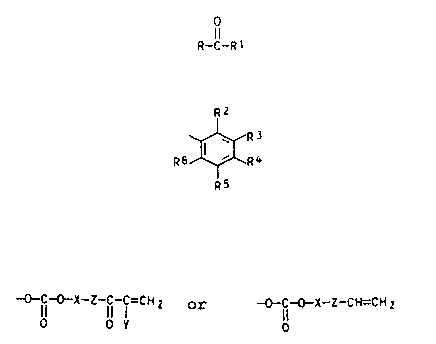Note: Descriptions are shown in the official language in which they were submitted.
2UU~~~
O.Z. 0050/40443
Radiation-sensitive, ethylenically unsaturated,
copolvmerizabie compounds and their preparation
The present invention relates to novel radiation
sensitive, ethylenically unsaturated phenone derivatives
and a process for their preparation.
W-sensitive aceto- and benzophenones are fre-
quently added as external initiators to radiation-
sensitive polymers (eg. G. Li Bassi, J. Rad. Cur. _14
(1987), 18). In general, however, such procedures are
not completely satisfactory since, after the initiator
has been mixed with the polymer, problems with the com-
patibility, the uniformity of the distributiOrc, the
volatility, the odor, the toxicity, the exudation and the
migration of the additive are encountered, frequently
leading to an undesirable, premature and non-uniform
reaction. In the actual exposure process, lower reac-
tivity is then observed owing to lower effective
initiator concentrations, and a number of troublesome
secondary reactions are observed after exposure.
It is known that some of the stated problems can
be solved if the radiation-sensitive initiator is copoly-
merized with monomers by a conventional process, ie. is
incorporated in a polymer chain. The photosensitive in-
itiator is linked t~ the base polymer by means of an
anchor group, ie. the spacer. The spacer also serves to
reduce the influence of the base polymer chain on the
photochemical behavior of the initiator.
In principle, therefore; copolymerizable in-
itiators have the following structure:
z
Initiator Spacer Reactive
double bond
Scheme I
U.S. Patents 3,214,492, 3,429,852, 3,622,848 and
4,304,895 describe acryloxy- or methacryloxy-substituted
aceto- and benzophenone derivatives, for example
.. 2005283
- 2 - O.Z. 0050/40443
0
I . I
' ' o
These can be copolymerized with ethylene or other vinyl
monomers to give polymers which can be cured by irradia-
tion, for example after thermal forming. In the model
according to Scheme I, the reactive double bond and the
initiator are separated by the carbonyloxy group as a
spacer in the case of these radiation-sensitive monomers.
From the photochemical point of view, these compounds are
of less interest since the acryloyl substituent st311 has
a very pronounced influence on the absorption of the in-
itiator fragment at long wavelengths.
A similar situation is also observed in the case
of the copolymerizable acetophenone acrylates described
in DE-A-35 34 645, o CHj
I
i-CH3
' 0
0
in which the photoreactivity is reduced by virtue of the
fact that the reactive acrylate group is too closely
coupled to the carbon atom a to the carbonyl group. The
reason for this behavior is that, during the
photochemically initiated a cleavage, a further, not very
reactive radical is formed in addition to the benzoyl
radical.
To avoid substantially reducing the photochemical
reactivity of the copolymerizable initiator compared with
the parent substance, the reactive double bond must be
decoupled mesomerically and inductively from the photo
initiator moiety.
In 2-acryloylthioxanthone (Eur. Polym. J. 2~
(1987), 985)
0
. I s I . o
2005283
- 3 - O.Z. 0050/40443
the separation has not yet been completely achieved. The
copolymer with methyl methacrylate is less reactive than
2-hydroxythioxanthone.
In 4-(4'-vinylbenzyloxy)-benzophenone,
0
I
I
described in DE-A-28 18 763, the styrylbenzyloxy radical
proves to be a good spacer.
In Uvecryl' P36, a commercial product from UCB, a
particularly long spacer consisting of four ethyleneoxy
units separates the benzophenone from the acryloxy
radical.
0
I ~ I
0
This compound, which is described in, for ex-
ample, Technical Bulletin 2480/885 (1985) of UCB or in
New Polym. Mat. 1 (19 87), 63, can be used in photopoly-
mers for coating materials. The synthesis is expensive,
and this product has only moderate photochemical reac
tivity since the spacer is too long.
The introduction of functional groups into the
spacer does not significantly influence the photochemical
s
behavior of the chromophore if they are separated from
one another by an alkyleneoxy group. The benzophenone
derivatives of the type
0
I . I . N_ xo
I
OH
mentioned in EP-A-279 475 are examples of this.
2005283
- 4 - O.Z. 0050/40443
According to the abovementioned prior art, an
ether or ester group should therefore form part of the
spacer (Scheme II).
Initiator ~0~ ; ~0~ Reactive
Io' double bond
Scheme II
A novel spacer scheme is obtained by considering
the influence and the function of the spacer as a sub-
stituent on the photochemically excited aceto- or benzo-
phenone fragment. For example, possible spacers are
those which, owing to their structure, can have a stabil-
izing or destabilizing effect.
In particular, the carbamoyl-substituted benzo-
phenones of the type
0
o
' ~N R
Scheme III
constitute an interegting class of substances from this
point of view. U.S. Patent 3,322,818 describes allyl- or
methallyl-substituted carbamoylbenzophenones. However,
they are only suitable as fungicides (cf. Scheme IV).
r Carbamoyl group
= 0
o
' ' NH-CNZ H-CHI
Initiator Spacer Double bond
Scheme IV
An allyl or methallyl group is unsuitable for
2005283
- 5 - O.Z. 0050/40443
copolymerizations. These benzoylphenyl allyl carbamates
are therefore of no practical importance in the polymer
sector.
Novel but photochemically less reactive monomers
having an extremely long spacer are described in British
Patent 2,100,722.
Finally, DE-A-3 820 463 claims monomers of the
type (Scheme V)
0 0
~
Initiator NH-
Scheme V
which are particularly reactive and conveniently obtain-
able from hydroxyaromatics and (meth)acryloylalkyl iso-
cyanates. The necessary isocyanates, for example
~O~NCO ( I EM ) or
I I0
~O~O~NCO (IOPM)
0
are, however, very toxic and expensive to prepare.
It is an object of the present invention to pro
vide radiation-sensitive, ethylenically unsaturated
phenone derivatives, for example acetophenone, benzo
phenone or thioxanthone derivatives, which are readily
obtainable and contain a spacer optimally tailored to the
phatoinitiator moiety.
A number of aceto- and benzophenone carbonates
are known for various obligations.
In the total synthesis of griseofulvin (A.C. Day
et al., J. Chem. Soc. 1 61, 4067), the compound
0
H3C0 OCHj
0~~
H jC ~ I H H;C ~ I ~CH;
CI
2005283
- 6 - O.Z. 0050/40443
is used as an intermediate. Further examples of
pharmacologically interesting carbonates of the above
mentioned type are described in J. Chem. Soc. C 1969,
1721; ibid 1970, 392; Tetrahedron Lett. 1979, 4363, and
Dutch Patent 7,008,636.
According to Japanese Preliminary Published
Applications 54-002323 and 57-181001, the halogenated
benzophenone carbonates
Cl 0
C1
0
ct ~ I ~ I ~o-Atkyt
ci
act as herbicides.
If, on the other hand, at least one OH group is
introduced ortho to C=0 in the parent benzophenone
structure, a substance belonging to the UV stabilizer
class is obtained. As is evident in the Example below,
chromophores
0 OH
0
I ~ ~_~ N ~ I
0
(cf. U.S. Patents 3,981,822, 4,115,348 and 4,174,321) or
a further structural element having UV-absorbing proper
ties, as described in USSR Patents 352,883 and 491,661 or
Japanese Preliminary Published Applications 58-159460 and
61-130 362, are frequently attached to the benzophenone
via the carbonate group.
Finally, the noncopolymerizable benzil dimethyl
ketals
0 OR
I
C-OR
0I
Alky l
2005283
- 7 - O.Z. 0050/40443
and the benzoin monomethyl ethers
0 OR
C-H
0I
Alkyl-0~0
mentioned in Japanese Preliminary Published Application
61-228007 are claimed for photopolymerization.
- Thus, only alkyl- and aryl-substituted aceto- and
benzophenone carbonates which do not contain a functional
group which permits copolymerization have been disclosed
to date.
It is an object of the present invention to pro-
vide copolymerizable phenone derivatives of the type
0
R
0 0
Scheme VI
We have found that this object is achieved by
ethylenically unsaturated copolymerizable, radiation-
sensitive organic compounds of the general formula (I)
0
R_~~_R 1 - ( I )
where
R is straight-chain alkyl of 1 to 4 carbon atoms, prefer-
ably methyl, ethyl or n-propyl, branched, unsubstituted
or substituted alkyl of 3 or 4 carbon atoms, such as
isopropyl,sec-hydroxyisopropyl,sec-dimethylaminopropyl,
sec-morpholinopropyl or tert-butyl, aryl, eg. phenyl,
tolyl or naphthyl, or a radical R1, and
R1 is a radical
R2
R3
R6 ~ ~ R4
RS
.. 2005283
- O.Z. 0050/40443
where R2 to Re are identical or different and are each H,
alkyl of 1 to 4 carbon atoms, eg. methyl, ethyl, n-
propyl, isopropyl or tert-butyl, phenyl, OH, OCH3, OCZHS,
SH, SCH3, SCZHS, F, C1, Br, CN, COOH, COOAlkyl where alkyl
is 1 to 17 carbon atoms, COOAryl, CF3, N(Alkyl)z,
N ( Alkyl ) ( Aryl ) , N ( Aryl ) z, N~ ( Alkyl ) 3Ae, N~H ( Alkyl ) zAe where
alkyl is of 1 to 4 carbon atoms and Ae is the anion of an
acid, eg. Cle, S042e, P043e, acetates, BFde, CF3S03 , SbFfi ,
AsFfi or PFfi, and at least one but not more than three of
the radicals Rz to R6 is a radical
-o- ~-o-x-z-II j-cH z or -o- j"'o x z-~N cH z
0 ov o
where X is a divalent, unsubstituted or substituted
alkylene radical -(CHz)m-, a radical
R
I
-C-
R~~ m
where m is from 1 to 10 and
R' and R " are identical or different and are
each aryl, eg. phenyl, C1-C4-alkyl, H, COOH, COOCH3 or
COOC2H5, a perfluorinated alkylene radical (-CFz)m where
m is 1 to 10, preferably a perfluoroethylene radical, an
oxaalkylene radical Qf the type - ( CHz ) n-O- ( CHz ) p- where n
and p are each from 1 to 5, preferably n and p are each
2, ie. -CZH~-O-CZH,-, a perfluorinated oxaalkylene radical
of the type - (CFz ) ~,-O- ( CFz ) p- where_ n and p are each 1 to
5, for example tetrafluoroethylene, or a polyoxaalkylene
radical which may be perfluorinated and has 2 to 20
s
oxygen atoms which are bonded to one another via at least
one -CHz-, -CFz- or -CHz-CH ( CH3 ) - group, or are each an
alkylene radical of the type - ( CHz ) m O-CO-O- ( CHz ) n-. -
( CHz ) n-O-CO-NH- ( CHz ) m- ~
- ( CHz ) n-~-CO-O- ( CHz ) m i - ( CHz ) m-CO-O- ( CHz ) n- Or - ( CHz ) m 0
3 0 CO- ( CHz ) n- where m and n are each from 1 to 10 , a
phenylene radical which is unsubstituted or substituted
by alkyl of 1 to 4 carbon atoms, eg. methyl, ethyl, n
X005283
9
propyl or isopropyl, OH, OCH3, OC2H5, SH, SCH3, SC2H5, C1, F,
N(Alkyl)2 or N(CH3)C6H5 in the o-, m- and/or p-position, or a
cycloalkylene radical of 5 to 10 carbon atoms, eg.
cyclohexylene or cyclooctylene, or a (bis)methylene-
cycloalkylene radical of 6 to 12 carbon atoms,
Y is H, alkyl of 1 to 6 carbon atoms or phenyl,
Z is 0 or NY
or, if R is aryl, one of the radicals R2 or R6 may be a
sulfur atom by means of which the aryl radical R is bonded in
the ortho-position with R1 and gives, for example, a
thioxanthonyl radical.
The present invention also proposes an ethylenically
unsaturated copolymerizable, radiation-sensitive organic
compound of the formula (I)
O
R-C-R1
where
R is aryl or a radical R1 and
R1 is a radical
R~
R R~
Rs
where
R2 and R6 are identical or different and each of the
radicals can be H or one of the radicals
X005283
9a
-0-C-0-X-Z-C-C=CH2 or
to Y
-O-;I-0-X-Z-CH=CH2 ,
0
R3 to R5 are identical or different and each is H, alkyl of 1
to 4 carbon atoms, phenyl, OCH3, OC2H5, F, C1, Br, CN,
COOAlkyl where alkyl is 1 to 17 carbon atoms, COOAryl, CF3,
N (Alkyl) 2, N (Alkyl) - (Aryl) , N (Aryl) 2, I~(Alkyl) 3A0 or
N~H(Alkyl)2 A~ where alkyl is of 1 to 4 carbon atoms, and A~
is the anion of an acid, and one of the radicals
-0-C-O-X-Z-C-C=CH2 or
[o to r
-0-il-0-X-Z-CH=CH2
0
where at least one of the radicals R2 to R6 is a radical
-O-C-0-X-Z-C-C=CH2 or
il [l I
0 0 Y
-0-li-0-X-Z-CH=CH2
O
where
A ~~
r
X005283
9b
X is a divalent, unsubstituted or substituted alkylene
radical -CH2)m or
R'
-C-
R"
r . m
where m is from 1 to 10 and
R' and R" are identical or different and each is H or alkyl,
an oxaalkylene radical -(CH2)n-0-(CH2)p- where n and p are
each from 1 to 5, or a polyoxaalkylene radical having 2 to 20
oxygen atoms which are bonded to one another via at least one
-CH2- or -CH2- CH(CH3)-group, or a radical -(CH2)m-0-CO-0
(CH2)n-, -(CH2)n-NH-CO-0-(CH2)~ , -(CH2)m-CO-0-(CH2)n or
- (CH2 ) m 0-CO- (CH2 ) n- where m and n are each from 1 to 10, an
unsubstituted or substituted cycloalkylene radical of 5 to 10
carbon atoms, a (bis)methylenecycloalkylene radical of 6 to
12 carbon atoms or an unsubstituted or substituted o-, m- or
p-phenylene radical,
Y is H, alkyl of 1 to 6 carbon atoms or phenyl and
Z is 0.
Surprisingly, the novel compounds have particularly high
photochemical reactivity in the short wavelength to
relatively long wavelength W range from 254 to 400 nm and a
good shelf like.
It is a further object of the present invention to
provide a process for the preparation of novel radiation-
sensitive acetophenone, benzophenone and thioxanthone
carbonates containing at least one terminal acrylate or
methacrylate group.
.A
~~ ~p0 5283
9C
The synthesis of aryl carbonates without a
copolymerizable terminal group is known (Japanese Preliminary
Published Applications 59-001 438 and 59-170 033). A good
overview is given in: a) Houben-Weyl, Methoden der Organische
Chemie, Vol 8, pages 75, 101-107, Thieme-Verlag 1952, b)
Kirk-Othmer, Encyclopedia of Chemical Technology, Vol. 4,
pages 758-771, John Wiley 1978, and c) Ullmann's Encyclopedia
of Industrial Chemistry, Vol. A5, pages 197-202, Verlag
Chemie 1986.
The most important preparation process for carbonates is
the reaction of carbonic ester chlorides with alcohols.
Theprocedure is described in detail in Houben-Weyl, Vol. 8
(see above), German Patent 1,080,546 and J. Org. Chem. 26
(1961), 5119. The carbonic esters are formed in good to very
good yields if the alcohol and the chlorocarbonic esters are
reacted with one another in
t .. ,.
L
200528
- 10 - O.Z. 0050/40443
a molar ratio of 1 . 1 in the absence of a solvent or in
excess alcohol as a solvent. Where the alcohol or the
phenol and/or the chlorocarbonic ester are present as
solids, aprotic solvents, eg. dichloromethane, dichloro-
ethane, acetonitrile, toluene, xylene, etc. are used.
There are in principle two routes for the syn-
thesis of the compounds stated in claim 1 (Scheme VII):
-- 2005283
- 11 - O.Z. 0050/40443
a~
z of
x
i
I O~ U
O ~O
i
i
O O
O
\_/
Z
O
Z
\ / N
+ N
O
N
O
v
\ /
\ U
N
2005283
- 12 - O.Z. 0050/40443
The hydroxyacetophenones and hydroxybenzophenones
required as starting materials can be prepared by known
processes. For example, 4-hydroxybenzophenone is ob-
tained in a yield of about 90% by Friedel-Crafts acyla-
tion of phenol with benzoyl chloride in nitrobenzene in
the presence of A1C13 or TiCl4 (Houben-Weyl 7/2a, page
186) or in the form of a pure isomer by oxidation of 4-
hydroxydiphenylmethane with 5,6-dichloro-2,3-dicyano-p-
benzoquinone (Houben-Weyl 7/2a, page 681).
- The syntheses of the amino-substituted benzo-
phenones, eg. 2-benzyl-2-(dimethylamino)-1-(4-hydroxy-
phenyl)-butan-1-one or 1-(4-hydroxyphenyl)-2-mE~~yl-2-
morpholinopropan-1-one, are described in EP-A-284 561 and
EP-A-117 233.
2-Hydroxythioxanthone can be prepared from thio-
salicylic acid and phenol by the process described in
British Patents 2,108,487 (1981) and 2,108,979 (1982).
The aromatic chloroformates (cf. J. Prakt. Chem.
(1971), 331, ibid 17 (1975), 62, 73 and 81) of the
general formula (IIIb) can be prepared in good yields
from a substituted phenol, -eg. 4-chloro-5'-fluoro-2'-
hydroxybenzophenone, 4-chloro-4'-hydroxybenzophenone,
2,4-dihydroxybenzophenone,4,4'-dihydroxybenzophenone,4-
fluoro-4'-hydroxybenzophenone, 2-hydroxybenzophenone, 4-
hydroxybenzophenone, 2-hydroxy-4-methoxybenzophenone,
2,3,4-trihydroxybenzophenone, 2-hydroxythioxanthone, 3-
hydroxythioxanthone or 4-hydroxyphenyl 2-hydroxyprop-2-
yl ketone (German Laid-Open Application DOS 3,534,645),
by phosgenation with phosgene by standard processes known
from the literature, cf. for example Houben-Weyl, Method-
en der Organischen Chemie, Vol. 8, Thieme-Verlag 1952,
trichloromethyl chloroformate (diphosgene), J. Prakt.
Chem. ~ (1930), 210, ibid ~ (1930), 233, Chem. Abstr.
81766, J. Org. Chem. ,~Q (1985), 715, J. Org. Chem. 41
(1976), 2070, Angew. Chem. ~ (197?), 267, crystalline
triphosgene, Angew. Chem. ~ (1987), 922, N,N'-carbonyl
diimidazole or N,N'-carbonyldi-s-triazole (Fieser _1
. ~. 200283
- 13 - O.Z. 0050/40443
{1967), 116).
Merck Kontakte 1 1 (1), 14-18, gives information
about the use of alternative methods of phosgenation, for
example reaction with chlorocarbonic esters.
The hydroxyalkylene (meth)acrylates and hydroxy-
alkylene {meth)acrylamides are formed by acylation or
esterification of suitable a, ~-alkanediols or amino-
alcohols, for example 1,2-ethanediol, 1,3-propanediol,
1,2-butanediol, 1,4-butanediol, 1,5-pentanediol, 1,6-
hexanediol, 1,4-cyclohexanediol, 1,2-cyclohexanediol,
ethanolamine, p-hydraxyaniline or diols such as poly-
tetrahydrofuran, polyethylene oxide, or polypr~rylene
oxide, with acyl chlorides, esters or anhydrides of
acrylic and methacrylic acid. The transesterification
reaction is of particular interest. For example, methyl
acrylate, ethyl acrylate, n-butyl acrylate, methyl meth-
acrylate, ethyl methacrylate, propyl methacrylate,~n-
butyl methacrylate and tert-butyl methacrylate are used
for transesterification. Methyl methacrylate is of par-
ticular interest. When the process is carried out in
practice, the acrylate or methacrylate can be used in
less than the stoichiometric amount or, preferably, in
excess. In general, from 1 to 1.5 times the molar
amount, based on the diol, of the acrylate or meth-
acrylate are used; an excess of from 1.5 to 5 times the
amount should be avoided in particular in order to keep
the amount of diacrylate to a minimum. In addition, the
reaction mixture may contain solvents, eg. benzene, tolu-
ene, xylene, chlorobenzene, dioxane, cyclohexane, n-
heptane, n-octane, n-nonane or n-decane. The reaction is
preferably carried out in the absence of solvents.
In general, a catalyst conventionally employed
for transesterifications is used in the transesterifica-
tion stage. Examples of suitable catalysts are alkali
metal alcoholates, such as sodium methylate, ethylate or
propylate, lithium methylate and, preferably, compounds
of titanium, tin and zirconium. Examples are titanium
2005283
- 14 - O.Z. 0050/40443
tetramethylate, tetraethylate, tetrapropylate and tetra-
butylate, dibutyltin oxide, dibutyltin dilaurate, di-
methoxydibutyltin and zirconium pentane-2,4-dionate.
The amounts of catalyst are frequently from
0.0005 to 0.5, preferably from 0.001 to 0.02, mole of
catalyst per mole of diol. The transesterification is
generally carried out at from 50 to 150°C, preferably from
80 to 120°C, with boiling of the reaction mixture, if
necessary the alkanol liberated during the
transesterification being distilled off from the reaction
mixture together with some of the acrylate or
methacrylate, as an azeotropic mixture.
After the transesterification reaction has been
carried out, the excess acrylates or methacrylates and
any solvent can be separated off from the reaction mix
ture, for example, by distillation, preferably under
reduced pressure. If desired, the monohydroxyalkylene
(meth)acrylate obtained can be purified, for example by
distillation under reduced pressure, by extraction or by
crystallization. However, it is also possible for the
reaction product of the transesterification stage to be
fed to the next stage of the process without further
purification or, if necessary, after removal of the
catalyst or of its salt by hydrolysis and filtration.
In carrying out the acylation with (meth)acrylic
anhydride, the educts are preferably used in stoichio-
metric amounts. The reaction is carried out in general
at from 80 to 140°C, preferably from 90 to 110°C. Par-
ticularly suitable catalysts are acids, in particular
conccentrated sulfuric acid, which are used in amounts of
from 0.1 to 2 mol %. After the catalyst and (meth)-
acrylic acid have been separated off, for example by
neutralization with an aqueous base, eg. sodium carbonate
solution or dilute sodium hydroxide solution, the crude
product can be used in the next stage without further
purification, if necessary after drying, for example with
sodium sulfate or magnesium sulfate.
. M 2005283
- 15 - O.Z. 0050/40443
To prevent premature polymerization of the a,8-
monoolefinically unsaturated reactants, a conventional
stabilizer is preferably added to the reaction mixture.
Examples of suitable stabilizers are hydroquinone, hydro-
quinone monomethyl ether, 2,6-di-tert-butyl-4-methyl-
phenol, para-nitrosophenol and/or phenothiazine. Fur-
thermore, it has proven extremely advantageous to pass
oxygen or air through the reaction mixture during the
acylation.
The hydroxyalkyl or hydroxyalkylene monovinyl
ether are obtainable in good yields by the process des-
cribed in Liebigs Ann. Chem. 601 (1956), 81.
The following (meth)acrylates, (meth)acrylamides
and hydroxyalkyl vinyl ethers which can be used according
to the invention may be mentioned by way of example:
~NHw/~OH
I I0
~NH~OH
I I0
iH;
~N~OH
I I0
iH3
~N~OH
I I0
~Ow/~OH
'I0
~O~OH
I I0
~Ow/~/OH
I I0
2005283
- 16 - O.Z. 0050/40443
~O~OH
I I0
~O~OH
I I0
~O~OH
I I0
0
~Ow/~0 H
I I0
0
~O~O~O~pH
I I0
0
O~NH~OH
0
~NHJCpH
I I0
~NH ~ - ~ H
I'0
II N i_v H
'' 0
0-O-OH
0
p-( }-OH
0
~0-C H 2-~-C H 2-OH
I I0
2005283
- 17 - O.Z. 0050/40443
~0-CHZ-~CHZ-OH
I I0
OH
0
0
off cf. also CA 86, 107063a
o (Deposited Doc. 1974,
o VINITI 2066-74)
~N-~-OH
0
~O~OH
~O~O~OH
~0-~--OH
~0-CHZ-~-CHZ-OH
For the reaction of the hydroxyacetophenones,
hydroxybenzophenones or hydroxythioxanthones, the corres
ponding ~-(meth)acryloyloxyalkyl chloroformates are
generally required. These can be prepared conveniently
and in good yields by processes known from the litera
ture, as described in , for example, Eur. Polym. J. 14
a
(1978), 205; J. Polym. Sci. Polym. Symp. ,~~ (1979), 41;
Bull. Soc. Chim. Belg. ~ (1984), 159.
Surprisingly, we have found that the novel aceto-
phenone, benzophenone and thioxanthone derivatives are
obtainable readily and in very good yield by the routes
A and B shown in Scheme VII. This is unexpected
particularly in view of the reactivity and
bifunctionality of the (meth)acrylate or vinyl ether
2005283
- 18 - O.Z. 0050/40443
component, since many different reaction products are
possible. Routes A and B differ in particular from the
variant described in DE-A-3 820 463, in which isocyanates
which are toxic and dif f icult to obtain are used under
neutral conditions.
Routes A and B are more economical, more effi-
cient and, because of the bifunctionality of the starting
materials, novel compared with the prior art (cf. Houben-
Weyl, Vol. 8, pages 75, 101-107).
The present invention furthermore relates to a
process for the preparation of compounds of the general
formula (I), wherein a compound of the formula (IIa) or
(IIb)
I I
I I
B~C~O-x-Z-il-i=CHZ or B~C~O-X-Z-CH=CHz
0 Y
(IIa) (IIb)
where
X, Y and Z have the abovementioned meanings and
B is one of the groups tosylate, alkoxy of 1 to 5 carbon
atoms, halogen, eg. C1 or Br, chlorocarbonyl, imidazolyl,
pyrazolyl or an ammonium, pyridinium, phosphonium or
sulfonium cation, preferably, for example, 2-
(acryloyloxy)-ethyl _ or 2-(methacryloyloxy)-ethyl
chlorocarbonate, 2-(methacryloyloxy)-ethyl
chloroglyoxylate or 2-(meth)acryloyloxyethyl methyl
carbonate, is reacted with a compound of the general
formula (IIIa)
0 R8
R~-CI I ~ R9 ( rIIa)
R12 ~ R10
R11
where
R' is straight-chain alkyl of 1 to 4 carbon atoms,
branched, unsubstituted or substituted alkyl of 3 or 4
carbon atoms, aryl or a radical R1 and
2005283
- 19 - O.Z. 0050/40443
Re to R12 are identical or different and are each H, alkyl
of 1 to 4 carbon atoms, phenyl, OH, OCH3, OCZHS, SH, SCH3,
SC2H5, F, C1, Br, CN, COOH, COOAlkyl where alkyl is of 1
to 17 carbon atoms, COOAryl, CF3, N(Alkyl)2, N(Alkyl)-
( Aryl ) , N ( Aryl ) 2, N~ ( Alkyl ) 3Ae or N~Fi ( Alkyl ) zAe where alkyl
is of 1 to 4 carbon atoms, and Ae is the anion of an acid,
eg. Cle S0,''~, PO,~, acetates, BF; , CF3S03 , AsFs , SbFs , PFs
etc . , or one of the radicals R8 or R12 is a sulfur atom
by means of which the aryl radical is bonded in the
ortho-position with R', with the proviso that at least one
of the radicals RB to R12 is hydroxyl, in an equimolar
ratio (if necessary with up to 20% excess) or, depending
on the number of hydroxyl groups in the radicals R° to
R12, in two or three times the equimolar ratio, in the
presence or absence of an inert solvent or solvent mix-
ture and of a basic catalyst, at from 0 to 100°C under
anhydrous conditions (route B).
The present invention furthermore relates to a
process for the preparation of compounds of the general
formula (I), wherein a compound of the formula (IVa) or
(IVb)
H0-X-Z-C-C=CH2 Or HO-X-Z-CH=CH2
0 Y
(IVs) _ (IVb)
where
X, Y and Z have the meanings stated in claim l, prefer-
ably the (meth)acrylates, methacrylamides or hydroxyalkyl
vinyl ethers stated above by way of example, is reacted
with a compound of the general formula (IIIb)
.z
0 R8
R~-C I ~ R9 ( IIIb)
R12 ~ R10
R11
where
R' is straight-chain alkyl of 1 to 4 carbon atoms, pref-
erably methyl, ethyl or n-propyl, branched, unsubstituted
200523
- 20 - O.Z. 0050/40443
or substituted alkyl of 3 or 4 carbon atoms, such as iso-
propyl, hydroxyisopropyl, dimethylaminopropyl,
morpholinopropyl or tert-butyl, or aryl, eg. phenyl,
tolyl or naphthyl, preferably phenyl, and
Re to R12 are identical or different and are each H, alkyl
of 1 to 4 carbon atoms, eg. methyl, ethyl, n-propyl, iso-
propyl or tert-butyl, phenyl, OH, OCH3, OCZHS, SH, SCH3,
SCZHS, F, Cl, Br, CN, COOH, COOAlkyl where alkyl is of 1
to 17 carbon atoms, COOAryl, CF3, N(Alkyl)2, N(Alkyl)-
(Aryl), N(Aryl)2, N~(Alkyl)3Ae, or N~I(Alkyl)2Ae where alkyl
is of 1 to 4 carbon atoms, and Ae is the anion of an acid,
eg. Cle, SO~~, P04~, acetates, BF4 , CF3S03 , Asi~'B'~; SbFs ,
PFB , etc . , or one of the radicals RB or R12 may be a
sulfur atom by means of which the aryl radical is bonded
in the ortho-position with R', with the proviso that at
least one of the radicals RB to RlZ is a group of the type
B-CO-O, where B has the meanings stated in formulae (IIa)
and (IIb), for example
0
o~C 1
0
0
' ~ ss ~ ' o~ct
0 iH3 _
0 ~ I i-CH;
C1 ' OH
0 IH3
0 ~ C-CH;
C1~0 ' I ~C1
I I0
0
0 ~ I CHg
C1 ' CHj
2005283
- 21 - O.Z. 0050/40443
o iH3
0 ~ C-CH 3
C 1 ~0 ~ I N
Ca~
0 iH3
0 ~ C-CH g
C1~ ~ I ON~H C10
H
OI~CH3
0 N~
0 ~ C~3 Clue
C t ~0 ~ I /
I
0
/ I / ~ o
Cl
0
0
I C1
0
0 O~C 1
I
0 -
I \ I Cl
0
a
0
/ / I 0
Cl ~ ~ C1
0
I ~ I 0
F 1
2005283
- 22 - O.Z. 0050/40443
0
0 O~C l
0
Cl
0
0 ~ ~ 0
C 1 ~0 ~ I ~ I O~C 1
0
0
HO ~ I ~ I 0~C l
0
0 O~C 1
I OCHg
in an equimolar ratio (if necessary in an excess of up to
10-30%) or, depending on the number of B-CO-0 groups in
the radicals Re to R12, in two or three times the equi-
molar ratio, in the absence of moisture and in the
presence or absence of an inert solvent or solvent mix-
ture and of a basic catalyst, at from 0 to 100°C, prefer-
ably from 20 to 50°C (route A).
Regarding the preparation process, the following
may be stated specifically.
The chloroformates used in the reaction react
readily with nucleophiles, including water. In the reac-
tion, it is therefore essential to exclude moisture by
using dried nonnucleophilic solvents, eg. acetonitrile,
dichloromethane, dichloroethane, tetrahydrofuran, tolu-
ene, xylene, chlorobenzene, ethyl acetate, chloroform,
etc., and if necessary to maintain an inert gas atmos-
phere, for example nitrogen, argon or carbon dioxide.
As a rule, a solution or suspension of the
hydroxy compound in an inert solvent, which may be omit
ted if the compound is a liquid at the reaction
temperature, is initially taken at from 0 to 100°C,
. 2005283
- 23 - O.Z. 0050/40443
preferably from 10 to 50°C, in the presence of a basic,
nonnucleophilic amine, preferably triethylamine, 4-
dimethylaminopyridine, imidazole, 1,4-
diazabicyclo[2.2.2]octane, 1,5-diazabicyclo[4.3.0]non-5-
ene, 1,8-diazabicyclo[5.4.0]undec-7-ene,
polyvinylpyridine, N,N'-dimethylpropyleneurea, N,N'-
dimethylethyleneurea, etc. The chloroformyl compound, if
necessary dissolved in an inert solvent, eg. dichloro-
methane, dichloroethane, acetonitrile, toluene, chloro-
benzene, xylene, etc., is then added dropwise while stir-
ring in the abovementioned temperature range. This pro-
cedure is particularly suitable for relativelg large
batches.
After stirring has been carried out for from 1 to
48, preferably from 1 to 20, hours at from 10 to 40°C,
filtration, washing and drying are carried out by stan-
dard methods and the product is isolated after recrystal-
lization, distillation or extraction.
The acetophenones, benzophenones and thioxan
thones into which functional groups have been introduced
according to the invention are suitable as photo
initiators, which are polymerizable or copolymerizable
with unsaturated compounds, for radiation-curable com
positions. _
Surprisingly, we have found that, from a certain
spacer length upward, the photoinitiators provided with
the carbonate group are more reactive after photochemical
excitation than the derivatives described in, for ex-
ample, DE-A-37 38 567. In contrast to the carbamoyl
derivatives described in DE-A-38 20 463, the carbonates
in the irradiated polymer do not tend to yellow and are
completely odorless and universally applicable since they
are readily compatible with many binders and binder
systems.
For all compounds stated in the Examples below,
the structure was confinaed in some cases by independent
syntheses and in all cases by correct 1H-NMR, IR and mass
2005283
- 24 - O.Z. 0050/40443
spectra and by conforming elemental analyses.
EXAMPLE 1
4-Chloroformylbenzophenone
A total of 3.4 kg of phosgene were passed in the
course of 5 hours into a solution of 4 kg of 4-hydroxy-
benzophenone and 190 g of benzyltrimethylammonium
chloride in 11.4 kg of o-xylene. During this time, the
internal temperature was increased from 95 to 120°C.
After the end of the introduction of phosgene, stirring
was continued for 30 minutes at 115°C. Working up was
carried out by expelling the excess phosgene with
nitrogen. The salt (catalyst) precipitated toward the
end of the reaction was filtered off and the solvent was
distilled off. 4.9 kg (93%) of yellowish 4-
chloroformylbenzophenone of melting point 67-72°C were
obtained. This crude product containing 12.69% of C1-
(theoretical value 13.60%) was used directly for the
subsequent reactions, without further purification.
The following chloroformyl compounds were prepar-
ed by a method similar to that stated in Example 1:
EXAMPLE 2
2-Chloroformylthioxanthone (C1: calculated -
12.19%; found = 12.03%) was obtained in 79% yield from 2-
hydroxythioxanthone.-
EXAMPLE 3
3-Chloroformylthioxanthone was obtainable in 63%
yield from 3-hydroxythioxanthone- (C1: calculated -
12.19%; found = 11.22%).
s EXAMPLE 4
4-Chloroformylphenyl 2-hydroxyprop-2-yl ketone
was obtained as a crude product (C1: calculated -
14.61%; found = 13.07%) in 75% yield from 4-hydroxyphenyl
2-hydroxyprop-2-yl ketone.
EXAMPLE 5
Phosgenation of 1-(4-hydroxyphenyl)-2-methyl-2-
morpholinopropan-1-one first gave the hydrochloride,
which could be converted into the free amine after the
2005283
- 25 - O.Z. 0050/40443
careful addition of 1,5-diazabicyclo[4.3.0]non-5-ene;
yield: 62% (C1: calculated = 11.37%; found = 11.21%).
EXAMPLE 6
4,4'-Benzoylphenyl ethyl carbonate
1.3 kg of 4-chloroformylbenzophenone in 2.2 kg of
tetrahydrofuran were added dropwise at room temperature
to a solution of 0.25 kg of ethanol and 0.53 kg of tri-
ethylamine in 1.33 kg of tetrahydrofuran. The mixture
was refluxed for 1 hour, cooled to 25-30°C and then
stirred into 20 kg of ice water. The aqueous phase was
extracted three times with dichloromethane and the
organic phase was dried over sodium sulfate and
evaporated down under reduced pressure. The residue was
recrystallized from methanol. 1.05 kg (78%) of pale
crystals of melting point 121-122°C were obtained.
The 4,4'-benzoylphenyl ethyl carbonate was iden-
tical to authentic material (Can. J. Chem. 5~C (1978),
1031).
EXAMPLES 7 TO 18
The asymmetric carbonates below were prepared by
a method similar to that stated in Example 6.
No . Compound yield [ % ~
0
7 \ I \ I 0 83
0~OCH3
0
8- I I ~ 58
0 0
0
0 0~OCH3
9 I ' I 0 65
0~OCH3
0
10 0 ~ I ~ I 0 67
H 3C ~ ~ CH;
2005283
- 26 - O.Z. 0050/40443
No . Compound Yield [ % ]
0 iH3
0 ~ C-CH; 75
H3C0~0 ~ I OH
0 IH3
12 0 ~ I i-CH; 69
HgC z0~ ~ OH
0 iH;
13 ~ I i-CH; 52
OH
0
14 0 ~ CH; $$
H;C0~0 ~ I CH;
0 CH;
15 0 ~ I i-CH; 73
H;CO~ ~ N
0
16 ~ I ~ I O~OCH; 72
S
0 -
17 ~ I ~ I O~OCZHg 77
S
0
18 ~ I ~ I 0 70
S ~ O~CZHg
2005283
- 27 - O.Z. 0050/40443
EXAMPLE 19
a, ~-Acryloylbutylene 4,4'-benzoylphenyl carbonate
13.0 kg of 4-chloroformylbenzophenone in 22 kg of
toluene were added dropwise at from 25 to 30°C to a
solution of 7.2 kg of butanediol monoacrylate and 5.3 kg
of triethylamine in 17 kg of toluene. The mixture was
stirred for 2 hours at room temperature and then washed
in succession with water, with sodium bicarbonate solu-
tion and then with water. The organic phase was dried
over~sodium sulfate and evaporated down under reduced
pressure from a water pump. 17.9 kg (97%) of a yellow-
ish, viscous liquid, which was found to be pure hry thin-
layer chromatography were isolated.
EXAMPLE 20
0.91 kg of 2-hydroxythioxanthone was suspended in
a solution of 0.5 kg of triethylamine and 4.3 kg of
toluene, and a solution of 0.81 kg of 2-chloroformyl
ethyl methacrylate in 1.7 kg of toluene was slowly added
dropwise to this mixture at from 20 to 24°C. After the
addition of 0.002 kg of phenothiazine, stirring was
carried out for 18 hours at room temperature. The or-
ganic phase was then washed with water, with sodium bi-
carbonate solution and again with water, dried over
sodium sulfate and evaporated down under reduced pressure
from a water pump, and the residue was recrystallized
from isopropanol.
0 . 97 kg ( 63% ) of yellow crystals of melting point
72-76°C was obtained.
EXAMPLES 21 TO 34
' The following compounds were synthesized by
methods similar to those described in detail in Examples
19 and 20s
20U~~83
28 - O.Z. 0050/40443
No . Compound Yield [ ~ ]
0
21 0 39
0~0~0
0
0
22 ~ ~ 0 94
I II
0~0~0
0
0
23 I \ I 0 0 95
0~0~0
0
24 I \ I 0 0 49
0~0~0
H3i 0
25 H3C-C ~ 0 64
OH ~ I D~~
I I0
H; i 0
26 H gC-C ~ 0 71
OH ~ I 0~0
0
H3i 0
27 H3C N ~ I 76
0
0 0
28 I \ I 0~~~ 58
5~/ 00
2005283
- 29 - O.Z. 0050/40443
No . Compound Yield [ $ ]
0
H
29 ~ I ~ I ~ ~ I N
0 0 ~ 0
0
30 I \ I 0 0 97
O~O~N H
0
31 I \ I 0 42
0
0
I I0
0
32 I \ I 0 0 88
0~0~0~0
I I0
0
33 ~ ~ a 0 79
I 0~0~0
0 -
34 ~ ~ 0 82
I
s
2005283
- 30 - O.Z. 0050/40443
EXAMPLES 35 TO 53
Use of the acetophenone, benzophenone and thioxanthone
derivatives as photoinitiators
From 0.1 to 0.3 g of the photoinitiator to be
tested, together with from 0.2 to 0.3 g of an amine and,
if required, a sensitizer, are dissolved in a model
polymer consisting of 62% by weight of a bifunctional
epoxyacrylate (acrylate derived from bisphenol glycidyl
ether), 35% by weight of hexanediol acrylate and 3% by
weight of butanol (viscosity adjustment, film formation)
and the solution is stirred for 1 hour. During this
time, the measuring cell is prepared. It consists of two
highly transparent NaCl windows separated by two films,
each of which is 25 ~m thick. The polymer sample to be
measured is now applied to one of the films and covered
with the other, and the stack is clamped with the NaCl
windows in a metal frame in such a way that a film about
10-30 ~m thick forms between the films.
This sandwich is exposed to a very high pressure
mercury lamp (HBO 200 W) at a distance of 51 cm, inter
ference filters furthermore permitting the selection of
only one particular wavelength (eg. 330, 365 or 404 nm).
Before exposure, an IR spectrum of. the sample
sandwich is recorded (t - 0) and this procedure is
repeated after exposure for 5 seconds. In the case of a
good photoinitiator, the intensity of the bands assigned
to the acrylate groups has decreased substantially while
those of the aromatic internal standard remain unchanged.
A number characteristic of the photoinitiator can be cal-
culated from this for each band.
This evaluation is carried out for the three most
intense acrylate bands and the values are added to give
the initiator characteristic. This characteristic is
purely a relative number and is from 1 to 15. The better
the initiation of the polymerization by an initiator, the
higher is its characteristic.
The characteristics correlate with the parameters
200523
31 - O.Z. 0050/40443
typical of photopolymers, such as pencil hardness and
Konig pendulum hardness.
The following values were found:
2005283
- 32 - O.Z. 0050/40443
U
.,i
N
H
O
O
O .r r~ r-' co w c~
U
-1 ~t1 C 07 C~ N
fd
s
H
U
v
a
0
v
x
N
H
N ~ v i
r-~
U
O
O
_~,
T~ V
O Z Z
O V V
O O
O ~ '"
V
\ / \ / ~ ~ _ ~ \ / \ /
s V-
O O O O
\ / \ / \ / \ /
s
'~' O
f~7 ?r
2005283
- 33 - O.Z. 0050/40443
U
N
.,i
O N ,~ o _
b U ~ ~n ~p ,n
..~ cd
+~ ~
~.~1 rt!
H U
H
O i i ,
N
i~
N
O
Z ~ n, en
U
U ~ U U
O_ ~ O U O \ / \ /
O U-U
O
\ /
U \ /
V ~~ \ /
O O
CL O\
\ / ~O
/Z O
v z o
s
0
0 0 0
\ / /
Q~ ~ cr c~ ~ ~r,
.~ ~ a s a
~ O
W ?.
2005283
- 34 - O.Z. 0050/40443
U
N
O O
b U ~ ~ rv
s _
. ,
+~ Sa .r - r o~
-
_
H U
0
C
0
N
y,.r N
N
N , U
i i
,~ ~
U
N
O
O ' O O
O
O
'd
O O O O
O
U
s \ / \ / \ / \ /
O O O O
V C~
\ / \ / \ /
Z
? ? ? ?
~C O
W ?,
.~ 200283
- 35 - O.Z. 0050/40443
U
.rl
N
S-~ H
O O
b ~ ~ ~1
i b ~ ~? °'
O~
H U
v
C
0
O C
N
w ~ x
0
H
cn r
a
0
U
d1
p, u~
~C O
wz
2005283
- 36 - O.Z. 0050/40443
Legend to Examples:
41 : According to DE-A-35 34 645
42 : Obtainable in good yields from Darocut 2959 and
isocyanatoethyl methacrylate
43 : Obtainable from Darocur 2959 and 2-chloroformyl-
ethyl methacrylate in 79% yield
44 : Obtainablefrom2-benzyl-2-dimethylamino-1-[4-(2-
hydroxyethyloxy)-phenyl]-butan-1-one and 2-
chlorofonaylethyl methacrylate in 84% yield
Examples 35 to 44 are Comparative Examples.