Note: Descriptions are shown in the official language in which they were submitted.
2C~~442
O.Z. 0050/40293
Preparation of a_cyl chlorides
Acyl chlorides can rs:adily be prepared by react-
ing the corresponding carboxylic acids with phosgene.
The reaction has to be catall~zed. Examples of catalysts
used are carboxamides, preferably N-alkylformamides (DE-
A-34 39 937).
In the case of the N,N-dialkylformamides, the
size of the alkyl group ranges from dimethylformamide to
formamides of 30 carbon atoms (EP-A 0 050 779, DE-A-29 50
115 and DE-A-19 31 074).
The course of the phosgenation of a carboxylic
acid to the acyl chloride and the working up of the mix-
ture is decisively influenced by the choice of the
catalyst system.
As an alternative to filtration of tar-containing
crude products, working up of the catalyst-containing
product by distillation would also be possible in some
cases. However, distillation of the resulting acyl
chlorides is not only an a:nergy-consuming and time-
consuming process but also h,as a number of other dis-
advantages.
Many relatively long chain acyl chlorides cannot
be distilled without partial decompostion. It is also
known that the distilled products may be contaminated
thxough decomposition of the catalyst present in the
bottom product of the distillation. Larger amounts of
catalyst residue constitute a safety risk during distil-
lation, because there is a danger of spontaneous decom-
positon at elevated temperatures.
In working up impurity-containing mixtures to
obtain the product, the activity of the catalyst is
greatly reduced both by filtration and by distillation.
In most cases, the catalyst u~ced becomes useless, ie. it
cannot be reused.
Both distillation and filtration of catalyst-
containing acyl chlorides thus constitute disadvantageous
methods of working up. Becaua~e of the catalyst loss due
2~~0~9~42
- 2 - O.Z. 0050/40293
to working up, the amount of: catalyst used must be as
small as possible.
In DE-A-29 50 155, t;he catalyst used is diiso
butylformamide, which is solulble in the reaction mixture
in every phase of the reaction. If a final distillation
of the acyl chloride is to be dispensed with, the amount
of soluble catalyst must be kept to a minimum to ensure
product purity. In the case of this catalyst system too,
the catalyst cannot be reused since it is discharged with
the product.
It is also known that the reactions with phosgene
take place more effectively the larger the amount of
catalyst. Conversely, small amounts of catalyst result
in either poor utilization of the gaseous phosgene used
or long gassing times.
DE-A-22 40 883 describes the preparation of acyl
chlorides using equimolar amounts of carboxylic acid and
catalyst. However, to separate off and recover the large
amount of catalyst, it is necessary finally to add
benzene in an amount corresponding to 3-4 times the reac-
tion volume and then to distill the solution of the
product in benzene.
The use of large amounts of catalyst is also des
cribed in JP-10 613/68 for the preparation of linoleyl
chloride using from 10 to 50 mol % of dimethylformamide,
as well as from 1 to 10 equivalents of dimethylformamide,
based on linoleic acid used. Z'he resulting acyl chloride
has to be distilled and in some cases were additionally
purified by treatment with active carbon. It is not
intended to reuse the large amounts of catalyst.
In the synthesis of ac:yl chlorides from carbox-
ylic acids and phosgene, it is known that the problem of
removing excess phosgene from the crude acyl chloride is
encountered.
According to the prior- art, phosgene-containing
acyl chloride can be freed from phosgene by stripping for
several hours with nitrogen and./or under slightly reduced
20004 ~ 2
3
pressure. This procedure is time-consuming and has a very
adverse effect on the space-time yield of the process.
In DE-A-29 50 155, the excess phosgene is distilled
over with the first part of the: acyl chloride distilled. In
addition to a deterioration of the space-time yield, which is
observed in this case too, this procedure requires additional
outlay for apparatus and analysis.
DE-A-22 40 883 discloses a working up process in
which the dilute reaction solution is washed briefly with ice
water before the distillation. In view of the sensitivity of
acyl chlorides to hydrolysis, thi:~ process presents problems on
the industrial scale.
In the process disclosed in JP-A-10613/68, too,
excess phosgene must be removed by working up the crude acyl
chloride by distillation.
It is an object of the present invention to provide
a process for the preparation of <~cyl chlorides which overcomes
the abovementioned disadvantages.
More particularly, the object of the invention is to
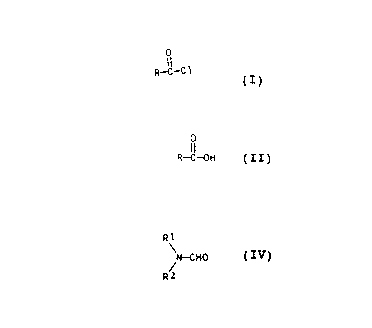
provided a process for the preparation of an acyl chloride of
the general formula (I):
0
II
R - C ~- C1 (I)
where R is Cg-C30-alkyl,, C3-C30-alkenyl or Cg-C30-alkynyl,
comprising reacting a carboxylic acid of the formula (II):
O
II
R - C -- OH (II)
where R has the above mentioned meanings, with phosgene:
COC12(III),
in the presence of a catalyst adduct of phosgene and
an N,N-dialkylformamidE~ of the formula (IV):
',
2:s,,.~
20004 2
4
R1
N - CHO (IV)
R2 /
where R1 and R2 independently of one another are each C1-C3-
alkyl.
This process is characterized in that it comprises:
- carrying out the reaction with the carboxylic acid
II and the phosgene III reactants in essentially equimolar
amounts while using said catalyst adduct in an amount of from
5 to 200 mold based on the carboxylic acid II;
- allowing the reaction mixture containing the acyl
chloride I product to separate into two phases;
- separating the lower phase formed by the catalyst
adduct from the upper product phase; and
- reusing the lower phase containing the catalyst
adduct.
In use, liquid or gaseous phosgene is added to the
initially taken reaction mixture, consisting of carboxylic acid
II and the adduct of phosgene and N,N-dialkyl-formamide of the
formula (IV). The time required fo:r passing in gaseous phosgene
can be restricted to 3-4 hours, the phosgene being virtually
quantitatively utilized. Thereafter, the mixture is allowed to
stand for from 1 to 2 hours and t:he phases are separated.
In this reaction, from 5 to 200, preferably from
10 to 100, particularly preferably from 10 to 30, mol %
of the adduct of phosgene and N,N-dialkylformamide IV,
preferably a 1 : 1 adduct, if necessary in excess N,N-
dialkylformamide, is used, the percentages being based on
the carboxylic acid II used.
The amount of phosgene I7:I is essentially equi-
molar with respect to the carboxylic acid II.
The phosgene may be mixed with an inert gas. The
reaction can be carried out under atmospheric, reduced or
superatmospheric pressure. The reaction temperature may
be from 0 to 200°C but is preferably from 30 to 100°C,
particularly preferably from 60 to 80°C.
200044 2
4a
If necessary, solvents may be added to the reac-
tion mixture. These must be inert under the reaction
conditions, examples being saturated aliphatic hydro-
carbons, ethers, acetonitrile, toluene, benzene or cyclo-
hexane.
At a temperature of from 0 to 150°C, preferably
from 20 to 50°C, the lower phase formed by the catalyst
is separated from the upper product phase. This can be
done by a plurality of methods., For example, the catal-
yst can be discharged into a storage vessel until re-
quired for further use. However, it is also possible to
syphon the acyl chloride, i . a . the upper phase of f the
30
a :.,
2~~Q442
- 5 - O.Z. 0050/40293
catalyst via a riser tube and to leave the catalyst in
the reactor until the next reaction.
The acyl chloride is obtained in a quantitative
amount and in high purity. It can be used without
further purification, for example distillation or fil
tration. A particular advantage of the novel process is
that the acyl chloride is free of phosgene at the end of
the reaction. Hence, there is no need for any measures
for removal of phosgene from the crude acyl chloride.
Another particular ad~;rantage of the novel process
is that the catalyst can be recovered without any prob-
lems and reused.
The novel process for the preparation of acyl
chlorides from aliphatic carboxylic acids is particularly
suitable for monocarboxylic acids, ie. for the prepara
tion of compounds of the general formula RCOX, where R is
an aliphatic hydrocarbon group and X is chlorine. The
aliphatic group may be straight-chain or branched,
saturated or olefinically or acetylenically unsaturated.
Aliphatic carboxylic acids of 8 to 30, in particular 12
to 22, carbon atoms are particularly preferred.
Suitable N,N-dialkylformamides are dimethyl-,
ethylmethyl-, methyl-n-propyl-, methylisopropyl-, di
ethyl-, ethyl-n-propyl-, ethylisopropyl-, di-n-propyl-,
n-propylisopropyl- and diisopropylformamide, di.methyl
and diethylformamide being pre:Eerred and diethylformamide
being particularly preferred.
EXAMPhES
EXAMPLI~ 1
In a thermostated 5 1 reactor, 2, 746 g ( 10 moles )
of technical grade stearic acid are initially taken at
70°C and 202 g (2 moles) of diethylformamide (DEF) are
added. 1,188 g (12 moles) of gaseous phosgene are then
passed in uniformly over 2.5 hours with thorough stir-
ring. The internal temperature during the addition of
phosgene is from 70 to 75°C. The waste gas from the
reaction is passed directly via a scrubber, in which
~~~0(~442
- 6 - O.Z. 0050/40293
unconsumed phosgene is hydrolyzed.
After the end of the addition of phosgene, stir-
ring is continued for 0.5 hour, the stirrer is switched
off and the reaction mixture i.s transferred to a separat-
ing funnel. The vapor space above the mixture is
phosgene-free (test cartridges from Dr~ger).
After 2 hours at 25°C, the lower catalyst phase
(342 g of activated DEF) is Eceparated off from the two-
phase reaction mixture. The upper phase contains 3,900 g
of stearic acid (9.89 moles - 98.9% yield, based on
technical grade stearic acid used) having a purity of 95%
(determined by IR spectroscopy) . The iodine color number
(ICN) of the aryl chloride is 10. Both the acyl chloride
and the catalyst are phosgene-free.
EXAMPLES :? TO 10
To determine the efficiency of the novel process,
Example 1 is repeated several times. The catalyst used
in each case is the catalyst phase of the preceding
experiment.
2 0 EXAMPL;B 2
The experimental method described in Example 1 is
used, and 2,746 g (10 moles) ~of technical grade stearic
acid and the catalyst phase (342 g) obtained in Example
1 are initially taken. 1,08! g (11 moles) of gaseous
phosgene are then introduced. The product phase contains
2, 930 g ( 99 . 9% yield) of stear5rl chloride having a purity
of 97% (determined by IR spectroscopy); the iodine color
number is 30.
Examples 3 to 10 are carried out similarly to
Example 2.
2~~Q~~42
- 7 - O.Z. 0050/40293
Exam- Acid Phosgene Yield Purity (IR) ICN
ple (moles) (moles)
3 10.0 10.0 99% 97% 20
4 10.0 10.0 100% 95% 10
5 10.0 10.0 100% 96% 10
6 10.0 10.5 100% 96% 10
7 10.0 10.0 100% 94% 25
8 10.0 10.0 100% 96% 15
9 10.0 10.0 100% 96% 15
10 10.0 10.5 100% 97% 15
All acyl chlorides and catalysts are obtained in
phosgene-free form.
EXAMPLE 11
542 g (2.0 moles) of technical grade stearic acid
and 44 g of diisobutylformamicle (0.28 mole) are initially
taken in a reactor at 65°C, and 208 g (2.1 moles) of
gaseous phosgene are passed into the melt in the course
of 2.5 hours with thorough stirring. The internal
temperature is kept at 65°C. Stirring is then continued
for 0.5 hour at 60°C.
The stearic acid is completely converted. The
reaction mixture is phosgene-free. Even after the mix-
ture has cooled to 20°C, the catalyst remains in solution
in the crude acyl chloride. The amount of catalyst-
containing stearic acid discharged is 624 g (maximum
possible yield of stearyl chlorides 579 g.). The purity
of the acyl chloride is 84% (IR). The product is brown
(ICNz 110).
E%AMPLE 12
In a thermostated 5 1 reactor, 2,028 g (6 moles)
of technical grade behenic acid are initially taken at
73°C and 223 g (about 1.2 moles) of activated diethyl-
formamide are added. 596 g (6.0 moles) of gaseous phos-
gene are then passed in uniformly over 2.75 hours with
thorough stirring. The internal temperature during the
addition of phosgene is from '76 to 80°C. The waste gas
2~t~Q442
- 8 - O.Z. 0050/40293
from the reaction is removed directly via a scrubber.
After the end of the reaction, the vapor space above the
reaction mixture is phosgene-.free.
Stirring is then continued for 1.0 hour at 77°C,
the stirrer is switched off and the reaction mixture is
cooled to 42°C. At this temperature, the lower catalyst
phase (208 g of activated DEF) is separated off from the
two-phase reaction mixture after 1.5 hours. The upper
phase contains 2,122 g of beh~enyl chloride (99.2% yield,
based on technical grade behenic acid used) having a
purity of 94% (IR). The iodine color number of the acyl
chloride is 20. Both the acy.l chloride and the catalyst
are phosgene-free.
EXAMPLIE 13
In a thermostated 5 1 reactor, 1,120 g (4 moles)
of technical grade talloleic acid (mixture of mono-
unsaturated and polyunsaturated C18-carboxylic acids) and
147 g (0.8 mole) of activated diethylformamide are
initially taken at 70°C. 390 g (3.94 moles) of gaseous
phosgene are then passed in uniformly over 2.0 hours with
thorough stirring. The internal temperature during the
addition of phosgene is from 72 to 74°C. The waste gas
from the reaction is removed directly via a scrubber.
After the end of the reaction,, the vapor space above the
reaction mixture is phosgene-free.
Stirring is then continued for 1.0 hour at 72°C,
the stirrer is switched off and the reaction mixture is
cooled to 20°C. At this temperature, the lower catalyst
phase (141 g of activated DEF) is separated off from the
two-phase reaction mixture after 1.5 hours. The upper
phase contains 1,188 g of talloleic acid chloride (99.5%
yield, based on talloleic acid used) having a purity of
92% (IR). The iodine color number of the acyl chloride
is 100. Both the acyl chloride and the catalyst are
phosgene-free.
COMPARATIVE EXAMPLE A
In a thermostated reacaor, 1,946 g (7 moles) of
2~~(~442
- 9 - O.Z. 0050/40293
technical grade stearic acid are melted at from 60 to
65°C and 12 . 8 g ( 0 . 175 mole ) of dimethylformamide ( DMF )
are added. Gaseous phosgene is then passed in uniformly
over 3.0 hours with thorough. stirring until an on-spec
acyl chloride is obtained. The internal temperature
during the addition of phosgene is kept at from 60 to
65°C. The required amount of phosgene is 940 g (9.5
moles).
The acyl chloride contains phosgene, and is de
phosgenated with dry nitrogen in the course of 12 hours
at room temperature. During this procedure, the catalyst
separated out as a solid. After filtration, 2,025 g
(97.6% yield) of stearyl chloride having a purity of 98%
are obtained.
COMPARATIVE EXAMPLE B
similar to DE.-A-29 50 155
1,355 g (5.0 moles) of technical grade stearic
acid are melted at 60°C and 2.7 g (0.0172 mole) of di-
isobutylformamide are added. The apparatus has a reflux
condenser (coolant temperature -20°C) with a downstream
scrubber. Gaseous phosgene is passed in at from 60 to
65°C with thorough stirring until conversion of the
stearic acid to the acyl chloride is complete. The phos-
gene is metered in such a way that there is only an
extremely small phosgene reflux in the reflux condenser.
781 g (7.9 moles) of phosgene are required. The addition
of the phosgene takes 9 hours.
The phosgene-containing stearyl chloride is de
phosgenated with dry nitrogen in the course of 3 hours at
60°C. The reaction mixture remains homogeneous. The
amount of crude acyl chloride discharged is 1,435 g
(maxi.mum possible yield of steaaryl chloride 1, 448 g) , the
purity being 96% (IR). The iodine color number of the
brown product is 100.