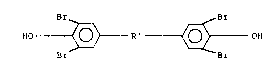Note: Descriptions are shown in the official language in which they were submitted.
~)0~iS88
SEMIPERMEABLE MEMBRANES BASED ON SPECIFIED
~E~RA~RQ~ENO~ TYP:~ POLY~TEF~
Qf the Inven~iQn
This application is a c~ontinuation-in-part
of application Serial ~o. 358,631, filed May 30,
1989; which was a continuation-ln-part of
application Serial No. 289,668, filed ~ecember 27,
1988.
This invention relates to semipermeable
membranes of polyesters of tetrabromobisphenol and
aromatic dicarbo~ylic acids as the predominant
nuclei components of the polyester. The invention
also relates to processes using said membranes for
the selective permeation of at least one component
from a fluid mi~ture containing said one component
in admixture with other components, in particular
for oxygen/nitrogen, and carbon dio~ide/methane
separations.
DescriDtion of the Prior Art
Permeable membranes capable of selectively
permeating one component of a fluid mixture, either
liquid or gas, are considered in the art as a
convenient, potentially highly advantageous means
for achieving fluid sepasations. For practical
commercial operations, permeable membranes must be
capable of achieving an acceptable level of
selectivity or separation of the gases or liquids
contained in the fluids feed stream while, at the
same time, achieving a desirably high productivity,
or rate, of component separat~ion.
Various types of permeable, or
semipermeable, membranes are known in the art for
D-16101-2
2~ iS~38
2 --
carrying out a variety of fluid separations. Such
membranes have been classified as being of the
isotropic, or homogeneous, or composite, or
asymmetric types and their structures are well known
to those skilled in this art.
As the ~dvantages of permeable and
semipermeable membranes have become increasingly
appreciated, the performance requirements have
likewise increased and the drive to find new
membranes for more applications has continued to
grow. Th2se demands have resulted in the art mo~ing
in the direction of very thin membranes having
desired permeability characteristics without
sacrifice of the separation, or selectivity,
characteristics of the membrane, or of the
permeation rate, or productivity, of separation
achievable.
At the current time permeable membranes are
known that are made from a wide variety of
materials, e.g. natural and synthetic polymers such
as rubbers, polysilo~anes, polyamines, brominated
polyphenylene oxide, cellulose acetate, ethyl
cellulose, polyethylene, polypropylene,
polybutadiene, polyisoprene, polystyrene, the
polyvinyls, polyesters, polyimides, polyamides, the
polycarbonates, and a host of other materials.
The following table shows the published
diameters of a few of the various gases commonly
separated with polymeric membranes.
Gas He H C02 N2 CH4
_ ~ 2
Diameter 2.6 2.89 3.3 3.46 3.64 3.8
(Angstrom)
D-161Ql-2
~06~i~8
In the case of oxygen and nitrogen the size
difference is rather small, therefore, most
polymeric membranes used commercially to separate
nitrogen from oxygen have molecular structures that
impede the flow of the gases, e.g., such as o~ygen,
~hrough the membrane. For that reason these
polymeric ~embranes need to be e~tremely thin,
generally about 2~0 to about 10,000 Angstroms thick,
preferably less than 2,000 Angstroms, to make the
separation economically viable. The thinner
membrane allows faster transport of the permeate
through the membrane.
Technology and physical factors limit how
thin one can prepare the membrane film or the
coating of a composite membrane, thus it would be
advantageous to develop new membrane polymers which
have higher permeation rates without greatly
sacrificing their ability to separate the desired
gas mi~tures. However the large body of gas
permeability coefficients and gas separation data in
the literature ~e.g., Polymer Handbook, 2nd ed. John
Wiley & Sons, 1975) generally shows that increasing
the permeability of gases, such as o~ygen, by
varying the polymer structure, decreases the
latter's separation characteristics, the ability to
separate o~ygen from nitrogen. The data also shows
that with the current state-of-the-art it is not
really possible to predict gas permeation rates or
gas selectivity even when rather minor changes are
made in the chemical structur~e of the membrane of
one polymer class, such as the polyesters or
polycarbonates, even where certain structural
D-16101-2
6~ii8~3
features remain constant. The literature also
indicates that variations in the membrane itself, be
it isotropic, asymmetric or composite in structure,
and its thickness can also have a marked effect on
permeation rate and selectivity. The inference
drawn from the literature is that the inclusion of a
large number of arbitrary modifications to the basic
polymer structure of one o~ more polymer classes in
many membrane patents is not fully instructive in
predicting the usefulness of the alternative
structures that had not been studied. It would
appear that careful consideration needs to be given
to defining both the chemical and physical
structures of membranes suitable for use in gas
separation processes.
Many of the factors which influence gas
permeability have been largely known for over two
decades, but the ability to quantitatively predict
the magnitude and even direction of a combination of
these factors in a specific polymeric membrane has
not been successful to this day. In the nineteen-
fifties and nineteen-sixties researchers knew that
the attrac~ive forces between polymer chains,
packing density, rotation around single bonds in the
polymer chain, and the relative rigidity (aromatic
structures) or flexibility (aliphatic structures) of
the polymer chain affected gas permeability. Rigid
highly aromatic polymer structures such as
bisphenol-A polycarbonates were examined in the
nineteen-si~ties and early nineteen-seventies in
attempts to obtain an optimum`combination of gas
permeability and gas separation or selectivity. For
D-lÇ101-2
88
-- 5
example, outstanding values of qas selectiYity for
oxygen/nitrogen were obtained, but this was not
combined with sufficiently high gas permeability and
the desire to attain higher gas permeability has
continued.
A publication in August 1375 by Pilato et
al. (Amer. Chem. Soc. Div. Polym. Chem., Polym,
Prepr., 16t2) (1975~ 41-46) showed that it is
possible to modify rigid aromatic polymer structurPs
such as polysulfones, polycarbonates and polyesters,
including certain bisphenol-phthalate polyesters not
within the scope of this invention, to increase the
gas permeation rate without significant decreases in
helium/methane and carbon dio~ide/methane
separations. More data by Pilato et al. show that
the incorporation of tetraisopropyl bisphenol A or
tetramethylbisphenol L (based on Limonene +
Dimethylphenol) in these polymers to try to increase
the gas flux resulted in decreased gas selectivity.
Therefore, even in the rigid polymer systems, it
appears that the general trend noted in the Polymer
Handbook holds; increasing the gas permeability
results in reduced gas selectivity. Based on this
work and the other publications, infra, it appears
that additional effort was necessary to achieve
higher gas permeability and still retain high gas
selectivity.
Also in August 1975 another unusually broad
disclosure appeared, U.S. Patent No. 3,899,309
(Reissue 30,351, July 29, 1980), which described
highly aromatic polyimides, polyamides and
polyesters. The patent alleged the combination of
D-16101-2
'~0~)65~8
main chain non linearity, high aromatic structure
and prevention of free rotation around main chain
single b~nds led to increased g,3s permeability. The
disclosure is so broad that one is not adequately or
fully instructed to enable a skilled person to
determine which particular structure or structures
would gi~e the more desirable qas permeability and
selectivity without e~tensi~e study and
e~perimentation.
In U.S. Patent Reissue 30,351, file May 18,
1976 by H. H. Hoehn et al. (reissued on July 29,
1980), which is the reissue of U.S. Patent No.
3,899,309 (issued on August 12, 1975) there are
broadly disclosed separation membranes of aromatic
polyimides, polyesters, and polyamides. The
invention broadly descri~ed and claimed in these
patents requires the polymer aromatic imide,
aromatic ester, or aromatic amide repeating unit
must meet certain requirements, namely:
(a) it contains at least one rigid
divalent subunit, the two main chain single bonds
extending from which are not colinear,
(b) is sterically unable to rotate
360 around one or more of said main chain single
bonds, and
(c) more than 50% of the atoms in the
main chain are members of aromatic rings.
~ hese requirements are set forth in the
reissue patent in the Abstract; at column 1, lines
40 to 53; in claim 1 and in all claims dependent
upon claim 1. The manner in which requirement (a)
is determined is sèt forth in column 2, lines 51 to
D-16101-2
~0~)6~
68; the determination of requirement (b) i5
described in column 3, lines 1 to 2B; and the
determination of requirement (c) is descri~ed in
column 3, lines 29 to 56; with c:olumn 3, lines 57 to
68 explaining how the requirements were determined
in the e~amples. Thus, for a polymer to be within
the orbit of the invention descxibed and claimed in
Re. 30,351 it must meet all three criteria or
requirements defined in the patent. Should it fail
to meet all three requirements it cannot be
considered a polymer falling within the orbit of the
invention. Requirement (b) of Re. 30,351 restricts
the membranes to those from polymers in which the
polymer chain contains at least one rigid monolinear
band between rigid subunits around which subunit the
polymer chain is sterically prevented from rotating
360 and specifically describes the manner in which
this can be ascertained by the use of a clearly
identified, readily available molecular model kit.
Thus, a polymer structure assembled from the
identified kit which is not sterically prevented
from rotati,ng 360C cannot be considered as being
within the scope of Re. 30,351.
Re. 30,351 defines the polyesters alleged
to meet the requirements (a), (b), and (c) at column
2, lines 21 to 34; column 6, lines 26 to 56; column
7, lines 19 to 29 and 42 to 53 and column 11 line 62
to column 12, line 68 (Tables III and IV), with
specific examples of polyesters and their membranes
being shown in Examples 1-5, 9-12 and 22. The use
of polyester membrane-s in the process is claimed in
claims 1 and 8 to 13; with claims 12 and 13 being
D-16101-2
;20~6S8~
-- 8
duplicates. The membranes of the invention are said
to be in film form or hollow fiber form, column ~,
lines lD to 15 and lines 43 to 46 and it is stated
they can be uniform membranes (column 4, lines 47 to
49) or asym~etric membranes (column 4, lines 99 to
S4).
In U.S. 3,822, 202, issued to H. H. Hoehn
Gn July 2, lg74, the same polyimide, polyester and
polyamide polymers are disclosed as suitable for use
as membranes but in this patent the membranes are
subjected to a heat treatment in an environment of
air or an inert gas under vacuum at a temperature
range of 150C up to just below the softening point
of the polymer. This results in the formation of a
true asymmetric membrane. In all of U.S. 3,822,202
there is no mention of composite membranes and the
only example in U.S. 3,382,202 employing a polyester
membrane is Example 21, which uses an air dried flat
film 2.15 mils thick. It is to be noted that there
is no specific disclosure in U.S. 3,822,202 of any
membrane produced from a polyester of a
tetrabromobisphenol and an aromatic dicarboxylic
acid or the use thereof in a fluids separation
process.
Most recently U.S. 4,822,382 issued to
J. K. Nelson on April 18, 1989. This patent
discloses separation membranes, in particular
composite membranes, having a separation layer
comprised of one or more poly(tetramethyl) bisphenol
A phthalates for use in separating a gas mi~ture.
The patent does not disclose other polyesters within
D-16101-2
~oo~
this class and the data in the examples show low
permeation rates of oxygen in air separations.
In European Patent Application 0 242 147,
published October 21, 1987, Aneda et al. there are
disclosed gas separation membranes based on
polycarbonate polymers derived from bisphenols and
their use in gas separation processes. The
membranes are alleged to have particular application
in separating o~ygen from nitrogen, but they are not
polyesters.
European Patent Application 0 244 146,
published November 4, 1987, Anand et al., disclosed
membranes based on polyestercarbonate polymers in
which the polymer backbone is a tetrabromo
diphenolic residue, and the use of the polymers in
gas separation processes, but they are not
polyesters.
9Oth of these European Patent Applications
are based on polycarbonate polymers containing the
carbonate group: - O - C - O -
ll
in the polymer chain. The presence of thiscarbonate link is an essential element of the
inventions disclosed and is to be distinguished over
the polyesters which contain the ester group:
- C - Q -.
Il
o
Japanese Une~amined Patent 53-66880,
published June 14, 1978, Shoji Ueno et al.,
discloses membranes based on àromatic polyesters
,
D-16101-2
iS8~3
produced frorn aromatic dicarbo~ylic acids and
bisphenols of the structure:
HO ~ O ~ X ~ ~ OH
R3 R9 R4 R3
wherein Rl_4 and R 1-4 are hydrogen, halogen or
hydrocarbon; and
X is either -O-, -SO2-, -CO-, -S-,
alkylene, or alkylidene.
All of the bisphenols disclosed and discussed as
suitable contain one of the defined X grsups as the
linking or bridging group. The Japanese publication
contains no disclosure or suggestion of any
bisphenol compound in which the linking group
contains either halogen atoms or a divalent
cycloalkyl group.
Summarv of the Invention
This invention comprises an improved gas
separation membrane consisting predominantly of a
polyester or copolyester based on (1) at least 50
mole percent or more, preferably 80 mole percent or
more and most preferably 100 mole percent of a
tetrabromobisphenol of the general formula:
Br Br
HO ~ R~ ~ OH (I)
Br Br
as hereinafter more fully defined, reacted with Sa3
80 mole percent or more of isophthaloyl dichloride
and/or 4-bromoisophthaloyl dichloride and (b) 20
D-16101-2
2~ S88
mole percent or less of terephtllaloyl dichloride
and/or 2-bromoterephthaloyl dichloride, or ~2~ at
least 50 mole percent or more, preferably 50 mole
percent or more and most preferably 100 mole percent
of said tetrabromobisphenol ~I) reacted with (~) 30
mole percent or less of isophthaloyl dichloride
and/or 4-bromoisophthaloyl dichloride and (b) 70
mole percent or more of terephthaloyl dichloride
and/or ~-bromoterephthaloyl dichloride.
Alternatively one can use the free acid or ester or
salt forms of the phthaloyl compounds in producing
the polyesters. This invention also comprises the
use of said membrane in processes for the separation
of oxygen from nitrogen and the separation of carbon
dioxide from methane.
Eçtailed ~escriPtion of the Invention
This invention provides novel improved
polyester permeable membranes having exceptional
oxygen/nitrogen and carbon dioxide/methane gas
separation properties ~ith enhanced o~ygen and
carbon dio~ide permeabilities.
The preparation of polyesters is well known
and several procedures can be used. Thus, it is
known that they can be produced by the reaction of a
dihydroxyl compound with an aromatic dicarboxylic
acid or an ester-forming derivative thereof such as
an acid chloride. The method for producing the
polyesters comprising the gas separation membranes
of this invention is not a part of this invention
and any polyesterification process can be used. A
typical proedllre employed for preparing the
polyester membranes of this invention is the
D-16101-2
X~06~38
12 -
reaction of t~e tetrabromo- bisphenol compound (I)
with terephth310yl chloride, isophthaloyl chloride
or mi~tures thereof. Such a process is disclosed in
U.S. Patent No. 3,388,097, issued June 11, 1968 to
Cramer et al. The phthaloyl compounds are used at a
mole ratio of terephthaloyl to isophthaloyl
compounds of 80:20 to 0:100, preferably 20:80 to
0:100, and most preferably 0:100 for polyesters
based on 50 mole percent or more of
tetrabromobisphenol (I) for o~ygen/nitrogen
separations (e.g. air separations). A mole ratio of
terephthaloyl to isophthaloyl compounds of 100:0 to
0:100, preferably 90:10 to 70:30, and most
preferably 85:15 to 75:25 for polyesters based on 50
mole percent or more of tetrabromobisphenol (I) for
carbon dio~ide/methane separations. In addition, as
is known to those skilled in this art, a small
amount of another suitable aromatic dicarbo~ylic
acid, the acid chloride or the ester can be used in
the polyes~erification process; further, a small
amount of the aromatic dicarboxylic acid component
can be replaced with an aliphatic dicarbo~ylic acid;
these small amounts added should be in quantities
that do not have any significant deleterious effect
on permeability and/or selectivity. Further, one
can use mi~tures of the tetrabromobisphenols of
Formula I with small amounts of other bisphenols or
other aromatic and/or aliphatic diols with up to
about 10 mole percent of tetrabromobisphenol (I)
~eing replaced by other bisphenols or such diols.
The preferred polyesters are khose produced by the
condensation polymerization of the
D-16101-2
;8~
- 13 -
tetrabromobisphenols of Formula I with terephthalic
acid, isophthalic acid, or mi~tures thereof, or of
the salts or esters thereof, such as the acid
chlorides. The Encyclopedia of Polymer Science &
Technology, Mark et al. Editors, John Wiley ~nd
Sons, Interscience Vivision, N~ N.Y., publishers,
1969, Volume ll, pages 1 to 168, contains a
description of the many processes known for the
preparation of polyesters. In view of the extensive
knowledge of these polymers, there is no need for
any detailed description of the specific reactants
that have been described above nor of the reaction
conditions required for the polyesterification
reaction. This technical material is well known to
those or ordinary skill in the polyester art.
The gas separation membranes of this
invention comprise a thin layer consisting
predominantly of a polyester or copolyester derived
fr~m a tetrabromobisphenol o the general formula:
Br Br
HO - ~ R~ ~ OH (I)
Br Br
I -
wherein R' is CF3-C-CF3 or divalent
cyclododecyl. I ~
The diol component of the polyesters or
copolyesters constitutes more than 50 mole percent
of tetrabromobisphenols (I), preferably at least
about 80 mole percent of the tetrabromobisphenols
(I) and can be 100 mole percent of said structure
(I) in admisture with other bisphenols of the
D-16101-2
2~6S~
- 14
structure (II), below. The diols, thus, can be
mi~tures of more than 50 mole p'ercent of the
tetrabromobisphenols SI) and less than 50 mole
percent of a bisphenol of the general formula:
HO - ~ R~ - ~ OH ~(~ ) 3
wherein R" is methyl or chlorine.
The tetrabromobisphenols (I) used in
producing the polyester yas separation permeable
membranes make up at least 50 mole percent or more
of the dihydroxyl compound used to produce the
polyesters, 8S stated above. The polyesters or
copolyesters are the reaction products of:
(1) at least 50 mole percent or more
of said tetrabromobisphenol (I) reacted with (a) 80
mole percent or more of isophthaloyl dichloride
and/or 4-bromoisophthaloyl dichloride and (b) 20
mole percent or less of terephthaloyl dichloride
and/or 2-bromoterephthaloyl dichloride as the
dicarbozylic acid compound, or
(2) at least 50 mole percent or more
of said tetrabromobisphenol (I) reacted with (a) 25
mole percent or less of isophthaloyl dichloride
and/or 4-bromoisophthaloyl dichloride and (b) 75
mole percent or more of terethaloyl dichloride
and/or 2-bromoterephthaloyl dichloride.
The polyester gas separation membranes of
this invention contain as the~predominant recurrin~
unit the group having the structural formula:
D-16101~2
6~8~3
- 15 -
o ~ R~ OOC ~ CO
wherein R''' is hydrogen or bromine and ~ is an
integer having a value of at least about 20 up to
about 200 or more, preferably from about 25 to about
175. The polyester preferably has a weight average
molecular weight of ~rom about 20,000 to about
150,000, most preferably from about 30,000 to about
125,000.
The gas separation membrane of this
invention can be of dense film or of any form known
to those skilled in the art. Further, it can be a
composite membrane, an asymmetric membrane, or a
homogeneous membrane or isotropic membrane. The
membranes may be in spiral form, flat sheet, tubular
form, or other configurations, as well as in hollow
fiber form. Those skilled in the art are aware of
the many methods available for their production and
~now how to prepare the membranes in any of these
forms. The preferred membranes of this invention
are the asymmetric or composite membranes, with
separation layers less than 10,000 Angstroms thick
preferably less than 5,000 Angstroms thick, most
preferably from about 200 to about 2,000 Angstroms
thick.
The isotropic and asymmetric type membranes
are generally comprised essentially of a single
permeable membrane material càpable of selective
o~ygen/nitrogen and carbon dioxide/methane
D-16101-2
20~6~88
- 16 -
separations. Asymmetric membranes are distinguished
by the existence of two or more morphological
regions within the membrane structurei one such
region comprising a thin relatively dense
semipermeable skin capable of selectively permeating
at least one component from the sas mi~ture
containing said at least one component in admi~ture
with other components, and the other region
comprising a less dense, porous, essentially
non-selective support region that serves to preclude
the collapse of the thin skin region of the membrane
during use. Composite membranes generally comprise
a thin layer or coating of the polyester
semipermeable membrane material superimposed on a
porous substrate.
Flat sheet membranes are readily prepared
from polyester solutions in a suitable solvent, e.g.
methylene chloride, by casting the solution and
evaporating the solvent, and thereafter drying and
curing the cast film, either under vacuum, at
elevated temperature, or a combination of both.
Such thin film membranes can vary in thickness from
about 0.5 ~il to about 10 mils or more, preferably
from about 1 mil to about 3 mils.
Flat sheet membranes are generally not the
preferred commercial form. In large scale
commercial applications hollow fiber permeable
membranes are generally more desirable because they
provide a significantly larger surface area per
volume unit when fabricated as modules. The porous
hollow fiber permeable membra`nes comprise a porous
hollow fiber support having a permeable membrane
D-16101-2
21)~6S138
layer on the surface thereof. The methods for their
production are well known (See Eor e~ample, "Hollow
Fibers Manufacture and Applications~, ed. J. Scott,
Noyes Data Corporation, N.J., 1981, p. 264 et seq.)
The tetrabromobisphenol type polyester
permeable separation membranes of this invention
exhibit a high separation factor for o~ygen over
nitrogen from air mixtures of at least about 5.6
coupled with a permeability rate or flux of at least
about 4 and a high separstion factor for carbon
dio~ide over methane in mi~tures containing said
gases. The ability of these membranes to separate
these components with such high combination of both
separation factor and permeability rate was
completely une~pected and is superior to the results
often exhibited by many existing membranes in the
art. Thus, for example, the polycarbonate memhranes
disclosed in EPO 0 242 147 and the polyester~
carbonate membranes disclosed in EPO 0 244 126 have
relatively low permeability rate. None of the
membranes in these EPO applications show a
combination of high selectivity and high permeation
rate. The data in the Table of EPO 0 244 126 show
low o~ygen permeability P for the polyestercarbonate
membranes of from 0.96 to 1.23 Barrers combined with
a separation factor or selectiYity for oxygen over
nitrogen of 6.7 or 7.2; these Yalues are not
considered in the art as a high combination of the
two values. Likewise in Table 1 of EPO 0 242 147
the data show low oxygen permeability P for the
polycarbonate membranes of from 0.8 to 1.448 Barrers
combined with a separation factor or selectivity for
D-16101-2
6~88
- lB -
o~ygen over nitrogen of 5.4 to 7.4; again not
considered a high combination of the two values.
The tetrabromobisphenol polyester membranes
of this invention, as shown by the experimental data
in the Examples show a combination of both high
selectivity and high permeation rate. As seen in
the data obtained and reported in the e~amples,
infra, o~ygen permeability P of the membranes of
this invention of from about 4.7 up to about 11.8
Barrers combined with a separation factor or
selectivity for o~ygen over nitrogen of from about
5.6 up to abo~t 7, are truly a combination of the
two high values.
It was found that high percentages of
isophthalic acid versus terephthalic acid polyesters
of tetrabromohe~afluoro bisphenol A ~I)
significantly increases the o~ygen/nitrogen
selectivity over that of polyesters with high
amounts of terephthalic acid without yielding low
oxygen permeability of less than 4.5 Barrers.
Preferably the isophthalic acid ester content should
be 80 mole percent or higher and most preferably 100
mole percent isophthalic acid ester. In contrast,
for carbon dio~ide/methane separations, surprisingly
the optimum combination of separation and
permeability is achieved when the terephthalic acid
ester content is about 75 mole percent or more and
isophthalic acid ester content is 25 mole percent or
less with the same tetrabromohe~afluorobisphenol.
The oxygen/ nitrogen separation is significantly
less efficient with high terephthalic acid ester
content. Therefore, for o~ygen/nitrogen separations
D-16101-2
588
-- 19 --
a high isophthalic acid ester content is preferred,
while for carbon dioxide/ methane separations a high
terephthalic acid ester content is preferred.
Copolyesters based on 50 mole % or greater
and preferably 60 mole ~ or greater of compounds of
formula (I), such as tetrabromohesafluoro bisphenol
A and one or more other bisphenols (compound III in
the table) can also provide useful gas separation
membranes with less favorable intrinsic permeability
and gas separation properties than the previously
mentioned tetrabromobisphenol polyesters. However,
many of these copolymers provide solubility
characteristics slightly more favorable than the
bromobisphenol polymers for preparing composite
membranes by coating onto polysulfone hollow fiber
as described in U.S. Patent No. 4,822,382 with some
sacrifice in selectivity and, usually, improvements
in permeability. Solubilities of the polyesters in
specific solvent and solvent systems are important
because the polysulfone hollow fiber is susceptable
to attack by many common solvents used to dissolve
many membrane polymers. Therefore, even if a
polyester has e~cellent intrinsic separation and
permeability properties, if it cannot be coated on a
substrate such as polysulfone or other porous hollow
fiber substrates, its usefulness becomes limited.
Chemically resistant porous hollow fibers as
substrates for these coatings would be ideal if
costs, coatability, and other factors are overcome
to make them useful for composite membranes. Of
course, asymmetric hollow fibèr membranes can be
made entirely from these polymers, but the costs
D-16101-2
~6~ 8
- 20 -
will be much higher. Methods ot:her than solution
coating can be possible, but need to be developed in
the future.
Polyesters based on tetrabromohexafluoro
bisphenol A have been disclosed previously in the
Hoehn patent U.S. 3,899,309 but it does not
specifically anticipate or susgest the unexpected
and unpredictable improvements provided for
oxygen/nitrogen ~nd carbon dio~ide~methane
separations achieved by the above claimed
structures. Claim 11 of U.S. 3,899,309 discloses an
isophthalate/terephtalate polyester based on
tetrabromohe~afluorobisphenol A. Column 8, lines
25-35, states that the preferred
isophthalate/terephthalate composition ratio is
70/30. Moreover, in column 16, line 17-18 the
tetrabromohexafluoro bisphenol A is not included in
the list of preferred diols ~bisphenols).
The data in the table below show that the
specific polyesters and copolyesters provide an
incomparable combination of e~tremely good
oxygPn/nitrogen separation factors and high oxygen
gas permeability when compared with previously known
examples in the literature.
D-16101-2
~0~ 38
- 2 1
~Q~ L~QNQ F LAS ~3RA-N~s_FOR OXYGEN/NITRQGEN S~PARATIONS
Perme~t~L1Ly SeParation
~j~phenolls~ Q l~rrel'S) F~tt9r
100~0 Iso~Tere P~Z~ 2/N2
1. T~rF6BA(I) 100~0 5.25 6.7
2. T~rF68A(I) 80~20 5.~0 6.4
3. TBrF6BA~I) 25~75 9.0 6.1
4. TClF6BA(III) 100~0 5.64 6.12
5. Dow~ T~rBA
Polycarbona~e 1.87 6.9
6. Dow~ Pol~carbonate l.B5 7.4
7. Dow~ Polyc~rbonate 1.07 6.9
8. Hoeh~ Po1yester 1.79 5.5
9. Hoehn~ Polyester 1.30 5.6
10. Polysulfo~e~ 1.2 5.9
11. Cellulose acet~te~ 1.0 5.5
Do~ U.S. Patent No. 4,B18,254
Hoehn U.S. Patent No. 3,B99,309, Re. No. 30,351
Co~mercial gas separation membranes
I Compound I of Experime~t 1, infra
iII Tetrachlorohexafluorobisphenol-A
Note in the table that the tetrabromohexafluoro
bisphenol A polyisophthalate of Run 1 has a high
oxygen/nitrogen separation factor of 6.7 and high
oxygen permeability of 5.2S Barrers compared to only
1.87 with a comparable separation factor of 6.9 of
Run 7. Other factors being equal the latter
membrane will require almost three times the
membrane area to the former e~ample, a decided
economic advantage. Other examples in the Hoehn
reissue patent have considerably lower gas
selectivity which are not competitive with Run 1 in
the table.
D-16101-2
38
- 2~
Carbon dio~ide/methane separations have
been difficult because factors w~hich lead to high
carbon dioxide permeability yield low carbon
dioxide/methane separation factors. The table below
shows that the commercially available membranes
based on cellulose acetate and polysulfone yield
good separations for this ~as pair but the
permeability for carbon di~ide is low and needs to
be higher for more commercially economical
operations. The tetramethyl bisphenol A
polycarbonate appears to have the best combination
of permeability and separation factor reported in
the literature but it is not as good as the
polyester of tetrabromohexafluoro bisphenols (I) of
this invention.
Structures of this invention e~hibit a
remarkable combination of very high permeability and
carbon dioxide/methane separations based on
pure/mi~ed gas measurements. In the optimum
structure for oxygen/nitrogen separations the
tetrabromohe~afluoro bisphenol A high isophthalic
acid ester yields the best separation and
permeability combinations. Surprisingly an
unusually high carbon dio~ide permeability is seen
in the 25/75 isophthalic~terephthalic acid ester
ratios with essentially no significant decrease in
gas selectivity for carbon dio~ide/methane. This
remarkable doublinq of the carbon dio~ide
permeability from the 100% isophthalic acid ester
structure where P~20.0 to 2~42 in the 25/75
isophthalic/terephthalic acid`ester structure
D-16101-2
2~06~88
- 23 -
without a significant decrease i.n gas selectivity
was not expected.
Comparison of Gas Membranes
QL ca~Qn ~ id~Methane SeparatiQn
Separation
hQnQ~ R~ ~n~ili~F a c~o r
Iso/Tere P(CO2)CO2/CH4
1. TBrF6BA(I) 100/0 20.0 50.0
2. TBrF6BA(I) 25/75 42.0 44.0
3. Dow TMB~ PC~ -- 16.3 26.7
4. Polysulfone 5.5 26.0
5. Cellulose
Acetate 6 30.0
~ Dow U.S. Patent No. 4,818,254
I Compound I of Experiment 1, infra
Note that the tetrabromohexafluoro bisphenol A 25/75
isophthalic/terephthalic acid ester membrane of this
invention has substantially improved properties over
the other known polymeric membranes and the same
polyester based on 100% isophthalic acid.
Although the data are limited on the
various combinations of isophthalic/terephthalic
acid ester.ratios in the copolymers they do show
that we can vary the permeability and gas
selectivity by varying the bisphenol and by analogy
with the above examples the isophthalic/terephthalic
acid ester ratios as shown in the e~perimental
section.
The reduced viscosities of the polyesters
were determined at 25C using a polymer solution
containing 0.200 g of polymer~per 100 ml of
chloroform and calculated by the equation
D-16101-2
~6~88
- 24 -
R (c)(B)
wherein A is the time it takes t:he sample of
chloroform solution to travel through the
viscometer, ~ is the time it ta)ses chloroform to
travel through the viscometer a~ld C is the weight of
the sample of chloroform solution.
The polyesters were film forming at a
reduced viscosity in chloroform of about 0.25 and
above. For gas permeable processes the polyester
having viscosities of about 0.25 or higher provide
adequately strong films of about 2 mils to about S
mils thick; preferred viscosities are from about
0.25 to about 1.6, most preferably from about 0.95
to about 1.3. The film thickness can vary from
about 1 mil to about 10 mils, preferably from about
2 mils to about 5 mils.
Porous hollow fiber polysulfone substrates
ar~ useful in the preparation of composite
membranes. Porous polysulfone hollow fibers are
produced from solutions of the polysulfone in a
solvent~nonsolvent mixture, as is knvwn in the art,
using the procedure described by I. Cabasso et al.
in "Composite Hollow Fiber Membrane", Journal of
Applied Polymer Science, ~, 1509-1523 and in
"Research and Development of NS-l and Related
Polysulfone Hollow Fibers For Reverse Osmosis
Desalination of Seawater~ PB 248,666, prepared for
the Office of Water Research and Technology,
Contract No. 14-30-3165, U.S. Department of the
Interior, July 197S. The well known tube-in- tube
jet technique is used for the spinning procedure,
with water at about room temperature being the
D-16101-2
;~0~;~8~3
- 25 -
outside quench medium for the fibers. The quench
medium in the center bore of the fiber was air.
Quenching is generally followed by extensive washing
to remove pore forming material. ~ollo~ing the
wash, the hollow fibers are dried at elevated
temperature by passage throuyh a hot air drying oven.
Advantageously, the walls of the porous
polysulfone hollow fibers are sufficiently thick so
that no special apparatus would be required for
their handling and they can be conveniently formed
into cartridges. The outside diameter of the porous
polysulfone hollow fiber can vary from about 1 mil
or less to about 100 mils or more, preferably from
about 2 mils to about 80 mils. The wall thickness
of the porous polysulfone hollow fiber can vary from
about 0.1 mil to about 25 mils or more, preferably
at least about 0.2 mil up to about 20 mils. The
spun polysulfone fibers are generally considered to
be substantially isotropic, however, some degree of
asymmetry is usually present. Porosity of hollow
fibers can be modified, by annealing techniques,
particularly by heat annealing. This is
conventionally performed by passing the dried porous
polysulfone hollow fiber through a hot air oven at a
temperature of from about 160C up to close to the
glass transition temperature of the polysulfone
(195~-200~C) for a period of less than about 30
seconds, preferably not more than about 10 seconds.
The gas permeability or permeation rate P
measurements of the flat film membranes evaluated in
the following e~amples were determined at 25C by
placing a small disc of the polymer membrane film of
D-16101-2
6~88
_ ~6 -
known thickness in a constant volume - variable
pressure perrneation cell. Both sides of the
membrane were degassed under vacuum overnight and
one side of the membrane was then e~pos~d to the gas
at 25 psig. The permeate gas was collected in a
reser~oir on the other side of the membrane and the
gas pressure was measured using a sensitive
transducer. The pressure build-up as a function of
time was recorded on a strip chart and the data was
used to determine the steady state permeation rate P.
The permeability rate P is reported in 3arrer units,
a Barrer unit being:
(cm3 (STP) cm/cm2-sec. cm Hg) X 10 10
The membranes were prepared from 2 to 10 weight
percent polymer solutions in methylene chloride and
were from about 2 to about 10 mils thick. The
solvent was removed under vacuum at 40~C and finally
at 125C for 5 days before evaluation.
Experiments 1 to 5 show the preparation of
intermediates used for producing polyester membranes
used in the e~amples of this invention. The
structures of the compounds were confirmed by both
proton and C-13 nuclear magnetic resonance analyses,
and melting points.
~xperiment 1
The procedure used was that described by
F.S. Holahan et al., Makromol. Chem., 103(1), 36-46
(1967).
To a two liter 3 necked flask eguipped with
a stirrer, addition funnel, c~ondenser, thermometer
and a 10% sodium hydro~ide trap there were added
201.76 grams of 4,4'-t2,2,2-trifluoro~l-(trifluoro-
D-16101-2
;~06~
methyl)ethylidene]bisphenol, 3G0 ml ethanol, and 140
ml water. To this reaction mi~ture was added with
good stirring 124.84 ml of bromine over a 3 hour
period at 15C. The reaction mixture was stirred
overnight. About 3 grams of sodium thiosulfate was
added to decompose the excess bromine. Three liters
of distilled water was added to precipitate the
product. The product was filtered and washed 3
times with water and dried in a vacuum oven at
80C. Yield of 4,4'[2,2,2-trifluoro-1-(trifluoro-
methyl)ethylidene]bis[2,6-dibromophenol] (Compound
I) was 388 grams. The prod~ct was recrystallized
from chlorobenzene to give an overall yield of 87%
m.p. e 256.5-258C. Literature m.p. ~ 256-257C.
Experiment 2
A one liter 3-necked round bottom flask was
eguipped with a stirrer, chlorine gas inlet fitted
sparge tube, a dry ice-acetone condenser, and an
outlet leading to a 10% sodium hydroxide trap and
charged with 67.25 9 of 4,4'-(hexafluoroisopropylidene)
diphenol and 600 ml of dichloromethane, and cooled
with an ice water bath to about 20C. Chlorine gas
was sparged in at a rate to maintain a saturated
solution; the temperature was controlled at about
20C. After 8 hours the dichloromethane was removed
using a rotary evaporator under vacuum to obtain 8Bg
(93~ yield) of 4,4'-[2,2,2,-trifluoro-1-
(trifluoromethyl)ethylidene]bis[2,6-dichlorophenol]
(Compound II). Recrystallization in methanol/water
gave an overall yield of B0~ ~f purified product,
m.p. 225-227C. (Literature mp 223-224C-see
experiment 1).
D-16101-2
~o~
- 23 -
To a 1000 ml. 3 necked round bottom flask
equipped with an addition funnel, thermometer,
thermowatch tempeIature regulator and a dry ice~
acetone condenser insulated with glass wool was
added 457.5 grams 2,6-dimethylphenol, 75 grams
methane sulfonic acid, and the reaction mi~ture was
heated to 95C. Then 75 grams of 1,1,1,3,3,3-he~a-
fluoro-2-propanone sesquihydrate was added dropwise
in one hour. The reaction mixture was heated to
148C in two hours. In 3 additional hours the
temperature was up to 160C. The progress of the
reaction was followed by isolating a 10 gram sample
and removing the acid with water and sodium
bicarbonate and the dimethyl phenol with methylene
chloride, drying, and taking a melting point. After
15 hours at 160 the melting point was 208-217C.
In 22 hours at 160 the melting point was
221-223~C. The reaction mi~ture was worked up by
pouring the warm semi-solid into a 4000 ml beaker
and washang it 5 times with 2000 ml portions water.
Then 400 ml of methylene chioride was added and the
sample washed with an additional 3 times with 20 ml
portions of water. Complete acid neutralization was
obtained by adding a few grams of sodium
bicarbonate. The methylene chloride layer was
separated along with some solid and the solvent and
residual dimethylphenol was removed on the rotary
evaporator via vacuum up to 165C. Yield of
4,4'-[2,2,2,-trifluoro-1-(trifluoromethyl)ethylidene3-
bis[2,6-dimethylphenol] (Compound III) was 117
grams. The sample was washed with 500 ml of
D-16101-2
~6~81~
- 29 -
methylene chloride and 500 ml toluene, and finally
with 150 ml of methylene chloricle, dried in a vacuum
oven at 80C. Yield 69 grams. The mp ~ 219-
221.5~C. Literature U.S. patent: 4,358,62q (11/9~B2)
mp ~ 218-219C.
eriment 4
To a 3000 ml 3 neck round bottom flask
equipped with a mechanical stirrer, a gas sparger,
thermometer, thermowatch temperature regulator,
hydrogen chloride lecture bottle connection, and a
10% sodium hydroxide trap for the hydrogen chloride
which escapes from the reactor, and an ice bath to
keep the temperature at 20VC there were added 273.45
grams cyclododecanone, 837.0 grams 2,6-dimethylphenol,
27.0 ml n-octyl mercaptan, 315.0 ml methylene
chloride. Hydrogen chloride was ~parged through the
solution for 7 1/2 hours at such a rate to obtain a
saturated solution. The solids obtained after 2
days at room temperature was filtered and washed 4
times with 2000 ml portions of methylene chloride.
Recrystallization twice from toluene gave a 19.7%
overall yield of the bisphenol, l,l-bis(3,5-dimethyl-
4-hydro~yphenol)cyclododecane (Compound IV), mp
240.5-242.5C. Literature U.S. patent 4,559,309
mp-239-240.5C.
Experiment 5
Procedure for the preparation of the
4-Bromoisophthaloyl and 2-bromoisophthaloyl
chlorides from their corresponding acids.
To a 500 ml 3-neck roundbottom flask
equipped with a mechanical stirrer, dropping funnel,
condenser, a silicone oil heating bath, a nitrogen
D-lÇ101-2
Z~06588
- 30 -
inlet and an outlet leading to a sodium hydrsxide
scrub solution were added 100 grams (0.408 mole) of
monobromoiso-or monobromo-terephthalic diacid and 1
ml pyridine. Then 202 ml (328.5 grams, 2077 moles)
of thionyl chloride were added dropwise. When all
material was added, the mi~ture was reflu~ed for 24
hours while hydrochloric acid and sulfur dio~ide
were given off. During this time a yellow solution
was obtained. On standing overnight no crystals
developed indicating that the diacid chlorides were
liquids. The exess SOC12 was distilled off and
the yellowish oily crude product was boiled with a
seven-fold excess of n-hexane. The hot solution was
filtered to remove unreacted diacids. The hexane
was distilled off. The samples were further
purified by distilling at a reduced pressure of 3-4
mm Hg at 125-132C.
In separate experiments, about 70 grams
each of a yellowish 4-bromoisophthaloyl chloride and
a purplish colored 2-bromoterephthaloyl chloride of
oily appearance were obtained and used directly in
the pGlymerizations.
The following e~amples serve to further
illustrate the invention. In the examples the
aromatic dicarbo~ylic acid derivatives used were
terephthaloyl chloride and isophthaloyl chloride or
mi~tures thereof, unless otherwise stated. Parts
are by weight unless otherwi~e indicated.
The flat membranes were prepared from 3 to
7 weight percent polymer solutions in methylene
chloride. A portion of the solution was poured onto
a glass plate and kept covered overnight with an
D-16101~2
2006sa~
_ 31 -
aluminum lid at ambient conditit~ns. The film was
stripped off the plate and dried in a vacuum oven at
40C ~or one day. Then the film was further dried
at 125~C in ~acuum for 5 days and its thickness
measured. The membrane was tested at 25C and 2
atmospheres pressure for pure gas, o~ygen and
nitrogen permeabilities.
The polyesters were prepared by known
interfacial polymerization procedures in a Waring
Blender and in a three-necked round bottom flask
with mechanical stirrin~ and cooling with an ice
bath. The stir rate was not always monitored, but
it was generally about 1000 rpm~ The rate of
addition of the acid chloride was based on the
control of the e~otherm. As is well known in the
literature ("Condensation Polymers by Interfacial
and Solution Methods, Chapter VII, Paul W. Morgan,
Interscience Publishers, 1965.), if everything else
is constant, the molecular weight is higher the
faster the acid chlorides are added to the reaction
mixture. Also faster stir rates are significantly
helpful and the use of a Morton flask appeared to
help obtai~ higher molecular weights.
ExamDle 1
A. Preparation of Polyarylate from
4,4~-t2,2,2,-trifluoro~ trifluoromethyl)ethylideneJ
bist2,6-dibromophenol] (Compound I) and 100%
Isophthaloyl chloride.
To a 3-necked 500 ml round bottom Morton
flask equipped with a mechanical ~tirrer,
thermometer, addition funnel, nitrogen inlet and
condenser there were added 26.07 grams of Compound
D-16101-2
~)6S~38
- 32 ~
I, 0.4 grams tetrabutyl ammoniurn hydrogen sulfate,
10.25 grams of 45.9% aqueous pol:assium hydroxide and
40 ml of distilled water, and 40 ml of methylene
chloride. With ice water cooling, a solution of
a. 12 grams of isophthaloyl chloride in 80 ml of
methylene chloride was added in about 15 minutes
with very fast stirring. After stirring for about 2
hours, 100 ml of methylene chloride was added and
the mi~ture acidi~ied by adding 0.5% sulfuric acid.
The polymer solution was washed three times with
1000 ml of distilled water. The polymer was
coagulated in methanol and dried in a vacuum oven at
80C overnight. The yield was 27.2 grams of
polyester. The reduced viscosity was 0.42.
B. A gas permeable flat membrane having a
thickness of 2.06 mils was prepared and evaluated
for the permeation of oxygen, nitrogen, carbon
dio~ide, methane and helium.
The oxygen P ~ 5.25 (xlO~lOcm3(STP)-
cm/cm2-sec-cmHg (Barrers). The oxygenJnitrogen
selectivity was 6.7.
The carbon dioxide P , 19.9 Barrers and the
carbon dio~ide/methane selectivity at 35 psia using
pure gases was 50.
The helium P - 57 Barrers and the helium/
methane selectivity was 133.
The nitrogen P ~ 0.7B7 Barrers and the
nitrogen/methane selectivity was 1.8.
~xample 2
A. Preparation of ~Polyarylate from
Compound I and 80~20 Isophthaloyl/terephthaloyl
chlorides.
D-16101-2
6~813
- 33 -
Essentially the same pzocedure as in
Example 1 but ~or two ch3nges in quantities of
reagents. 10.734 g.ams of 45.9~, aqueous potassium
hydroxide and ~.5 gram~ of isophthaloyl chloride and
1.625 grams of terephthaloyl chloride were charged.
The yield of polyester was 28 grams; the reduced
viscosity was 0.37. This polymer has an iso/tere
ratio of 80/20.
B. A gas permeable flat membrane having a
thickness of 1.24 mils was prepared and evaluated
for the peImeation of oxygen, nitrogen, carbon
dioxide, methane and helium.
The oxygen P . 5.7 (Barrers~. The oxygen/
nitrogen selectivity was 6.4.
The carbon dio~ide P ~ 24 Barrers and the
carbon dioxide/methane selectivity using pure gases
was 48.
The helium P ~ 57 Barrers and the helium/
methane selectivity was 113.
ExamPle 3
A. Preparation of Polyaryla~e from
4,9'-[2,2,2,-trifluoro-1-(trifluoromethyl)ethylidene]-
bis[2,6-dibromophenol~ (Compound I) and 25/75
Isophthaloyl/terephthaloyl chlorides.
To a 3-necked 500 ml round bottom Morton
flask equipped with a mechanical stirrer,
thermometer, addition funnel, nitrogen inlet and
condenser there were added 52.15 grams of Compound
I, 0.8 grams of tetraoutyl ammonium hydrogen
sulfate, 19.94 grams of 45.9~ aqueous potassium
hydroxide a~d 160 ml of distilled water, and 80 ml
of methylene chloride. With ice water cooling, a
D-16101-2
20~16S813
_ ~4 -
soluti~n of 12.18 grams terephtlnaloyl chloride and
4.06 grams of isophthaloyl chloride in 160 ml
methylene chloride was added in about 15 minutes
with very fast stirring. After stirring 80 minutes
100 ml of methylene chloride was added and the
mixture acidified by adding 0.5% sulfuric acid. The
polymer solution was washed three times with 500 ml
of distilled water. The polymer was coagulated in
methanol and dried in a vacuum oven at aooc
overnight. The yield was 54.5 grams of the
polyester. The reduced viscosity was O.B9.
B. Following the procedure described in
E~ample 1 a gas per~eable flat membrane 2.7 mils
thick was evaluated. A combination of high values
from both the permeability rate and the selectivity
was found to exist in both gas separation processes.
The oxygen P . 9.0 Barrers. The oxygen/
nitrogen selectivity was 6.1.
The carbon dioxide P . 42 Barrers and the
carbon dioxide/methane selectivity was 42 based on
pure gases at 35 psi~.
The helium P Y 75 Barrers and the helium/
niL,~ rselectivity was 75.
The nitrogen P ~ 1.5 Barrers and the
nitrogen/methane selectivity was 1.5.
The tetrabromobisphenol A polycarbonate
resin of Example 1 of EPA 0 242 147 showed the
oxygen P - 0.8 Barrer and an oxygen/nitrogen
selectivity of 7.4 in Table 1 of that application.
The tetrabromobisphenol A polyestercarbonate resin
of Example 4 of EPA 0 244 126' showed the oxygen P 8
D-16101-2
6~;~8
- 35 -
1.23 Barrers and an o~ygen/nitrogen selectivity of
7.2 in the Table.
The data ror the bisphe!nol polyester of
this invention shows a far superior combin2tion of
permeation and separation factol f Qr o~ygen/nitrogen
compa~ed to the values reported in the t~o
references. The polyester membrane of this
invention showed a permeation l:L.25 times higher
than that of the polycarbonatP and 7.32 times higher
than that of the polyestercarbonate of the
references.
.
A. Preparation of Polyarylate from
Compound I and 100% 4-Bromoisophthaloyl chloride.
Synthesis procedure was essentially the
same as in Example 1 but for two changes. 4 Bromo-
isophthaloyl chloride (22.554grams) was used and all
quantities are one-half of Example 1. The reduced
viscosity was 0.29 in chloroform.
B. A gas permeable flat membrane having a
~hickness of 3.91 mils was prepared and evaluated
for the permeation of oxygen, nitrogen, carbon
dioxide, methane and helium.
The oxygen P ~ 4.7 Barrers and the oxygen/
nitrogen selectivity was 6.B.
The carbon dioxide P - 19.4 Barrers and the
car~on dioxide/methane selecti~ity was 49 at 35 psia
using pure gases. Miged gas gave the carbon dioxide
P ~ 17.2 ~arrers and a selectivity of 48 at 167 psia
using a 50/50 mi~ture of gases.
The helium P , 51 ~arrers and the helium~
ni~ selectivity was 130.
,!i3~
D-16101-2
)65~8
The nitrogen P , 0.613 Barrers and the
nitrogen~methane selectivity was 1.7.
ample 5;
A. Preparation of Polyarylate from
Compound I and 100~ 2-Bromoterephthaloyl chloride.
Sy~thesis procedure is essentially the same
as in Example 1. Two changes were made,
2-brominated terephthalic acid chloride was used in
place of the isophthaloyl chloride of E~ample 1.
Only half the molar quantities of E~ample 1 were
used. The yield was 31.7 grams and the RV was 0.51.
B. Since the films looked very cloudy,
possibly due to high level of crystallinity, no
permeation measurements were made.
xample ~
A. Preparation of Polyarylate from
Compound I and 100~ Terephthaloyl chloride.
Used essentially the same procedure
described in Example 1. The only difference was
8.12 grams of terephthaloyl acid chloride was used
instead of isophthaloyl acid chloride in Example 1.
The yield was 29 grams of a polymer insoluble in
methylene chloride; it appeared to be of crystalline
structure.
~xample 7
A. Preparation of Polyarylate from an
80~20 Mole Ratio Mi~ture of Bisphenol Compounds I
and III and a 75/25 Mole Ratio Mixture of
terephthaloyl chloride and isophthaloyl chloride.
D-15101-2
~06S88
The procedure followed was that described
in Example 3. The yield of p~lyester was 27.8
grams; the reduced viscosity ~was 0.39.
B. Following the procedure described in
E~ample 1 a gas permeable flat membrane 2.3 mils
thick was evaluated. A combination of high values
for both the permeability rate and the selectivity
was found to exist in the gas separation processes
The oxygen P - 9.3 Barrers. The
oxygen/nitrogen selectivity was 5.8.
The helium P ~ 73 Barrers and the helium/
nitrogen selectivity was 46.
Example 8
A. Preparation of Polyarylate from a
70/30 Mole Ratio Mi~ture of Bisphenol Compounds I
and III and a 75/25 Mole Ratio Mi2ture of
terephthaloyl chloride and isophthaloyi chloride.
The procedure followed was that described
in Example 3. The yield of polyester was 24.93
grams; the reduced viscosity was 0.34.
B. Following the procedure described in
Example 1 a gas permeable flat membrane 4.1 mils
thick was evaluated. A combination of high values
for both the permeability rate and the selectivity
was found to exist in the gas separation processes
The o~ygen P . 11.5 Barrers. The
o~ygen/nitrogen selectivity was 5.6.
The helium P ~ 88 Barrers and the helium/
nitrogen selectivity was 43.
D-16101-2
~6~
- 38 --
E~ample 9
A. Preparation of Polyarylate from a
60/90 Mole Ratio Mi~ture of ]3isphenol Compounds I
and IV and a 75/2~ Mole Ratio Mi~ture of
terephthaloyl chloride and isophthaloyl chloride.
The procedure followed was that described
in E~ample 3 using 10.73 grams of ~6 weight %
potassium hydro~ide and 40 more ml of water. The
yield of polyester was 25~74 grams; the reduced
viscosity was 0.57.
B. Following the procedure described in
E~ample 1 a gas permeable flat membrane 4.2 mils
thick was evaluated. A combination of high values
for both the permeability rate and the selectivity
was found to exist in the gas separation processes.
The oxygen P , 8.83 Barrers and the ~ygen/
nitrogen selectivity was 5.66.
The helium P ~ 68.5 Barrers and the helium/
nitrogen selectivity was 49.
Example LQ
A. Preparation of Polyarylate from a
70/30 Mole Ratio Mixture of Bisphenol Compounds I
and II and a 75/25 Mole Ratio Mi~ture of
terephthaloyl chloride and isophthaloyl chloride.
The procedure followed was that described
in E~ample 9. The yield of polyester was 27.1
grams; the reduced viscosity was O.S9.
B. Following the procedure described in
Example 1 a gas permeable flat membrane 4.1 mils
thick was evaluated. A combination of high values
for both the permeability rate and the selectivity
was found to e~ist in the gas separation processes.
D-16101-2
~OO~i~S8
. ~g .
The oxygen P 8 9 . 95 Barrers and the oxygen/
nitrogen selectivity wa~ 5.8:2.
The helium P - 78 Barrers and the helium
nitrogen selectivity was 46.
C. ~he pure gas c,arbon dioxide P , 42
Barrers and the carbon dio~ide/methane selectivity
was 46. Mixed gas separation of a 50/50 CO2/CH4
mi~ture at 3 atmospheres pre~ssure had a selectivity
of 44 indicating no significant plasticization from
carbon dio~ide. ~his composition has a combination
of both higher carbon dio~ide permeability and
better CO2/CH4 selectivity than reported in the
art. If plasticization of these polyarylates does
not occur, as indicated, these membranes are an
unexpected and unpredictable advance in the
CO2/CH4 separations field.
xample 11
Preparation of Polyarylate from a 60i40
Mole Ratio Mixture of ~isphenol Compounds I and II
and a 75/25 Mole Ratio Mixture of terephthaloyl
chloride and isophthaloyl chloride.
The procedure followed was that described
in Example 9. The yield of polyester was 18.4
grams; the reduced viscosity was 0.61. Following
the procedure described in Example 1, permeable flat
membranes can be prepared.
Example 12
A. Preparation of Polyarylate from a
75/25 Mole Ratio Mixture of ~isphenol Compounds I
and III and a 75/25 Mole Ratio Mi~ture of
terephtha-loyl chloride and isophthaloyl chloride.
D-16101-2
~(36~;~38
- 40 ~
The procedure followed ~as that described
in Example 9. The yield of polyester ~as 26.3
grams; the reduced viscosity was 0.38.
B. ~ollowing the procedure described in
Example 1 a gas permeable flat membrane about 4 mils
thick was evaluated. ~ combination of high values
for both the permeability rate and the selectivity
was found to exist in the gas separation processes.
The oxygen P ~ 11.8 Barrers and the o~ygen~
nitrogen selectivity was 5.53.
The helium P 8 85.1 Barrers and the helium/
nitrogen selectivity was 40.
xample 13
Preparation of Polyarylate from a 60/40
Mole Ratio Mixture of Bisphenol Compounds I and II
and a 75/25 Mole Ratio Mi~ture of terephthaloyl
chloride and isophthaloyl chloride.
The procedure followed was that described
in Example 9 using 4.06 grams each of isophthaloyl
chloride and terephthaloyl chloride. The yield of
polyester was 25.2 ~rams; the reduced viscosity was
0.29. Following the procedure described in
E~ample 1 a gas permeable flat membrane can be
prepared.
~xamplE_19
Preparation of Polyarylate from a 60/40
Mole Ratio Mi~ture of Bisphenol Compounds I and IV
and a 75/25 Mole Ratio Mi~ture of terephthaloyl
chloride and isophthaloyl chloride.
The procedure followed was that described
in E~ample 1 using 6.091 grams of isophthaloyl
D-16101-2
~:00~i~;i88
- 41 -
chloride and 2.03 grams of terephthaloyl chloride.
The yield of polyester was 25.3 grams; the reduced
viscosity ~as 0.75. Following the procedure
descrihed in Ex3mple 1 a gas permeable flat membrane
can be prepared.
~parative Run 1
A. Preparation of Polyarylate from
compound II and 100~ Isophthaloyl Chloride.
Following essentially the procedure in
Example 2, a polyester was produced from 18.96 gms
of compound II and B.12 gms of isophthaloyl chloride
with a yield of 19.9 gms. The reduced viscosity was
0.46 in chloroform.
B. A gas permeable flat membrane having a
thickness of 1.88 mils was prepared and evaluated
for separation of oxygen and nitrogen.
The oxygen P ~ 5.69 Barrers and the oxygen/
nitrogen selectivity was 6.1.
The permeability values (P in Barrers) and
the oxygen/nitrogen selectivity and the helium/
nitrogen selectivity values for the polyesters of
this invention (first nine entries) and of
comparative data from the literature (last eight
entries) as derived by the instant inventors are
summarized in T~BLE 1.
D-16101-2
~0Q16~88
-- 42 --
_ _ Selectivjty__ - P (8arrers)
_____ 02/N2C02/CH4 He/N2 He~CH4 N2/CH~ Z C02 He N2
6.7 50 72 133 1~8 5.25 19.9 57 0.787
2 6.4 48 66 S .7 24 58 D .88
3 6.1 42 50 75 1.5 9 42 75 1.5
4 6.8 49 75 130 1.7 4.7 19.4 51 0.68
7 5.8 46 9.3 73 1.6
8 5.6 43 11.5 8B 2.1
9 5.66 35 44 8.83 40 68.5 1.56
5. 8246 46 9. 95 42 78 1.71
12 5.53 40 11.8 85.1 2.1
Comp. Run 1 6.1 5.64
Ex 1 (EPA-7) 7.4 0.8
Ex 6 ~EPA-7) 5.0 3.9
Bis-A Polyether 5.75 4.9
Ex 4 (EPA-6) 7.2 1.23
Ex 2 ~EPA-7) 6.3 1.448
Ex 6 (EPA-7) 5.û 26.7 3.9 16.3
Ex 7 ( Texas)5. 4 9.7
Fo~tnotes;
EPA-7 - EP0 Appl;cation 0 242 147
EPA-6 = EP0 Application 0 244 126
Texas = Tetrabromohexafluorobisphenol A polycarbonate,
University of Texas, ~. J. Koros and M. ~. Hel1ums,
September 26-27, 1989.
D - 1 6 1 0 1 - 2