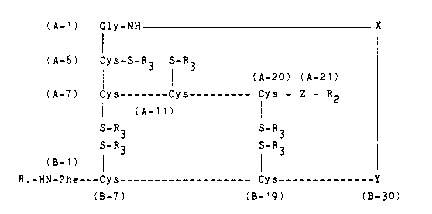Note: Descriptions are shown in the official language in which they were submitted.
fi
HOECHST AKTIENGESELLSCHAET HOE 8~/F 020 Dr.ME/rh
Description
Process for renaturing incorrect recombinants of insulin
precursors
Insulin is a molecule which consists of 2 polypeptide
chains linked to one another via disulfide bridges. The
A chain consists of 21 amino acids and the B chain of 30
amino acids. These two chains are linked to one another
in the precursor molecule, the proinsulin, by a peptide,
the C-peptide. The C-peptide in human proinsulin consists
of 35 amino acids. In the context of the maturation
process of the hormone, the C-peptide is split off by
specific proteases and the proinsulin is thus converted
into insulin (Davidson et al., Nature 333, 93-96, 1988).
In addition to the naturally occurring C-peptides, a
large number of ~oining possibilities between the A ohain
and B chain are described in the literature (Yanaihara et
al., Diabetes 27, 149-160 (1978), Busse et al., Bio-
chemistry 15, 1649-1657 (1971), and Geiger et al., Bio-
chem. Biophys. Res. Com. 55, 60-66 (1973)).
In the context of genetic engineering, it is now possible
to prepare insulin from microorganisms modified by
genetic engineering. If E. coli is used as the micro-
organism, the product is frequently expressed as fusion
protein, that is to say the product is coupled with a
protein endogenous to the bacteria, for example with ~-
galactosidase. This fusion protein precipitates out in
the cell and is in this way protected from proteolytic
degradation. After breakdown of the cell, the fusion
protein content i8 split off chemically or enzymatically
and the 6 cysteines of the insulin precursor are con-
verted into their S-sulfonates (-S-S03- ) by means of
oxidative sulfitolysis. In a subsequent ("renaturing or
recombination~) step, natural preproinsulin must be
produced from this so-called preproinsulin S-sulfonate by
formation of the 3 correct disulfide bridges - that is to
~ L~ 6
say -S-S- bridges from A6 to All, from A7 to B7 and from
A20 to Bl9 in the corresponding insulin peptide sequen-
ces.
According to the process described in EP-B-0,037,255,
this step is carried out, for example, by reaction of the
starting S-sulfonate with a mercaptan in an amount which
results in 1 to 5 SH radicals per SS03- radical in an
aqueous medium at a pH of 7 to 11.5 and an S-sulfonate
concentration of up to 10 mg per ml of aqueous medium,
preferably in the absence of an oxidizing agent.
Yields of in some cases more than 80% are said to be
obtained here.
In addition to the (desired) renaturing products with
correct disulfide bridges, more or less substantial
amounts of ~undesired) "incorrect" recombinants, that is
to say insulin products with disulfide bridges which are
only partly correct or not correct at all and also with
intermolecular disulfide bridges, are always also formed
- depending on the reaction conditions and in particular
the concentration circumstances - in this and practically
all other known renaturing or recombination processes in
which insulin precursors with opened disulfide bridges
are converted into products with the correspondingly
closed disulfide bridges.
When the insulin precursor products obtained by the known
renaturing processes are worked up to insulin without the
"incorrect" recombinants being removed - this working up
being effected by known techniques (chemically or
enzymatically) - no (natural) insulin is formed from the
"incorrect" recombinants.
It is therefore advantageous or necessary to remove the
~incorrect" recombinants from the renaturing products
with the correct disulfide bridges before working up the
corresponding renaturing products to give insulin. This
~0(~ 4fi
-- 3 --
can be effected, for example, by known chromatographic
processes. In another particularly advantageous process,
the removal is effected by adjusting the reaction mixture
to pH 4 to 6 - preferably in the presence of a small
amount of a physiologically acceptable surface-active
substance - the ~correct recombinants remaining virtu-
ally completely in solution and the "incorrect" recom-
binants being precipitated (c.f. DE-A-35 01 641).
The "incorreck" recombinants removed are then advantage-
ously converted back by sulfitolysis into the correspond-
ing S-sulfonate, which is subjected to renewed folding,
it often being necessary to remove by-products formed
during the sulfitolysis by chromatography before the
renaturing. The ~incorrect recombinants can in this way
largely be converted back into "usable" product.
Sulfitolysis of the "incorrect" recombinants with subse-
quent chromatography and renewed folding of course means
a not inconsiderable expenditure.
In the efforts to avoid or at least reduce this expendi-
ture, it has now been found that this is possible byreacting the "incorrect" recombinants of insulin precur-
sors with excess mercaptan in an aqueous medium in the
presence of an organic redox system or at least one
organic compound which forms such an organic redox system
under the reaction conditions.
The "incorrect" recombinants can in this way be converted
in high yields directly into the "correct" renaturing
products or "correct" recombinants without sulfitolysis,
which represents a considerable advantage compared with
the prior art.
There is also no prior art at all which would have made
obvious in any manner bypassing of sulfitolysis with the
subsequent folding stage merely by reaction with excess
mercaptan and the addition of an organic redox system.
~OC~
-- 4 --
Possible incorrect recombinants of insulin precursors
for the process accordin~ to the invention are preferably
the products which are formed as by-products on recom-
bination of insulin precursors with opened -S-S- bridges
of the following formula I:
(A-1) Gly-NH -- X
I
(A-6) Cys-S-R3 S-R3
I l (A-20) (A-21)
(A-7) Cys-------Cys--------- Cys - Z - R2
I (A~ (I)
S-R3 S-R3
S-R3 S-R3
(3-1) 1 l
R1-HN-Phe---Cys------------------~ Cys--------------Y
~B-7) (B-19) (B-30)
in which R~ = H or
an amino acid or peptide radical which can
be split off chemically or enzymatically,
R2 = OH or an amino acid or peptide radical,
preferably OH,
R3 - H or
a Cy~-5 protective group,
preferably the -SO3- or the tert.-butyl
group,
X = a radical which ~oins the insulin A and B
chains, preferably an amino acid or peptide
radical,
Y = the radical of a genetically encodable
amino acid, preferably Thr, Ala or Ser, in
particular Thr,
Z = the radical of a genetically encodable
amino acid, preferably Asn, Gln, Asp, Glu,
Gly, Ser, Thr, Ala or Met, in particular
Asn, and
~l-A2Q and Bl-B29 = peptide sequences of insulin
which are non-mutated or mutated by re-
placement of one or more amino acids,
- s -
preferably the non-mutated peptide sequen-
ces of human, porcine or bovine insulin, in
particular of human or porcine insulin.
If R, = H in formula I, the products are products which
are derived from proinsulin; if Rl = an amino acid or
peptide radical which can be split off chemically or
enzymatically, the products are products which are
derived from preproinsulin.
Amino acid radicals which can be split off chemically are
those which are split off, for example, by means of BrCN
or N-bromosuccinimide; these are, for example, methionine
(Met) or tryptophan (Trp).
Amino acid radicals which can be split off enzymatically
are those which can be split off, for example, by means
of trypsin (such as, for example, Arq or Lys).
Peptide radicals which can be split off chemically or
enzymatically are peptide radica's having at least 2
amino acids.
All the amino acids possible for Rl are preferably those
of the group of naturally occurring amino acids, that is
to ~ay mainly Gly, Ala, Ser, Thr, Val, Leu, Ile, Asn,
Gln, Cys, Met, Tyr, Phe, Pro, Hyp, Arg, Lys, Hyl, Orn,
Cit and His.
R2 is OH or - sLmilarly to Rl - likewise an amino acid or
peptide radical, the meaning of OH being preferred. The
amino acids (including those which form the peptide
radical - consisting of at ~east 2 amino acid radicals)
preferably originate - as for Rl - from the group of
naturally occurring amino acids.
R3 is hydrogen or a cysteine-sulfur protective group, the
-S03- or the tert.-butyl group being preferred cysteine-
sulfur protective groups.
;~0~ 6
X is a radical which joins the insulin A and B chains,
preferably an amino acid or peptide radical.
If X is an amino acid radical, the radical of Arg or Lys
is preferred; if X is a peptide radical, the radical of
a naturally occurring C-peptide - in particular the
human, porcine or bovine insulin C-peptide - is
preferred.
Genetically encodable amino acids - for Y - are (in each
case in the L form): Gly, Ala, Ser, Thr, Val, Leu, Ile,
Asp, Asn, Glu, Gln, Cys, Met, Arg, Lys, His, Tyr, Phe,
Trp and Pro.
Preferred genetically encodable amino acids are Thr, Ala
and Ser, in particular Thr.
Z can - like Y - also denote the radical of a genetically
encodable amino acid, but in this case Asn, Gln, Asp,
Glu, Gly, Ser, Thr, Ala and Met, in particular Asn, are
preferred.
Al - A20 and Bl - B29 can in principle be the peptide
sequences which are non-mutated or mutated by replacement
of one or more amino acids and originate from all pO8-
sible insulins; the mutants can be produced by known
processes of genetic engineering (site directed mutagene-
sis~. However, the non-mutated peptide sequences of
human, porcine or bovine insulin, in particular of human
or porcine insulin ~the Al - A20 and B1 - B29 sequences
of human and porcine insulin are identical) are
preferred.
The "incorrect" recombinants formed as by-products during
recombination of insulin precursors with opened -S-S-
bridges of the formula I are removed from the "correct"
recombinants in a known manner - preferably by precipita-
tion at pH values of ~ to 6 in accordance with the
process of the abovementioned DE-A-35 01 641 - and are
X0(~46
then dissolved directly, or after prior freeze-drying, in
water or an aqueous solution for use for the process
according to the invention.
The concentration of the "incorrect" recombinants in the
aqueous starting solution can vary within a wide range;
preferred concentrations are about 0.1 to about 100 mg,
in particular about 0.1 to about 10 mg/ml, the mg values
relating to the "incorrect~ recombinants as dried solid.
Suitable mercaptans for the reaction according to the
invention are in principle all the possible organic
compounds with SH groups; mercaptoethanol, thioglycolic
acid, dithioerythritol, qlutathione and cysteine, in
particular mercaptoethanol and cysteine, are preferred.
The mercaptans can be employed individually or as a
mixture.
The amount of mercaptan to be employed can vary within
wide limits; an excess of mercaptan corresponding to a
ratio of mercaptan-SH groups~cysteine-S units (in the
~incorrect~' recombinants) of at least about 5 ifi pre-
ferred. This ratio has an upper limit imposed practicallyonly by economic considerations. An upper limit of about
100 i8 advantageous.
Because of the wide range of variation of the excess
mercaptan, the number of cysteine-S units in the "in-
correct" recombinant employed does not have to be deter-
mined completely accurately.
Preferred possible organic redox systems are pairs of
compounds, one component of which is an organic compound
having the structural element of the formula II
OH OH O
1~ (II)
O OH OH
-- C -- C _ C --
ZOG~ 6
-- 8 --
or an aromatic o- or p-dihydroxy compound and the other
component of which is an organic compound having the
structural element of the formula II in oxidized form =
structural element of the formula II'
O O O
5- C - C - C - (II')
or is an o- or p-quinone.
The free valencies of the structural element of the
formula II and II' can be satisfied by hydrogen or
organic groups, such as, for example, C1-C4-alkyl groups.
However, ~he structural element can also be part of a
ring having preferably 4, 5 or 6 carbon ring atoms and if
appropriate also one or two hetero atoms, such as, for
example, O, it being possible for the ring in turn to be
substituted by groups which are inert under the reaction
conditions, such as, for example, alkyl or hydroxyalkyl
groups.
Examples of compounds having the structural element of
the formula II are:
reductone OH OH O
H - C = C - C - H
reductic acid
pH OH
HC - CH
\C'
o
H2
methylreductic acid
OH OH
H~ -- CH
C O
H CH3
X~ 6
g
ascorbic acid OH OH
(vitamin C) OH HC CH
HOCH2 - CH - CH C - O
O
The formulae here are in each case written only in one of
the tautomeric forms.
All the compounds are reducing. In the oxidized form, the
structural element of the formula II becomes that of the
formula II'.
Possible aromatic o- and p-dihydroxy compounds are in
principle all the possible aromatic compounds having two
OH groups in the o- or p-position, it merely being
necessary that the o- or p-quinone formation from the o-
and p-dihydroxy compounds cannot be prevented by any
particular substituents or the like. Examples of aromatic
o- and p-dihydroxy compounds are
1,2-dihydroxybenzene = pyrocatechol,
1,4-dihdyroxybenzene = hydroquinone,
methyl-hydroquinone, naphtho-1,4-hydroxyguinone and
anthra-hydroquinone; on oxidation, the corresponding
quinones are formed therefrom.
In the reaction according to the invention the particular
organic redox systems consisting, for example, of the
pairs of compounds of ascorbic acid + dehydroascorbic
acid, pyrocatechol + o-quinone, hydroquinone + p-quinone,
naphthohydroquinone + naphthoquinone and the like, can
thus be employed in virtually any desired ratio (prefer-
ably in an approximately equimolar ratio). However, it is
also possible for only the particular individual com-
ponen~s of these pairs of compounds - that is to say, for
example, only ascorbic acid or only dehydroascorbic acid
or only hydroquinone and the like - to be added, because
the other particular component belonying to the redox
pair of compounds ~dehydroascorbic acid or ascorbic acid
or p-quinone and the like) forms in the reaction medium.
x~ >~
-- 10 --
Preferred organic redox systems are the combinations
consisting of the pairs of compounds
ascorbic acid + dehydroascorbic acid,
pyrocatechol + o-quinone and
hydroquinone + p-quinone
and preferred individual compounds which form such a
redox system under the reaction conditions are the
individual components of these pairs of compounds.
Ascorbic acid and/or dehydroascorbic acid are especially
preferred.
The amount employed of the compound(s) which form(s) the
organic redox system can vary within wide limits. The
number of mol of compound(s) which fo~m(s) the organic
redox system can be chosen as being between about
1/10,000 and 10,000, preferably between about 1/10 and
10, based on one gram equivalent of mercaptan (= mole-
cular weight of the mercaptan employed in g/number of SH
groups in the mercaptan molecule)~
It is advantageous also to add urea to the reaction
solution, concentrations corresponding to about 0.1 to 1
M ~M = molar), in particular 0.1 - 0.5 M, being pre-
ferred.
The reaction according to the invention is advantageously
carried out in the alkaline pH range, preferably between
about 7 and 12, in particular between about 9.5 and 11.
To maintain the desired pH, it is advantageous to add a
buffer substance, the nature and ionic strength of the
buffer having a certain influence on the folding yield.
It is advantageous to keep the ionic strength low, a
3~ range of about 1 mM (mM = millimolar) to 1 M (M = molar),
in particular one from about 5 mM to 50 mM, being pre-
ferred. Buffer substances which can be used are, for
example, borate buffer, carbonate buffer or glycine
buffer, the latter being preferred.
XO~ ~4fi
-- 11
A general range of reaction temperature which may be
stated is one between about 0 and 45C; a range from
about 4 to 8DC is preferred.
Covering the renaturing solution with a layer of certain
gases, such as, for example, oxygen, nitrogen or helium,
has no noticeable influence on the renaturing yield.
The reaction time is in general between about ~ and 24
hours, preferably between about 6 and 16 hours.
The renaturing product of the reaction according to the
invention is - if the "incorrect" starting recombinant
originates from recombination of an insulin precursor
with opened S-S bridges of the abovementioned formula I -
an insulin precursor having correct disulfide bonds
(~correct" recombinant) of the formula III
(A-1) Gly-NH X
I
(A-6) Cys-S-S
I ¦ (A-20) (A-21)
(A-7) Cys---Cys--------- Cys - Z - R2
¦ (A-11) l (III)
S S
(B-1) l l
Rl-HN-Phe---Cys--------------- Cy~------------Y
(~-7) tB-19) (B-30)
in which Rl, R2, X, Y and Z have the same meaning as in
formula I.
When the reaction has ended (which can be ascertained,
- for example, by high performance liquid chromatography),
the mixture is worked up in a known manner.
The "correctly" folded product, preferably of the formula
III, can then be converted into the corresponding insulin
enzymatically or chemically by known techniques.
~o~
- 12 -
The following example is intended to illustrate the
invention in more detail. Before the (invention) example,
the preparation of the starting substance is also des-
cribed by way of example.
A) Preparation of the starting substance
1. Folding of "miniproinsulin"
~Miniproinsulin-S-SO3~, that is to say an insulin
precursor in which the A and B chains of the insulin
are linked via an arginine and the B chain is leng-
thened N-terminally, is employed. The freeze-dried
material (60% pure) is dissolved at a solids con-
centration of 0.5 g/l in 50 mM glycine buffer, pH
10.7, which corresponds to a precursor concentration
of 0.3 g/l. 630 ml of 1 M mercaptoethanol and 630 ml
of 1 M ascorbic acid are added to the batch (100 1),
and the mixture is then stirred slowly in a cold
chamber at 8C for 16 hours. The folding yield,
determined by high performance liquid chromatography
against a standard, is 0.228 g/l (76% of theory).
2. Precipitation of aggregates ("incorrect" recombinants)
1 g of polyethylene-polypropylene glycol is added to
the folding batch and the total batch is divided into
5 batches of 20 ml, with which a pH precipitation
series between pH 5.0 and pH 7.0 is set up in 0.5 pH
value steps. After establishing the pH values, the
mixtures are left to stand at room temperature for 15
minutes and the precipitates are then centrifuged off.
The supernatants are quantified by means of high
performance liquid chromatography in order to deter-
mine the precipitation losses, and the precipitates
are combined ~nd freeze-dried (weight: 15 g of solid,
content 40% pure).
Loss of correctly folded product as a function of the
pH:
20~~
- 13 -
pH 5.0 0~
pH 5.5 6%
pH 6.0 10
pH 6.5 9
pH 7.0 4~
This series shows that the optimum precipitation pH is
5Ø
B) Example according to the invention: Renaturing
15 g of freeze-dried precipitate are taken up in 5 1 of
~ M urea. 13.2 ml of mercaptoethanol (14.35 M) are added
(final concentration about 35 mM) and the mixture is left
at room temperature for 10 minutes. The 5 1 of solution
are introduced into 25 1 of 50 mM glycine buffer, 188 ml
of 1 M ascorbic acid are added and the pH is brought to
10.7. The batch iæ then stirred gently at 8C for 5
hours. The folding yield is 0.146 g/l (73% of theory).
The course of the reaction i~ monitored by means of high
performance liquid chromatography.
The total yield of the "miniproinsulin" folding (c.f. A1)
can thus be increased from 76~ of theory to about 85% of
theory.