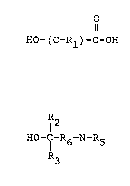Note: Descriptions are shown in the official language in which they were submitted.
Z00391~i
~'
`~ Dl~SCALING COMPOSITION AND METHODS OF USE
This application is a continuation-in-part of my co-pending
i U.S.A. application, Serial Number 195,851, filed May 19, 1988
'
This invention relates to chemical compositions and methods
of use thereof which are useful in removing and preventing
reformation of water scale on various surfaces.
' ,:
In an even more particular respect, the invention relates to
~- 10 such compositions which are especially adapted for use in heat-
~` exchange systems utiliæing water as a recirculating heat-exchange
!, ' .I medium.
"Scale" is a general term which describes precipitates from
aqueous fluids which deposit on surfaces in contact with water,
~15 generally as a result of exceeding the limit of solubility of such
materials in the water, e.g., by evaporation of the water or by
- ~ otherwise concentrating these components. Such scale materials
. ~ include compounds such as sulfates, oxides, carbonates, halides and
the like. Examples include calcium carbonate and iron oxides.
To be effective, a descaling composition should break down
components of scale. Primarily, the descaling composition should
remove carbonates and sulfates.
A number of methods have been devised to prevent or remove
scale. Foriexample,~Rubin, in U.S. Patent No. 3,075,924 teaches
~25 a detergent composition which includes a long chain aliphatic
sulfate and an alkylamine acetic acid.
'f',~
~: ~
~ .~ . ,
200391~:i
,
~,
Clark et al, in U.S. Patent No. 3,053,897, disclosed
compositions for the removal of rust and scale. Clark teaches the
~,use of triethanolamine in combination with an alXali and an
aliphatic hydroxy acid. Clark also teaches the use o~ alkali metal
salts of sulfonated fatty acids.
Conklin et al, in U.S. Patent No. 2,847,384, disclosed a
descaling composition which includes a non-ionlc wetting agent
which is a mixture of polyoxythylene-propylene polymeric compounds
with sodium xylyene sulfate.
Petroy, U.S. Patent No. 3,699,047, employed zinc ion in a
scale inhibiting composition which may also include an
alkyleneamine-acetic acid compound.
Monson, in U.S. Patent No. 2,589,195, disclosed an inhibiting
scale composition which includes a triethanolamine which is reacted
with an ethylene oxide to form an amine having a number of ethylene
oxide units. The terminal hydroxy groups are acylated with a fatty
~: acid.
l ~ Further examples of prior art descaling compositions are
. "~disclosed in the Abadi patents, U.S. 4,435,303 and 4,595,517.
~; ~As yet, however, such prior art does not provide economically
effective methods for preventing scale formation, particularly in
large industrial applications where recirculating water containing
scale-forming components is used as a circulating medium in heat-
~- ~exchange equipment, e.g., boilers, coolers, etc. Instead, the
`".'.t,'' ~common approach in the ! prior àrt is to employ expensive and
complicated deionizing and other water purification pretreatment
processes to reduce the initial level of scale-forming components
.~ -~in the aqueous heat-exchange medium. Also it is common practice
¦to bleod sizabl- guantitios o~ th- aquoous hcat--xohange m-dium
Z003915
from the system to avoid build up of concentrations o~ the scale-
forming components which exceed their solubility limits in the
cooling or heating water. The water removed to control the
concentration of scale-forming components in such heat-exchange
systems is called "blowdown".
It would be highly desirable to reduce the amount o~ blowdown
encountered in operating conventional heat-exchange systems,
particularly evaporative cooling systems which utilize water as the
heat-exchange medium, as the problem is disposing o~ the blowdown
has become increasingly serious and the cost of the makeup water
constitutes a significant cost element in the operation of such
systems.
Additionally, it would be desirable to provide improved
', descaling compositions for use in a wide variety of end-use,~ 15 applications in which water conservation or waste disposal is a
significant factor. For example, it would be highly desirable to
achieve such results in the operation of direct heat-exchange
systems such as automotive radiators, and the like. Also, reducing
~; the solubility of scale-forming components in water is important
in residential use, e.g., tub and tile cleaners and the like, and
;~ the metal scavenging effect of such compositions would provide
desirable results in widely diverse products such as plant roods,
medicines, etc.
Therefore, the principal object of the present invention is
to provide an improved composition for solubilizing scale and
scale-forming components in aqueous systems. -
~ :;'`. .
Another object of the invention is to provide methods for
operating heat-exchange systems which reduce the amount of blowdown -~ `
required to prevent scale formation.
-3-
},~
:
200~915
Still other objects of the invention are to provide
compositions for solubilizing scale-forming components in aqueous
systems and utilizing such capabilities to improve the qualities
of various household, industrial and medical products.
Briefly, in accordance with the invention, a composition is
provided which consists essentially of a hydroxycarboylic acid, an
amine and a monocyclic aryl surfactant.
According to another embodiment of the invention, methods for
removing water scale from surfaces are provided which include the
step of contacting the surfaces with water containing a minor
effective amount of this composition.
According to a more specific embodiment of the invention, a
method is provided for operating a heat-exchange system which
utilizes water as a heat-exchange medium. These methods include
the step of circulating in the system a water heat-exchange medium
containing scale-forming components and an effective amount of this
composition, i.e., in an amount sufficient to maintain the scale-
forming components in solution.
- The composition of the present invention is in the form of a
~20 concentrate which can be further diluted as required by the
particular end-use of the composition.
The hydroxycarboxylic acids used in the practice of the
invention have the structural formula
1 ~ ! O
~-~ HO-(C-Rl)-C-OH
wherein Rl is hydrogen, a branched or straight chain alkyl,
cycloalkyl, or aryl substituent having one or more carbon atoms and
.
:~
Z0039~
.
; forms a stable C-Rl group which does not inactivate the carboxylic
acid group. The preferred hydroxycarboxylic acid is hydroxyacetic
acid wherein C-Rl is a methane radical.
The alkonolamines used in the practice of the lnventlon have
the structural formula
R
'1 12
,, ~O-C-R6-N-R5
i~ wherein R6 is a branched or straight chain alkyl group having ~rom
0-5 carbon atoms, and R2, R3, R4 and R5 are selected from the group
consisting of hydrogen, a branched or straight chain alkyl group,
a cycloalkyl group or aryl group, such that the cumulative electro-
chemical effect of the substituent groups does not create an overly
'~ positive charge and negate the basicity of the alXanolamine.
2`~ 15 Preferred alkanolamines of this general formula consist of
methanolamine and triethanolamine.
The alkalimetal monocyclic aryl sulfonates use in the
concentrate composition include sodium and potassium salts of the
sulfurio acids of tolerance and xylene, e.g. 2,3-dimethyl
~2-0 benzenesulfonate: 2,4-dimethylbenzenesulfonate; 2,5-
; dimethylbenzesulfonate; 3,4-dimethylbenzesulfonate, 4-
ethylbenzemesulfonte and the like. Sodium dimethyl benzene
sulfonates and mixtures thereof are preferred.
The compositions of the invention can be prepared by
~25 dissolving the alkanolamine in a suitable solvent, suitably an
aqueous alcoholic solvent, and then mixing the amine solution with
the surfactant. The mixing steps are preferably carried out under
high shear conditions until complete solution and reaction occurs.
"~
` : ~S~ ~`
2003915
,.
,,.;
Suitable alcohol solvents include those having the structural
formula
1 1
HO-C-R2
R3
wherein Rl, R2, R3 are each selected from the group de~ined above
for Rl R5. Preferred alcohols of this general formula are
methanol, ethanol, propanol and ethylene glycol.
In my previous co-pending U.S.A. application S/N 195,851, I
described compositions in which the amine-sulfonate composition
further reacted with. However, I have now determined that such
further steps can be omitted. one may to obtain a useful, desirable
product if the carboxylic acid is added directly to the
composition. -
I have further determined that alcohol used to dissolve the
-~ amine is largely dissipated during the exothermic mixing with the
sulfonate. Substantial alcohol remaining in the concentrate
composition does not materially increase the effectiveness of the
product.
The volume percentage of the hydrocarboxylic acid to the total
;~ mixture is in the range of about 25-60%, preferably about 35-45~.
The efficacious ratio of hydrocarboxylic acid to alhauolamine is
in the range of 0.7 - 10, preferably about 2.0 - 3.5. -~
In use,~the agueous chemical composition of the present
~25 invention is added to water in a recirculating agueous system.
Agueous systems which are non-recirculating may be recirculated to
increase ths rate of reaction. If a relatively higher reaction
; rate is not reguired, non-recirculating systems may remain 80.
-6-
Z0039~
Solutions of about 0.5% to 5%, preferably ~rom 0.5% to 1.0% by
volume, of the present invention to water are usually suf~icient
to maintain a previously cleaned aqueous system. To clean a scaled
and/or rusted aqueous system, solutions o~ up to 50%, by volume,
of the aqueous chemical composition in water may be employed.
Higher concentrations and higher reaction temperatures increase the
rate of the descaling and antioxidizing reaction. It has been
found, however, that concentrations in excess of 50% by volume do
10 not exhibit statistically significant further increases in reaction
rate.
To further illustrate the preferred practice of the invention,
the following examples are presented.
,~
,~
, ~ .
: ~
~ .
.
, ~ .
:
:
,.
~:~
',~;
,~ ~ :
. ~ .
200391 ~
EXAMPLE 1
A dilute ethanol-water solvent is prepared by agitating a
mixture of water and ethanol (0.015% by volume~. To this ethanolic
aqueous solvent is added an equal volume o~ triethanolamine and the
resultant mixture is agitatad for an additional 30 minutes at room
temperature and pressure, resulting in complete solution of the
amine. To this amine solution is added about 30% by volume of
sodium xylene sulfonate and shear agitation is continued for an
additional approximately 30 minutes. The reaction begins at room
temperature but the temperature of the reaction mixture rises to ~ m
approximately lOO-F during this mixing period. To this mixture is
added about 40~ by colume of hydroxyacetic acid and mixing is
continued for an additional 10 minutes.
' ,' :
,~
,,,~
,
,
~ Z003915
EXAMPLE 2
For use in residential evaporative cooler, egual parts o~ the
concentrate of Example 1 and water are mixed in the evaporative
cooler sump.
~ 9- ~,~
200:~9~5
EXAMPLE 3
For use in an industrial cooling tower, ~rom about 0.5 to 1.0%
by volume of the composition of Example 1 is added to the
recirculating water in the system.
,~
:, .
-
' , ' '
,
,.
~ ' .. : ,: .
~ .~-' :"'.' '
~ ~ 1 0 ~ ,
. :
:
'~ -
20039~5
EXAMPLE 4
A suitable residential tub and tile cleaning solution is
prepared by physically admixing the following components in the
indicated percentages by volume.
COMPONENTS PERCENTAGE
Water 56
Concentrate 33
Butylcellosolve .05
Monoethanolamine 10.95
Cherry scent trace
~ ,~
I,;
:~ ;
., ~
.
-11- .
",, ~
.~.~ - . . .
Z0039~S
EXAMPLE S
A suitable buffered descaling composition is prepared by
physically admixing the following component~ in the indicated
percentages by volume.
COMPONENTS PERCENTAGE
Hydrochloric acid 70
Product of Example 430
Cherry scent trace
~ ~ , ....
., - . ~
,~, :; :,
,::
`''~
`'~
-12-
~: : -
,`~
,~
~`~4
Z003915
~-
EXAMPLE 6
A suitable buffered descaling composition is prepared byphysically admixing the following components in the indicated
percentages by volume.
COMPONENTS PERCENTAGE
Sulfuric acid 70
Product of Example 1 30
,:
I ~
I :- ~ .
~ ~ ,
.~
~ 13-