Note: Descriptions are shown in the official language in which they were submitted.
~0~4304
HOECHST ARTIENGESELLSCHAFT HOE 88/F 338 Dr. RH/PP
Description
New angucyclinones from Streptomycete~, a process for the
preparation thereof ~nd the u~e thereof
It is known that Streptomyces spec. synthesizeæ under
conventional culture conditions an angucyclinone with the
name ochromycinone tBowie J.H., Johnson A.N. Tetrahedron
Letters I6, 1449 (1967)~
:' `'`'
It has now been found, surprisingly, that Streptomyces
spec. DSM 4769 produces new angucyclinones.
Hence the invention relates to~
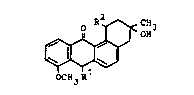
1. A compound of the formula I
~ . .,:
OCH3R
in which a) Rl and R2 are hydroxyl, ; ;~
b) Rl is an oxo group and R2 is hydroxyl or
c) Rl and R2 are an oxo group. ~.~
, ,' , " . ,',: .'
. . . -
2. A process for the preparation of the compound of the -~
formula I, which comprises cultivation of Strep-
. ~:
tomyces spec. DSM 4769 until the compound of the
formula I accumulates in the culture medium, and -
i~olation of the compound where appropriate.
3. The use of the compound of the formula I as a
sub~tance having therapeutic activity. ;`~ ~-
The invention is described in detail hereinafter,
especially in its preferred embodiments. The invention is
,-. . ~ :: . . .:
'''~,' '.'',..",''''~
:: :: . , :
'' .,'"'~"., ". .~
2U~4304 :
- 2 -
furthermore defined in the patent claims. The compound of
the formula I can be prepared with the aid of
Streptomyces spec. DSM 4769. The strain was deposited on
Aug. 26, 1988, at the Deutsche Sammlung von
Mikroorganismen und Zellkulturen (German Microorganism
and Cell Culture Collection) under the stated number in
accordance with the conditions of the Budapest Treaty.
. ,. .: ~: . . :~
Streptomyces spec. DSM 4769 has the following ~ -
characteristic features~
~ . ~
Spore color: red
Spore chain: close spirals
Spore surface: smooth
Melanin production: positive
~''
It is also possible in place of Streptomyces spec.
lS DSM 4769 to use its mutants and variants as long as they
are in fact able to prepare the compound of the formula
I. Mutants of this type can be generated in a manner
known per se by physical means, for example irradiation
such a8 with ultraviolet or X-ray6, or chemical mutagens
such as, for example, ethyl methane sulfonate (EMS), N-
methyl-N'-nitro-N-nitrosoguanidine (MNNG) or 2-hydroxy-
4-methoxybenzophenone (MOB).
Suitable and preferred as sources of carbon for the
aerobic fermentation are assimilable carbohydrates and
sugar alcohols such ns glucose, lactose or D-mannitol, as
well as carbohydrate-containing natural product such as
malt extract. Suitable and preferred nitrogen-containing
nutrients aret amino acids, peptides and proteins a8 well
a8 the degradation products thereof, such a8 peptones or
tryptones, also meat extracts, milled seeds, for example
of corn, wheat, beans, soybean or the cotton plant, dis-
tillation re~idues from the production of alcohol, meat
meals or yeast extracts, a8 well as ~mmonium salts and
nitrates. The nutrient solution can additionally contain,
for example, chlorides, carbonates, sulfates or
.. ~
.: .... :,.,~ . i .
' ''' '' '"''''
:;, ' ..'."......
200~304
phosphates of the alkali metals or alkaline earth metals,
iron, zinc and manganese as additional inorganic salts.
The production of the compound of the formula I
takes place especially well in a nutrient solution which
contains glycerol in concentrations of 0.5 to 6 ~,
preferably 2 to 4 %, as well as soybean meal in con-
centrations of 0.1 to 4 %, preferably 0.5 to 2 %, in each
case based on the weight of the total nutrient solution.
The fermentation is carried out aerobically, that is
to say, for example, submerged with shaking or stirring
in shaken flasks or fermenters, where appropriate
introducing air or oxygen. The fermentation can be
carried out in a temperature range of about 18 to 40C,
preferably at about 25 to 30-C, in particular at 28 to
30-C. The microorganism is cultivated under the stated
conditions until the stationary phase is reached, for
about 60 to 120 hours, preferably 70 to 75 hours.
The cultivation is advantageously carried out in
~everal stages, i.e. initially one or more precultures
are prepared in a liguid nutrient medium and are then
transferred into the actual production medium, the main
eulture, for example in the ratio 1 s 10 by volume. The
preculture is obtained, for example, by transferring a
~porulated mycelium into a nutrient solution and allowing
it to grow for about 48 to 72 hours. The sporulated
mycelium can be obtained by allowing the strain to grow
for ~bout 7 days on a solid or liguid nutrient medium,
for example yeast/malt agar.
The progre~s of the fermentation can be monitored by
means of the pH of the culture or of the mycelium volume,
by thin-layer chromatography or testing the biological
~ctivity.
The angucyclinones of the general formula I are
present in the culture broth. Hence it is expedient for
, '..;''~ '' ':,'~'. ..",
200~30
- 4 - :
the isolation of the substance to ~eparate the mycelium
from the culture broth, for example by filtration or
centrifugation. The compound of the formula I can then be
isolated from the supernatant, expediently in the pH
range 2 to 8, preferably at pH values from 5 to 7. The
substance can be extracted with conventional agents, for
example polar solvents, for example lower alkanols. ~ ~
However, it is advantageous to pass the liquid over an ~ ;
adsorber resin such as, for example, those based on poly-
styrene. The elution can then be carried out with a polar
solvent, preferably lower alkanols such as, for example,
methanol, which are possibly also mixed with water. The
solvent can be removed from the eluate by distillation,
and the agueous residue containing the angucyclinones can -~
be dried. ~ - ;
The compounds b and c of the formula I can be
obtained not only microbiologically but also chemically -~
from the compound Ia. For this, the compound Ia is
dissolved in solvents such as chloroform, methylene
chloride, tetrahydrofuran, ethyl acetate or (C1 to C~
alcohols and ~tirred in the air with or without addition
of air, with exclusion of light, for 1 to 20 days. The -
duration of this reaction depends on the amount of air in -
the reactlon mixture. The compound Ib is obtained a8
final product. It is possible in a subseguent reaction to
prepare the compound of the formula Ic by irradiation j
with W light (366 nm) of the resulting reaction solution ''"~'~ "~'' ~ r~
in a guartz flask for a period of 12 to 48 hours. ~ f~,
The angucyclinones of the general formula I are
colorless amorphous solids which are readily soluble in
methanol, acetone, DNS0, dioxane and chloroform but
insoluble in water and alkanes. The compounds of the
general formula I can be incorporated in pharm~ceutical
formulations appropriate for their stability. The `~
antibacterial and antiviral action and the action against ;
protozoa can be demonstrated in the agar diffusion test
or by cell culture tests in vitro. The compounds shcw a
.;
200430~
-- 5 --
particularly good action in particular against
Staphylococcu~ aureus and Staphylococcus pyogenes as well
as against adenoviruses, HSVI and HSVII viruses as well
as Trichomonas vaginalis ~protozoa). ~;~
~he invention i~ explained further in the examples
which follow. As in the foregoing description, percentage
data relate to weight.
E~amples:
1. a) Preparation of a suspension of spores of
the producer strain
100 ml of nutrient solution (4 g of yeast `~
extract, 10 g of malt extract, 4 g of glucose,
1 1 of tapwater, pH before sterilization 7.3) in
a 500 ml Erlenmeyer flask are inoculated with the
strain DSN 4769 and incubated on a rotating
shaker at 120 rpm and 27-C for 72 hours. Subse-
quently 20 ml of culture liquid sre uniformly
distributed in a 500 ml Erlenmeyer flask contain- ;~
ing the nutrient medium of the abovementioned
compo~ition to which 20 g of agar/l have been ;added for solidification, and are decanted. The
cultures are incubated at 27C for 10 to 14 days.
The spores which have resulted after this time in ~;;
a flask are rinsed out w~th 500 ml of deionized ~,,,r,
water which contains one drop of a commercially
available non-ionic surfactant (Triton X100, from
Serva), and immediately used further or stored at
-22-C. `~ -
b) Preparation of a culture or preculture of
the producer strain in an Erlenmeyer flask ~-
A 500 ml Erlenmeyer flask containing 100 ml of a
nutrient solution composed of 2 % meat meal, 10 %
malt extract, 1 % calcium carbonate and water ad
..... ...
: . . - .
:-,.
.... .
2()0~30
-- 6 --
100 % (pH 7.2 before autocla~ing) is inoculated
with a culture grown in a slant tube or with
0.2 ml of suspension of DSM 4769 gpores and i8
incubated on a shaker at 120 rpm and 27~C.
Maximum antibiotic production is reached after 72
hours. A 48-hour old submerged culture (5 %) from
the same nutrient solution suffices to inoculate
10 and 100 1 fermenters. `~
2. Preparation of the angucyclinones
A 10 1 fermenter inoculated with DSM 4769 is
operated under the following conditions~
Nutrient medium: 30 g/l glycerol
2 g/l casein peptone ~-
1 g/l R2HPO
1 g/l NaCl
0.5 g/l MgSO4 . 7H2O
5 ml/l trace element
solution
Trace elements: 3 g/l CaCl2 . 2H2O ,~
1 g/l FeC~O,H5 -
0.2 g/l MnSO~ .. ,,,.,.~.. ,,,,!~,
0.1 g/l ZnCl2
0.025 g/l CuSO~ . 5H2O
0.02 g/l Na2B4O7 lOH2O
0.004 g/l CoCl
0.01 g/l Na2MoO4 2H2O ,
Incubation times 72 hours
Incubation temperature: 30~C
Stirrer speeds 250 rpm
Aerations 4 1 of air/min.
~' . -. ::
Foam production can be ~uppressed by repeated
addition of a few drops of ethanolic polyol
solution. The production maximum is reached after
about 70 hours (pH = 5.3).
.;'~ " ' ''
... ..
- . . .
.. ~. . : .. .
2004304
3.a Isolation of the an~ucyclinones
The fermenter contents of 8 1 are filtered and
the filtrate is subsequently extracted with ;
3 x 6 1 of methylene chloride. The syrup (4 g) -
remaining after concentration in a rotary evapor-
ator is chromatographed on ~ilica gel (eluent ~ -
CHCl3:MeOH 30:1). The result i~ the compound of
the formula I ~ ;
a) with R1 -OH 330 mg
R2 -OH ~ `
b) with Rl =0 220 mg
RZ - OH ~--
c) with R1 =0 220 mg -~
R2 =o
b) Pre~aration of the compound of the formula I with
Rl =0 and R2-OH
. ~: . :.... ,.,.-
50 mg of the compound of the formula I with R1 -
OH and R2 -OH are stirred in 10 ml of methylene
chloride in the air with exclusion of iight for
24 days. The solvent iB evaporated in vacuo, and
the residue i~ chromatographed on silica gel `",",' '," !,.j.,:.,."'."
(eluent CHCl3/MeOHs 30:1). 42 mg of the desired
compound are obtained.
',,',' ':.~" `''',",''~':',',
c) preparation of the compounds b and c of the
formula Is ~ -
100 mg of the compound of the formula Ia with
R1 OH and R2 OH in CHCl3 (technical) in a quartz
fla~k are irradiated with W light (366 nm) for ; ~;
24 hours. The residue after concentration in a ~ ~-
rotary evaporator is chromatographed on silica
gel (eluent CHCl3/MeOHs30/l). 7.3 mg of the ~ 5
compound Ib with Rl ~O and R2 -OH and 53 mg of the
compound Ic with R1 =0 and R2 -O are obtained.
;:004304
-- 8 -- : :
Compound of the formula I
13C-NMR in CDCl3 / 1H-NMR 1n CDCl3
a b c
R1 -OH R1 =o R1 =o
C R2 -OH R2 -OH R2 =o ,~
1 65,7 196,7 5-4 ~-:: ::
2 44,1 53,8 2,2 - :.`-.
3 69,0 77,15
4 45,2 44,1 2,9 -.;-;
4A 136,4 146,4
135,7 133,6 7,5 . `
6 128,2 135,2 8,2 .
6A 137,2 135,0 .
7 63,3 181,1 .
7A 129,4 120,3
8 156,8 159,9
9 114,8 117,3 7,3
129,5 129,8 7,7 `~
11 120,2 119,5 7,9
11A 143,4 137,7 ~ `
12 187,5 lB4,3
12A 128,2 135,1
12B 140,0 134,3
13 29,1 29,7 1,5
14 56,0 56,4 4,05
3