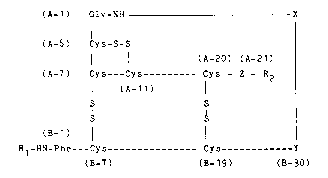Note: Descriptions are shown in the official language in which they were submitted.
2~ 2~5
HOECHST AKTIENGESELLSCHAFT HOE 89/F 021 Dr. ME/rh
Description
Pro~ess for the preparation of an insulin precursor
Insulin is a molecule which consists of 2 polypeptide
chains linked to one another via disulfide bridges. The
A chain consists of 21 amino acids and the B chain of 30
amino acids. These two chains are linked to one another
in the precursor molecule, the proinsulin, by a peptide,
the C-peptide. The C-peptide in human proinsulin consists
of 35 amino acids. In the context of the maturation
proeess of the hormone, the C-peptide is split off by
specific proteases and the proinsulin is in this way
converted into insulin (Davidson et al., Nature 333, 93-
96, 1988). In addition to the naturally occurring C-
peptides, a large number of bonding possibilities betweenthe A chain and B chain are described in the literature
(Yanaihara et al., Diabetes 27, 149-160 (1978), Busse et
al., Biochemistry 15, 1649-1657 (1971) and Geiger et al.,
Biochem. Biophys. Res. Com~ 55, 60-66 (1973)).
In the context of genetic engineering, it is now possible
to prepare insulin from microorganisms modified by
genetic engineering. If E. coli is used as the micro-
organism, the product is often expre~sed as fusion
protein, that is to say the product is coupled with a
protein endogenous to the bacteria, for example with ~-
galactosidase. This fusion protein precipitates out in
the cell and i8 in this way protected from proteolytic
degradation. After breakdown of the cell, the fu&ian
protein content is split off chemically or enzymatically
and the 6 cysteines of the insulin precursor are con-
verted into their S-sulfonates (-S-S03- ) by means of
oxidative sulfitolysis. Natural preproinsulin must be
produced from this so-called preproinsulin S-sulfonate in
a subsequent step, the 3 correct disulfide bridges being
formed.
2~ 5
-- 2 --
This step is carried out, for example, hy the process
described in EP-B-0,037,255 by reaction of the starting
5-sulfonate with a mercaptan in an amount which results
in 1 to 5 SH radicals per SS03- radical, in an aqueous
medium at a pH of 7 to 11.5 and a S-sulfonate concentra-
tion of up to 10 mg per ml of aqueous medium, preferably
in the absence of an oxidizing agent.
However, to obtain high folding yields - that is to say
high yields of preproinsulin with the "correct" peptide
sequence linkages (-S-S- bridges from A6 to A11, from A7
to ~7 and from ~20 to Bl9) - it is necessary to maintain
the stated - narrowly limited - SH/SS03- ratio, which
requires a not inconsiderable care in carrying out the
process and in particular a - not entirely simple -
quantitative determination of the SS03- groups in the
starting S-sulfonate.
Surprisingly, it has now been found that high folding
yields - which are virtually independent of the SH/SS03-
ratio within wide limits - are obtained if the procedure
~0 is carried out at a higher - that is to say above 5:1 -
SH/SS03- ratio in the presence of an organic redox system
or compounds which form such an organic redox system
under the reaction conditions; instead of the SS03-
groups, it is also possible for other S-protective groups
to be present in the corresponding starting substance; in
addition, the peptide sequences of the insulin A and B
chain can also be modified by replacement of one or more
amino acids.
The higher SH/SS03- (or S-protective groups) ratio means
that - as long as only a certain minimum amount of
mercaptan i8 exceeded - the procedure can be carried out
virtually independently of the level of mercaptan excess
without substantial impairment in the yield; exact
quantitative determination of the Ss03- (or S-protective~
groups in the corresponding insulin starting substance is
in this way also superfluous, which represents a con-
82~S
-- 3 --siderable advantage of the process.
Carrying out the process virtually independently of the
level of the mercaptan excess is made possib~e by the
presence of an organic redox system or of compounds which
form such an organic redox system under the reaction
conditions.
Although it is known that reduced proinsulin - that is to
say proinsulin, the S-S bridges of which, for example,
have been split reductively with a mercaptan to give SH
groups - can be reoxidized to the original proinsulin and
that this reoxidation is accelerated by the presence of
dehydroascorbic acid - cf. D.F. Steiner and J.L. Clark,
Proc. Natl. Acad.Sci. USA 60, 622-629 (1968) - even if a
redox system of dehydroascorbic acid and ascorbic acid
should be formed from dehydroascorbic acid under the
reaction conditions described therein, it is a matter
there of oxidation of reduced proinsulin with unprotected
S groups, whereas in the present case according to the
invention only insulin precursors having protected S
groups are suitable starting substances. In addition, the
literature reference by D.F. Steiner and J.L. Clark loc.
cit. mentioned contains no indication or suggestion at
all in the direction of the use and the action of an
organic redox system in recombination of insulin precur-
sors containing S-protective groups by means of mercap-
tans.
In detail, the invention thus relates to a process for
the preparation of an insulin precursor of the formula I
200~5
-- 4 --
(A-1) Gl~-NH X
I
(A-6) Cys-S-S
1 l (A-20) (A-21)
(A-7) Cys---Cys~ ---- Cys - Z - R2
¦ (A~ (I)
S S
S S
(B-1) 1 1
R1-HN-Phe---Cys--------------- Cys------------Y
(B-7) (B-19) (B-30)
in which R1 = H or
an amino acid or peptide radical which can
be split off chemically or enzymatically,
S R2 = OH or an amino acid or peptide radical,
preferably OH,
X = a radical which bonds the insulin A and
chain, preferably an amino acid or peptide
radical,
Y = the radical of a genetically encodable
amino acid, preferably Thr, Ala or Ser, in
particular Thr,
Z = the radical of a genetically encodable
amino acid, preferably Asn, Gln, Asp, Glu,
Gly, Ser, Thr, Ala or Met, in particular
Asn, and
Al-A20 and Bl-B29 = peptide sequences of insulin
which are non-mutated or mutated by re-
placement of one or more amino acid radi-
cals, preferably non-mutated peptide
~equences of human, porcine or bovine
insulin, in particular human or porcine
insulin,
by reaction of a precursor having protected Cys-S groups
with a mercaptan in aqueous medium; the process comprises
reactinq a precursor having protected Cys-S groups, of
s
- s -
the formula II
(A-1) Gly-NH X
I
(A-6) Cys-S-R3 S-R3
I l (A-20) (A-21)
(A-7) Cys-------Cys--------- Cys - Z - R2
¦ (A~ (II)
S-R3 S-R3
S-R3 S-R3
(B-1) ¦ l
R1-HN-Phe---Cys------------------- Cys--------------Y
(~~7) (B-19) (B-30)
in which Rl, Rz, X, Y, Z, Al-A20 and Bl-B29 have the same
meaning as in formula I and
R3 = a Cyæ-S protective group,
preferably the -S03- or the tert.-butyl group,
with a mercaptan in an amount corresponding to a (mer-
captan) SH/(insulin precursor) Cys-S-R3 ratio of more than
5 in the presence of an organic redox system or at least
one organic compound which forms such an organic redox
system under the reaction conditions.
If R2 in formula I and II = H, the substances are proin-
sulin or products derived from proinsulin; if R1 = an
amino acid or peptide radical which can be split off
chemically or enzymatically, the substances are prepro-
insulin and products derived therefrom.
Amino acid radicals which can be split off chemically are
those which can be split off, for example, by means of
BrCN or N-bromosuccinimide; these are, for example,
methionine (Met) or tryptophan (Trp).
Amino acid radicals which can be split off enzymatically
are those which can be split off, for example, by means
of trypsin (such as, for example, Arg or Lys).
Peptide radicals which can be split off chemically or
- 6 ~ 2~5
enzymatically are peptide radicals having at least 2
amino acids.
All the amino acids possible for Rl are preferably from
the group of naturally occurring amino acids, that is to
say mainly Gly, Ala, Ser, Thr, Val, ~eu, Ile, Asn, Gln,
Cys, Met, Tyr, Phe, Pro, Hyp, Arg, Lys, Hyl, Orn, Cit and
His.
R2 is OH or - similarly to Rl - likewise an amino scid or
peptide radical, the meaning of OH being preferred. The
amino acids (including those which form the peptide
radical - consisting of at least 2 amino acid radicals)
preferably originate - as for Rl - from the group of
naturally occurring amino acids.
X is a radical which bonds the insulin A and B chains,
preferably an amino acid or peptide radical.
If X is an amino acid radical, the radical of Arg or Lys
is preferred; if X is a peptide radical, the radical of
a naturally occurring C-peptide - in particular of human,
pork or bovine insulin C-peptide - is preferred.
Genetically encodable amino acids - for Y - are (in each
case in the L form): Gly, Ala, Ser, Thr, Val, Leu, Ile,
Asp, Asn, Glu, Gln, Cys, Met, Arg, Lys, His, Tyr, Phe,
Trp and Pro.
Preferred genetically encodable amino acids are Thr, Ala
and Ser, in particular Thr.
Z can - like Y - likewise denote the radical of a geneti-
cally encodable amino acid, but in this case A~n, Gln,
Asp, Glu, Gly, Ser, Thr, Ala and Met, in particular Asn,
are preferred.
Al - A20 and B1 - B29 can in principle be the peptide
sequences, which are non-mutated or mutated by
- 7 - Z ~ S
replacement of one or more amino acids, of all possible
insulins; the mutants can be produced by known processes
of genetic engineering (site directed mutagenesi~).
Howe~er, the non-mutated peptide sequences of human,
S porcine or bovine insulin, in particular of human or
porcine insulin (the A1 - A20 and Bl - B29 sequences of
human and porcine insulin are identical) are preferred.
The radical R3 which occurs only in formula II denotes
virtually any desired Cys-S-protective group, but prefer-
ably the -S03- or the tert.-butyl group, the -S03- group
being of somewhat greater importance here.
The starting substance of the formula II can on principle
be employed within a wide concentration range - advan-
tageously between about 10 ~g and 10 mg/ml of solution -
lower concentrations as is known leading to higher
renaturing yields, 8 ince at low protein concentrations
the tendency towards aggregation is reduced. Preferred
concentrations are between about 0.1 mg and 0.5 mg/ml.
Suitable mercaptans for the reaction according to the20 invention are in principle all the possible organic
compounds having SH groups; mercaptoethanol, thioglycolic
acid, glutathione and cysteine, in particular mercap-
toethanol and cysteine, are preferred. The mercaptans can
be employed individually or as a mixture.
The amount of mercaptan is (to be) chosen 80 that the
ratio of its SH groups to the Cys-SR3 groups of the
starting material of the formula II is, as far as pos-
8 ible, greater than 5.
The upper limit of this ratio is set virtually only by
economic considerations; an upper limit of about 100 is
advantageous. A ratio of about 10 - 50, in particular
about 10 - 30, is preferred. The mercaptan concentration
in the reaction batch then depends on the amount of
starting material of the formula II employed and the
zo~ s
-- 8 --
chosen SH/Cys-SR3 ratio.
Preferred possible organic redox systems are pairs of
compounds, one component of which is an organic compound
having the structural element of the formula III
OH OH O
11
-- C = C -- C --
O OH OH (III)
- ~ - C = ~ -
or an aromatic o- or p-dihydroxy compound and the other
component of which i6 an organic compound having the
structural element of the formula III in oxidized form =
structural element of the formula III'
O O ~
C ~ C (III')
or an o- or p-quinone.
The free valencies of the structural element of the
formula III and III' can be satisfied by hydrogen or
organic groups, such as, for example, Cl-C4-alkyl groups.
However, the structural element can also be part of a
ring having preferably 4, 5 or 6 C ring atoms and if
appropriate also one or two heteroatoms, such as, for
example, O, it being possible for the ring in turn to be
substituted by groups which are inert under the reaction
conditions, such as, for example, alkyl or hydroxyalkyl
groups.
Examples of compounds having the structural element of
the formula III are:
reductone
OH OH O
~1
H - C = C - C - H
- g - ;~ 2~r.
reductic acid OH OH
HC CH
H2C~ ~ ~o
H2
methylreductic acidOH OH
HC CH
\C ~ O
H CH~
ascorbic acid OH OH
(vitamin C)OH HC - CH
HOCH2 - CH - CH C - O
~ O~
S The formulae are written here in each case in only one of
the tautomeric forms.
All the compounds are reducing. In the oxidized form, the
structural element of the formula III becomes that of the
formula III'.
Suitable aromatic o- and p-dihydroxy compounds are in
principle all the possible aromatic compounds having two
OH groups in the o- or p-po~ition, it merely al~o being
necessary that the o- or p-quinone formation from the o-
and p-dihydroxy compounds cannot be prevented by any
particular substituents or the like. Examples of aromatic
o- and p-dihydroxy compounds are
1,2-dihydroxybenzene = pyrocatechol,
1,4-dihydroxybenzene = hydroquinone,
methyl-hydroquinone, naphtho-1,4-hydroquinone and anthra-
hydroquinone; the corresponding quinones are formedtherefrom on oxidation.
In the reaction according to the invention, the par-
ticular organic redox systems, consisting, for example,
- 10 - ~ ~('8 X ~S
of the pairs of compounds ascorbic acid + dehydroascorbic
acid, pyrocatechol + o-quinone, hydroquinone + p-quinone,
naphthohydroquinone + naphthoquinone and the like, can
thus be employed in virtually any desired ratio (prefer-
ably in approximately the equimolar ratio). However, itis also possible for the particular individual components
of these pairs of compounds - that is to ~ay, for ex-
ample, only ascorbic acid or only dehydroascorbic acid or
only hydroquinone and the like - to be added, because in
each case the other component (dehydroascorbic acid or
ascorbic acid or p-quinone and the like) belonging to the
redox pair of compounds i6 formed in the reaction medium.
Preferred organic redox systems are the combinations con-
~isting of the pairs of compounds
ascorbic acid + dehydroascorbic acid,
pyrocatechol + o-quinone and
hydroquinone + p-quinone,
and preferred individual compounds which form such a
redox system under the reaction conditions are the
individual components of these pairs of compound~.
Ascorbic acid and/or dehydroascorbic acid are especially
preferred.
The amount employed of the compound(s) which form(s) the
organic redox system can be varied within wide limits.
The number of moles of the compound(s) which form(s) the
organic redox system can be chosen between about 1/10,000
and 10,000, preferably between about 1/10 and 10, based
on one gram equivalent of mercaptan (= molecular weight
of the mercaptan employed in g/number of SH groups in the
mercaptan molecule).
The reaction according to the invention is advantageously
carried out in the alkaline pH range, preferably between
about 7 and 12, in particular between about 9.5 and 11.
To maintain the desired pH, the addition of a buffer
substance is advantageous, the nature and ionic strength
X~ 32~5
of the buffer having a certain influence on the folding
yield. It is advantageous to keep the ionic strength low,
a range of about ~ mM (mM = millimolar) to 1 M (M =
molar~, in particular one of about 5 mM to 50 mM, being
preferred. Examples of buffer substances which can be
used are borate buffer, carbonate buffer or glycine
buffer, the latter being preferred.
A range between about 0 and 45C can be fitated as a
general range for the reaction temperature; a range from
about 4 to 8C is preferred.
Covering the renaturing solution with a layer of certain
gases, such as, for example, oxygen, nitrogen or helium,
has no noticeable influence on the renaturing yield.
The duration of the reaction is in general between about
2 and 24 hours, preferably between about 6 and 16 hours.
When the reaction has ended (which can be ascertained,
for example, by high performance liquid chromatography),
the mixture is worked up in a known manner, such as is
also described, for example, in the abovementioned EP-B-
0,037,255.
The "correctly" folded product of the formula I can then
be converted into the corresponding insulin enzymatically
or chemically by known techniques.
The following examples are now intended to explain the
invention further, and also to illustrate the advantages
over the prior art.
Preproinsulin-S-S03~ obtained by genetic engineering was
used as the starting material for the xenaturing experi-
ments.
E. coli was used as the expression system. In E. coli,
the gene for proinsulin is coupled with a part of the ~-
2~ 2~S
- 12 -
galactosidase gene and i6 synthesized as fusion protein,
which precipitates in the cell and is deposited in the
polar caps ~by the process of DE-A-3,805,150). After
breakdown of the cell, the fusion protein content is
split off by means of cyanogen halide (in accordance with
the process of DE-A-3,440,988) and is then sub~ected to
oxidative sulfitolysis (R.C. Marshall and A.S. Ingles in
A. Darbre (publishers) ~'Practical Protein Chemistry - A
Handbook" (1986), pages 49 - 53), in order to convert the
6 cysteines into their S-sulfonate form. The prepro-
insulin-S-SO3~ (the prefix ~pre~ means that the proinsulin
is extended on it~ N-terminus by 5 amino acids) prepared
in this way is then concentrated by means of ion
exchangers in a manner which i5 known per ~e, precipita-
ted and freeze-dried. According to determination by high
performance liquid chromatography, the freeze-dried
starting material obtained in this way has a content of
60%.
1. Dependence of the folding yield on the mercaptan/
ascorbic acid ratio
Preproinsulin-S-SO3~ (60%) is dissolved in a con-
centration of 0.33 mg/ml in 20 mM glycine buffer, pH
10.5, which corresponds to a preproinsulin-S-SO3~
concentration of 0.2 mg/ml. 20 ml are employed per
batch, to which 480 ~1 of a 0.1 M cysteine solution (~
20-fold molar exce6s per S-S03- group) and between 0
and 480 ~1 of a 0.1 M ascorbic acid solution are
added. The renaturing temperature is 8C and the
duration of the reaction is 16 hours.
30Ascorbic acid (0.1 M) Folding yield
0 25~ ] comparison
24 ~1 28% ~
48 ~1 53% according to the
96 ~1 78% invention
35480 ~1 81%
This example shows the influence of the redox compound
z~
_ 13 -
on the folding yield. Whereas predominantly only
formation of incorrectly folded proteins occurs in the
batch without ascorbic acid, the folding yield in-
crease~ in the presence of the redox compound.
2. Dependence of the folding yield on the
mercaptan/ascorbic acid exces~
The amount weighed out, volume, buffer, reaction time
and temperature and pH correspond to experiment 1. In
each case equimolar amounts of cysteine and ascorbic
acid are added, the molar excess per S-S03- group being
between 2.5 and 100.
Amount of cysteine Excess per Folding yield
and ascorbic acid added S-S03- group
60 ~1 2.5 38%~ comparison
15 120 ~1 5 70% J
240 ~1 10 83~
480 ~1 20 85~ according to
1200 ~1 50 81~ the inven-
2400 ~1 100 72~ tion
This example shows that - as soon as a certain minLmum
amount of mercaptan is exceeded - the folding yield is
largely independent of the mercaptan excess within a
wide range.
3. Dependence of the folding yield on the pH
The amount weighed out, volume, buffer concentration
and reaction time and temperature correspond to
experiment 1. In the experiment, 480 ~1 of 0.1 N
cysteine solution and 480 ~1 of 0.1 M ascorbic acid
solution are added ~~ 20-fold molar excess per S-S03-
group)
32~5
- 14 -
pH Folding yield
1~.0 79~
10.5 81%
10.0 66%
9.5 53%
9.0 46%
8.5 33%
8.0 24%
4. Dependence of the folding yield on the
10type of mercaptan
The amount weighed out, volume, buffer concentration,
reaction time and temperature and pH correspond to
experiment 1. In the experiment, in each case 480 ~1
of the corresponding 0.1 M mercaptan solution and
480 ~1 of 0.1 M ascorbic acid solution are added
(z 20-fold molar excess per S-S03- group)
Mercaptan Folding yield
Cysteine 81%
Mercaptoethanol 86%
Glutathione 7s%
Thioglycolic acid 74%
3-Mercapto-1,2-propanediol 76%
5. Dependence of the folding yield on the buffer
fiubstance and on the ionic ~trength of the buffer
The amount weighed out, volume, reaction time and
temperature and pH correspond to experiment 1. In the
experiment, in each case 480 ~1 of the corresponding
0.1 M mercaptan solution and 480 ~1 of 0.1 M ascorbic
acid solution are added (~ 20-fold molar excess per
S-S03- group).
Buffer Folding yield
10 mM glycine 89~
100 mM glycine 82%
10 mM borate 82%
~ 2
15 -
100 mM borate 51%
10 mM carbonate 88~
100 mM carbonate 72%
6. Dependence of the folding yield on the reaction tL~e
S The amount weighed out, volume, buffer concentration,
pH and reaction temperature correspond to experiment
1. In each case 480 ~1 of a 0.1 M mercaptoethanol
solution and 480 ~1 of a 0.1 M ascorbic acid solution
are added (- 20-fold molar excess per S-S03- group)
10Reaction time (hours) Folding yield
0.25 25%
0.5 40%
1 46%
2 61%
4 70%
6 77%
8 83%
24 79%
7. Dependence of the folding yield on the preproinsulin-
20S-sulfonate concentration
The volume, buffer composition, pH, reaction time and
temperature correspond to experiment 1. In each case
enough mercaptoethanol stock solution and ascorbic
acid stock solution are added to the amount of prepro-
insulin-S-~ulfonate employed for a ~ 20-fold molar
excess per S-S03- group to exist.
Preproinsulin-S-S03~ concentration Folding yield
0.1 ml/ml 86%
0.2 ml/ml 84%
0.3 ml/ml 78
0.4 ml/ml 65~
0.5 ml/ml 55%
1.0 ml/ml 19~
2.5 ml/ml 5%
~2~)G~4~,
_ 16 -
8. Dependence of the folding yield on the ascorbi~ acid/
mercaptoethanol excess using modified starting
material
A precursor molecule in which the A and B chain of the
insulin are linked only by an arginine is employed.
The production of this molecule by genetic engineering
is carried out as described at the start of the
descriptions of the experiments. The freeze-dried
material has a content of 60% and is dissolved in a
concentration of 0.5 mg/ml in 20 mM glycine buffer, pH
10.5, which corresponds to a precursor concentration
of 0.3 mg/ml. The reaction time and temperature
correspond to experiment 1 and the molar excess of the
ascorbic acid/mercaptan mixture varies between 2.5-
and 50-fold.
Excess per S-S03 group Folding yield
2.5 56%~ comparison
61%1
74~ according to
75% the invention
55~
9. Dependence of the folding yield on the redox system
The amount weighed out, volume, buffer composition, pH
and reaction time and temperature correspond to
experiment 1. A ~ 20-fold molar excess of mercapto-
ethanol/S-SO3~ group and a 20-fold molar excess of the
corresponding redox partner are added~
Redox partner Folding yield
Ascorbic acid 77%
Dehydroascorbic acid 70%
Pyrocatechol 66%
Hydroquinone 40%
Benzo(-p-)quinone 23%