Note: Descriptions are shown in the official language in which they were submitted.
2(~ 3 - ~
.
SECUR}TY MAFXTNG ' ' ; -
Ihis invention relates to security markin6 and in pareicular to the ~ -~
use of marking substances which include a pho~och~o~ic c~mPou~d. These
substances can be arranged to take a colourless ~orm when applied So
an article requiring authentication, ~or e~a~Ple a persanal
identification document. The photochromic compound can be conver~ed to
~ coloured form by irradlation ~ith W, the opcically detectable
colour change in the markin~ substance then serving to authentlCate
the document.
Security markin~ substancec as outl~ned above are known ~nd reference
is directed by way of exa~ple to W0 88/06306; JP 62227194 and
WO 88/01288.
. :., . ;~
It is known that exposure to W over a perlod of time can destroY the
photo~hromic effect ~n cert~n co~pounds. The life of a particular
~arking substance will depend UPon the flu~ of W eo which it is ; ;~
subjected ~hether durlng the authene~catlon procedure or extraneously.
The W pplled durin~ the authentication step is liable eo be a
critical factor in deter~ining the ll~e of a marking subseance only in
the case o~ ite~s r quiring very frequent authenelcation. E~traneou4
W froo sunlight will b~ an i~po~tant f~ctor in many cases ~here no
control can be exerclsed over the m~nner in which the ~rked iten is
stored.
. ~ .
It is one object of t~is inv-ntion to provide s method of ~rkin~
offerin~ increa~ed lifeti~es.
~ .' . ' . ~:~ ' ..
In applications where a relatively hidh de~ree of secusity is
required, it would b~ adv~ntageous to provide a oore demanding
~uthentic~ion step, prefer~bly without sign~ficantl~ incre~sins the
co~plexity of the authentic~tion apparstus. It is ehus a f~rther
ob~ect of the present invention eo provide an i~proved ~ethod of
markin6 oPfer~ng closor control over the e~sential require~onts of the
auehentication stcp.
, .' ,'' ~ '.-`
' ~': ,' . . ' ,
..,: : . ..
, , . . . , .. .. ~, , . , : . ~ . . , .,: . .. . .
Z~)4~3
.
.
Accordingly, the present invention con~istg in one aspec~ in a ~ethod
of ~arking an ite~ with.. a security mark ~hich exhibit~ ~ ~eversible
and opticRlly detectable ch~nge on e~posure to l;ght of a specific
wavelength, co~pr$sing the steps of applying to the art~cle a ~arking
sub~tance comprising a photochro~ic coopound convert~ble ~rom ~ne
photochro~e t~ another on exposure to light o~ said wavelength, said
co~pound ~eing covered by 8 controlled absorption layer cont~ning a
sstursble absorber at said wavelength, whereby there is defined a
threshold intensity of lieht at said wavelength bene~th wh~ch ~ :
threshold thore is ~y ~eason o~ absorption in said controlled
absorption layer no detectable chsnge In the mark within a given time
Per$od, a detectable change occuring substantially immediately at
intensities above said threshold. : :::
~ '''.
Preferably, the saturable absorber comprises a laser dye having at
s8id wavelength sn absorbtion cross section in the excited state whiCh . . . .
small compared with ehe absorbtioa cross section in the gro~nd ~tate. .
In another respeCt, the present invention consis~ in a security cark
CO~prising 8 body 0~ oarking substance Cocprising a photochromic
oo~pouna 8nd a sunlight protection lay~r covering said ~arkin~
9ubstanco, the sunli~ht protection layer comprisin~ a saturable ;:~
ab80rber.
It should be reco~nised th~t the te~m ~light~ as ~sod herein is not to
b~ rogarded a9 restrietod to v~sible li6ht.
A variety of saturab~e absorber compound5 ~re known. They have the
property that below a ehr~shola lighe Snt~nsity, ~n equilibrium is -~
e~tablished betw~ n moleculgr processes of photon absorptioa and
relaxation, such Shat th~ transmissivity at the specific wavelen~th of ;~
operat~on is very low. Above the threshold Sntensity, the photon
~bsorption process ~s saturated. with 11 absorber ~olecule~ being : .
held ~n the h~eher en~rgy st~te. Above this threçhold inten5itY.
therefore, the substance has gi~n$~C4nt t~an4~S5ivitY at the
specif~c wa~elensth.
- . .
2~ 4~3
~ ~ .
Saturable absorbers are known `for use ~s h~gh speed swit~he~ in l~ers i~
~nd reference ~s directed in tA~s re~ard to ~Lasers - Lipe ~3pl~fiers
and oscill~tors~ - D~eter ~oss - 1969 Acade~lc Press. . :~
}n the practice o~ the present invention, lt can be arranged, by
appropriate selection o~ a saturable absorber and the eoncentration
and thickness of the controlled sbsorPtion laye~, that ehe - .
transmissivity o~ the ~ayer to W at norn~ ight inten~ieie~ i9 -~
very low but ehat at a UV intensity achievable - ulth careful design -
in authentication apparstw , the t~nsoissivity is high. ; ~ ..
The invention will ~ow be desc~lbed by w~y of exa~ple with reference
to the accompanyin~ drawings in which~
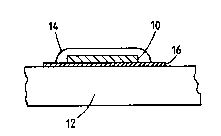
Flgure 1 i~ a di~gra~tlc section throu8h a secu~ty mark in
accordance with this invention, and
.
Flgure 2 ls a diagra~atic sect~on thrvugh an alternative security nark
accord~ng to thls inv~ntio~
Ref~rrlng to F16ur~ 1. a ~arklng subst~nce 10 is applied to a
substrate 12 by printin~ or any other suitable ~pplication technique. ;
The marking ~ubstance includes a photochromic coopound which is
pr ferably a ~ulgide but whlch could tak~ other forms such a~
splropyran, Thc detailed 5tructure o~ the photodhrvmic compound ~orm$
no part of the preseQt invention ~nd it will suf~ice to ~ake re~renc~
to the known ~re, ex4sples Or wh~ch a~ GB-A-1 464 603, GB-A-2 002 752 .
~nd GB-A-Z 051 813,
The photochromic compound is incorporated ln the oarking ~ubstance by
any of a ~ariety o~ appropr~te technigue~. For e~ample, the
photochrom~c co3pound may be dissol~e~ or disperset in a solution of
polymer~c material which is cast, eoated or otherwise ~pplied to the -~
substrate. For exa~ple, a photochromic fulg~de can ~e dissol~ed in a -~
solutlon o~ cell~lo~e ~cotate ~n acetone wi~h a fil~ Or the ~arking
~';'; ' ',''''''"''.,'.,''','`'''''',
: :' ~' :,. . ,.'-~. ::
~ -. ,:, :- . .
.: . .: ., : . , ~ i .
: : : ~ : ::
Z1~4~3 ~ ~ ~
substance being fo~med upon evaporation of scetone; Alter*stive
polymers are polyeste~s e.g. polyethylene terePhthPlate; acrylates,
e.g. PMMA; ole~in poly~ers or co-poly~Qrs: vinyl polymers. e.t. P~A or
PV~; polycarbonates and polyamides. Alternative organic sol~ents are
toluene And chlorinsted hydroC~rbons.
In the illustrated example, ~he mark~ng substance 10 co~prises a 2.5%
concentratton of the photochromic Fulgide Aberchrome 540 (a~ailable
fro~ Aberchro~ics Li~ited, ¢ard~ff) in PMMA cast from a solution ~n
ethyl acetaeo. The for~ed security ~ark h~s an area of 1 sq C3 and the ~`
thickness o~ the ~arking substance is 5 uicrons. ;~
There is provided over the marking substance 10 a controlled
absorption layer l4. This eo~prises a 10% concentration o~ a saturable
absorber in an sppropriate ~atrlx s~ch as polyvinyl alcohol. In th$s
exa~ple the saturabl~ absorber oolecule is triazinyl stilbene. For
detailed in~ormat$on concerning the proproperties of this known
satur~ble absorbe~. reference io dir~cted to textbooks such as ~Laser
Dy~s ~ Properties o~ oryanic co~pounds for dye lasers~ - Mitsuo M~eda
1987 AcQdenic Press, ospoc~ally Sable 20. The thickness of the
controllod absorption layer i~ 5 microns and it is calc~lat~d th~t ~t
noroal sunlight intensities of UV, the controll d absorption layer ~ ~ -
will dec~e~se th~ x of W incident upon the na~kiing substance by
f~ctor o~ 1~2. ;;~
In tho ~uthentication step, the security nark i~ irradiat~d with high ~ ;
intensity W so as to convert the photoch~o~ic co pound to a coloured ~ n
~or~. ~hi~ chi~n6ing colour nay be observed visually. The intensity o~
UV e~ployod in the activation ~tep is in ~xcess of thc ~hreshold ~t
which the 8aturable absorber i~iisaturate~. mug at an inten5ity of ;~
15 kw suitably focussed, the cont~olled absorption layer has ~
trans~iss~vity of ~pproxi~ate~y 80%. me high intensity UV may be
prcvlded. for examplc, by a rlashlR~p with appropr~ate focusing, or
other hieh intens$ty puls~d llght sourCQS.
: .: :
~.
.
2(~4a~3
. ~,, ,
~he use of a saturable absorber ~n thë ma~ner accord~ng to thls ~ -~
invention has the adva~tage that the liPeti~e o~ the secur~tY mark on
exposu~e to sunlight is substantially increased. ~owever, the -
controlled absorption layer has little effect upon visible - for
example, green or red - lig~t so that the photochro~c compound can be
ret~rned rspidly to i S colourless for~ by irradiatlon at the
appropriate w~velen~th. This can be done by exposure to sunllght ~r ~
another white li~ht Qource~ S~nce the trans~i~s~vitY of the controlled - ~ d
absorption layer ~s relatively high above the threshold intensity, the . ; :
ti~e taken to colour the security mark in the actlvation step is not
substantially increased.
In a modification, illustrated in Fi~ure 2, 8 base coat 16 is
interposed between the markin~ substance 10 and the substrate 12. This
bsse coat ~y t~pic~lly co~prlse a one to two micron layer of
polyvinyl chloride which se~ves to enhance optical contrast and to
protect the photochromic compound from poss~bly ds~sgin~ effeces of
the substrate. The ~se of suc~ a base coat would, for exa~ple. be
$mportant in the csse of paper or other fibre based substrates h8ving
relatively high moisture level~ and containing fillers and other
reaCt~nts having a pos~ibly doleterious ef~ect upon the photochro~ic
conpound.
It should be under~tood that th$s invention has been described by way `~
of exa~ple only and a var~ety of modi~ications ~re poss~ble without
depart~ng fro~ the scopa o~ the inv~nt~on.
Other s~turable 8bsorbers cgn be eoploy~d and the~e can be provided in .
a controlled absorption layer in a variety of tochniques. ~ne exa~ple
o~ an alternative saturablo absorber ~olecule useful with fulgide
photochroucs ~s 1,4-~is (o-methylstyryl) benzene. Th1s c~n be for3ed ~,~
~-: :. ,.: . . :: . :
as a sCr~en ink using a hydrocarbon sol~ent.
.: ;,, ~
.. .. . ......
':' ~,: ,:;;
' :...: "
. . . :
- :-'
~ : ~
2(~4~3
., ^
The wavelength band of 360 to 390mm is appropriate for a wide range of ~:
photochromic fulgides. Beyond ehe speci~ic saturable sb~orbers
ment~oned, it will be posgible to employ a ran~e of lasor dyes
absorbiny in the wa~elength range o~ interest and having ~n absorPtion
cross section in the excited seate which is s~ll coopared to th~t mn
the ~round s~a~e and thu~ bein~ capable of saturation. Reference is
dir~ct~d in this r~rd to M~4d~ f~f~ ~n~ tn ~n~hnnk nf :
fl~ore~cent spectra of aromstic molecules~ Isador Berl~an - 19
Academic iress. ;
It is not necessary for the entire secur~ty mark to be co~ered b~ a
single, contiguous controll~d absorption layer; separate block~ of the
~arking substance may be covered by respecei~e controlled absorption
layers. The marking subst~nce need not be a poly~eric film it cay
t8ke the form of. for exa~Ple, a paint or ink cont~inin~ a
photochromic compo~nd or may in apProPriate cases consist of a
photochromic compound alone.
~ :