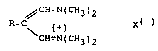Note: Descriptions are shown in the official language in which they were submitted.
Z005330
HOECHST AK~IENGESELLSCHAFT HOE 88/F 362 Dr.JA/PP
.; .
- - Description
Explosion-proof 1-dimethylamino-3-dimethylimino-2-aryl-
l-propene salts, proces3es for their preparation and
their use
As is known, 1-dimethylamino-3-dimethylimino-2-aryl-1-
propene perchlorates are important intermediates for the
synthesis of heterocycles, such as, for example, pyrimi-
dines (cf. Zaschke, Z. Chem. 17 (1977) p. 63 and 293;
Arnold, Collect. Czech. Chem. Commun. 38 (1978) 1371;
Zaschke, J. prakt. Chemie 321 (1979) 619) and pyridines
(cf. C.A. 109:64513w, (1988)). Preparative methods for
this class of compounds, which is also known by the short
name trimethine perchlorates, are also known. It is
furthermore known that these perchlorates, which are
- slightly soluble in water, are explosive to highly
explosive products when dry (cf. Chem. Eng. News 52
(1974) No. 31, p. 3), the handling of which in their
possible use as intermediates is extremely dangerous.
~heir industrial use is therefore virtually precluded.
Accordingly, the ob~ect of the present invention was to
provide trimethine salts which can be handled in an
explosion-proof manner, can be prepared without difficul-
ties in the purity required, and can otherwise be usedlike the perchlorates, but without risk of explosion, as
intermediates, inter alia, for the syntheses of the
abovementioned heterocycles.
Surprisingly, it has now been found that, while avoiding
the use of perchlorate as anion, explosion-proof trimeth-
ine salts of low to slight solubility in water can be
obtained by reacting water-soluble trimethine salts in
aqueous medium with non-oxidizing acids which can form
trimethine salts of low to slight solubility in water and
are inert towards the trimethine cation, or with water-
soluble salts of these non-oxidizing acids and isolating
, : , . . . .
- , ,,
.. . ..
,,
.:. ,, . , . :
"
~005330
. -- 2 --
the trimethine salts of low to slight solubility which
precipitate in the aqueous phase, preferably by filtra-
tion or by centrifugation, and, if desired, purifying
them further by washing and/or, if desired, by reprecipi-
tation and, if desired, drying them.
The invention accordingly relates to explosion-proof
trimethine salts of low to slight solubility in water
which have the formula I
,~CH-N(cH3)2
R^C (+) X( ) (I)
CH=N(cH3)2
in which R and X have the following meaningss
R is aryl or heteroaryl which are unsubstituted or mono-
or polysubstituted by halogen, (C1-C~)-alkyl, (C1-
C12)-alkoxy or hydroxyl, in which aryl is preferably
phenyl, naphthyl, biphenylyl, anthracenyl or tetra-
linyl and heteroaryl is preferably pyridinyl,
pyrimidinyl, pyrazinyl or pyridazinyl, halogen is
preferably F, Cl or Br, in particular F or Cl, and
the radic~ls (C1-C12)-alkyl and (C1-C12)-alkoxy are
unsubstituted or mono- or polysubstituted by halo-
gen, in particular F or Cl;
X is the anion equivalent of a non-oxidizing acid radical
which is inert towards the trimethine cation and can
form trimethine salts of slight solubility in water,
preferably from the group p-toluenesulfonate,
naphthalene-2-sulfonate, naphthalene-l-sulfonate,
naphthalene-1,5-disulfonate, BF4~-), SbF8~-), PF5(-),
AlF6~-), in particular PF6(-) and naphthalene-2-sul-
fonate.
The invention furthermore relates to a process for the
preparation of explosion-proof trimethine ~alts of the
abovementioned formula I by formylation of compounds of
, . . . . . .
.
,
',: . ' , . : .
.. ..
- 200s~30
- 3 -
the formula II
R-CH2-COOH (II)
in which R is as defined in formula I under the condi-
tions of the Vilsmeier-Haack reaction, followed by
hydrolysis of the formylation product in aqueous medium
with the formation of water-soluble primary trimethine
salts, which comprises adding mono- or polybasic acids of
the formula III
(H)nX (III)
in which X is as defined in formula I and n is a number
from 1 to 3, preferably 1 or 2, in particular 1, or
water-soluble salts thereof in at least stoichiometric
amounts to the aqueous reaction mixture containing the
~ater-soluble primary trimethine salts and separating off
- 15 the resulting trimethine salts of the formula I, of
slight solubility in water, in a preferably fine-crystal-
line form.
Preferred acids of the formula III or water-soluble salts
thereof are p-toluenesulfonic acid, sodium p-toluene-
sulfonate, sodium tetrafluoroborate, sodium hexafluoro-
antimonate, sodium hexafluorophosphate, sodium hexa-
fluoroaluminate, naphthalene-2-sulfonic acid, sodium
naphthalene-2-sulfonate, sodium naphthalene-l-sulfonate,
disodium naphthalene-1,5-disulfonate, or mixtures of
these salts or scids, in particular with the acids or
salts based on them. Hexafluorophosphate and naphthalene-
2-sulfonate are particularly preferred.
The trimethine salts of the formula I prepared according
to the invention are preferably formed in a fine-crystal-
line form and can be easily separated off from the motherliquor, washed with water and, if desired, subsequently
dried, after which they are typically obtained in a
purity of over 99%. To purify them further, they can, if
,
' ' " ., ' ' ...... , ~ . : .
.
,, , . ,
, . . :
:, , ': ,' , . . .
",' " ' ', ' ' '
,: , , . . ~ . , ,
, "" '., ' :
~005330
- 4 -
desired, additionally be recrystallized from suitable
solvents, which is however, in general not necessary for
their further use as intermediates for the synthesis of
heterocycles, since they are already excellent synthetic
components even without additional purification. Guiding
tests in this direction have also shown that, when the
compounds according to the invention of the formula I are
used as starting materials for pyrimidine syntheses, the
anion of the compounds of the formula I apparently only
plays a minor role in the outcome of the syntheses, even
if compared with the corresponding known perchlorates,
which is highly surprising and advantageous. The com-
pounds of the formula I are characterized, inter alia,
for example, by determining their melting point and
recording their IR absorption spectrum.
Thé invention is illustrated in more detail by the
examples which follow.
Example 1
In a stirred apparatus, 54 ml (0.6 mol) of phosphorus
oxychloride are slowly added dropwise to 73.9 g (1.0 mol)
of dimethylformamide (DMF) with stirring and cooling at
such a rate that the temperature of the reaction mixture
does not exceed 20C. The resulting ~olution is cooled to
-10C, and 30 g (0.2 mol) of p-hydroxyphenylacetic acid
are added in portions with stirring. The resulting syrup-
like mixture is first maintained at room temperature for
1 hour, then stirred at 60C for 2 hours and at 80C for
3 hours and then poured onto 500 g of ice. After the
exothermic hydrolysis reaction resulting from the contact
with the ice is complete, the resulting aqueous solution
is stirred with activated carbon, filtered, and 38 g
(0.22 mol) of sodium hexafluorophosphate, dissolved in
60 ml of water, are added to the clear solution obtained.
A light yellow precipitate is formed immediately, subse-
quently separated off, washed with water and dried. It
consi~ts of the compound of the formula I l-dimethyl-
,,, ,,, , . , , ~ .
, ~ '
"
,
.,
,
~005330
- 5
amino-3-dimethylimino-2-[4-hydroxyphenyl]-1-propene
hexafluorophosphate, m.p.: 198-199C.
The yield is 62.5 g (= 86% of theory).
The compound can be obtained analytically pure by recrys-
tallization from methanol/diethyl ether (6:4 v/v) and has
the following characteristics. m.p.: 212C (incipient
decomposition). The IR spectrum can be seen from
Figure 1.
Example 2
Example 1 is repeated, except that 53 g (0.23 mol) of
sodium naphthalene-2-sulfonate, dissolved in 1.2 1 of
water, are added instead of 38 g (0.22 mol) of sodium
~ hexafluorophosphate, dissolved in 60 ml of water, to the
clear aqueous solution obtained. A light yellow precipit-
ate is formed immediately, subsequently separated off,
washed with water and dried. It consists of the compound
of the formula I l-dimethylamino-3-dimethylimino-2-[4
hydroxyphenyl]-l-propene-naphthalene-2-sulfonate, m.p.:
262-263C.
The yield is 69 g (= 81~ of theory).
The compound can be obtained analytically pure by recrys-
tallization from methanol and has the following charac-
teristics. m.p.: 268C (incipient decomposition). The IR
spectrum can be seen from Figure 2.
Example 3
Example 2 is repeated, except that the same amount of
sodium naphthalene-l-sulfonate instead of sodium naph-
thalene-2-sulfonate is added. The light yellow precipit-
ate formed is separated off, washed with water and dried.
It consists of the compound of the formula I l-dimethyl-
amino-3-dimethylimino-2-[4-hydroxyphenyl]-l-propene
.. ..
.
.. . . . . .
',
~0~)5330
- 6 -
naphthalene-l-sulfonate, m.p.: 235-237C.
The yield is 38.5 g (= 41~ of theory).
The compound can be obtained analytically pure by recrys-
tallization from methanol and has the following charac-
teristics: m.p.: 248C (incipient decomposition). The IRspectrum can be seen from Figure 3.
, , . , ~ .
~ ,
- :