1.22
D.t.P,~. ~ 0.4a 0.40 o.~o 0.4a 0.4a o.~o
H202 hDDED X 2.50 2.50 2.50 2.50 2.$0 2.50
C~NSlSt~!lC~ 7. 11.1 la.O Ie.O 10.0 10.0 IQ.a
TE~PERATURE C 70 78 70 7a 70 70
RETENTIOH tlll6 hr. 1 5 1 0 1.5 1.5 1.5 1.5
pH (INITIAL~ - 11.0 11.0 11.0 11.0 11.1 ll.a
pH (EI~AL) - 9,1 8.8 9.6 9.5 10.5 10.2
THIRO STA6E
H~2S~3 X 3.oo 3.~a 3.0D 3.00 3.0a 3.00
~S04 ~ ~.05 0.05 ~.as S.OS O.OS o.es
N~OH ~ 1.2S 1.00 e.7s 1.25 I.oo l.oo
D.T.P.A. Z o.~a 0.4~ e,4a 0.4e 0.40 o.~a
H202 AODED X 2.s0 2.50 2.5a 2.50 2.S1 2.5a
CONSlYtENC~ 7 2a.0 21.1 2e.1 20.a 20.0 20.0
tEl~PERATURE C 70 70 70 10 7a 70
RETENrloll Tï~ hr. 1.5 1.5 1.5 l.5 1.5 1.5
pH (IHITIAL) - 11.1 11.0 Il,a 11.2 ~ 1.0
pH (~ AL) - la.l 10.3 lo.~ Ia.s 10.5 1~.6
2 5 TOTAL
. .
H202 ~ADDED) X 5.22 5,0a s.za s.oa 5.0B 5.20
H202 ~RESIDUAL) ~ 2.38 2.34 Z.41 2.48 2.g5 3.19
H202 ~CONSUIIED) I 2.62 2.66 2.59 2.52 2.15 I.a
OPTICAU PROPERTIES
8FFORE REVERSION:
9R16HTNE6S ~Iso-4s7n-)z62,11 77.5 71.2 77.8 78.5 79.2 77.8
7.3 95.2 95.1 95.0 95.3 9S.Q 94.6
A- - -0.74 -2.71 -2.tS -2.75 -2.92 -2.20 -2. J7
5~ - 9.37 ~.77 8.5~ 8.52 8,Z5 7.98 7.~4
AFTER REVERSION t
8R16HTNE9S ~ISO-451n-)T 61.9 7$.9 16.0 76.5 77.1 77.1 77.0
l- ~ e7.~ 9~.1 g~.l 94.1 94.1 9~.1 93.8
A- -I . 25 ~3.11 -3.11-2. 49 -~, 93 -2. 79~2. ~2
8- 8.73 8.19 9.~9 7.39 7.39 7,39 6.9~
13~0348
.
- 18
,, . ..... , . ~ , . . .....
TABLE 4: CHEnlCAL CH~R6ES AND BLEACHlt6 ~ONDITIONS FO~
THE SEQUENCE SODlUn 30ROHYDRIDE-PER5X106-PEROXIOE ~aPP)
EIRST StA6E
~aOH X l.Q3 I.oa ~.aa l.oa
D.T.P.A. 7. 0,~0 a.40 0.~0 e.~o
NaBH~ X a. 3a o. 3~~ . 30 O. 30
coHslsTENcr S la.l 17.0 10.8 la.
tE~lPERATURE ~ tO 70 7a 7a
RETENTION TI~IE ~in, 2a 2B 20 2a
pH (INITIAL) - ll.S 11.5 11.7 II.S
pH l~l~Al) - 10.0 10.3 10.~ 10.
10 SECOND STA6E
N~25iO3 l 3.00 3.00 3.00 3.eO
llgSO~ ~ o.es e.as a.ss o.as
NIOH I 0. 7a 1. 22 1. 39 1. 39
D.T.P.~ 0 0.~0 ~.~a o.~a
H202 ADDED S 1.52 l~Qa l.sa 2.50
COH315tENCï 7. 1~.~ 10.0 IQ.O 10.2
TEI~PER~tURE C 7a 7a 7a 7a
15 RETENTION tl~E hr. 1.5 1.5 I.S 1.5
pH ~INltlAl) - lI.a 11.0 11.1 11.0
pH ~EIN~E) - 18.0 11.1 10.1 10.5
tHlRD StA6E
N~25i~3 X 3.80 3.00 3.00 3.80
~9~O~ l a.os 0.05 ~.OS e.o5
20 H~OH 7 1.15 1.50 1,00 1,00
D.T.P.A. X 8.~0 0.40 1.~2
H202 ~DDED S 8.sa 1~8a 1.50 2.50
CONSlSTEltCr Z IC.O 19.1 IB.O 10.0
TE~IPERAIIIRE ~ Ja 7a 70 7a
RETENTION tlllE hr. 1.5 1.~ I.S l.S
pH ~INITIAL~ - 11.1 11.0 11.1 11.1
pH ~EINAEI - la.7 11.7 10.5 IO.S
25 TOtAL
.
H202 ~DDED) ~ I.OZ 2.00 3,00 5.0Z
H202 (RESIDUAL) 7. - 0.90 1.66 2.85
H202 (CO~SU~ED) ~ - I .10 1. 3~ 2.15
OPTICAL PROPERTIES
30 BEFORE RE~ERSION:
3Q16HthESS (ISa-~57nn)X62.0 74.3 76.9 77.6 78.2
L~ - 87.3 9~.2 9~,.6 9S.1 95.0
A- - -O. 7~ -2.~2 -2.51 -2. 62 -2. 28
B{ ~ 8.07 9.48 8.6a 6.35 7.98
A~tER RE~ERSION .
~RT6~1TNESB (ISa-~57n~)~61.g 13.3 75.1 7~.5 77.0
35 I.t .97.~ 9~.5 g3.g 94.8 94.1
At - -1.25 -3~01 -2.69 -2.72 -2.78
Q~ - B.739.?!1 0.~7 6.60 7.39
13~0348
.. . . . .
TABLE 5: CHOllICA- CHAR6E~ AND B~EACHIN6 60NO1~15NS f0R
THE SELUE!~CE SO01Ut~ HZDRO9ULflTE-PER0XlDE-PEROXlDE (hPP)
FIR~T STA8~
D.l.P.A. X 0.~ 0.~ o.~a 0.$3 0.41
N~2820~ X 0.50 0.S0 O.S0 0.50 2.52
CUNSlSTEh'Cr X 5.2 5.0 5.0 5.0 $.
TE~IPER~TURE C 71 70 70 70 70
REtENT~aN Tln8 hr. O.S 0.5 O.S 0.5 0.
pH (INITIAL) - 5.~ 5.4 5.S 5.4 5.
pH lFlNAL) - ~.9 ~.5 ~.7 5.2 5.
1O SECoNa STASÉ
t~2510~ X 3.0a 3.ea 3.02 3.09 ~.08
n~so~ X ~.05 O.O~ e.os 0.0S ~.05
N~0H X 0.70 1.22 1.39 1.74 2.09
D.T.P.A. 2 1.~0 0.4a 2.40 0 ~0 140
H202 ADDE~ Z a.s~ a l,sa 2.s0 4.00
CORSISTEN~Y Z IO.O le.l t0.Q t0.0 lll.9
TEnRER~TURE C 70 70 70 70 70
15 R~T~TION TlllE hr. 1.5 1.5 I S 1.5 I.S
pH ~INlTlAl) ~ la.9 11.9 It.B 11.1 11.1
~H ~FINAE1 - 9.7 8.7 a.s 8.6 8.a
THIRD STA6E
. .
N~28103 Z 3.08 3 ED 3.00 3.U 3.01
llgS~ 2 ~.05 ~.05 2.15 ~.O5 ~.~S
20 RILH l 0.75 1 Z5 1.58 1 75 2.00
D.T.P.A~. 2 a.49 0.41 0 40 0 49 0.49
H202 ADDED l a.so 1.00 1.50 2.53 4.ao
ca~ x la.o IS.~ Io.~ Io.o Io.e
TEnPERATURE C 70 7a t0 7a 70
RETENTIOII ttnE hr. 1.5 I.S l.S 1.5 1.5
pH ~IN~TIALI - II.O 11.9 II.O 11.0 lI.e
pH IFINAl~ - 10.3 9.3 9.8 11 4 1~.1
25 t0ZA~
H202 ~ADDED) Z l.eo 2.0a 3.00 s.oa 8.00
11202 (RESIOUAL) ~ 1.26 0.78 1.45 2.9r 4.45
H202 (C0NSUnE0) ~ l.74 1.22 1.55 2.03 3.5S
0PTIC~E RR0 1ltlES
30 9EFORE REYERSION ~
8RI6NT~IE9~ tlSO-4~7n-)l62.S71.7 75.7 77 78.9 79.6
l~ - 88.1g3.79~.5 9~.9 95.2 95.0
A~ - -0.~7-1.85-2.15 -2.31 -2.08 -1.92
9- - 9.2~l~.919.18 6.87 8.~2 6.87
~f~ER RE~IER610N:
LR16HT~IE55 (190-457n-)X61.971.a 74.7 76.2 78.2 78.2
35 ~t _ 88.1193.~9~.~ 94.7 95.1 9~.9
At - -8.~7-Z.l9-2.38 -1 Hl -1.93 -1.72
- 9.6~11.369.69 ~.~68.1~3 7 61
.. _ _ . . . . . _ . .. ..
~ 13~0~48
.. . . .. . . ..
TABLE 6: CHE~ICAL CH1~RSES AND ~LEACHIN6 CONOltlON3 fO~
THE SE~UENC~ SOOlUn H~DROSUEflTE-PEROXlD~-PERaXlOE (HPR)
Fl~sr SrA6~
D.T.P.A. X 0.~0 0.41 ~.4a o.~a D.40
N~2$20~ ~ 1.9a I.ea l.aa l.oa l.aa
CONSIST~I~C~ Z 5. a ~ a 5. 0 S. 0 5 . I
Tf.11RERATVRE O 70 7a 7a 7o 7a
RETENTIOH TIllE hr. 0.5 0.5 a.s o.s ~s
~H IINITIAE) - 5.~ 5.s 5.4 s,l 5 3
pH tflHAl) - 5.1 5.1 5.0 $.9 s.a
SECOhD STA6E
N~2SiO3 x 3.ao 3.0a 3.0a 3.0a 3.ea
n9so4 ~ o~o~ a.os o~os 2.95 0.0S
II-OH X 1.39 1.39 1.57 1.7~ 2.26
D.T,P.A. ~ a.4a 0.40 0,40 1.~0 0,4D
H20Z AD~ED 7 0.50 1.00 1.S0 2.sa ~.aa
CONSlSlEH~r I 10.0 la.l lo.a 10.1 10.
TEHPER~TURE C 70 io 70 7a 70
RETENTIOII TltlE hr. 1.5 I.S 1.5 1.5 1.5
pH ~INITIAL~ - 11.1 11.0 11.0 ID.9 10.8
pH (FJN~l~ - 9.5 9.1 9.1 8.7 8.8
T~IRD STA6E
Na2SiO3 1 3.~1 3.0a 3.aa 3.aa 3.ao
r9so~ ~ o~os 0.~5 e.os o.as O.IS
NaDH X l.ao 1.25 1.25 1.75 2.00
O.r.P.A. Z ~.40 e.~o 0.40 0 ~0 0.40
H202 ADDED X 0.50 1.00 - I.SO 2.50 4.00
CONSISTENCY 2 20.1 2e.0 2a.a 211.121.0
TEnPER~TURE C 71 70 7D 70 71
ROTEIITIOR Tl~l~ hr. 1.5 1.5 l.5 1.5 1.5
plt (l~lTlAl) - 11.1 11.0 Ia~s
pH tflNAL~ - 10.2 10.2 9,9 tO.5 10.6
TOT~L
H202 (~DDED1 X 1.80 2.00 3.0a S.lO s.oa
H202 ~RESIDUAE) I 1.1g 1.81 1.60 2.71 ~.~6
H202 t~ON~UnED) ~ 0.81 1.19 1.40 2.21 2.1
30 OPTICAE PROPERTIES
8~PORE REV~RS10~1 ~
~Q16HtNESS t150-451nu)X62.571.4 75.7 16.8 79.0 80.5
O-l93.4 94.s 94.5 ss.a 95.3
0.37-2.08-1.8~ -I.S6 -1.97 -2.~3
8~ - 9.2S1~,819.17 8.45 J.49 6.68
35 AftER REVERSION:
SR16HTIIESS ~ISO-457n~)~61.970.4 74.7 7s.7 77.1 7B.4
~- - 88.193.19~.~ 94.4 94.8 95.
A~ - -6.37-1.S7-1.93 -1.S9 -1.60 -2.~1
~t _ 9.6~11.149.69 ~.95 ~.~8 7.S~
. . . _ . .