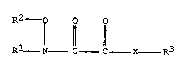Note: Descriptions are shown in the official language in which they were submitted.
` ~: `
:
2()021~21 ~:
` .
CR-8686
INHIBITION OF KETOL-ACID REDUCTOISOMERASE BY
OXALYLHYDROXAMATE ~ERIVATIVES ;
5~CK~ uNn OF T~ rQ;~
Fi~l~ of the Inv~$; ~n ,~
This invention relates to the process of ~ ~
selectively inhibiting ketol-acid reductoisomerase ` ;
(EC 1.1.1.86; KARI) through the use of oxalylhydroxamate
10 derivatives, thereby effecting herbicidal activity on P
plants and inhibiting microbial growth. The invention
also discloses specific oxalylhydroxamate compounds.
Additionally, a process for assaying ketol-acid
reductoisom~rase at low levels is disclosed and can be
15 used to assess the activity of inhibitors of this
enzyme.
Summary of the 8ack~round
Plants, unlike animals, are able to synthesize the
branched-chain amino acids. These amino acids are
20 essential for animals because they lack the enzymes ~ `~
necessary for branched-chain amino acid synthesis `and ;; `
must therefore obtain such amino acids from dietary .
sources. Plants, however, contain three common enzymes ;
specific to the synthesis of valine, leucine, and
isoleucine, which are the branched-chain amino acids.
The first of these sequential enzymes is acetolactate
synthase, the second is ketol-acid reductoisomerase, and
the third is dihydroxyacid dehydratase. Isoleucine
biosynthesis requires a fourth enzyme, threonine ~ "
30 deaminase, which provides a substrate for the first -
common enzyme, acetolactate synthase. Three additional ;
enzymes are required for leucine biosynthesis, and these `~
are isopropylmalate synthase, isopropylmalate ~"
dehydratase, and isopropylmalate dehydrogenase. The
biosynthesis of all three branched-chain amino acids
;"" '~ :,., '.
'"''~'''''"`'' '
';'.~,': ' ",
Z()QZl~Z~
- 2
also requires a transaminase, which is not restricted to
~ranched-chain amino acid synthesis nor is it unique to
plants.
It is known that selective inhibition of the first
enzyme in the biosynthetic pathway for branched-chain
amino acids, acetolactate synthase (EC 4.1.3.18), is the
basis for the growth inhibition of plants, bacteria,
yeast, and fungi by various sulfonylurea and
imidazolinone herbicides. LaRossa, et al., J. B;ol.
Chem. 259:8753 ~1984); Falco, et al., Ge~eti~ 109:21
(1985); Chaleff, et al., ~e~ e 224:1443 (1984); Ray,
Pla~ Physiol~ 75:827 (1984); Shaner, et al., ~l~n~
~hys~ol~ 76:545 (1984); Muhitch et al., ~la~t Physiol.
83:451 (1987). Although the sulfonylurea and
imidazolinone herbicides are quite selective in
inhibiting acetolactate synthase, the site on this
enzyme to which the herbicides bind is not intrinsically
required for function. Schloss, et al., ~atUL~ (Lo~don~
331:360 (1988). This increases the likelihood that
resistance to these herbicides in weeds will be obtained
by the mutation of this enzyme.
Ketol-acid reductoisomerase (EC 1.1.1.86; KARI) is
the second common enzyme specific to the biosynthesis of
branched-chain amino acids for plants, bacteria, yeast,
and fungi. There are currently no disclosed compounds
known to be selective inhibitors of this enzyme. This
invention discloses a process for the selective
inhibition of ketol-acid reductoisomerase through the
use of oxalylhydroxamate derivatives. Selective
30 inhibition of ketol-acid reductoisomerase results in -
herbicidal activity and inhibition of microbial growth,
and yet is not injurious to animals because animals lack -
this enzyme.
Ketol-acid reductoisomerase is magnesium dependent.
Hydroxamates without the oxalyl moiety are known to
~- ;- - . . . . . . .
. :. , . :. .-. .. . .~ , .- .. :
3 ` `
inhibit certain metal-dependent enzymes. Hydroxamate
substrate and reaction-intermediate analogs are known to
inhibit lipoxygenase (iron dependent), aminopeptidases :
(zinc dependent), collagenase ~zinc dependent), elastase
S (zinc dependent), thermolysin (zinc dependent), urease
(nickel dependent), and enolase (magnesium dependent).
See, respectively, Summers, et al., J. Med ~h~m. 30:574
(1987); Corey, et al., J. Am~ Che~ so~ 106:1503
(1984); Cherot, et al., ~ol. Pharma~ol~ 30:338 (1986);
Fournie, et al., J. Med. ChenL 28:1158 (1985); Chan, et
al., J. R;ol. Chem~, 257:7955 (1982); Wilkes, et al.,
J. Riol . Chem. 258:13517 (1983); Baker, et al.,
B;~chemi5t~Y 22:2098 (1983); Coletti-Previero, et al.,
BiQ~hS2L ~i~Rhys. Res. Co~m~n~ 107:465 (1982); Blumberg, ~;
et al., ~ife Sc;. 28:301 (1981); Moore, et al., Rioch~
~in~hy~ Res. ~Qmmun. 136:390 (1986); Kessler, et al.,
Tnfect. Tmmu~. 38:716 (1982); Holmes et al.,
3i~h~=ist~ 22:236 (1983); Holmes, et al., BiQchemi~try
20:6912 (1981); Nishino, et al., Bioche~ Ly 17:2846
(1978); Yamaya, et al., Pla~t Physio~ et~s~l 67:1133 -~
(1981); Nagarajan & Fishbein, E~d. PrQ~ 36:700 (1977);
Kobashi, et al., Bioc~im. Bio~hys . Act~ 227:429 (1971);
and Anderson, et al., B;ochemi~Ly 23:2779 (1984).
The inhibition reactions of lipoxygenase,
aminopeptidases, collagenase, elastase, and thermolysin
are thought to proceed by reaction-intermediate mimicry.
In the case of urease, the hydroxamate inhibitor acts as ;
a nonspecific, metal chelator, and the particular -
structure of the hydroxamate is unimportant for this ~ ;
reaction, unlike the structural requirements for ketol~
acid reductoisomase inhibition. Inhibition of enolase,
which is a magnesium-dependent enzyme (as is ketol-acid ~
reductoisomerase), probably depends on both metal ~-
chelation and reaction-intermediate mimicry. The ~-~
structural requirements for the hydroxamate inhibitor of
.... ..
~; .. .
:.:
!:;` . ` . ` ; ` ` ` . ~ - ~ ; , -
Z()(~
. - .
enolase (phosphonoacetohydroxamate) differ from those of
ketol-acid reductoisomerase, however, and the
phosphonoaceto moiety is essential for inhibition of
enolase. As discussed below, the inhibition of ketol-
acid reductoisomerase requires the presence of an oxalylmoiety on the hydroxamate for potency.
A hydroxamate is also ~nown to be an inhibitor of
an enzyme that is not metal dependent, as is the case
for the inhibition of triose phosphate isomerase by the
hydroxamate of phosphoglycollate, wherein the
hydroxamate presumably mimics the ene-diol reaction
intermediate of this enzyme as the sole basis for
inhibition. Collins, J. Bio~. C~em. 249:136 (1974).
Specific instances of the inhibition of particular
enzymes, other than ketol-acid reductoisomerase, by
oxalyl- and oxamylhydroxamates are are known in the art.
Oxamylhydroxamate (H2N-CO-CO-NH-OH) inhibits virus,
bacterial, and tumor cell replication at high (> 10-4 M)
concentrations. Gale et al., Ex~er;entia (Rasel) 24:194 -
20 (1968); Hynes, et al., J. Med. Che~. 16:576 (1973). The
mode of action is thought to be similar to hydroxyurea,`- -
which acts as a radical scavenger, inactivating -
ribonucleotide reductase, a radical-containing enzyme
essential to the biosynthesis of deoxynucleotides (DNA :
25 biosynthesis). Gale, Cancer Res. 26:2340 (1966);
Kjoeller Larsen/ et al., Eur. J. Bioc~e~. 125:75 ~1982).
~hus, the reaction does not proceed by metal chelation
or reaction-intermediate mimicry. (That
oxamylhydroxamate inhibits growth by virtue of its
inhibition of ribonucleotide reductase is consistent
with the observation that deoxyribonucleosides protect
~Ehrlich ascites tumor cells against inhibition. Gale,
r ~_if~ L~ - lL 24:57 (1968).) This radical-
scavenger mode of action is probably shared by several
derivatives of oxamylhydroxamate, in which the amide
2(1()z¢'2.~ ; ~
nitrogen bears various alkyl or aryl substituents
(R-NH-CO-CO-NH-OH), that have been described as having
fungicidal (R s 4-tolyl~ or bactericidal (R = PhCH2-)
activity. Petyunin, et al., ~hlm. Farm. Z~. 12 :106
(1978).
Oxamylhydroxamate is also known to inhibit
histidine decarboxylase and glutamate carboxylyase.
Gale, et al., ~iocheml Pharma~l. 19:628 (1970); Howle &
Gale, p-oc. Soc. FXp. Riol A M~, 131:6~7 (1969).
Inhibition of histidine decarboxylase and glutamate
carboxylyase is not dependent on the oxalyl moeity of
oxamylhydroxamate, because a number of other
structurally unrelated hydroxamates are equally
effective as inhibitors. Similarly, N-methyl
oxalylhydroxamate methyl ester, which is one of the
inhibitors of ketol-acid reductoisomerase as disclosed
in the present invention, has been described as an
inhibitor of urease, but it is no better an inhibitor
than are a variety of other hydroxamates. Kobashi, et `;
al., R;ochim Bio~hys. Acta 227:429 (1971). This
indicates that the oxalyl moiety is not critical to
urease inhibition, which differentiates it from the ~
selective ketol-acid reductoisomerase inhibition, -
wherein the oxalyl moiety is an essential feature for `-
25 potent inhibition. As discussed above, the inhibition .
reaction for urease is thought to proceed through
nonspecific metal chelation, whereas the suggestion in ;
the present invention is that oxalylhydroxamate
derivatives inhibit ketol~acid reductoisomerase by, in
part, their metal chelation properties and, to a greater
extent, by their mimicry of the reaction intermediate of
the rearrangement catalyzed by ketol-acid
reductoisomerase.
None of the other oxalylhydroxamates has been
35 previously described as an enzyme inhibitor or as an ;
Z()Q2~2~
inhibitor of a biological process. Additionally, the
present invention discloses specific oxalylhydroxamate
derivatives that currently are not known in the art.
Oxalylhydroxamate, oxalylhydroxamate methyl ester, and
N-methyl oxalylhydroxamate methyl ester have been
previously disclosed. Kobashi, et al., BiQ~hlmL
B;o~hys. ~cta 227:429 (1971).
Ketol-acid reductoisomerase can be assayed from
purified bacterial preparations, as described by Arfin,
et al., J. Biol. Chem. 244:1118 (1969). The present
invention discloses a process for assaying ketol-acid
reductoisomerase at low levels through the use of
selectively radiolabeled substrates. `
SUM~A~RY OF THE INVENTTON
The present invention provides a process for
inhibiting the enzymatic activity of ketol-acid
reductoisomerase to effect herbicidal activity or to -~
inhibit microbial growth, which comprises contacting,
respectively, a plant or microorganism with an effective
amount of a chemical compound of the formula
R2 o o o
R1 N - ¦ I X R3
or an agriculturally acceptable salt thereof, wherein R1
is selected from the group consisting of H, alkyl,
alkenyl, alkynyl, aryl, alkylaryl, alkenylaryl, and
alkynylaryl substituents having from 1 to about 300
atoms, wherein one or more carbon atoms can be
optionally substituted with a heteroatom selected from
the group consisting of N, S, O, Si, and P, and wherein
one or more hydrogen atoms can be optionally substituted
with a substituent selected from the group consisting of
35 F, Cl, Br, I, and OH; R2 is selected from the group ; ;
zn~z~2~
consisting of the group consisting of H, alkyl, alkenyl, :
alkynyl, aryl, alkylaryl, alkenylaryl, and alkynylaryl
substitutents having from 1 to about 50 atoms, and acyl
substituents having from l to about 300 atoms, wherein
S one or more carbon atoms can be optionally substituted
with a heteroatom selected from the group consisting of
N, S, O, Si, and P, and wherein one or more hydrogen
atoms can be optionally substituted with a substituent
selected from the group consisting of F, Cl, Br, I, and :
OH; R3 is selected from the group consisting of H,
alkyl, alkenyl, alkynyl, aryl, acyl, alkylaryl,
alkenylaryl, and alkynylaryl substitutents having from 1
to about 300 atoms, wherein one or more carbon atoms can
be optionally substituted with a heteroatom selected `~:
15 from the group consistinq of N, S, O, Si, and P, and ...
wherein one or more hydrogen atoms c~n be optionally
substituted with a substituent selected from the group : -
consisting of F, Cl, Br, I, and OH; and X is selected
from the group consisting of O, S, and NY, wherein Y is :
selected from the group consisting of H, OH, and
independently selected R3; provided that the combination `
of X and R3 is hydrolytically labile.
Additionally, the present invention discloses :: -
chemical compounds of the formula
R2 0 0 0 :: `
11 11
Rl _ N C C X R3 ::
~ :.
wherein Rl is selected ~rom the group consisting of H,
methyl, ethyl, propyl, isopropyl, butyl, sec-butyl,
isobutyl, t-butyl, phenyl, 2-pentyl, 3-pentyl, 2-hexyl, : .
3-hexyl, Cl-C6 perfluoroalkyls, and benzyl wherein one ~;- `
or more o-, m-, or p- positions can be optionally ~-:
35 substituted with a substituent selected from the group ;~ ~
Z(~)OZ-~`2~
;-: 8 `
consisting of F, C1, Br, I, methoxy, methyl, and
trifluoromethyl; R2 is selected from the group
consisting of H, methyl, ethyl, propyl, isopropyl,
butyl, sec-butyl, isobutyl, t-butyl, carbamoyl,
N,N-dimethylcarbamoyl, N,N-dimethylthiocarbamoyl,
N,N-diphenylcarbamoyl, N,N-diphenylthiocarbamoyl, `
acetyl, thioacetyl, propanoyl, thiopropanoyl, ;~.
trimethylsilyl, t-butyldimethylsilyl, and benzoyl
wherein one or more o-, m-, or p- positions can be
optionally substituted with a substituent selected from
the group consisting of F, Cl, Br, I, methoxy, methyl,
and trifluoromethyl; R3 is selected from the group
consisting of H, methyl, propyl, isopropyl, butyl,
sec-butyl, isobutyl, t-butyl, 2-pentyl, 3-pentyl,
2-hexyl, 3-hexyl, and C1-C6 perfluoroalkyls; and X is
selected from the group consisting of O and S; provided
that when R1 is H, R2 cannot be H; and further provided
that when R2 is H, Rl and R3 cannot both be CH3.
The present invention also includes a process for
assaying ketol-acid reductoisomerase, which comprises
mixing an aliquot selected from the group consisting of
a plant extract and a microbial extract with radioactive `
[1-14C]-acetolactate, NADPH, and Mg2+, in a buffered
solution having a pH above about 6 and below about 9;
adding an acid to the assay mixture, after an
appropriate incubation time; removing volatile
components from the acidified mixture; and measuring the `~
remaining radioactivity.
pFT~TT~ED l')F.SCRTPTTON OF T~F TNVF.I~'rTON
30l The present invention provides processes for ~
inhibiting the enzymatic activity of ketol-acid :
reductoisomerase to effect herbicidal activity or to
inhibit microbial growth, which comprises contacting,
respectively, a plant or microorganism with an effective
35 amount of a chemical compound of the formula ;~
'.~''.....
',: "',"",
Z()Q2(~
` `-.
R2 o o o
Rl N C C X--R3 ~ ~ ~
S ``
or an agriculturally acceptable salt thereof, wherein R1
is selected from the group consisting of H, alkyl, :
alkenyl, alkynyl, aryl, alkylaryl, al~enylaryl, and .-~
alkynylaryl substituents having from 1 to about 300 ;: :
10 atoms, wherein one or more carbon atoms can be `
optionally substituted with a heteroatom selected from
the group consisting of N, S, O, Si, and P, and wherein
one or more hydrogen atoms can be optionally substituted .~
with a substituent selected from the group consisting of - .
15 F, Cl, Br, I, and OH; R2 is selected from the group
consisting of the group consisting of H, alkyl, alkenyl,
alkynyl, aryl, alkylaryl, alkenylaryl, and alkynylaryl
substitutents having from 1 to about 50 atoms, and acyl`:
substituents having from 1 to about 300 atoms, wherein ::
20 one or more carbon atoms can be optionally substituted ::
with a heteroatom selected from the group consisting of.~.
N, S, O, Sl, and P, and wherein one or more hydrogen
atoms can be optionally substituted with a substituent
selected from the group consisting of F, Cl, Br, I, and
25 OH; R3 is selected from the group consisting of H, :`
alkyl, alkenyl, alkynyl, aryl, acyl, alkylaryl,
alkenylaryl, and alkynylaryl substitutents having from 1
to about 300 atoms, wherein one or more carbon atoms can ::
~ be optionally substituted with a heteroatom selected ~ :
30 from the group consisting of N, S, O, Si, and P, and : .
wherein one or more hydrogen atoms can be optionally
substituted with a substituent selected from the group ::
consisting of F, Cl, Br, I, and OH; and X is selected ~:
from the group consisting of O, S, and NY, wherein Y is
selected from the group consisting of H, OH, and
Z~)02~21
. .
. .
independently selected R3; provided that the combination `
of X and R3 is hydrolytically labile.
The combination of X and R3 must be hydrolytically
labile, wherein X-R3 will be substituted by OH in the
presence of water, either spontaneously or through the
action of naturally occurring esterases and amidases.
When R2 is an acyl substituent, the number of atoms is
not critical to the process of the present invention
because the acyl substituent is hydrolytically labile
and ultimately will be cleaved from the
oxalylhydroxamate and will be replaced by H. Because Rl
and R2 are near to each other, there is competition for
space and, the larger the Rl substituent, the more R2
will be constrained in size, and vice versa. The alkyl,
lS alkenyl, alkynyl, acyl, alkylaryl, alkenylaryl, and
alkynylaryl substituents of Rl, R2, and R3 may be
linear, branched, cyclic, or mixtures thereof. The
alkenyl and alkenylaryl substituents have one or more `-
unsaturated positions (double bonds). The alkynyl and
20 alkynylaryl substituents have one or more triple bonds. -
Optionally, Rl can be joined to either R2 or R3 to
form a ring; R2 can be joined to either Rl or R3 to form
a ring; or R1 and R2 and R3 can be joined to form two
fused rings.
A preferred embodiment is wherein R1 has from 1 to
about 150 atoms, with 1 to about 100 atoms being more
preferred, and with 1 to about 50 atoms being most
preferred; R2 has from 1 to about 50 atoms, with 1 to
about 30 atoms being more preferred, and with 1 to 20
atoms being most preferred; and R3 has from 1 to about
150 atoms, with 1 to about 100 atoms being more ` -~
preferred, and with 1 to about 50 atoms being most
preferred. ~
Preferably, X is O. Preferred oxalylhydroxamate -
35 derivatives for the process of the present invention ~; ~
, ,"..,, ,-.
.: .. ,
:: . . ~.
",` ""
include compounds wherein R1 is selected from the group --
consisting of hydrogen, methyl, ethyl, isopropyl,
t-butyl, and benzyl, with hydxogen, methyl, ethyl,
isopropyl, and benzyl being most preferred. Another
preferred embodiment is wherein R2 is selected from the
group consisting of hydrogen, methyl, and t-butyl, with
hydrogen being most preferred. Preferred R3
substituents are those selected from the group
consisting of hydrogen and methyl, with hydrogen being
most preferred.
The most preferred compounds for the process of ~ -
this invention are, in descending order of preference, `
N-isopropyl oxalylhydroxamate ~wherein R1 is isopropyl,
R2 and R3 are H, and X is O), N-ethyl oxalylhydroxamate
~wherein Rl is ethyl, R2 and R3 are H, and X is O), and
N-methyl oxalylhydroxamate ~wherein R1 is methyl, R2 and
R3 are H, and X is O).
An effective amount of the chemical compounds
discussed above is contacted with a plant or a
microorganism to result in, respectively, herbicidal
activity or inhibition of microbial growth. By an
effective amount, it is meant that amount causing 50 %
or greater mortality in plants or resulting in readily
observable toxic effects, such as chlorosis, leaf
curling, and leaf burning, or, respectively, that amount
causing a greater than three-fold increase in the time
required for microbial cell reproduction.
Additionally, the present invention discloses
chemical compounds of the formula
R2 o o o
11 11 ..
Rl _ N C C X R3
~ . .. ,., .- ,. -, - . . ~, . - .... . .
Z )02Q2'1
12
wherein R1 is selected from the group consisting of H,
methyl, ethyl, propyl, isopropyl, butyl, sec-butyl,
isobutyl, t-butyl, phenyl, 2-pentyl, 3-pen~yl, 2-hexyl,
3-hexyl, C1-C6 perfluoroalkyls, and benzyl wherein one
or more o-, m-, or p- positions can be optionally
substituted with a substituent selected from the group
consisting of F, Cl, Br, I, methoxy, methyl, and
trifluoromethyl; R2 is selected from the group
consisting of H, methyl, ethyl, propyl, isopropyl,
10 butyl, sec-butyl, isobutyl, t-butyl, carbamoyl, --
N,N-dimethylcarbamoyl, N,N-dimethylthiocarbamoyl,
N,N-diphenylcarbamoyl, N,N-diphenylthiocarbamoyl,
acetyl, thioacetyl, propanoyl, thiopropanoyl, ~`~
trimethylsilyl, t-butyldimethylsilyl, and benzoyl ~
15 wherein one or more o-, m-, or p- positions can be ~ -
optionally substituted with a substituent selected from
the group consisting of F, Cl, Br, I, methoxy, methyl,
and trifluoromethyl; R3 is selected from the group
consisting of H, methyl, propyl, isopropyl, butyl, sec-
butyl, isobutyl, t-butyl, 2-pentyl, 3-pentyl, 2-hexyl,
3-hexyl, and C1-C6 perfluoroalkyls; and X is selected `~
from the group consisting of O and S; provided that when
R1 is H, R2 cannot be H; and further provided that when
R2 is H, R1 and R3 cannot both be methyl.
An additional compound is disclosed wherein R1 is
ethyl and R2 is carbamoyl and C-2 of ethyl and N of
carbamoyl are joined to form a ring.
Preferred oxalylhydroxamate derivatives include
compounds wherein Rl is selected from the group `;
consisting of isopropyl and ethyl, with ethyl being most
preferred. Another preferred embodiment is wherein R2
is H.
Synthesis of oxalylhydroxamate derivatives is given --
in Examples 2-3. ; :
....,.: ..
'.' ~' '~'.',~
~... ,.,:;,.
. ` :
20(1~)21.
.`
13
Turning to the inhibition mechanism of ketol-acid ~. :
reductoisomerase, a possible reaction pathway for the
enzyme is illustrated below. - ~.
: - - .:.
2. 2- "
.' ". ,M~g ~ -
1l IOH
CH3C--f--C02- - CH3--C--C C02- ~ `
CH3 CH3 : :.
2- 2~
M~g ,Mg
- O O ~ O 'O ~ -'
CH3--f _c_ C02- - CH3 :,--C--CO~
CH3 CH3 ~`
~ ~ .
IV~
.``: -
2 - 2 -
,M~g ,Mg
HO 1I NADI'N NADP HO OH :- :
CH3--IC C CO2 - CH3 f I co2 ~ ~:
CH3 CH3 H ~ ~
V VI ;` ~:
In this possible reaction pathway, one of the
physiological substrates, a-acetolactate (I), is
converted to the product 2,3-dihydroxyisovalerate ~VI).
:
~2
14
The other physiological substrate and product are ~ `-
-aceto-a-hydroxybutyrate and 3-methyl-2,3-
dihydroxypentanoate. The remaining structures (II-V)
are tightly bound intermediates of the reaction, which
S never leave the enzyme. Although it is known that `
2-oxo-3-methyl-3-hydroxybutanoate (V) is reduced by the
enzyme when the independently synthesized compound is
exogenously supplied to the enzyme, a concentration of
this intermediate never builds up during the normal
10 reaction, suggesting that it is tightly bound by the `
enzyme. One analog of intermediate (V),
N-methyloxalylhydroxamate (VIIa, below), is of
particular interest because it has characteristics - ` `~
(apparent in resonance form VIIb, below) similar to
15 earlier intermediates in the reaction pathway (IV and ~ -
III). Furthermore, as ketol-acid reductoisomerase is a ` `-
magnesium-dependent enzyme, and magnesium is likely to
be involved in the rearrangement of (I) into (V), the
analog (VIIa, below) has the added advantage of being an
20 excellent metal chelator. Thus, there is the potential `~
for ketol-acid reductoisomerase inhibition by ;~
oxalyhydroxamates due to both reaction-intermediate `~
mimicry and hydroxamate coordination of the essential `~
magnesium. ` `;
The important structural features for inhibition of
ketol-acid reductoisomerase are best illustrated by
considering the structures below. ~ ;~
" , . ~
.. ' ~ -,' `'
. ~ ., . "; " ~
Z(~02021
' '.'~
I . 2
M~, Mg
. O O ' - O O
I 11 1 1'
C~3 N C--CO, ~ N---'C- CO2 -
VIls~ VIIb :
H10 ¦¦ Hf 11
H--N C--CO~ ~ H--N C--C2--CH3
VIII IX
HO Ol H O
H--N C CH3 H--N C C02
X X I
CH, 1 1I H 1 1l 1
H--N C--CO2 - H--N C C ~13
XII XIII
' ' :
Substitutions on the nitrogen (i.e., R1) of
oxalylhydroxamate are well tolerated, as numerous
substituents either increase the affinity of the enzyme
for the compound relative to oxalylhydroxamate or reduce
it only slightly (Examples 4-16). By contrast,
substituents on the oxygens of the hydroxamate (XII) or
! carboxylate (IX) consistently reduce the enzyme's
affinity for the compound (Example 4). However,
elimination of either carboxylate or N-hydroxyl oxygen
has a much more dramatic effect (i.e., 104- to 105-fold ~-~
reduced affinity for the enzyme). Oxamate (XI) or
pyruvyl hydroxamate (XIII) are not time-dependent
~'. ~ ;'''~
;: :
zon2~.z~
16
inhibitors of ketol-acid reductoisomerase, and the
affinity of the enzyme for these analogs is reduced
relative to their hydroxyl-bearing counterpart ~VIII), ~
by greater than 105 and 104, respectively (Example 4). -
Similarly, completely replacing the carboxylate of
oxalylhydroxamate with a methyl moeity results in the
rather poor inhibitor acetohydroxamate (X), which has an
affinity reduced by greater than 105 relative to ~``
oxalylhydroxamate ~Example 4). Structurally, an oxalyl
10 moiety is necessary for ketol-acid reductoisomerase ;~
inhibition. Simple metal chelators or hydroxamates,
such as compounds (X) and (XIII), will not potently
- inhibit this enzyme. - - `
Herbicidal activity results when plants are brought - ~-
in contact with the inhibitors of this invention at
concentrations of between 10 and 100 ppm (Examples
23-30). The herbicidal oxalylhydroxamate compounds may ~`
be contained in the medium in which the plants are grown .
or may be applied to the surfaces of growing plants.
Herbicidal action results in the lack of growth after
germination. Topical applications at a rate greater `~
than 0.4 kilogram~/hectare may result in other symptoms ~ `
of plant toxicity such as chlorosis, leaf burn, and `~
curling of the leaves (Examples 38-46).
The process of the present invention inhibits -~
microbial growth through the inhibition of ketol-acid
reductoisomerase by oxalylhydroxamate derivative
Bacterial growth is affected by the addition of filter- -
I sterilized solutions of these oxalylhydroxamate `~
derivatives to sterile growth media solutions lacking
,. ~ .,
branched-chain amino acids. These solutions contain
0.02 % thiamine (required for scher;ch;a coli K ;-;`
strains) and 0.2 % glucose as a carbon source. An -~-
inoculum of bacteria, with sufficiently few bacteria in
it so that the inoculated solution is initially almost
'''~ "'`'''''''"'
':~ .`,, `. -,
... ~, ....
~ .. ,.-,.....
Z()OZ(~2iL
17
free of turbidity, is added. The turbidity of samples
lacking inhibitor increases with time as a result of
bacterial growth, which can be correlated with increases
in bacterial numbers. Solutions containing greater than
1 mM of the oxalylhydroxamate inhibitors display no
turbidity after 8 to 27 hours, and thus little or no
bacterial growth has taken place. Lower concentrations
of inhibitor allow some bacterial growth, albeit a time-
dependent lag in cell growth is observed (Examples
17-20). Isoleucine and valine (branched-chain amino
acids) may be added to eliminate the time-dependent lag
in cell growth and, this demonstrates the selective
inhibition of branched-chain amino acid biosynthesis in
the intact organism (Examples 21-22).
The present invention also includes a process for
assaying ketol-acid reductoisomerase, which comprises,
in sum, mixing an aliquot selected from the group
consisting of a plant extract and a microbial extract
with radioactive [1-14C]-acetolactate, NADPH, and Mg2+,
in a buffered solution having a pH above about 6 and
below about 9; adding an acid to the assay mixture,
after an appropriate incubation time; removing volatile
components from the acidified mixture; and measuring the -
remaining radioactivity.
The ketol-acid reductoisomerase enzyme currently
may be assayed from purified bacterial preparations, as
described by Arfin et al., J. B;ol. Che~. 244:1118
(1969), but this method is not well-suited to plant
e~tracts because of the high level of enzyme required
and the nonspecific oxidation of NADPH. The assay of
the present invention is suitable with low levels of the
enzyme and avoids the consequences of nonspecific
oxidation of NADPH.
The present assay measures the time-dependent
increase in acid-stable radioactivity due to the
- r
zno~n2l - .
18 `
reduction of [1-19C]-acetolactate (acid-sensitive ` ~
substrate, 48 cpm/nmol) to [1-14C]-2,3- `~` `;~ `
dihydroxyisovalerate (acid-stable product) by ~ `
reductoisomerase in the presence of NADPH and magnesium ~ `
(with liberation of 14C02). ~ ~
The reaction is initiated by mixing an aliquot of a `
ketol-acid reductoisomerase-containing solution
(containing 4 X 10-5 mmol~min of activity in 10 to
20 IlL; more or less activity, in any convenient volume,
can be used in the assay by changing the assay time
proportionately) to a 1 mL assay solution (final volume)
containing 100 mM HEPES-NaOH, pH 7.4, 10 mM MgC12, ~
400 llM NADPH, 125 ~M [1-14C]-acetolactate ` - ~-
(48 cpm/nmole), and various concentrations of the ~ - oxalylhydroxamate inhibitor to be tested.
[1-14C]-Acetolactate can be obtained by the
conversion of [1-14C]-pyruvate (Du Pont-New England
Nuclear) by use of acetolactate synthase, as described
by Schloss, et al., Riochelni5try 24:4952 (1985).
Alternatively, [1-19C]-pyruvate can be obtained from
D-ribulose 1,5-bisphosphate and 14C02 by the action of
ribulosebisphosphate carboxylase, phosphoglycerate ~ ;3
mutase, enolase, pyruvate kinase, ADP, Mg2+, and
2,3-bisphosphoglycerate at pH 8. With the exception of
14Co2 (Du Pont-New England Nuclear), these reagents can
be obtained commercially from Sigma Chemical Co.
After incubating the samples for an appropriate `~
time, which is the time necessary to give detectable
levels of product and depends upon the specific activity
and quantity of the radioactive material (herein 5 hours
at 26C), the ~nzymatic reaction is terminated and ~
~14C]-acetolactate is converted to acetoin and 19Co2 by ;-
adding 0.1 mL 5096 trifluoracetic acid. ` -
The volatile components of the acidified~ solution ` ~ ~ ;
are removed, preferably by evaporation at 100C under a ; -~ ~
' ' , .' '' ~'
.: . :::
2~)02~2~
lg
stream of nitrogen. Other methods of removal include,
e.g., drying, gassing, and prolonged standing. The
resulting residue is resuspended in 50 % trifluoroacetic
acid, and the suspension is re-evaporated. The final
residue is resuspended in 1 mL water and 10 mL
Scintiverse I~ (Fischer Scientific Co., Pittsburgh, PA)
is added, and the radioactivity is measured by
scintillation counting.
The enzymatic activity of ketol-acid
reductoisomerase is 50 % inhibited under these
conditions with the preferred oxalylhydroxamate
inhibitors (Examples 5-16) at concentrations between 9
and 20 nM for bacterial ketol-acid isomerase and between
50 and 100 nM for plant ketol-acid isomerase. The `
lowest concentration that yields 50 % inhibition of the
enzyme is 9 nM and is approximately half of the enzyme
concentration employed in the assay. This represents
the theoretical limit for an exceptionally potent
inhibitor, i.e., one molecule of inhibitor for each
molecule of enzyme.
EXZ~MPT.ES
The following Examples illustrate, but do not `~
limit, the processes and compounds of the present
invention. Examples 1-3 illustrate the synthesis of
various oxalylhydroxamates, with the claimed compounds
in Examples 2-3. Example 4 assesses the time-dependent
nature of the inhibition of ketol-acid reductoisomerase ` `~
and also shows that inhibition of ketol-acid
reductoisomerase by oxalylhydroxamate derivatives is
functionally irreversible. Examples 5-16 disclose the
process of the present invention for assaying ketol-acid
reductoisomerase and demonstrate that both bacterial and
plant enzymes are inhibited by oxalylhydroxamate
derivatives. Examples 17-20 show that growth of
35 bacteria is inhibited by oxalylhydroxamate derivatives. ~
`:. ~'
2~)0Z~2~
` 20
- . ~
Examples 21-22 demonstrate that inhibition of bacterial
growth (a consequence of ketol-acid reductoisomerase
inhibition) is prevented in the presence of branched-
chain amino acids, and Examples 23-30 illustrate the
herbicidal effect of oxalylhdroxamate derivatives on
pla.nts (a consequence of ketol-acid reductoisomerase
inhibition) as well as the prevention of thei~
herbicidal effect on plants by the presence of branched- `:
chain amino acids. Examples 31-37 prove that the : - .
10 process of the present invention inhibits only ketol- :~ `
acid reductoisomerase, and not one or more of the other
common ezymes in the branched-chain amino acid
biosynthetic sequence. Examples 38-46 demonstrate the
post-emergence herbicidal activity in whole plants
brought about by the inhibition of ketol-acid
reductoisomerase using topical applications of
oxalylhydroxamate derivatives. -~
The following abbreviations are employed throughout ~ `
the Examples~
OHA: oxalylhydroxamate `
KARI: ketol-acid reductoisomerase ~-
NADP: nicotinamide adenine dinucleotide phosphate
NADPH: nicotinamide adenine dinucleotide
phosphate, reduced form -~
FAD: flavin adenine dinucleotide
HEPES: N-2-hydroxyethyl piperazine-N'-ethane
sulfonic acid
TRICINE: N-tris-(hydroxymethyl) methyl glycine
TLC: thin layer chromatography .
30` Specific substituent values for compounds used in ::
the Examples are:
oxalylhydroxamate (Rl = H; R2 = H; R3 = H; X = O)
oxalylhydroxamate Methyl ester (R1 = H; R2 = H; R3 :~
= CH3; X = O) ; ~; `
N-methoxy OHA (Rl= H; R2 s CH3; R3 = H; X = O) .~
'' :' ' ~'''`,
Z002(?~1
;- 21
N-methoxy OHA methyl ester (Rl = H; R2 = CH3; R3 =
CH3i X = O)
N-methyl OHA (R1 = CH3; R2 and R3 = H; X = O)
N-methyl OHA methyl ester (R1 = CH3; R2 = H; R3 =
5 CH3; X 8 O) ..
N-ethyl OHA (R1 = C2Hs; R2 and R3 = H; X = O)
N-ethyl OKA methyl ester (R1 = C2Hs; R2 = H; R3 = ~ ~
CH3; X = O) ~-`
N-isopropyl OHA (R1 = C3H7; R2 and R3 = H; X = O)
N-isopropyl OHA methyl ester (R1 = C3H7; R2 = H; R3
= CH3; X - O)
N-t-butyl OHA (R1 = C4Hg; R2 and R3 = H; X = O)
N-t-butyl OHA methyl ester (R1 = C4Hg; R2 = H; R3 =
CH3; X = O)
N-benzyl OHA (R1 = C7H7; R2 and R3 = H; X = O)
N-benzyl OHA methyl ester (R1 = C7H7; R2 = H; R3 = ;~
CH3; X = O)
N-t-butoxy OHA (R1 = H; R2 = C4Hg; R3 = H; X = O) ~ ~-
N-t-butoxy OHA methyl ester (R1 = H; R2 = C4Hg; R3
= CH3; X= O)
Exam~le 1
Synthesis o~5~3lylh~droxa~ate~and
Oxalylhys~s~mate ~ethyl Este- :-
To 1 g of hydroxylamine hydrochloride (14.3 mmol)
in 100 mL of methanol were added 4.01 mL of
triethylamine (28.~ mmol) and 1.32 mL of
methyloxalylchloride (14.3 mmol). After allowing the
reaction to proceed for 30 minutes at room temperature,
30 1.43 mL of 10 N NaOH (14.3 mequivalents) were added.
Three hours after the addition of base, the sample was
diluted with an equal ~olume of water ~pH = 9.9) and-~
1 mL of concentrated hydrochloric acid was added to
adjust the pH to 6.5. Following dilution of the sample
35 to 1 liter with water, it was applied to a 2.5 X 59 cm
2()02(~Z l
22
column of AGl-X8 (Chloride form, BioRad Labs, Richmond,
CA 94804). The column was eluted with 200 mL of water,
followed by 2 liters of 50 mM HCl, and 23 mL fractions ;
of the acid eluant were collected. Hydroxamates were "
detected by the addition of 0.2 mL of each fraction to
0.1 mL of 1 % FeC13 in 1 N HCl. Hydroxamates give a red
color in this test and two hydroxamate peaks were
detected, one contained in fractions 10-14, and another
contained in fractions 41-55. The fractions containing
each hydroxamate were pooled s~parately and lyophilized
to give 159 mg of OHA methyl ester (first peak) and ~
103 mg of OHA (second peak). Titration of the OHA with ;
0.1 N KOH (to pH 7) gave an equivalent weight of 111 vs.
the expected value of 105, with a pX of 2.4.
1 5
~xample 2 `
Synthesis of N-Ethyl Oxalylhydroxama~e and ~-F~thyl
Oxalylhydroxama~ Methyl-E~
N-Fthylhydroxylamine was prepared from the NaBH3CN
20 reduction of acetaldehyde oxime according to the ;
procedure of Borch, et al., J. ~m. Che~. Soc. 93:2897
(1971). `
Methyl N-ethyl oxalylhydroxamate was prepared by
the dropwise addition of a solution of 0.82 g
N-ethylhydroxylamine, 1.87 ml triethylamine, and 20 ml
chloroform to a solution of 1.64 g methyloxalylchloride ;~
dissolved in 100 mL diethyl ether at room temperature.
The solution was stirred 2 hours then filtered to remove
precipitated triethylamine hydrochloride szlt.
30 Evaporation under reduced pressure of solvent and - -;;
unreacted triethylamine and methyloxalylchloride
afforded 1.91 g of a visccus pale yellow oil. One spot -
with a Rf = 0.83 was observed on silica gel TLC plates
using ethanol as a deve'oping solvent, and it tested
positive for hydroxamic acid with acidic FeC13. The lH
`':' ;,"~',
''.'',;;'`,"'''"''
. - , . .. - - . .. . , .... , . . . -; . . ~,. . . . ....
20~)2nZ~
. ,
23 -~
NMR in CDC13 corresponded to N-ethyl OHA methyl ester:
1.40 ppm (triplet, 3H), ~ 3.90 (s, 3H), ~ 3.96 (q, 2H).
N-Ethyl OHA was prepared by alkaline hydrolysis of
0.205 g N-ethyl OHA methyl ester in 10 ml water with 1
equivalent of potassium hydroxide. Hydrolysis was
monitored by the decrease of the resonance at 3.90 ppm
in the lH NMR spectrum.
N-Ethyl OHA and its methyl ester are compounds `-
disclosed in the present invention.
,
Synthesis of N-Methyl. N-Iso~ro~yl N-Methoxy.
N-Benzyl. N-t-Butyl and N-t-Butoxy Oxalylhydroxamates `
and Their Methyl Esters
15The methyl ester of N-benzyl OHA was prepared by
the procedure described in Example 2 startin~ with `-
N-benzaldehyde oxime. The methyl ester was a cream-
colored solid (m.p. 102-104C).
The methyl esters of N-methyl, N-isopropyl,
20 N-methoxy, N-butyl and N-t-butoxy OHA were prepared by -
the procedure described in Example 2, with the exception
that the hydrochloride salts of the hydroxylamines were
initially dissolved in chloroform with two equivalents
of triethylamine. This chloroform solution was added to
methyloxalylchloride in diethyl ether. After 6 hours,
the mixture was filtered to remove precipitated
triethylamine hydrochloride salt, and the solvent was
removed under reduced pressure. The methyl esters were
purified by vacuum distillation.
compound preSsure lmm) h.p (C)
N-methyl OHA 0.12 92-94
N-isopropyl OHA 0.10 95-97
N-t-butoxy O~A 0.09 74-76
35 N-t-butyl OHA 0.10 74-76
;~; " '
z~2n2~ `
24 `
The corresponding acids were obtained as their
potassium salts by alkaline hydrolysis of the methyl
ester with one equivalent of potassium hydroxide. -
All of the compounds in Example 3, with the
exception of N-methyl OHA methyl ester, are disclosed as
part of the present invention.
, :.
~Yam~ls 4 ~,,
~ssessmen~ of the T~me-De~e~dent Nature of the,
~hl~ion of Ke~QL-Aci~-Rg~tolsome~e by Use ~_z
ContinuQ~ Assay
Inhibition of the purified E_ col; KARI by all
oxalyhydroxamate derivatives is time dependent. At a ` ~;
15 concentration of 0.1 ~M of N-isopropyl OHA, 66 nM KARI -~
was inactivated with a half-time of about 2 minutes when
it was added last to an assay solution containing 100 mM ~ -
HEPES, p~ 7.4, 0.13 mM acetolactate, 10 mM MgCl2, and .
0.2 mM NADPH. The assay was continuously monitored at
340 nm by use of a recording spectrophotometer. The
loss of absorbance due to oxidation of NADPH was ` :
followed. OHA, N-methyl OHA, and N-benzyl OHA gave
similar time courses of inactivation but at
concentrations of about 1 ~M. Similarly, the methyl
ester of OHA and the N-methoxy OHA exhibited time
dependent inactivation, but at still higher
concentrations (approximately 10-5 and 10-4 M, ~ ;
respectively). Inhibition of the enzyme was complete at
these inhibitor concentrations after incubation for a ;~
30 sufficient duration. If the enzyme was incubated with ~;
OHA prior to assay, complete inhibition was observed
upon initiating the assay by addition of substrate if
Mg2+ was present during the pre-incubation. Dilution of
the enzyme-OHA comple~ 100-fold into the assay solution
did not reverse the inhibition within a 10-hour period.
`
2002n2~l
This demonstrates that these oxalylhydroxamate
derivatives are functionally irreversible inhibitors.
By contrast, inhibition of KARI by oxamate (XI, above),
acetohydroxamate (X), or pyruvylhydroxamate(XIII), was
not time dependent, and gave 50 % inhibition at
concentrations of 10-3 M for oxamate and
acetohydroxamate and 10-4 M for pyruvylhydroxamate.
This demonstrates that these latter inhibitors are
reversible.
E~amRl~S 5-16
I~_V;tro Inhib;~iQn_Qf_~. coli and ~ doDsis thaliana
Ketol-Acid Reductoisomerase
The in vitro inhibition of KARI from bacteria (E.
col;) and plants (~L2~ido~si~ ~h~lia~a) by N-alkyl
oxalylhydroxamates and the corresponding esters is
demonstrated in Examples 5-16. ~_ ~Qli KARI was
purified using a modification of the procedure of Arfin
et al., ~. Riol . Chem, 244:1118 (1969). ~_ coli and
Al~kL~ç~sis KARI activity was measured by the
radiometric assay for KARI disclosed in the presen~
invention.
This assay measures the time-dependent increase in
acid-stable radioactivity due to reduction of [1-14C]-
acetolactate (acid-sensitive substrate) to [1-14C]-2,3-
dihydroxyisovalerate (acid-stable product) by KARI in
the presence of NADPH and magnesium. The reaction was
initiated by adding enzyme (4 X 10-5 ~mol/min contained ` -
in a convenient volume, e. g. 10 to 20 ~L) to a 1 mL ~ `
assay solution (final volume) containing 100 mM
HEPES-NaOH, pH 7.4, 10 mM MgC12, 400 ~M NADPH, 125 ~M
~1-14C~-acetolactate (48 cpm/nmole), and various ~;
concentrations of the inhibitor to be tested. After
incubating the samples 5 hours at 26C, the enzymatic
reaction was terminated and acetolactate was converted
, :. .
z()o~n2~
26
~'
to acetoin and CO2 by adding 0.1 mL 50% trifluoracetic
acid, with liberation of the radiolabel as 14co2. The - `
solution was evaporated at 100C under a stream of
nitrogen, resuspended in acid, and re-evaporated. The
resulting solid was resuspended in 1 mL water, then
10 mL Scintiverse I~ (Fischer Scientific) was added, and ``
radioactivity was measured by scintillation counting.
The ICso is the concentration of inhibitor that gives
50 % inhibition under these conditions. The results of `
these experiments are listed below in Table 1.
Although all of the compounds tested were effective -
inhibitors for both the E_ coli and ~ahi~sLa KARI, -
the best ICso value for the E_ coli KARI under the assay
conditions was 9 nM, and the best ICso value for the
plant enzyme was 50 nM. Given the complex, time-
dependent nature of these inhibitors, as shown in
Example 4, fixed-time inhibition values, such as these
ICso values, tend to underestimate the potency of the
inhibitor.
. `:
2()02~1
. -
27 ~ --
Table 1 -~
Extracts and with Pur;flecl E. coli Ketol-Acid
5 Example Compound E_ col; Arahi~QR~is
IC50 IC50
(~M) (~M)
OHA 0.02 0.10
10 6 N-methyl OHA 0.03 0.10
7 N-methyl OHA
methyl ester 0.06 0.35
8 N-ethyl OHA 0.02 0.05
15 9 N-ethyl OHA
methyl ester 0.08 0.25
, .,
.N-isopropyl OHA 0.014 0.25
11 N-isopropyl OHA -.
methyl ester 0.04 0.30 ~ :
12 N-benzyl OHA 0.009 0.45
13 N-methoxy OHA 0.4 30
25 14 N-methoxy OHA
methyl ester 14 90 ;. ~.
~ .
N-t-butoxy OHA 40 130
~ ` 16 ~N-t-butoxy OHA
methyl ester 60 400
G-owth Tnhihit;on of E. col; Ml 52 .~ .
Growth inhibition of E_ col; M152 by N-alkyl .~
35 oxalylhydroxamates and their methyl esters is ,~ ;
~, , .~ :, i .,.'.
.,, ., ..",..........
.-:: :;: ',
'''''; .''.".'''
2()02~
28
demonstrated in Table 2. Although the M152 strain of
coli was used due to its simple nutritional requirements
~i.e., no amino acids are required for growth), there is
no reason that any microorganism that can grow in the
absence of branched-chain amino acids could not have
been used.
Growth experiments with E_ ~Qli M152 were conducted
under sterile conditions in culture tubes containing
1.8 mL minimal medium M63 (Difco Laboratories Inc.,
Detroit, MI) supplemented with 0.01 % thiamine and 0.2
glucose. Filter-sterilized inhibitor solutions were ~
added to the autoclaved medium to yield final ~:
concentrations ranging from 1 ~M to 10 mM. Bacteria
(0.2 mL of ~_ ~Qli M152) in sta~ionary phase, which had
15 been cultured overnight in minimal medium containing -
0.2 % glucose and 0.01 % thiamine, were added to fresh `
medium with or without (control) inhibitor. Samples
were initially clear but the turbidity or samples
lacking inhibitor (controls) increased with time due to
the increase in cell mass. The bacteria were grown in
an incubator-shaker at 37C and 250 rpm. Bacterial
growth was qualitatively scored as the increase in
turbidity relative to the control containing no ~
herbicide. The lowest concentration of inhibitor at -
which no growth occurred at 8, 19, and 27 hours after
the tubes were inoculated with bacteria is listed in
Table 2. A concentration-dependent lag in growth was
observed at levels of inhibitors greater than 1 ~M.
Of the compounds tested, the N-isopropyl OHA
derivative was the most potent because effects on the
growth lag were observed at micromolar levels.
200zn2 l
29
Table 2
Concentra~lg~ and ~ime DeDendency of Growth
Inhi~itlon of E. coli M152
Hour post-inoculation at which
5no growth was observed at the
concentration shown
Example Compound 8 19 27
17 N-ethyl OHA1 mM 10 mM
18 N-isopropyl OHA 1 mM 1 mM 10 mM
19 N-methyl OHA ~-
methyl ester 1 mM 10 mM 10 mM
N-methoxy OHA 10 mM
ExamDles 21-22
~vention of ~-Alkyl Oxalylhydrox2mate-Mediated
Inhibit;on of E. coli M152 Growth by Branch~d-chain
Ami rl o Ac ids `: : `
Prevention of ~_ ~Qli M152 growth inhibition by -~
N-alkyl OHA with branched-chain amino acids is
demonstrated in Examples 21-22. ~-
E_ sQli cells were grown using conditions listed
for Examples 17-20, except some samples containing 1 ~M
or 1 mM N-isopropyl OHA were supplemented with sterile
valine and isoleucine solutions to a final concentration
of 1.5 mM. Growth was determined visually in a
qualitative fashion, as the increase in turbidity. The
results, listed in Table 3, demonstrate valine and ~-
, isoleucine prevent the inhibition of bacterial growth by - ~`
30 1 ~M and 1 mM N-isopropyl OHA. `~
This illustrates the selective inhibition of ;~
bacterial branched-chain amino acid biosynthe~is by
N-alkyl OHA in intact organisms. ~ '
''"''''''''''''''''''''`''`''''
,......
,r~ ~
20~12n2~ :
Prevent;Q~ of N-~lkyl Oxalylhydrox ~te-Mediate~ I~hib;tion of F.
coli M152 Growth by Br~ched-Chain Amino Ac;d~ : ~
5 Example Compound conc. amino acid Growth ~:
cmpd. (turbidity~
(mM) (1.5 mM) 3 hr 6 hr 8 hr
none - none ++ +++ ++++ : :
21 N-i~opropyl O~A O.001 ~one - - -
N-i.~opropyl OHA 0.001 Ile . - + ++++
N-i~opropyl OHA O.001 Ile, Val ++ +++ ++++ .
22 N-i-~opropyl OHA 1.0 Ile
N-i30propyl OHA 1.O Ile, Val ++ ++~ ++~+ : '' :
lS Ile icoleucine; Val - valine ~
' ~`.''`
~m~l~s 23-30
Tnhihition of ArabidQ~is thalian~ Growth by -
Oxalvlhydroxamate Der;vat;ves and the ~reventiQn_Qf
Tnhibit;~ yLE~u~ -Chai~ ~miDQ-~cids
Examples 23, 25-30 in Table 4 illustrate the -
he-bicidal effect of N-alkyl and N-alkylaryl
oxalylhydroxamates on ArahilQ2ai~ thal;ana, and Example
24 demonstrates the prevention of the herbicidal effect
25 by the presence of branched-chain amino acids. -
The ~&bl~s~sis-assay for herbicide sensitivity is -~
the same procedure described by Haughn et al., ~Ql_
Ge~. Genet 204 430 (1986). Seeds were surface
~ i sterilized with 25% bleach and 0.02% Triton-X100 then
30 transferred to bacteriological petri plates (90 mm x -
23 mm) containinq minimal medium and 8 g/L agar to a
density of 10 or 19 seeds per plate. Filter-sterilized
solutions of OHA compounds and amino acids were added to
the desired concentrations after the medium was
autoclaved. The seeds were next placed in a dark room
' :.,
`Z002f~2 ~
.
31
for 3-4 days for etiolation to occur and then
transferred to a growth room. The herbicidal effect of
the OHA compounds was scored as the % mortality of
plants in the presence of OHA compounds relative to
control plants in the absence of OHA compounds. Seeds
germinated, but the resulting plants died in the ;
presence of OHA compounds. The fraction of plants that -
died is listed as ~ mortality in Table 4.
~ahle 4
Gro~h~nhih;ti~n of Arabidopsis thalia
Example Compound Conc. Presence of
(ppm) Ile and Val Mortality
(1.5 mM)
15 23N-isopropyl OHA 100 - > 80
24N-isopropyl OHA 100 + < 10 ;-~
N-isopropyl OHA 10 - 50
26N-methyl OHA 100 - ~ 90
methyl ester -
20 27N-methyl OHA 10 - < 50 -
methyl ester
28N-methoxy OHA 100 - > 90 ;~
29N-benzyl OHA 100 50
30N-benzyl OHA 10 - 20 `
-; ;-~
Ile = isoleucine; Val = valine; + = presence of 1.5 mM
each of Ile and Val; - - the absence of Ile and Val;~
Exam~l e -37
Specif;c;ty of the Inh;~itiQn o,f Plant Re~Ql-Acid
Reductoisomera9e i n the Rranc~led-Cha; n Aln; no Ac; d '`
Riocynthet;c Pathway -~;
Data showing the insensitivity of the other common
enzymes, excluding KARI, in the branched-chain amino
acid biosynthetic pathway is given in Table 5.
2002n2~ `
32 `-
Acetolactate synthase activity was measured by the `-
continuous assay method described by Schloss, et al.,
3iochemistry 24:4952 (1985). Acetolactate synthase
(10 ~L) was added to a 1 mL assay solution equilibrated
S at 25C containing 100 mM Tricine-NaOH ( pH = pK), 50 mM
sodium pyruvate, 10 mM MgCl2, 0.1 mM thiamine
pyrophosphate, 0.1 mM FAD, and inhibitor. The decrease `
in pyruvate concentration was monitored at 333 nm for at
least 10 minutes.
10~,~-Dihydroxyacid dehydratase activity was measured
by a modification of the fixed-time assay deccribed by
~iritani and Wagner, ~ethods Enzymo. 17A:755 (1970).
Assay solution (l mL) containing 50 mM Tris-HCl (pH =
8.0), 10 mM MgCl2, 100 mM ,~-dihydroxyisovalerate,
enzyme, and inhibitor was incubated for 30 minutes at
37C. Trichloroacetic acid (0.25 mL of a 10 % solution)
was added to stop the reaction and 0.5 mL saturated 2,4-
dinitrophenylhydrazine (in 2 N HCl) was added to form
the hydrazone derivative of the -keto acid produced in
the enzymic reaction. The mixture was incubated 10
minutes at room temperature, then 1.75 mL of 2.5 N NaOH
was added to solubilize the hydrazone and precipitate
unreacted 2,4-dinitrophenylhyrazine. Samples were
degassed 10 minutes, centrifuged for 2 minutes, and
their absorbance at 550 nm recorded.
The results of these experiments ill~strate the
insensitivity of the other common enzymes that are
involved in branched-chain amino acid biosynthesis to
~ N-alkyl OHA derivatives. These data, together with the
data in Examples 21-30, demonstrate the selective
inhibition of ~A~I in intact organisms (i. e., -
prevention of inhibition by valine and isoleucine), and
they demonstrate that KARI is the only en7yme in this
pathway that is sensitive to N-alkyl OHA.
2002~
33
;:
;'
Spe"ci$i~ty of ~h~ Inhiki~l~n in the Branched-chain
Amino Ac,d Bi~ynthet;~ Path~a~y -~
~
Example Enzyme Source Inhibitor Conc. % Inhib. , ,
(mM) -;'~
31 acetolactate E. coli OHA 0.1 < 5 -~
synthase II
32 acetolactate E. coli N-isopropyl 1.0 < 5
synthase II OHA -~
33 acetolactate E. coli N-isopropyl 1.0 < 5
synthase II OHA methyl ester ~ "
34 dihydroxy- Spinach OHA 1.0 < 5
acid dehydratase '~
dihydroxy- Spinach N-methyl 1.0 < 5 -~
acid dehydratase OHA '~
36 dihydroxy- Spinach N-isopropyl 0.1 < 5 ''~
acid dehydratase OHA ;
37 dihydroxy- Spinach N-isopropyl 1.0 16 ,'
acid dehydratase OHA -'~
~xam~les 38-~6 '`""'~
HerbiCid-l Actiyity of N-Alkyl ~; ' '
Oxa1ylhy,dLoxam~ Derivatives
Herbicidal activity of N-alkyl OHA derivatives was ~'
also shown by topical application of a herbicide `'~
solution to growing plants. Arabi~R_is thalLana (a ~''"'`
l broadleaf plant), Rrassica kaber (a broadleaf plant), ~'`',`
and ~53Yhl~:hlQi s395c~alll (a barnyard grass) were grown ~ ",,,~
in pots containing Metro-mix~ 350 (W. R. Grace & Co.,
Cambridge, MA) for at least 1 week before topical
application. Several O}IA herbicidal solutions were ''~
applied as a fine mist to a final concentration of 10, ^ ,,,', ''''
35 2, and 0.4 kilograms/hectare of the herbicide. Effects '~
:-"'- (-
~.~,,, ,.,,,", ,~
2no2n2~
34
were recorded after one week and included toxic effects
such as plant death, chlorosis, leaf curling, and leaf
burn.
Of the herbicides tested, which are listed in Table
6, the N-isopropyl OHA methyl ester was the most potent
with plant death observed at 10 kg/hectare for ~
~h~11~a and deleterious effects of the herbicide
observed at 0.4 kg/hectare. ~No effect was seen in
barnyard grass, which was probably due to the higher
sensitivity of broadleaf plants.)
' ,' ~' ' .
; ~
2002n2~
~5
: -
~s~3i5i~ ==~it~ of N-Alky
Oxalylhy~Loxamat~A Derivatiye~
Example Plant inhibitor [inhibitor] inhibitor
5(kg/hectare) effects after ~:
1 week
~8 A. thaliana N-isopropyl 10 leaf burn, :
OHA methyl ester leaf curling,
chlorosis, . :
plant death ~ -
39 A. thaliana N-isopropyl 2 leaf curling,
OHA methyl ester chlorosis
: . ~ .
15 40 A. thaliana N-methyl 10 chlorosis
OHA methyl ester
41 B. kaber N-isopropyl 10 leaf burn, lear `
OHA methyl ester curling,
chlorosis
2 0 - . . ~
42 B. kaber N-isopropyl 2 leaf curling, ~..... `
OHA methyl ester chlorosis
. .
43 B . kaber N-isopropyl 0 . 4 leaf curling,
OHA methyl ester chlorosis
"............ ............ ............................................ ......... ~ ~".
44 B. kaber N-methyl 10 chlorosis -.::
OHA methyl ester `~ ;~
1 45 barnyard N-isopropyl 10 no effect ::~
grass OHA methyl ester
46 barnyard N-methyl 10 no effect : -~
grass OHA methyl ester ~ .
::' ;;,.:~:, ~
., . ... ~
: ~:, ., ..;