Note: Descriptions are shown in the official language in which they were submitted.
Z10l0746~ : ~
, ,
, . -
,, :~ ,:: ` ` ,,
,.. '
~. .
METH0D AND APPARATUS FOR PRODUCING ~ --
BORON CARBIDE C~YSTALS
. . :
Thls invention relates to a method and ~ -
apparatus for producing boron carbide crystals. More
qpecifically, the invention is directed to the
production of boron carbide crystals of a submicron
siz~.
Boron carbide (B4C) is a ceramic material
having a high degree of hardness, good structural ~ `;
integrity at high temperatures, and chemical inertne3s.
These properties make boron carbide a useful material
for fabricating devices such as armor plating, sand
blasting nozzles, bearings, dies, control rods ~or
nuclear reactors, and rePractory liner~. In many of
these applications it i9 de3irable to use a high purity,
5 monodispersedl boron carbide powder in which the ;
crystals are less than one (1) micrometer in size. The
narrow particle size di~tribution gives the product
certain advantages. One advantage is optimum
reactivity. Another is that the material can be
hot-pressed to yield a uniform, fine-grained material
that is free of pores, excess carbon, and low mélting
metallic carbide impurities.
34,924-F _1 ~;
- ~ ~ - . . . , .. . , .. . . .. , ". . . . . . .. . .. .. .
ZQO~6~
, ~
-2-
The usual method ~or producing boron carbide
crystals i~ to place a particulate mixture of a boric
oxide compound and a carbon compound in a crucible and
pass the crucible through ~he hot zone of a high
temperature furnace. A major problem with this method
is that the mixture iq heated to it~ reaction
temperature at a rate which produce~ only a very broad
range of crystals in the micron size range, and
essentially no crystals that are smaller than one
(1) micrometer in size.
The invention is directed to an apparatus and
method for producing a quantity o~ boron carbide
crystal3 in which a major portion of the crystals are o~
sub-micrometer size.
The apparatu~ used in the practice o~ thi~
invention i~ a modified verqion of a graphite reqistance
push-type Yurnace that operates at extremely high
temperatures. The furnace unit includes a floor member
and a roof member, with the space between these two
members defining a hot zone. Heat ii delivered to the
hot zone by heater means located inside the furnace unit
adjacent to the roof member.
Another component of the furnace unit is a
vertical feed tube, which iq poqitioned above the
furnace hot zone. The feed tube is designed for feeding
a nitrogen-free particulate mixture of a boric oxide
compound and a carbon compound into the hot zone. A
coollng fluid is ciroulated around the feed tube, which
cools the tube enough to maintain the boric oxide ~eed
compound below it~ melting point. The furnace unit also
includeq a group of boat members de~igned to move along
34,924-F -2-
2~074~i0
-3-
... . .
the floor of the furnace hot zone in a path that passes
direotly below the feed tube.
In a typical operation of the furnace unit, as
the particulate mixture falls from the feed tube through
the furnace hot zone, the temperature of the hot zone is
maintained above 1570C. At this temperature the boric
oxide compound will react with the carbon compound to
form boron carbide crystal~. As each boat member moves
underneath the the feed tube, the boat is filled with a
load of the boron carbide crystals, which are carrisd in
the boat to a collection point outside of the furnace
hot zone. ;
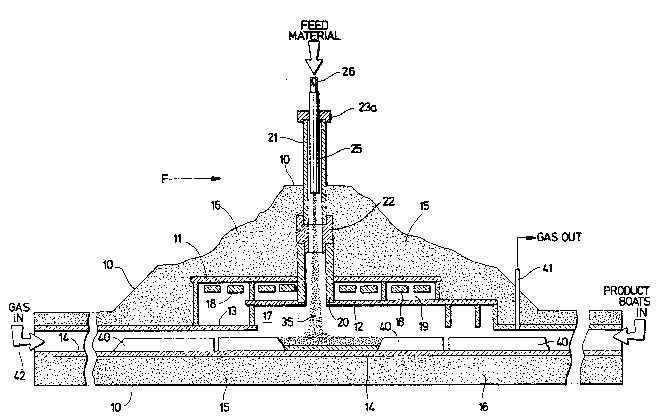
Figure 1 is a front elevation view, mostly in
~chematic, of a high temperature furnace u~ed in making
boron carbide according to this invention.
Figure 2 is a detail view, partly in schematic,
of component~ of the furnace ~hown in Fig. 1 which are
used to feed a starting material for boron carbide into
the furnace.
In the drawing7 referring particularly to
Figure 1, the high temperature furnace of thi~ invention
i9 generally designated by the letter F. The outside of
the furnace is defined by a metal qhell 10. The inside
part of the furnace i~ defined generally by a roo~
~ection and a floor member. The roof section consist~
of three deck~, namely, an upper deck 11, intermediate
deck 12, and lower deck 13, with the floor member 14
being located below deck 13. The roof section decks and
trle floor member are constructed of graphite. The space
between the shell and the rooP section, and the shell
and the floor member provides an insulation section 15.
34,924-F -3-
-. . . . , ................... . . . ....... ; ., . ~
, . .. ! ' ', . . . ..
2~)~746
In the furnace illustrated herein the insula-
tion section 15 is filled with lampblack 16~ The space
between the deck 12 and floor member 14 defines the hot
zone 17 of the ~urnace. Heater boards 18, which are
fabricated of graphite, are positioned directly above
the hot zone 17 in a space 19 between the upper deck 11
and the intermediate deck 12. A DC current is passed
through each board as the heating medium.
Above the hot zone 17 of the furnace is a
vertical chute ~tructure that consi3ts of three pieces.
The lower part of the chute is de~ined by a sleeve 207
that fastens into the deck 12 and extends up through
deck 11. The upper part of the chute is defined by a
leeve 21, of qmaller diameter than sleeve 20. The
lower end of aleeve 21 i~ coupled to the upper end o~
sleeve 20 by a transition piece 22. The top end of
sleeve 21 is fitted with a packing gland 23 and gland
nut 23a, which provide a seal aqqembly.
As shown particularly in Fig. 2, a cooling
jacket i~ mounted lengthwise inqide the sleeve 21 of the
chute. The jacket is defined by an inside tube 24,
which is enclosed within an outside tube 25. A vertical
feed tube 26 is mounted lengthwise inqide tube 24, and
an annulu~ between these tubes defines a passage 27 for
circulating a cooling fluid along the outside of the
feed tube. The cooling fluid enter~q passage 27 through
an inlet fitting 28. Another annulus between the out-
3 side of tube 24 and the in_ide of tube 25 defineq apa~sage 29 for the cooling fluid to leave the jacket
through an outlet fitting 30.
In the practice of this invention, the starting
material for producing boron carbide is a particulate -
~, "
. ..
34,924-F _4_
.
.
2~ 6~ -
:'`.`: -;
mixture of a boric oxide compound and a carbon compound.
A device for ~eeding the particulate mixture into the
feed tube 26 i3 located above the upper end of the ~eed
tube. As shown in Figure 2, one type of feeder device
that may be used is a screw feeder 31. An outlet spout -~
31a on the screw feeder iq connected to the top end of a
glass sight tube 32 with a flexible coupling 33. At its - -
bottom end the sight tube is connected to the top end of ``
feed tube 26 by another flexible coupling 34.
O~eration
A typical example will now be given to
illustrate the production of boron carbide crystals
according to the practice of this invention. The
particulate mixture consisted of a physical blend of
technical grade boric acid (U.S. Borax), size -200 mesh
tles9 than 74 micrometers) and 50 percent compressed
acetylene carbon black (Gul~ Oil Co.). The mixture was
blended for 30 minutes in a modified mortar mixer coated
with an epoxy film. The mixture was prepared to give an
excess of boron over carbon of approximately 20 percent,
based on the reaction stoichiometry:
2B203 + 7C ~ B4C + 6CO
4 mol~ B: 7 mols C = 100%
4 x 1.2 mol~ B: 7 molq C = 20% exceqs B
The mixture was heated in ti~anium pan~q for
3.5 hourq at 350C to dehydrate it to B203 + C. The
pan~ measured 2 in. high x 24 in. wide x 72 in.
(5 cm x 61 cm x 183 cm) long and they were closed with
titanium covers having 1/2 in. (1.3 cm) dia. holes -
therein to allow water vapor to escape from the mixture
during heating. A~ter cooling the dried mixture is
' ' " '`
:
34,924-F -5-
2~q6~
loosely agglomerated, but it can be easily broken up
into <10 me~h (1.68 mm) aggregates.
At this point in the preparation, the mixture
has a bulk density of about 15 lbs/cu. ft.
(240.3 kg/m3), since it contains a ~ubstantial amount oP
entrapped air. The entrapped air includes about
80 percent N2, which is undesire-able because it can
react to form boron nitride. To correct t~he problem, a
vacuum i~ pulled on the mixture to deaerate it, and
argon i~ used to fill the evacuated feed mixture. The
deaerating ~tep thus reduces the N2 concentration in the
boron carbide product to about 0.3 to 0.4 percent. In
situationi where the feed mixture i3 not deaerated, the
nitrogen content of the submicrometer boron carbide
crystals can be as high as 1 to 3 percent.
Following the deaerating step9 the particulate
mlxture 35 is loaded into a hopper 36, which is mounted
on the screw conveyor 31 at the end opposite from the
outlet spout 31a. During the production operation,
argon, as a purge gas, is directed into the hopper
through a purge line 37, and into the screw conveyor
through a purge line 38. The screw qhaft 31b in the
conveyor is driven by a motor 39, which has a variable
speed drive. The argon purge gas provides an inert
environment for the reaction of the boric oxide and
carbon compounds in the mixture 35.
As the mixture 35 moves from the conveyor 31
Lnto the feed tube 26, it passes through the glass sight
tube 32. The sight tube thu~ provides a "window" for
the furnace operator to periodically view the flow of
the reactive mixture into the furnace, and take
corrective action if it become~ necessary. From the
34,924-F -6-
~o~
_
. ..
. .
screw conveyor, the mixture ii3 delivered into the feed ~-
tube at a rate of not more than 0.3 lbs/min.
(0.7 kg/min), and preferably 0.1 to 0.2 lbi~3imin.
(0.2-0.4 kg/min). A~ the mixture falls through the feed
tube, water (or some other isuitable cooling fluid) is
5 continuou~ly circulated through the passagei~ 27 and 29 ~-
of the cooling jacket.
Cooling the feed tube ais described herein
keeps the temperature inside the tube below 300C, which
ii~ the i~oftening point o~ boric anhydride. If the
particulate mixture is not cooled as it moves through
the feed tube, it will rapidly convert to a semi-liquid
pha~e and plug off the tube. From the feed tube 26, the
1~ particulate mixture 35 Pallis downwardly through the hot
zone 17 oY furnace F and into a product boat 40, which
iq bein3 puished along the floor 14 of the furnace. As
shown in Figure 1, there is a continuous string oP the
product boati3, and they are moved by a suitable conveyor
i~yistem (not shown).
The boats are pu~hed through the hot zone 17 at
a rate of about 2 in. to 3 in. per minute (5 to
18 cm/min.). Ais the par-ticles 35 enter the hot zone 17
at the discharge end 26a of the feed tube 26, the
temperature ii~ about 1500C. The temperature increases
to about 2000C at the point where the particles fall
into the produ¢t boats. When each product boat 40
passes directly below the discharge end of the feed
3 tube, it is filled with a load of boron carbide
cryistals, and the product is carried to a collection
point (not shown) outside of the hot zone.
During the production operation, the hot ~ ~
zone 17 is filled with carbon monoxide and argon gas, to -
34,924-F -7~
~0~7460
--8
provide a desireable inert environment for the reaction
of the i~tarting material to boron carbide~ Ai~ ishown in
Figure 1, the argon i~ introduced into the hot zone at
two point~; one point is through the feed inlet pipe 26
and the other point i~ at the le~t end of the zone, a~
indicated by arrow 42. The argon !Ytream entering the
hot zone from the left end also serves another purpose.
Thi_ streiam move~ in a direction countercurrent to the
path followed by the product boats 40 a~ they leave the
hot zone. The ga_ _tream thus acts ai~ a barrier to
prevent the particlei~ in the mixture 35 from being swept
out of the hot zone before the boron carbide reaction i
completed.
A key to obtaining boron carbide cry~tal~ in a
_ub-micrometer size iq to be able to heat the starting
material above it~ initiation reaction temperature very
rapldly. In the practice of thii~ invention, therefore,
it i9 critical that the temperature of the hot zone be
maintained above 1570C. Another critical factor i3 the
time it take~ to heat the particles above the reaction
temperature. In the operation de~cribed herein the
temperature of the hot zone will be 1600C to 2100C and
the rate at which the particleq are heated a~ they move ~
through the hot zone is at lea~t 200C per isecond. The ~ ;
amount of boron carbide cryiqtal~ produced was about one
(1) pound per hour (0.46 hg/hr), of which about
90 percent by weight of the cry3tals were of
3ub-micrometer ~ize. The actual size wai~ 0.1 to
0.3 miorometer~. ~
~'":' ,"
' '
: . .: -
;'' " ' '
34,924-F -8-
:
: '
"