Note: Descriptions are shown in the official language in which they were submitted.
2008315
TITLE
OLEFIN COPOLYMERS AND PROCESSES FOR PREPARING SAME
FIFT~n OF T~F INVFNTION
5This invention relates to novel ethylene copolymers and
process for preparing the same and more particularly to novel
ethylene copolymers excellent in flowability in spite of the
fact that they are narrow in molecular weight distribution
(Mw/Mm) in comparison with conventionally known ethylene
copolymers, and to process for preparing the same.
In a further aspect, the invention relates to novel
propylene random copolymers and process for preparing the
same and more particularly to novel propylene random
copolymers having low melting points in comparison with
conventionally known propylene random copolymers and, in
particular, excellent in heat-sealing properties and anti-
block properties, and to process for preparing the same.
In another aspect, the invention, relates to olefin
polymerization catalysts capable of polymerizing olefins with
excellent polymerization activity even when the amount of
aluminoxane used is reduced and capable of giving olefin
polymers having high molecular weights.
BACKGROUND OF T~F INVENTION
'$
2 72932-62
2008315
~ hen molded into articles such as film, copolymers of
ethylene and a-olefins of 3 to 20 carbon atoms are found to
have excellent mechanical strength such as tensile strength,
tear strength or impact strength and also excellent heat
resistance, stress crack resistance, optical characteristics
and heat-sealing properties in comparison with conventional
high-pressure low density polyethylenes, and are known as
materials particularly useful for the preparation of
inflation film or the like.
Generally speaking, the ethylene copolymers mentioned
above have such excellent characteristics that when said
copolymers come to be narrower in molecular weight
distribution represented by the ratio (Mw/Mn) of weight
average molecular weight (Mw) to number average molecular
weight (Mn), the molded articles obtained therefrom, such as
film, are found to be less tacky. However, when these
ethylene copolymers having a narrow molecular weight
distribution are melted, there are such drawbacks that their
flowability represented by the ratio (MFR1o/MFR2) of MFR1o
under a load of 10 kg to MFR2 under a load of 2.16 kg as
measured at 190C is small, with the result that they become
poor in moldability.
Therefore, if ethylene copolymers which are small in
value of Mw/Mn and narrow in molecular weight distribution
and, moreover, large in value of MFR1o/MFR2 and excellent in
~J
3 2~831S
flowability come to be obtained, such ethylene copolymers are
certainly of great commercial value.
On the other hand, polypropylene has wide applications
in the field of plastics because of its excellent physical
properties. For example, polypropylene is widely used as
packaging film material. In the applications of the type,
however, because of its relatively high melting point,
polypropylene is generally copolymerized with ethylene or a-
olefins of 4 to 20 carbon atoms in order to improve heat-
sealing properties at low temperature, and is used in theform of propylene/a-olefin copolymer.
Packaging films formed from these known propylene/a-
olefin copolymers are still not sufficient in heat-sealing
properties, though they are excellent in transparency and
scratch resistance in comparison with those formed from low
density polyethylene, and accordingly it is hoped that
propylene/a-olefin copolymers excellent in heat-sealing
properties even at lower temperatures will come to be
obtained.
It is well known that the above-mentioned propylene/a-
olefin random copolymers may be improved in heat-sealing
properties by increasing the proportion of ethylene or a-
olefin of 4 to 20 carbon atoms to propylene in the copolymer.
However, if the proportion of ethylene or a-olefin of 4 to 20
carbon atoms is increased in the copolymerization, the
2~08315
resulting propylene/a-olefin copolymer increases in amount of
the solvent-soluble component, whereby the resultant
copolymer come to be poor in anti-blocking properties and
also in stiffness.
Such propylene/a-olefin random copolymers excellent in
heat-sealing properties, anti-block properties and stiffness
as mentioned above are available only when they have a low
melting point in spite of the fact that the proportion of a-
olefin in the copolymer is small.
Incidentally, olefin polymerization catalysts composed
generally of titanium compounds or vanadium compounds and
organoaluminum compounds have heretofore been used for
preparing ethylene copolymers. In recent years, however,
catalysts composed of zirconium compounds and aluminoxane
have been proposed of late as new Ziegler polymerization
catalysts. Japanese Patent L-O-P Publn. No. 19309/1983
discloses a process for polymerizing ethylene and one or two
or more C3-C12 ~-olefins at a temperature of from -50C to
200C in the presence of a catalyst composed of a transition
metal containing compound represented by the following
formula
(Cyclopentadienyl)2MeRHal
20~8~315
wherein R is cyclopentadienyl, C1-C6 alkyl or halogen, Me is a
transition metal, and Hal is halogen, and a linear
aluminoxane represented by the following formula
A120R4 (Al (R) ~ ) n
wherein R is methyl or ethyl, and n is a number of 4-20, or a
cyclic aluminoxane represented by the following formula
1 o L=~ Al (R) ~ ) n+2
wherein R and n are as defined above. The publication cited
above describes that ethylene should be polymerized in the
presence of small amounts, up to 10% by weight, of somewhat
long chain a-olefins or mixtures thereof in order to control
a density of the resulting polyethylene.
Japanese Patent L-0-P Publn. No. 95292/1984 discloses an
invention relating to a process for preparing a linear
aluminoxane represented by the following formula
R /R \ R
Al ~ t Al ~ n Al\
R \ J R
6 2~08;~15
wherein n is 2-40, and R is C1-C6 alkyl, and cyclic
aluminoxane represented by the following formula,
~ ( Al (R) - ) n+2
wherein n and R are as defined above. The publication cited
above describes that olefins are polymerized in the presence
of the aluminoxane prepared by the process of said
publication, for example, methyl aluminoxane in admixture
with a bis(cyclopentadienyl) compound of titanium or
zirconium, whereupon at least twenty-five million g of
polyethylene per 1 g of the transition metal per hour is
obtained.
Japanese Patent L-O-P Publn. No. 35005/1985 discloses a
process for preparing olefin polymerization catalysts,
wherein an aluminoxane compound represented by the following
formula
Rl Rl
Al - O~ Al ~ -) n Al
R0 R1 `R0
wherein R1 is C1-C1o alkyl, and R0 is R1 or represents -O- by
linkage therewith, is first allowed to react with a magnesium
compound, the resulting reaction product is then chlorinated,
7 ;~0831S
followed by treating with a Ti, V, Zr or Cr compound. This
publication cited above describes that the catalysts prepared
by the process of said publication are particularly useful
for the copolymerization of mixtures of ethylene and C3-C12 a-
olefins.
Japanese Patent L-O-P Publn. No. 35006/1985 discloses a
combination of ta) mono-, di- or tri-cyclopentadienyl of two
or more different transition metals or derivatives thereof
and (b) -aluminoxane as a catalyst system for preparing
reactor-blend polymers. Example 1 of the above-cited
publication discloses that polyethylene having a number
average molecular weight of 15,300, a weight average
molecular weight of 36,400 and containing 3.4% of the
propylene component has been obtained by polymerization of
ethylene with propylene in the presence of a combination of
bis(pentamethylcyclopentadienyl)zirconium dimethyl and
aluminoxane as the catalyst. Example 2 of the said
publication discloses polymerization of ethylene with
propylene in the presence of a combination of
bis(pentamethylcyclopentadienyl)zirconium dichloride,
bis(methylcyclopentadienyl)zirconium dichloride and
aluminoxane as the catalyst, whereby a blend of polyethylene
and ethylene/propylene copolymer is obtained, said
polyethylene having a number average molecular weight of
2,000, a weight average molecular weight of 8,300 and the
- 2~08~1~
propylene content of 7.1 mol%, and comprising a toluene-
soluble portion having a number average molecular weight of
2,200, a weight average molecular weight of 11,900 and the
propylene content of 30 mol% and a toluene-insoluble portion
having a number average molecular weight of 3,000, a weight
average molecular weight of 7,400 and the propylene content
of 4.8 mol%. Similarly, Example 3 of the said publication
discloses a blend of LLDP and an ethylene/propylene
copolymer, said LLDPE comprising a soluble portion having a
molecular weight distribution (Mw/Mnj of 4.57 and the
propylene content of 20.6% and an insoluble portion having
the molecular weight distribution of 3.04 and the propylene
content of 2.9 mol%.
Japanese Patent L-O-P Publn. No. 35007/1985 discloses a
process for polymerizing ethylene alone or together with a-
olefins of at least 3 carbon atoms in the presence of a
catalyst system containing metallocene and a cyclic
aluminoxane represented by the following formula
~ Al ~ -) n
R
wherein R is alkyl of 1-5 carbon atoms, and n is an integer
of 1 to about 20, or a linear aluminoxane represented by the
following formula
9 20~1~315
R2Al-O~ Al - ) n AlR2
wherein R and n are as defined above. According to the said
publication, the polymers obtained by the above-mentioned
process are alleged to have a weight average molecular weight
of from about 500 to about 1,400,000 and a molecular weight
distribution of 1.5-4Ø
Japanese Patent L-O-P Publn. No. 35008/1985 discloses
polyethylene or copolymers of ethylene and C3-Clo a-olefins,
both having a broad molecular weight distribution, obtained
by using a catalyst system containing at least two kinds of
metallocenes and aluminoxane. The said copolymers disclosed
in the above-mentioned publication are alleged to have a
molecular weight distribution (Mw/Mn) of 2-50.
Japanese Patent L-O-P Publn. No. 130314/1986 discloses
polypropylene high in isotacticity obtained by polymerization
of propylene in the presence of a catalyst system comprising
a sterically fixed zirconium-chelate compound and
aluminoxane.
J. Am. Chem. Soc., 109, 6544 (1987) discloses formation
of a high molecular weight isotactic polypropylene obtained
by polymerization of propylene in the presence of a catalyst
system comprising ethylenebis(indenyl)hafnium dichloride or
its hydride and aluminoxane, said isotactic polypropylene
-
1 o 20~8~15
having a narrow molecular weight distribution (Mw/Mn) of
2.1-2.4.
Japanese Patent L-O-P Publn. No. 142005/1987 discloses a
stereoblock polypropylene having Mw/Mn of 5.0-14.9 obtained
by polymerization of propylene in the presence of a catalyst
system comprising tetramethylethylenebis(cyclopentadienyl)
titanium chloride and aluminoxane. The polypropylene thus
obtained is short in isotactic chain length and is a rubbery
polymer.
The present inventors have come to accomplish the
present invention on the basis of their finding that ethylene
copolymers which are small in Mw/Mn and narrow in molecular
weight distribution and, moreover, large in MFR1o/MFR2 and
excellent in flowability are obtained by copolymerization of
ethylene with a-olefins of 3-20 carbon atoms in the presence
of olefin polymerization catalysts composed of specific
hafnium compounds and organoaluminum oxy-compounds.
Furthermore, the present inventors have found that when
propylene and a-olefins of 4-20 carbon atoms are
copolymerized in the presence of olefin polymerization
catalysts composed of specific hafnium compounds and
aluminoxane, there are obtained propylene/a-olefin copolymers
which are narrow in molecular weight distribution and small
in amount of the a-olefin copolymerized therewith, but are
low in melting point in comparison with conventionally known
1 1 20~8~15
propylene/a-olefin copolymers, on which the present invention
has been based.
Accordingly, the present invention is to solve such
problems associated with the prior art as mentioned above,
and an object of the invention is to provide ethylene
copolymers which are small in Mw/Mn and narrow in molecular
weight distribution and, moreover, which are large in
MFR1o/MFR2 and excellent in flowability, and processes for
preparing the same.
A further object of the invention is to provide
propylene/a-olefin copolymers which are narrow in molecular
weight distribution and small in amount of the a-olefin
copolymerized therewith but have a low melting point and,
moreover, which are excellent in heat-sealing properties and
also excellent in anti-block properties and stiffness, and
processes for preparing the same.
Another object of the invention is to provide olefin
polymerization catalysts, which produce polymers which are
narrow in molecular weight distribution in homopolymerization
or narrow in molecular weight and composition distribution in
copolymerization with high polymerization activities even by
the use of small amounts of aluminoxane, furthermore, produce
easily polymers which are high in molecular weight.
DISCTOSU~F. OF T~F INV~NTION
12 2 0 O a 3 1 S
The ethylene copolymers of the present invention are
those having (a) structural units derived from ethylene and
(b) structural units derived from a-olefin of 3-20 carbon
atoms, which are characterized in that they have
(i) a density of 0.85-0.92 g/cm3,
(ii) an intrinsic viscosity [~] of 0.1-10 dl/g as measured
in decalin at 135C,
(iii) a (Mw/Mn) ratio of a weight average molecular weight
(Mw) to a number average molecular weight (Mn) of
1.2-4 as measured by GPC, and
(iv) a (MFR1o/MFR2) ratio of MFRlo under a load of 10 kg to
MFR2 under a load of 2.16 kg of 8-50 as measured at
1 90C .
The processes for preparing ethylene copolymers of the
present invention are characterized in that ethylene and ~-
olefins of 3-20 carbon atoms are copolymerized so that a
density of the resulting copolymers becomes 0.85-0.92 g/cm3,
in the presence of catalysts formed from
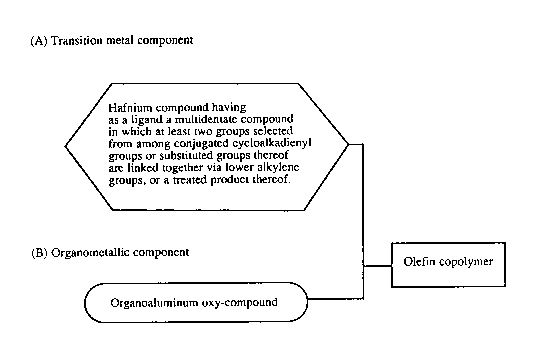
[A] hafnium compounds having multidentate coordination
compounds as ligands, in which at least two groups
selected from among conjugated cycloalkadienyl groups
or substituted groups thereof are linked together via
lower alkylene groups, or hafnium compounds obtained by
treating the above-mentioned hafnium compounds with
alkylsilylated silica gel, and
-- ~0~8~15
1 3
[B] organoaluminum oxy-compounds.
The first propylene/a-olefin random copolymers of the
present invention are those having (a) structural units
derived from propylene and (b) structural units derived from
a-olefins of 4-20 carbon atoms, which are characterized in
that they have
(i) 90-99 mol% of said structural units ~a) and 1-10 mol% of
said structural units (b),
(ii) an intrinsic viscosity [~] of 0.5-6 dl/g as measured in
decalin at 135C,
(iii) a melting point [Tm], as measured by a differential
scanning calorimeter, falling within the range of the
formula 90<Tm<155-3.5 (100-P) wherein P is the propylene
component (mol%) contained in the copolymer,
(iv) a (Mw/Mn) ratio of less than 3.5 between a weight
average molecular weight (Mw) and a number average
molecular weight (Mn) as measured by GPC, and
(v) a boiling trichloroethylene-insoluble matter in an
amount of less than 5% by weight.
The processes for preparing the first propylene/a-olefin
copolymers of the present invention are characterized in that
propylene and a-olefins of 4-20 carbon atoms are
copolymerized at a temperature of 40-100C so that the
resulting copolymers have 90-99 mol% of structural units (a)
derived from propylene and 1-10 mol% of structural units (b)
14 2 O ~ 8
derived from the a-olefin, in the presence of catalysts
formed from
[A] hafnium compounds having multidentate coordination
compounds as ligands, in which at least two groups
selected from among conjugated cycloalkadienyl groups
or substituted groups thereof are linked together via
lower alkylene groups, and
[B] organoaluminum oxy-compounds.
The second propylene/a-olefin random copolymers of the
present invention are those having (a) structural units
derived from propylene, (b) structural units derived from
ethylene and (c) structural units derived from a-olefins of
4-20 carbon atoms, which are characterized in that they have
(i) 90-99 mol% of said structural units (a), 0.5-9.5 mol% of
said structural units (b) and 0.5-9.5 mol% of said
structural units (c),
(ii) an intrinsic viscosity [~] of 0.5-6 dl/g as measured in
decalin at 135C,
(iii) a melting point [Tm], as measured by a differential
scanning calorimeter, falling within the range of the
formula 70<Tm<155-5.5 (100-P) wherein P is the propylene
component (mol%) contained in the copolymer, and
(iv) a boiling trichloroethylene-insoluble matter in an
amount of less than 5% by weight.
_ ZOCi8315
The processes for preparing the second propylene/a-
olefin copolymers of the present invention are characterized
in that propylene, ethylene and a-olefins of 4-20 carbon
atoms are copolymerized so that the resulting copolymers have
90-99 mol% of structural units (a) derived from propylene,
0.5-9.5 mol% of structural units (b) and 0.5-9.5 mol% of
structural units (c), in the presence of catalysts formed
from
[A] hafnium compounds having multidentate coordination
compounds as ligands in which at least two groups
selected from among conjugated cycloalkadienyl groups
or substituted groups thereof are linked together via
lower alkylene groups, and
[B] organoaluminum oxy-compounds.
In accordance with the present invention, there are
provided olefin polymerization catalysts formed from
[A] hafnium compounds having multidentate coordination
compounds as ligands in which at least two groups
selected from conjugated cycloalkadienyl groups or
substituted groups thereof are linked together via lower
alkylene groups,
[B] organoaluminum oxy-compounds, and
[C] organoaluminum compounds.
-
16 2 O ~ 8 ~ 1 5
In accordance with the present invention, furthermore,
there are provided olefin polymerization catalysts formed
from
[A] hafnium compounds having multidentate coordination
compounds as ligands in which at least two groups
selected from among conjugated cycloalkadienyl groups
or substituted groups thereof are linked together via
lower alkylene groups,
[B-1] organoaluminum oxy-compoun~ds formed from tri-n-alkyl
aluminum, and
[B-2] organoaluminum oxy-compounds in which at least one
hydrocarbon group other than n-alkyl group is bonded to
Al atom.
BRIFF DFSCRIPTION OF T~F DRAWINGS
Fig. 1 is an illustration of process for the preparation
of the olefin copolymers of the present invention.
Fig. 2 is a graph showing the relationship between the
propylene content and melting point of the propylene random
copolymer of the present invention.
Figs. 3-5 are stepwise illustrative of the method of
evaluation of heat-sealing properties of the propylene random
copolymer of the present invention.
BFST MODF OF PRACTICING T~F INVF.NTION
17 Z ~ ~ 8 3 1 5
The ethylene copolymers of the present invention and
processes for preparing the same are illustrated below in
detail.
The process for the preparation of the ethylene
5 copolymers of the present invention is illustratively shown
in Fig. 1
The ethylene copolymers of the invention are random
copolymers of ethylene and a-olefins of 3-20 carbon atoms.
The ethylene copolymers have a density of 0.85-0.92 g/cm3,
0 preferably 0.85-0.91 g/cm3 and especially 0.85-0.90 g/cm3.
In this connection, the density of these ethylene
copolymers was measured by gradient tube density
determination using the strand of ethylene copolymer used at
the time of determining MFR2 under a load of 2.16 kg at 190C.
In these ethylene copolymers, are present desirably
structural units ~a) derived from ethylene in an amount of
60-96 mol%, preferably 6S-95 mol% and especially 70-94 mol%,
and structural units (b) derived from ~-olefin of 3-20 carbon
atoms in an amount of 4-40 mol%, preferably 5-35 mol% and
especially 6-30 mol%.
The composition of the copolymer is determined by
measuring a spectrum of 13C-NMR of a specimen obtained by
dissolving about 200 mg of the copolymer in 1 ml of
hexachlorobutadiene in a test tube of 10 mm0 under the
conditions of a measurement temperature of 120C, measurement
1 8 20~8~15
frequency of 25.05 MHz, spectrum width of 1500 Hz, pulse
repetition time of 4.2 sec. and pulse width of 6 sec.
Alpha olefins of 3-20 carbon atoms used in the present
invention include propylene, 1-butene, 1-pentene, 1-hexene,
4-methyl-1-pentene, 1-octene, 1-decene, 1-dodecene, 1-
tetradecene, 1-hexadecene, 1-octadecene, 1-eicosene, etc.
The ethylene copolymers of the present invention
desirably have an intrinsic viscosity [~] of 0.1-10 dl/g,
preferably 0.5-6 dl/g as measured in decalin at 135C.
The molecular weight distribution (Mw/Mn) as obtained
by gel permeation chromatography (GPC) of the ethylene
copolymers of the invention is 1.2-4, preferably 1.4-3.5 and
especially 1.5-3Ø As is evidenced by the foregoing, the
ethylene copolymers of the invention are narrow in molecular
weight distribution and excellent in anti-block properties.
In this connection, a value of (Mw/Mn) obtained in the
invention was determined by the following procedure in
accordance with Takeuchi, "Gel Permeation Chromatography,"
Maruzen, Tokyo.
(1) Using a standard polystyrene having the known molecular
weight (a monodispersed polystyrene produced and sold by Toyo
Soda K.K.), molecular weight M and GPC (Gel Permeation
Chromatography) count of the sample are measured to prepare a
correlation diagram calibration curve of the molecular weight
1 9 Z~ 8~15
M and EV (Elution Volume). The concentration of the sample
used is maintained at 0.02% by weight.
(2) GPC chromatograph of the sample is taken by GPC
measurement, and a number average molecular weight Mn and a
weight average molecular weight Mw, in terms of polystyrene,
are calculated from the calibration curve mentioned in the
above procedure (1) to obtain a value of Mw/Mn. In that
case, the conditions under which the sample is prepared, and
the conditions under which GPC measurement is conducted are
as follows:
[Preparation of sample]
(a) The sample is put in an Erlenmeyer flask together with
o-dichlorobenzene so that the same amounts to 0.1% by weight.
(b) The Erlenmeyer flask is heated at 140C and stirred for
about 30 minutes to dissolve the sample in o-dichlorobenzene.
(c) The solution is subjected to GPC.
[GPC measurement conditions]
The measurement was conducted under the following
conditions.
(a) Apparatus 150C-ALC/GPC manufactured by Waters Co.
(b) Column GMH Type manufactured by Toyo Soda K.K.
(c) Amount of sample 400 ~l
(d) Temperature 140C
(e) Flow rate 1 ml/min
2 o Z~)~38~15
In the ethylene copolymers of the invention, a
(MFRlo/MFR2) ratio of MFRlo at 190C under a load of 10 kg to
MFR2 at 190C under a load of 2.16 kg is 8-50, preferably 8.5-
45 and especially 9-40.
Such ethylene copolymers having the MFRlo/MFR2 ratio
falling within the range of 8-50 as mentioned above are quite
favorable in flowability at the time when they are melted.
In contrast thereto, thé aforementioned known ethylene
copolymers having the Mw/Mn ratio of 1.2-4 will come to have
the MFR1o/M~R2 ratio in the range of 4-7, and hence they are
poor in flowability at the time when they are melted.
As mentioned above, the ethylene copolymers of the
present invention have such excellent characteristics that
they have a small molecular weight distribution (Mw/Mn), and
molded articles resulting therefrom are less sticky and, at
the same time, they are large in MFRlo/MFR2 and excellent in
flowability at the time when they are melted.
The ethylene copolymers of the invention as illustrated
above may be prepared by copolymerization of ethylene with ~-
olefins of 3-20 carbon atoms so that the resulting copolymers
have a density of 0.8S-0.92 g/cm3 in the presence of
catalysts formed from
[A] hafnium compounds having multidentate coordination
compounds as ligands in which at least two groups
selected from among conjugated cycloalkadienyl groups
2 1 2~83ls
or substituted groups thereof are linked together via
lower alkylene, or hafnium catalyst components obtained
by treating the above-mentioned hafnium compounds with
alkylsilylated silica gel, and
5 [B] organoaluminum oxy-compounds.
The catalyst components [A] used in the invention are
hafnium compounds having multidentate coordination compounds
as ligands in which at least two groups selected from among
conjugated cycloalkadieny~ groups or substituted groups
thereof, e.g. indenyl group, substituted indenyl group and
partially hydrated compounds thereof, are linked together via
lower alkylene groups, or compounds obtained by treating the
above-mentioned hafnium compounds with alkylsilylated silica
gel.
The above-mentioned hafnium compounds include, for
example, the following compounds.
Ethylenebis(indenyl)dimethyl hafnium,
Ethylenebis(indenyl)diethyl hafnium,
Ethylenebis(indenyl)diphenyl hafnium,
Ethylenebis(indenyl)methyl hafnium monochloride,
Ethylenebis(indenyl)ethyl hafnium monochloride,
Ethylenebis(indenyl)methyl hafnium monobromide,
Ethylenebis(indenyl)hafnium dichloride,
Ethylenebis(indenyl)hafnium dibromide,
2~8:~1S
Ethylenebis(4,5,6,7-tetrahydro-1-indenyl)dimethyl
hafnium,
Ethylenebis(4,5,6,7-tetrahydro-1-indenyl)methyl hafnium
monochloride,
Ethylenebis(4,5,6,7-tetrahydro-1-indenyl)hafnium
dichloride,
Ethylenebis(4,5,6,7-tetrahydro-1-indenyl)hafnium
dibromide,
Ethylenebis(4-methyl-1-indenyl)hafnium dichloride,
Ethylenebis(5-methyl-1-indenyl)hafnium dichloride,
Ethylenebis(6-methyl-1-indenyl)hafnium dichloride,
Ethylenebis(7-methyl-1-indenyl)hafnium dichloride,
Ethylenebis(5-methoxy-1-indenyl)hafnium dichloride,
Ethylenebis(2,3-dimethyl-1-indenyl)hafnium dichloride,
Ethylenebis(4,7-dimethyl-1-indenyl)hafnium dichloride,
Ethylenebis(4,7-dimethoxy-1-indenyl)hafnium dichloride.
The above-mentioned hafnium compounds may contain small
amounts of zirconium or titanium. In such a case, the
content of zirconium or titanium is less than 1% by weight,
preferably less than 0.7% by weight and especially less than
0.5% by weight.
The hafnium catalyst components used in the present
invention may include compounds obtained by treating the
above-mentioned hafnium compounds with alkylsilylated silica
gel. More particularly, the said hafnium catalyst components
_ 23
2(~08:~15
may be hafnium compound solutions which are obtained, for
example, by passing a solution of the above-mentioned hafnium
compound in an organic solvent such as toluene through a
column packed with alkylsilylated silica gel, wherein said
hafnium compound is brought into contact with the
alkylsilylated silica gel.
The organic solvents used in that case are preferably
aromatic hydrocarbons such as toluene, benzene and xylene.
The alkylsilylated silica gel used may includes those
obtained by treating silica gel with dimethyl dichlorosilane,
ethylmethyl dichlorosilane, trimethyl chlorosilane, trimethyl
bromosilane, divinyl dichlorosilane, diethyl dichlorosilane
or methylpropyl dichlorosilane. The hafnium concentration in
the hafnium compound solution is usually from 1 x 10-5 to 5 x
10-3 mol/l, and the amount of the alkylsilylated silica gel
used is usually 20-500 g per 1 mmol of the hafnium compound.
A temperature at which the hafnium compound solution is
brought into contact with the alkylsilylated silica gel is
usually 0-50C.
When the hafnium catalyst components obtained by
treating the above-mentioned hafnium compounds with the
alkylsilylated silica gel are used as the catalyst components
[A], ethylene copolymers excellent in transparency are
obtained.
2 4 ZO(~8;~15
The catalyst components [B] used in the process of the
present invention are organoaluminum oxy-compounds. The
organoaluminum oxy-compounds used as the catalyst components
may be shown as benzene-soluble aluminoxanes represented by
S the following general formulas (1) and (II).
R2Al -~-OAl t-m- OAlR2 - (I)
I O I ( Ogl ) m+2 (II)
wherein R may be the same or different and is a hydrocarbon
group such as methyl, ethyl, propyl or butyl, preferably
methyl or ethyl and especially methyl, and m is an integer of
at least 2, preferably at least 5. The above-mentioned
aluminoxanes may be prepared, for example, by the procedures
as exemplified below.
(1) A procedure which comprises reacting a suspension
in a hydrocarboh medium of a compound containing water of
absorption or a salt containing water of crystallization, for
example, magnesium chloride hydrate, copper sulfate hydrate,
aluminumsulfate hydrate, nickel sulfate hydrate or serous
chloride hydrate, with trialkylaluminum.
20~8315
(2) A procedure which comprises reacting
trialkylaluminum directly with water, water vapor or ice in a
medium such as benzene, toluene, ethyl ether and
tetrahydrofuran.
The aluminoxanes as illustrated above may contain small
amounts of organometallic co~ponents.
Further, the organoaluminum oxy-compounds used in the
present invention may be those which are insoluble in
benzene. The benzene-insoluble organoaluminum oxy-compounds
0 are illustrated hereinafter.
The benzene-insoluble organoaluminum oxy-compounds used
in the invention may be prepared by (i) reaction of
organoaluminum compounds with water or (ii) reaction of
solutions of aluminoxane, for example, hydrocarbon solutions
thereof, with water or active hydrogen-containing compounds.
The benzene-insoluble organoaluminum oxy-compounds are
considered to have alkyloxyaluminum units represented by the
formula~ Rl ~ wherein Rl is hydrocarbon of 1 ~ 12 carbon
t -Al-Ot
atoms and Al component soluble in benzene at 60C is in an
amount, in terms of Al atom, of less than 10%, preferably
less than 5% and especially less than 2%, thus they are
insoluble or sparingly soluble in benzene.
Solubility in benzene of the organoaluminum oxy-
compounds of the present invention is determined by
26
20~83~5
suspending the organoaluminum oxy-compound equivalent to Al
of 100 mg atom in 100 ml of benzene, stirring the suspension
at 60C for 6 hours, filtering the thuS treated suspension at
a temperature elevated to 60C using G-5 glass filter
5 equipped with a jacket, washing 4 times the solids portion
separated on the filter with 50 ml of benzene kept at 60C
and then measuring the amount of Al atoms (X mmol) present in
the total filtrate.
In the alkyloxyaluminum units mentioned above, R1 is
methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl,
pentyl, hexyl, octyl, decyl, cyclohexyl, cyclooctyl, etc.
Among these, preferred are methyl and ethyl, particularly
methyl.
In addition to the alkyloxyaluminum units of the formula
15 - ~ OAl -~ , the benzene-insoluble organoaluminum oxy-compounds
J
of the invention may contain oxyaluminum units represented by
the formula ~-OAl ~ -. In the above-mentioned formulas,
~ R2 J
iS as defined previously, R2 is hydrocarbon of 1 ~ 12 carbon
atoms, alkoxyl of 1 ~ 12 carbon atoms aryloxy of 6 ~ 20
carbon atoms, hydroxyl, halogen or hydrogen, and R1 and R2
2 5 represent the groups different from each other. In that
case, the benzene-insoluble organoaluminum oxy-compounds are
preferably those containing the alkyloxyaluminum units
2~3315
~ OAl ~ in the proportion of at least 30 mol%, preferably at
~ Rl J
least 50 mol% and especially at least 70 mol%.
The organoaluminum compounds (i) used for preparing such
benzene-insoluble organoaluminum oxy-compounds as mentioned
above are those represented by the formula RlnAlX3_n wherein
R1 is hydrocarbon of 1 ~ 12 carbon atoms, X is halogen,
alkoxyl of 1 ~ 12 carbon atoms, aryloxy of 6 ~ 20 carbon
atoms or hydrogen, and n is 2 ~ 3.
Such organoaluminum compounds (i) as mentioned above
include trialkylaluminum such as trimethylaluminum,
triethylaluminum, tripropylaluminum, triisopropylaluminum,
tri-n-butylaluminum, tri-sec-butylaluminum, tri-tert-
butylaluminum, tripentylaluminum, trihexylaluminum,trioctylaluminum, tridecylaluminum, tricyclohexylaluminum and
tricyclooctylaluminum; dialkylaluminum halides such as
dimethylaluminum chloride, dimethylaluminum bromide,
diethylaluminum chloride, diethylaluminum bromide, and
diisobutylaluminum chloride; dialkylaluminum hydrides such as
diethylaluminum hydride and diisobutylaluminum hydride;
dialkylaluminum alkoxides such as dimethylaluminum methoxide
and diethylaluminum ethoxide; and dialkylaluminum aryloxides
such as diethylaluminum phenoxide. Of these organoaluminum
compounds, preferred are those of the above-mentioned general
28
20~8315
formula in which Rl is alkyl and X is chlorine, and
particularly preferred is trialkylaluminum.
In this connection, isoprenylaluminum of the general
formula (i-C4H9)XAly(C5HlO)z wherein x, y and z are each
positive integer, and z > 2x may also used as the
organoaluminum compound (i).
The organoaluminum compounds (i) as illustrated above
may be used either singly or in combination.
The active hydrogen-containing compounds (ii) used in
preparing the benzene-insoluble organoaluminum oxy-compounds
of the present invention include alcohols such as methyl
alcohol and ethyl alcohol, and diols such as ethylene glycol
and hydroquinone.
When water is used in preparing the benzene-insoluble
organoaluminum oxy-compounds of the present invention, the
water may be used after dissolving or suspending in
hydrocarbon solvents such as benzene, toluene and hexane,
ether solvents such as tetrahydrofuran, and amine solvents
such as triethylamine, or may be used in the form of water
vapor or ice. As the water, moreover, there may also be used
water of crystallization of salt such as magnesium chloride,
magnesium sulfate, aluminum sulfate, copper sulfate, nickel
sulfate, iron sulfate and cerrous chloride, or water of
adsorption adsorbed to inorganic compounds such as silica,
alumina and aluminum hydroxide or polymers.
29
ZC~08
As mentioned above, the benzene-insoluble organoaluminum
oxy-compounds of the present invention may be prepared by
reaction of the organoaluminum compound (i) with water, or by
reaction of a solution of aluminoxane, for example, a
S hydrocarbon solution thereof, with water or the active
hydrogen containing compound. In preparing the benzene-
insoluble organoaluminum oxy-compound from the organoaluminum
compound and water, the organoaluminum compound is brought
into contact with water in a solvent, for example, a
hydrocarbon solvent, and in that case, the water is added to
the reaction system so that the organoaluminum atoms
dissolved in the reaction system become less than 20% based
on the total organoaluminum atom. In obtaining the benzene-
insoluble organoaluminum oxy-compounds according to the
procedure as mentioned above, it is desirable that the water
is brought into contact with the organoaluminum compound in
the proportion of 1 ~ 5 moles, preferably 1.5 ~ 3 moles of
the water to 1 mole of the organoaluminum compound.
The above-mentioned reaction for forming the benzene-
insoluble organoaluminum oxy-compounds is carried out in
solvents, for example, hydrocarbon solvents. The solvents
used include aromatic hydrocarbons such as benzene, toluene,
xylene, cumene and cymene, aliphatic hydrocarbons such as
butane, isobutane, pentane, hexane, octane, decane, dodecane,
hexadecane and octadecane, alicyclic hydrocarbons such as
Z008315
cyclopentane, cyclooctane, cyclodecane and cyclododecane,
such hydrocarbon solvents, for example, petroleum fractions,
as gasoline, kerosine and gas oil, halides of the above-
mentioned aromatic hydrocarbons, aliphatic hydrocarbons and
5 alicyclic hydrocarbons, especially chlorides and bromides
thereof, and ethers such as ethyl ether and tetrahydrofuran.
Among these hydrocarbon media as exemplified above,
particularly preferred are aromatic hydrocarbons.
A concentration in terms of Al atom of the
organoaluminum compound in the reaction system is desirably 1
x 10-3 to 5 gram atom/l preferably 1 x 10-2 to 3 gram atom/l,
and a concentration in the reaction system of water such as
water of crystallization is usually 1 x 10-3 to 20 mol/l,
preferably 1 x 10-2 to 10 mol/l. In that case, it is
desirable that the organoaluminum atoms dissolved in the
reaction system are less than 20%, preferably less than 10%
and especially in the range of from 0 to 5% based on the
total organoaluminum atom.
Contact of the organoaluminum compound with water may be
carried out, for example, by the following procedures.
(1) A procedure wherein a hydrocarbon solution of
organoaluminum is brought into contact with a hydrocarbon
solvent containing water.
3 1 2(~8:~15
(2) A procedure wherein water vapor is blown into a
hydrocarbon solution of organoaluminum, thereby bringing the
organoaluminum into contact with the water vapor.
(3) A procedure wherein a hydrocarbon solution of
S organoaluminum is mixed with a hydrocarbon suspension of a
compound containing water of adsorption or a compound
containing water of crystallization, thereby bringing the
organoaluminum into contact with the water of adsorption or
water of crystallization.
(4) A procedure wherein a hydrocarbon solution of
organoaluminum is brought into contact with ice.
The above-mentioned reaction of the organoaluminum with
water is carried out usually at a temperature of from -100 to
150C, preferably -50 to 100C and especially -30 to 80C.
The reaction time, though it may largely vary depending upon
the reaction temperature, is usually from 1 to 200 hours,
preferably 2 to 100 hours.
In preparing the benzene-insoluble organoaluminum oxy-
compounds from a solution of aluminoxane and water or a
active hydrogen-containing compound, the aluminoxane present
in the solution of aluminoxane is brought into contact with
water or the active hydrogen-containing compound.
The solution of aluminoxane is a solution of aluminoxane
in such a solvent as used in forming the above-mentioned
benzene-insoluble organoaluminum oxy-compounds, preferably
-
32 20~8315
aromatic hydrocarbons such as benzene and toluene, and this
solution may contain other components so long as they do not
affect adversely the reaction between the aluminoxane and
water or the active hydrogen-containing compound.
The amount of water or the active hydrogen-containing
compound used in the above-mentioned reaction is 0.1 to 5
moles, preferably 0.2 to 3 moles based on 1 gram atom of
aluminum present in the solution of aluminoxane. A
concentration in the reaction system of aluminoxane in terms
of aluminum atom is usually 1 x 10-3 to 5 gram atom/l,
preferably 1 x 10-2 to 3 gram atom/l, and a concentration in
the reaction system of water is usually 2 x 10-4 to 5 mole/l,
preferably 2 x 10-3 to 3 mole/l.
Taking, as an example, the reaction of the solution of
aluminoxane with water, said solution of aluminoxane is
brought into contact with water or the active hydrogen-
containing compound, for example, by the following methods.
(1) A method which comprises bringing the solution of
aluminoxane into contact with a hydrocarbon solvent
containing water.
(2) A method which comprises blowing water vapor into the
solution of aluminoxane, thereby bringing the aluminoxane
present in the solution of aluminoxane into contact with the
water vapor.
3 3 2~08315
(3) A method which comprises mixing the solution of
aluminoxane with a hydrocarbon solution of a compound
containing water of adsorption or a compound containing water
of crystallization, thereby bringing the aluminoxane present
S in the solution of aluminoxane into contact with the water of
adsorption or water of crystallization.
(4) A method which comprises bringing the solution of
aluminoxane into contact directly with water or ice.
The above-mentioned procedures may also be applied to
the case wherein the active hydrogen-containing compound (ii)
is used instead of water.
The reaction of the solution of aluminoxane with water
or the active hydrogen-containing compound as illustrated
above may be carried out usually at a temperature of from -
50 to 150C, preferably 0 to 120C and especially 20 ~100C. The reaction temperature, though it may largely vary
depending upon the reaction temperature, is usually 0.5 ~ 300
hours, preferably about 1 ~ 150 hours.
In preparing the ethylene copolymers by using the olefin
polymerization catalysts as mentioned above, a concentration
in the polymerization system of the hafnium compound in terms
of hafnium atom is usually 10-8 to 10-2 gram atom/l,
preferably 10-7 to 10-3 gram atom/l.
The above-mentioned organoaluminum oxy-compounds are
desirably used in an amount in terms of aluminum atom in
3 4 Z~8;~15
present in the reaction system of 10-4 to 1o-l gram atom/l,
preferably 5 x 10-4 to 5 x 10-2 gram atom/l.
The polymerization temperature employed is from -50 to
150C, preferably from 0 to 120C.
The olefin polymerization mentioned above is carried out
usually in vapor phase or liquid phase. In the liquid phase
polymerization, the solvent used may be inert hydrocarbon, or
the olefin itself may also be used as the solvent.
The hydrocarbon used in that case includes aliphatic
hydrocarbons such as butane, isobutane, pentane, hexane,
heptane, octane, decane, dodecane, hexadecane and octadecane,
alicyclic hydrocarbons such as cyclopentane,
methylcyclopentane, cyclohexane and cyclooctane, aromatic
hydrocarbons such as benzene, toluene and xylene, and
petroleum fractions such as gasoline, kerosine and gas oil.
The polymerization pressure employed is usually from
ordinary pressure to 100 kg/cm2, preferably from ordinary
pressure to 50 kg/cm2, and the polymerization may be carried
out batchwise, semi-continuously or continuously. A
molecular weight of the resulting polymer may be modified by
the addition of hydrogen and/or by regulation of the
polymerization temperature employed.
Hereinafter, the first propylene/a-olefin random
copolymers and process for preparing the same of the present
invention are illustrated in detail.
3s
20~8:~15
The propylene/a-olefin random copolymers of the present
invention are random copolymers of propylene and a-olefins of
4 ~ 20 carbon atoms. The propylene/a-olefin random
copolymers of the invention desirably contain structural
units (a) derived from propylene in an amount of 90 ~ 99
mol%, preferably 92 ~ 98 mol%, and structural units (b)
derived from a-olefin in an amount of 1 ~ 10 mol%, preferably
2 ~ 8 mol%. When the structural units (a) derived from
propylene contained in the propylene/a-olefin random
copolymer are less than 90 mol%, said copolymer tends to
become poor in anti-blocking properties and stiffness and, on
the other hand, when said units (a) are in excess of 99 mol%,
said copolymer tends to increase in melting point and become
poor in heat-sealing properties.
a-olefins of 4 ~ 20 carbon atoms used herein include 1-
butene, l-pentene, l-hexene, 4-methyl-1-pentene, 3-methyl-1-
pentene, l-octane, l-decene, l-dodecene, l-tetradecene, 1-
hexadecene, l-octadecene and l-eicosene. Among these,
particularly preferred is l-butene.
The propylene/olefin random copolymers of the present
invention desirably have an intrinsic viscosity [~] of 0.5 ~
6 dl/g, preferably 1 ~ 5 dl/g as measured in decalin at
135C. If this intrinsic viscosity is less than 0.5 dl/g,
the copolymers tend to become poor in anti-blocking
properties and toughness and, on the other hand if said
36
Z(~8;~15
intrinsic viscosity exceeds 6 dl/g, the copolymers tend to
become port in moldability.
Further, the propylene/a-olefin random copolymers of the
invention have a melting point [Tm] as measured by a
differential scanning calorimeter falling within the range of
90 < Tm < 155 - 3.5 (100 - P), preferably
100 < Tm < 150 - 3.5 (100 - P)
wherein P is the content (mol%) of propylene in the
copolymer.
In Fig. 2, there is shown as a straight line A the
schematic relationship between the melting point Tm of the
above-mentioned propylene/a-olefin random copolymer and the
propylene content (mol%) present in said copolymer. In this
Fig. 2, there is also shown as a straight line B the
relationship between the melting point Tm of the known
propylene/a-olefin random copolymer and the propylene content
(mol%) present in said copolymer.
As is clear from Fig. 2, the melting point of the first
propylene/a-olefin random copolymers of the present invention
is lower by 10 ~ 20C than that of the known propylene/a-
olefin random copolymers when the amount of a-olefin
copolymerized of the former is the same as that of the
latter. Accordingly, films obtained from the first
propylene/a-olefin random copolymers of the invention are
excellent particularly in heat-sealing properties at low
2(:~8315
temperatures, and the films exhibit excellent heat-sealing
properties even when they have small amounts of the
copolymerized a-olefin, and hence they are excellent in anti-
blocking properties and have excellent stiffness.
In the present invention, the propylene/a-olefin random
copolymer was allowed to stand in a differential scanning
calorimeter (DSC) at 200C for 5 minutes, cooled up to 20C
at a rate of 10C/min, and allowed to stand at 20C for 5
minutes, and then the temperature was elevated from 20C to
200C at a rate of 10C/min to obtain a temperature (Tm) at a
maximum endothermic peak which was then taken as a melting
point of said propylene/a-olefin random copolymer.
The molecular weight distribution (Mw/Mn) of the first
propylene/a-olefin random copolymers of the invention as
obtained by gel permeation chromatography (GPC) is less than
3.5, preferably less than 3.0 and especially less than 2.5.
As stated above, the first propylene/a-olefin random
copolymers of the invention are narrow in molecular weight
distribution and, from this point, they have excellent anti-
blocking properties.
The determination of melting point was conducted byusing about 2.5 mg of the specimen and DSC of Perkin Elmer-7
Model at heat-up rate of 10C/min.
The first propylene/a-olefin random copolymers of the
invention are desirably to have a soluble portion in boiling
38 2 ~ ~ 8 3 1 5
n-pentane in an amount of less than 3% by weight, preferably
less than 2% by weight and especially less than 1% by weight.
Further, the first propylene/a-olefin random copolymers
of the invention are desirably to have an insoluble portion
in boiling trichloroethylene in an amount of less than 5% by
weight, preferably less than 3% by weight and especially less
than 1% by weight.
The amounts of the insoluble portion in boiling
trichloroethylene and the soluble portion in boiling n-
pentane were determined by such a manner that about 3 g ofthe finely pulverized specimen was extracted for 5 hours with
180 ml of each of the solvents in a cylindrical filter paper
by using a Soxhlet's extractor, and the extraction residue
was dried with a vacuum dryer until it reached a constant
weight to obtain the weight thereof, whereby a difference in
weight between the dried residue and the original specimen is
calculated.
The first propylene/a-olefin random copolymers of the
present invention as illustrated above may be prepared by
copolymerizing propylene and a-olefin of 4 ~ 20 carbon atoms
at a temperature of 40 ~ 100C so that the structural units
(a) derived from propylene are present in an amount of 90 -
99 mol% and the structural units (b) derived from a-olefin
are present in an amount of 1 ~ 10 mol%, in the presence of
catalysts formed from
39
2~8315
[A] hafnium compounds having as ligands multidentate
compounds in which at least two groups selected from
among conjugated cycloalkadienyl groups or substituted
groups thereof are linked together via lower alkylene
groups, and
[B] organoaluminum oxy-compounds.
In that case, the copolymerization may be carried out by
employing the same conditions as used in the preparation of
the aforementioned ethylene copolymers.
0 The first propylene/a-olefin random copolymers of the
present invention are excellent particularly in heat-sealing
properties at low temperatures and hence are used as heat-
sealing agents.
Hereinafter, the second propylene random copolymers and
process for preparing the same of the present invention are
illustrated in detail.
The second propylene random copolymers of the invention
are random copolymers of propylene, ethylene and a-olefins of
4 ~ 20 carbon atoms. In the propylene random copolymers, the
structural units (a) derived from propylene are present in an
amount of 90 ~ 99 mol%, preferably 92 ~ 98 mol%, the
structural units (b) derived from ethylene in an amount of
0.5 ~ 9.5 mol%, preferably 1 ~ 9 mol%, and the structural
units (c) derived from a-olefin in an amount of 0.5 ~ 9.5
mol%, preferably 1 ~ 9 mol%. If the structural units (a)
-
4 o 20~8;315
derived from propylene present in the said copolymers are
less than 90 mol%, the copolymers tend to become poor in
anti-blocking properties and stiffness. On the other hand,
if the structural units (a) exceed 99 mol%, the copolymers
tend to increase in melting point and become poor in heat-
sealing properties.
a-olefins of 4 ~ 20 carbon atoms used herein include 1-
butene, 1-pentene, 1-hexene, 4-methyl-1-pentene, 3-methyl-1-
pentene, 1-octane, 1-decene, 1-dodecene, 1-tetradecene, 1-
hexadecene, 1-octadecene and 1-eicosene. Among these,
particularly preferred is 1-butene.
The second propylene random copolymers of the invention
desirably have an intrinsic viscosity [~] as measured in
decalin at 135C of 0.5 ~ 6 dl/g, preferably 1 ~ 5 dl/g. If
this intrinsic viscosity is less than 0.5 dl/g, the
copolymers tend to become poor in anti-blocking properties
and toughness and, on the other hand, if said intrinsic
viscosity exceeds 6 dl/g, the copolymers tend to become poor
in moldability.
Further, the second propylene/a-olefin random copolymers
of the invention have a melting point [Tm] as measured by a
differential scanning calorimeter falling within the range of
70 < Tm < 155 - 5.5 (100 - P), preferably
90 ~ Tm < 150 - 5.5 (100 - P)
4 1 2(~8;~15
wherein P is the content (mol%) of propylene in the
copolymer.
The schematic relationship between the melting point Tm
of the above-mentioned propylene random copolymer and the
S propylene content (mol%) in said copolymer is the same as
shown in Fig. 2.
In this manner, the melting point of the second
propylene random copolymers is lower by 10 ~ 20C than that
of the known propylene/a-olefin random copolymers when the
amounts of ethylene and a-olefin copolymerized of the former
are the same as those of the latter. Accordingly, films
obtained from the second propylene random copolymers of the
invention are excellent particularly in heat-sealing
properties at low temperatures, and the films exhibit
excellent heat-sealing properties even when they have small
amounts of ethylene and a-olefin copolymerized, and hence
they are excellent in anti-blocking properties and have
excellent stiffness.
Further, the molecular weight distribution (Mw/Mn) of
the second propylene random copolymers of the invention as
obtained by gel permeation chromatography (GPC) is less than
3.5, preferably less than 3.0 and especially less than 2.5.
As stated above, the second propylene random copolymers of
the invention are narrow in molecular weight distribution
4 2 20~8315
and, from this point, they have excellent anti-blocking
properties.
The second propylene random copolymers of the invention
are desirably to have a soluble portion in boiling n-pentane
in an amount of less than 5% by weight, preferably less than
3% by weight and especially less than 2% by weight.
Furthermore, the second propylene random copolymers of
the invention are desirably to have an insoluble portion in
boiling trichloroethylene in an amount of less than 5% by
weight, preferably less than 3% by weight and especially less
than 1% by weight.
The second propylene random copolymers of the present
invention as illustrated above may be prepared by
copolymerizing propylene, ethylene and a-olefins of 4 ~ 20
carbon atoms at a temperature of 40 ~ 100C so that the
structural units (a) derived from propylene are present in an
amount of 90 ~ 99 mol% in the resulting copolymers, the
structural units (b) derived from ethylene in an amount of
0.5 ~ 9.5 mol%, and the structural units (c) derived from a-
2 0 olefin in an amount of 0.5 ~ 9.5 mol%, in the presence ofcatalysts formed from
[A] hafnium compounds having as ligands multidentate
compounds in which at least two groups selected from
among conjugated cycloalkadienyl groups or substituted
Z~8~15
groups thereof are linked together via lower alkylene
groups, and
[B] organoaluminum oxy-compounds.
In that case, the copolymerization may be carried out by
employing the same conditions as used in the preparation of
the aforementioned ethylene copolymers.
The second propylene random copolymers of the invention
as illustrated above are excellent particularly in heat-
sealing properties at low temperatures, and hence are used as
0 heat-sealing agents.
Hereinafter, the first olefin polymerization catalysts
of the present invention are illustrated in detail.
The first olefin polymerization catalysts of the
invention are formed from
[A] hafnium compounds having as ligands multidentate
compounds in which at least two groups selected from
among conjugated cycloalkadienyl groups or substituted
groups thereof are linked together via lower alkylene
groups,
[B] organoaluminum oxy-compounds, and
[C] organoaluminum compounds.
In the olefin polymerization catalyst mentioned above,
the hafnium compounds [A] and organoaluminum oxy-compounds
[B] used may be the same as those mentioned previously.
-
4 4 2~38:~15
The organoaluminum compounds [C] used herein may be
those having in the molecule at least one Al-C bond, for
example, the compounds as mentioned below.
That is, (i) organoaluminum compounds represented by the
5 general formula (Rl)mAl(OR2)nHpXq wherein R1 and R2, which may
be the same or different, are each hydrocarbon of usually 1
to 15 carbon atoms, preferably 1 to 10 carbon atoms, X is
halogen, m is 1 _ m _ 3, n is O _ n _ 2, p is O < p _ 2, and
q is O < q < 2, and m + n + p + q = 3, and (ii) alkylated
complex compounds of metals of Group 1 of the periodic table
and aluminum represented by the general formula M1Al(R1)4
wherein M1 is Li, Na or K, and R1 is as defined above.
Of the organoaluminum compounds mentioned above,
particularly preferred are those having hydrocarbon groups
other than n-alkyl group. Hydrocarbon groups other than n-
alkyl group may include alkyl having a branched chain such as
isoalkyl, cycloalkyl and aryl. The organoaluminum compounds
as illustrated above may include, for example,
trialkylaluminum such as triisopropyaluminum,
triisobutylaluminum, tri-2-methylbutylaluminum, tri-3-
methylbutylaluminum, tri-2-methylpentylaluminum, tri-3-
methylpentylaluminum, tri-4-methylpentylaluminum, tri-2-
methylhexylaluminum, tri-3-methylhexylaluminum and tri-2-
ethylhexylaluminum; tricycloalkylaluminum such as
tricyclohexylaluminum; triarylaluminum such as
4 5 2~8315
triphenylaluminum and tritolylaluminum; dialkylaluminum
hydrides such as diisobutylaluminum hydride; and
alkylaluminum alkoxides such as isobutylaluminum methoxide,
isobutylaluminum ethoxide and isobutylaluminum isopropoxide.
5 Of these organoaluminum compounds, preferred are those having
branched alkyl groups, particularly trialkylaluminum
compounds. Furthermore, isoprenylaluminum represented by the
general formula (i-C5Hg)xAly(C5HlO)z wherein x, y and z are
each a positive integer, and z > 2x, is also useful.
Compounds capable of forming the above-mentioned
organoaluminum compounds in the polymerization system, for
example, halogenated aluminum and alkyl lithium, or
halogenated aluminum and alkyl megnesium, may be added to the
polymerization system.
In polymerizing olefin by using the first olefin
polymerization catalysts of the present invention, the
hafnium compound [A] is desirably used in an amount, in terms
of hafnium atoms present in the polymerization reaction
system, of 10-8 ~ 10-2 gram atom/l, preferably 10-7 ~ 10-3
gram atom/l, the organoaluminum oxy-compound [B] is desirably
used in an amountj in terms of aluminum atoms present in the
polymerization reaction system, of less than 3 mg atom/l,
preferably 0.01 ~ 2 mg atom/l and especially 0.02 ~ 1 mg
atom/1, and the organoaluminum compound [C] is desirably used
in such an amount that the proportion of aluminum atoms
-
46
ZC~ 15
derived from said organoaluminum compound [C] in the reaction
system to the total aluminum atoms of the organoaluminum oxy-
compound [B] and organoaluminum compound [C] is 20 ~ 99%,
preferably 25 ~ 98% and especially 30 ~ 95%.
In the reaction system, the ratio of the total aluminum
atom of the organoaluminum oxy-compound [B] and
organoaluminum compound [C] to halfnium atoms of the hafnium
compound [A] is usually 20 ~ 10000, preferably 50 ~ S000 and
especially 100 ~ 2000.
The olefin polymerization may be carried out by
employing the same condition as used in the preparation of
ethylene copolymers as aforesaid.
Hereinafter, the second olefin polymerization catalysts
of the present invention are illustrated in detail.
The second olefin polymerization catalysts of the
invention are formed from
[A] hafnium compounds having as ligands multidentate
compounds in which at least two groups selected from
among conjugated cycloalkadienyl groups or substituted
groups thereof are linked together via lower alkylene
groups,
[B-1] organoaluminum oxy-compounds formed from tri-n-
alkylaluminum, and
Z008315
47
[B-2] organoaluminum oxy-compounds in which at least one
hydrocarbon group other than n-alkyl is linked to
Al atom.
In the olefin polymerization catalysts mentioned above,
the hafnium compounds [A] used are the same as those
mentioned previously.
The catalyst components [B-1] used in the second olefin
polymerization catalysts of the invention are organoaluminum
oxy-compounds formed from tri-n-alkylaluminum.
0 n-alkyl group in the tri-n-alkylaluminum mentioned above
includes methyl, ethyl, n-propyl, n-butyl, n-octyl and n-
decyl. Among these, particularly preferred is methyl.
The catalyst components [B-2] used in the second olefin
polymerization catalysts of the invention are organoaluminum
oxy-compounds in which at least one hydrocarbon group other
than n-alkyl is linked to Al atom.
The hydrocarbon group other than n-alkyl includes alkyl
having branched chain such as isoalkyl, cycloalkyl and aryl.
The above-mentioned organoaluminum oxy-compounds [B-2]
in which at least one hydrocarbon group other than n-alkyl is
linked to Al atom may be formed from organoaluminum compounds
in which at least one hydrocarbon group other than n-alkyl is
linked to Al atom. Such organoaluminum compounds as used in
the above case include, for example, trialkylaluminum such as
triisopropylaluminum, triisobutylaluminum, tri-2-
48
20~315
methylbutylaluminum, tri-3-methylbutylaluminum, tri-2-
methylpentylaluminum, tri-3-methylpentylaluminum, tri-4-
methylpentylaluminum, tri-2-methylhexylaluminum, tri-3-
methylhexylaluminum and tri-2-ethylhexylaluminum;
tricycloalkylaluminum such as tricyclohexylaluminum;
triarylaluminum such as triphenylaluminum and
tritolylaluminum; dialkylaluminum hydrides such as
diisobutylaluminum hydride; and alkylaluminum alkoxides such
as isobutylaluminum methoxide, isobutylaluminum ethoxide and
0 isobutylaluminum isopropoxide. Of these organoaluminum
compounds, preferred are those having branched alkyl groups,
particularly trialkylaluminum compounds. Furthermore,
isoprenylaluminum represented by the general formula (i-
C4Hg)xAly(C5Hlo)z wherein x, y and z are each a positive
integer, and z > 2x, is also useful.
In polymerization olefin by using the second olefin
polymerization catalysts of the present invention, the
hafnium compound [A] is desirably used in an amount, in terms
of hafnium atoms present in the polymerization reaction
system, of 10-8 ~ 10-2 gram atom/l, preferably 10-7 ~ 10-3
gram atom/l, the organoaluminum oxy-compound [B-1] in an
amount, in terms of aluminum atoms in the polymerization
reaction system, of less than 3 mg atom/l, preferably 0.01 ~
2 mg atom/l and especially 0.02 ~ 1 mg atom/l, and the
organoaluminum oxy-compound [B-2] in such an amount that the
4 9 Z008315
proportion of aluminum atoms derived from the organoaluminum
oxy-compound [B-2] in the reaction system to the total
aluminum atom of the organoaluminum oxy-compound [B-l] and
organoaluminum oxy-compound [B-2] is 20 ~ 95%, preferably 25
~ 90% and especially 30 ~ 85%.
In the reaction system, the ration of the total aluminum
atom of the organoaluminum oxy-compound [B-l] and
organoaluminum oxy-compound [B-2] to hafnium atoms of the
hafnium compound [B] is usually 20 ~ 10000, preferably 50 ~
5000 and especially 100 ~ 2000;
In that case, the olefin polymerization may be carried
out by employing the same conditions as used in the
preparation of ethylene copolymers as aforesaid.
FFFF.CT OF T~F INVFNTION
Novel ethylene copolymers of the present invention are
small in Mw/Mn and narrow in molecular weight distribution
and, moreover, large in MFRlo/MFR2 and excellent in
flowability. Accordingly, the ethylene copolymers of the
invention have excellent processability and are excellent in
anti-blocking properties and the like properties.
Novel propylene copolymers of the invention are low in
melting point in comparison with known propylene/a-olefin
random copolymers even when said novel propylene copolymers
are low in a-olefin content, and hence they have excellent
5 20~315
anti-blocking properties and stiffness. In the present
invention, there are also provided processes for preparing
these novel copolymers mentioned above readily and
efficiently.
The olefin polymerization catalysts of the invention
exhibit high activities even when relatively small amounts of
organoaluminum oxy-compounds are used therein, and by the use
of said catalysts, olefin polymers large in molecular weight
and narrow in molecular weight distribution and composition
distribution are obtained.
The present invention is illustrated below with
reference to examples, but it should be construed that the
invention is in no way limited to those examples.
F.x~ e 1 (Preparation of ethylene copolymer)
(Preparation of methylaluminoxane)
Methylaluminoxane was prepared in accordance with the
procedure described in Polymer Commun., 29, 180 (1988).
(Synthesis of ethylenebis(indenyl)hafnium dichloride)
A nitrogen-purged 200 ml glass flask was charged with
2 0 5.4 g of bis(indenyl)ethane [synthesized on the basis of
Bull. Soc. Chim., 2954 (1967)] and 50 ml of THF, and the
flask was cooled with stirring to -30 ~ -40C. To the flask
was added dropwise 31.5 ml of n-Bu Li (1.6M solution),
stirred successively at -30C for 1 hours, and the
2 5 temperature was elevated spontaneously to room temperature,
5 1 200831S
thereby anionizing the bis(indenyl)ethane. Separately, a
nitrogen-purged 200 ml glass flask was charged with 60 ml of
THF, and the flask was cooled to below -60C, followed by
gradual addition of 6.7 g of HfCl4 (contained 0.78% by weight
of zirconium atoms as contaminants). Thereafter, the flask
was heated-up to 60C and stirred for 1 hour. To the flask
was added dropwise the anionized ligand, and stirred at 60C
for 2 hours, followed by filtration with a glass filter. The
filtrate was concentrated at room temperature to about 1/5 of
the original volume. By this operation conducted, solids
were separated. The separated solids were filtered with a
glass filter, followed by washing with hexane/ethyl ether and
vacuum drying to obtain ethylenebis(indenyl)hafnium
dichloride.
The hafnium compound thus obtained contained 0.40% by
weight of zirconium atoms.
(Polymerization)
A thoroughly nitrogen-purged 2 liter glass flask was
charged with 950 ml of toluene and 50 ml of 1-octene, and
ethylene gas was passed therethrough at a rate of 160 l/hr.
The temperature in the system was elevated to 55C, and 1.88
mmoles in terms of aluminum atom of methylaluminoxane and 7.5
x 10-3 mmole of ethylenebis(indenyl)hafnium dichloride were
added to the system to initiate polymerization. The
polymerization was carried out at 60C for 10 minutes under
20~8;}1S
atmospheric pressure while continuously feeding ethylene gas.
The polymerization was stopped by the addition of small
amounts of methanol, and the polymerization solution obtained
was poured in large amounts of methanol to separate polymer.
5 The separated polymer was dried at 130C for 12 hours under
reduced pressure to obtain 23.2 g of a polymer having a
density of 0.866 g/cm3, the ethylene content of 81.3 mol%,
[~] of 1.71 dl/g, Mw/Mn of 2.59, MFR2 of 2.12 g/10 min and
MFR1o/MFR2 ratio of 13.1.
1 0 F.x~m~l e 2
A thoroughly nitrogen-purged 2 liter glass flask was
charged with 1 liter of toluene, and a mixed gas of ethylene
and propylene (140 l/hr and 40 l/hr respectively) was passed
therethrough. The temperature in the system was elevated to
lS 75C, and 1.88 mmoles in terms of aluminum atom of
methylaluminoxane and 7.5 x 10-3 mmol of
ethylenebis(indenyl)hafnium dichloride were added to the
system to initiate polymerization. The polymerization was
carried out at 80C for 10 minutes under atmospheric pressure
while continuously feeding the above-mentioned mixed gas to
the system. Thereafter, the operation was conducted in the
same manner as in Example 1 to obtain 17.5 g of a polymer
having a density of 0.887 g/cm3, the ethylene content of 84.0
mol%, [~] of 1.50 dl/g, Mw/Mn of 2.50, MFR2 of 0.80 g/10 min
and MFRlo/MFR2 ratio of 12.7.
53
20g8~15
Co~p~r~tlve Ex~mp1e 1
A copolymer of ethylene and propylene (prepared by using
a catalyst composed of VOCl3 and aluminum ethyl
sesquichloride) having a density of 0.87 g/cm3, MFR2 of 2.9
g/10 min and Mw/Mn of 2.16 was found to have MFR1o/MFR2 ratio
of 5.90.
F.x~m~l e 3
(Preparation of hafnium catalyst)
A glass column of 35 mm in inside diameter was filled
with a suspension in toluene of 40 g of dimethylsilylated
silica gel (Art. 7719, a product of MERCK) deaerated at room
temperature for 4 hours. Subsequently, 200 ml of the toluene
solution (Hf = 2.07 mmol/l) of ethylenebis(indenyl)hafnium
dichloride prepared in Example 1 was gradually poured into
the column. A hafnium solution (Hf = 0.17 m mol/l) eluted by
this operation was used as a catalyst component.
(Polymerization)
The polymerization was carried out in the same manner as
in Example 1 except that the polymerization was carried out
at 70C for 35 minutes using 6.6 x 10-3 mg atom of hafnium
atom, to obtain 42.4 g of a colorless transparent polymer
having a density of 0.855 g/cm3, the ethylene content of 76.2
mol%, [~] of 1.89 dl/g, Mw/Mn of 2.48, MFR2 of 1.49 g/10 min
and MFR1o/MFR2 ratio of 10.1.
2~8315
F.X~ ~1 e 4
The polymerization was carried out in the same manner as
in Example 2 except that the hafnium catalyst component
prepared in Example 3 was used in an amount of 6.6 x 10-3 mg
5 atom in terms of hafnium atom to obtain 17.0 g of a colorless
transparent polymer having a density of 0.883 g/cm3, the
ethylene content of 83.5 mol%, [~] of 1.61 dl/g, Mw/Mn of
2.54, MFR2 Of 0.73 g/10 minj and MFR1o/MFR2 ratio of 12.2.
F.X;~IT~1 e 5
0 The polymerization was carried out in the same manner as
in Example 1 except that the polymerization temperature
employed was 40C and the polymerization time employed was lS
minutes to obtain 20.5 g of a polymer having a density of
0.868 g/cm3, the ethylene content of 82.0 mol%, [~] of 1.79
dl/g, Mw/Mn of 2.81, MFR2 Of 0.90 g/10 min and MFR1o/MFR2
ratio of 32Ø
F.x~m~l e 6 ~Preparation of propylene copolymer)
(Polymerization)
A thoroughly nitrogen-purged 2 liter stainless steel
autoclave was charged at room temperature with 500 ml of
toluene, 3 moles of propylene, 0.1 mole of 1-butene and 5 mg
atom in terms of Al atom of methylaluminoxane. The
temperature in the polymerization system was elevated to
45C, and 1.25 x 10-3 mmole of the ethylenebis(indenyl)hafnium
2(~08:~1S
dichloride obtained in Example 1 was added to the system to
carry out polymerization at 50C for O.S hours. The
polymerization was stopped by the addition of methanol to the
polymerization system.
The polymer slurry obtained was poured into large
amounts of methanol, the slurry was recovered by filtration
and washed with an isobutyl alcohol/hydrochloric acid
solution to remove the catalyst components therefrom. The
recovered slurry was then vacuum dried overnight at 80C and
200 ~ 300 mmHg to obtain 27.5 g of a polymer having the 1-
butene content of 2.2 mol%, [~] of 3.02 dl/g as measured in
decalin at 135C, a melting point of 124C as measured by
DSC, a boiling trichloroethylene-insoluble content of 0% by
weight, boiling n-pentane-soluble content of 0.3% by weight
and Mw/Mn of 2.41 as measured by GPC.
F.X~ e 7
The polymerization was carried out in the same manner as
in Example 6 except that the amount of 1-butene charged was
changed to 0.25 mol to obtain 29.1 g of a polymer having the
1-butene content of 5.6 mol%, [~] of 2.95 dl/g, a melting
point of 116C, a boiling trichloroethylene-insoluble content
of 0% by weight, a boiling n-pentane-soluble content of 0.4%
by weight and Mw/Mn of 2.33.
F.x;~ l e 8
5 6 2()0831S
(Polymerization)
A mixed gas composed of 96.7 mol% of propylene, 2.1 mol%
of 1-butene and 1.2 mol96 of ethylene was prepared.
A thoroughly nitrogen-purged 2 liter stainless steel
5 autoclave was charged with 500 ml of toluene and then cooled
to 0C, and the autoclave was further charged with 3 moles of
the mixed gas prepared above and 5 mg atom in terms of A1
atom of methylaluminoxane. The temperature in the
polymerization system was elevated to 45C, and 1.25 x 10-3 m
1 0 mole of the ethylenebis(indenyl)hafnium dichloride obtained
in Example 1 was added to the system to initiate
polymerization at 50C for 0.5 hours. The polymerization was
stopped by the addition of methanol to the polymerization
system. The polymer slurry obtained was poured into large
15 amounts of methanol, the slurry was recovered by filtration
and washed with an isobutyl alcohol/hydrochloric acid
solution to remove the catalyst components therefrom. The
recovered polymer was then vacuum dried overnight at 80C and
200 ~ 300 mmHg to obtain 51.3 g of a polymer having the 1-
20 butene content of 1.4 mol%, the ethylene content of 1.1 mol%,[11] of 3.32 dl/g as measured in decalin at 135C, a melting
point of 121C as measured by DSC, a boiling
trichloroethylene-insoluble content of 0% by weight, boiling
n-pentane-soluble content of 0.9% by weight and Mw/Mn of
2 5 2.45 as measured by GPC.
2(~g8;~1~S
F.~ pl e 9
The polymerization was carried out in the same manner as
in Example 8 except that the mixed gas composed of 95.1 mol%
of propylene, 3.9 mol% of 1-butene and 1.0 mol% of ethylene
was used to obtain 48.5 g of a polymer having the 1-butene
content of 2.7 mol%, the ethylene content of 0.8 mol%, [~] of
3.29 dl/g, a melting point of 118C, a boiling
trichloroethylene-insoluble content of 0% by weight, a
boiling n-pentane-soluble content of 1.1% by weight and
Mw/Mn of 2.40.
F.X~pl e 10
(Polymerization)
A thoroughly nitrogen-purged 2 liter stainless steel
autoclave was charged with 750 ml of toluene and then
saturated with propylene gas. To the autoclave were added
7.5 mg atom in terms of Al atom of methylaluminoxane and 1.88
x 10-3 mmole of the ethylenebis(indenyl)hafnium dichloride.
Polymerization was carried out at 50C for 0.5 hour at the
total pressure of 7 kg/cm2 G while continuously feeding
propylene gas to the autoclave. The polymerization was
stopped by the addition of methanol to the polymerization
system. The polymer slurry obtained was poured into large
amounts of methanol, the slurry was recovered by filtration
and washed with an isobutyl alcohol/hydrochloric acid
72932-62
_ 58
'~008~1~
solution to remove the catalyst components therefrom. The
recovered slurry was then vacuum dried overnight at 80C and
200 - 300 mmHg to obtain 107.1 g of an isotactic
polypropylene having Mw/Mn of 1.89 as measured by GPC, a
melting point of 132C as measured by DSC, [~] of 2.82 dl/g
as measured in decalin at 135C, a boiling trichloroethylene-
insoluble content of 0% by weight and a boiling n-pentane-
soluble content of 0.2% by weight.
F.x~ 1 e 1 1
A mixed gaS composing 98.5 mol% of propylene gas and 1.5
mol% of ethylene gas was prepared. The opera~ion subse~uerlt
thereto was carried out in the same manner as in Example 6 to
obtain 53.3 g of a polymer having the ethylene content of 1.3
mol%, [~] of 3.50 dl/g, a melting point of 125C, a boiling
trichloroethylene-insoluble content of 0% by weight, a
boiling n-pentane-soluble content of 1.0% by weight and
Mw/Mn of 2.39.
F.v~ 1 u~t; on
Heat-sealing properties of the propylene polymers
obtained hereinabove were evaluated in the following manner.
Pre~ r~t i on of f;1 m
On a press plate were placed an aluminum sheet of a 0.1
mm thick, a polyester sheet ~a product sold under the trade-
mark Rumiler by Toray) and a polyimide resin sheet (a
- 59 72932-62
2008315
product sold under the trade-mark Capton by Du Pont), a
square of 15 cm x 15 cm of the center of which had been cut
off, in that order, and 0.8 g of the specimen was placed in
this center (the cut-off portion of the polyimide resin
sheet), and then Rumiler~, an aluminum sheet and a press
plate were superposed thereon in that order ~see Fig. 3).
The specimen thus interposed between the press plates
was placed in a hot press kept at 200C and preheated for
about 5 minutes, followed by subjecting three times to
pressure application ~20 kg/cm2 G) and release operation in
order to remove air bubbles present in the specimen.
Subsequently, the pressure was increased finally to 150
kg/cm2 G and the specimen was heated for 5 minutes under
pressure. After releasing the pressure, the press plate was
taken out from a press machine and transferred to separate
press machine kept at 30C in its press in portion and then
cooled for 4 minutes at a pressure of 100 kg/cm2, followed by
releasing the pressure and taking out the specimen therefrom.
Of the films thus obtained, those having a uniform thickness
of 50 - 70 ~m were used as the films for measurement.
MeA~llrement of he~t-se~llng strength
The films thus prepared are placed for 2 days in a
hygrostat kept at 50C (aging~. In practicing the aging,
sheets of paper are applied to both sides of the film so that
the films do not come in contact with each other. The films
72932-62
- 2008315
thus aged are cut up into strips of a lS mm thick, and two
sheets of the strip are placed one upon another and then
interposed between two sheets of Teflo ~ Film of a 0.1 mm
thick, followed by heat sealing. The heat sealing is carried
5 out while maintaining the temperature of a lower portion of a
hot plate of heat sealer constant at 70C and varying only
the temperature of an upper portion of hot plate suitably at
intervals of 5C. The heat-sealing pressure employed is 2
kg/cm2, the heat-sealing time employed is 1 second, and a
width of heat seal is 5 mm (accordingly a sealed area is 15
mm x 5 mm).
Heat-sealing strength is determined by obtaining a
peeling strength of the heat sealed film subjected to peeling
test at a rate of 30 cm/min at each heat-sealing temperature
as mentioned above. (See Fig. 4.)
Following the above-mentioned procedure, a peeling
strength at each of the heat-sealing temperatures preset at
interval of 5C is obtained, and the plots of heat-sealing
temperature-peeling strength are connected by means of a
curved line. On the basis of the curved line, a heat sealing
temperature corresponding to a peeling strength of 800 g /15
mm is taken as a completely heat-sealed temperature (see Fig.
5).
Table 1 below shows completely heat-sealed temperatures
of the propylene polymers obtained in the foregoing examples.
6 1 20~)8315
T~hle 1
Example 1 2 3 4 5 6
Completely heat-sealed 134 125 117 122 120 127
temperature (C)
1 0
Example 12
(Preparation of organoaluminum oxy-compound [B-l])
A thoroughly nitrogen-purged 400 ml flask was charged
with 37 g of Al2(S04) 3-14H2O and 125 ml of toluene and cooled
to 0C, followed by dropwise addition of 500 mmol of
trimethylaluminum diluted with 125 ml of toluene. The
temperature of the flask was elevated to 40C, and reaction
was continued at that temperature for 10 hours. After the
completion of the reaction, solid-liquid separation of the
reaction mixture was effected by filtration, and the toluene
was removed from the filtrate to obtain 13 g of a white solid
organoaluminum oxy-compound. The molecular weight of the
compound obtained was 930 as measured in benzene by the
cryoscopic method, and m value shown in the catalyst
- 25 component [B-l] was 14.
(Polymerization)
A thoroughly nitrogen-purged 2 liter stainless steel
autoclave was charged with 500 ml of toluene, and the system
62 2~315
was purged with propylene gas. Successively, to the
autoclave were added 1 mmol of triisobutylaluminum, 1 mg atom
in terms of Al atom of the organoaluminum oxy-compound
obtained above, and 1 x 10-3 mmol of the
ethylenebis(indenyl)hafnium dichloride obtained in Example 1,
and the temperature of the system was elevated to 45C.
Thereafter, polymerization was carried out at 50C for 1
hours, while feeding propylene gas to the polymerization
system so that the total pressure became 7 kg/cm2 G, to
0 obtain 4S.0 g of an isotactic polypropylene having [~] of 2.5
dl/g as measured in decalin at 135C, Mw/Mn of 2.2, a
melting point of 132C, a boiling trichloroethylene-insoluble
content of 0% by weight and a boiling n-pentane-soluble
content of 0.2% by weight.
Co~p~r~t;ve F.x~mrl e 2
The polymerization of Example 12 was repeated except
that the triisobutylaluminum was not used to obtain 5.1 g of
isotactic polypropylene having [~] of 1.9 dl/g, Mw/Mn of 2.1
and a melting point of 131C.
F.x~m~le 13
The polymerization of Example 12 was repeated except
that in place of the triisobutylaluminum there were used 1 m
mol of tri-2-ethylhexylaluminum and 0.5 mg atom in terms of
Al atom of commercially available aluminoxane to obtain 38.2
63
2~0B31S
g of isotactic polypropylene having [~] of 2.3 dl/g, Mw/Mn
of 2.4, a melting point of 131C, a boiling
trichloroethylene-insoluble content of 0% by weight and a
boiling n-pentane-soluble content of 0.3% by weight.
F.XAmP1 e 14
A nitrogen-purged 1 liter glass autoclave was charged
with 335 ml of toluene and 15 ml of octene, followed by
elevating the temperature of the system to 70C while blowing
ethylene gas thereinto. Successively, to the autoclave were
0 added 0.4 mmol of triisobutylaluminum, 0.2 mg atom in terms
of Al atom of commercially available aluminoxane and 3 x 10-3
mmol of the ethylenebis(indenyl)hafnium dichloride obtained
in Example 1 to initiate polymerization. The polymerization
was carried out at 70C for 30 minutes while continuously
feeding ethylene gas to the polymerization system to obtain
16.8 g of an ethylene/l-octene copolymer having Mw/Mn of
3.18, MFR2 of 0.09 g/10 min, MFRlo/MFR2 of 30.7 and a density
of 0.879 g/cm3.
F.XAmP1 e 15
(Preparation of organoaluminum oxy-compound [B-2])
A thoroughly nitrogen-purged 400 ml flask was charged
with 4.9 g of A12(SO4)3-14H2O and 125 ml of toluene and cooled
to 0C, followed by dropwise addition of 200 mmol of
triisobutylaluminum diluted with 125 ml of toluene. The
-
6 4 20~8;}15
temperature of the reaction system was elevated to 40C, and
reaction was continued at that temperature for 24 hours.
After the completion of the reaction, solid-liquid separation
of the reaction mixture was conducted by filtration, the
toluene was removed from the filtrate, and a molecular weight
of the organoaluminum oxy-compound [B-2] obtained was
measured in benzene by the cryoscopic method to find that it
was 610.
(Polymerization)
A thoroughly nitrogen-purged 2 1iter stainless steel
autoclave was charged with 500 ml of toluene, and the system
was then purged with propylene gas. Successively, to the
autoclave were added 1 mg atom each in terms of Al atom of
the organoaluminum oxy-compound [B-l] and the organoaluminum
oxy-compound [B-2] and 1.0 x 10-3 mmol of
ethylenebis(indenyl)hafnium dichloride, and the temperature
of the polymerization system was elevated to 30C.
Thereafter, polymerization was carried out at 30C for 20
minutes while feeding propylene gas to the total pressure
became 5 kg/cm2 G to obtain 9.5 g of isotactic polypropylene
having [~] of 4.1 dl/g, Mw/Mn of 3.5, a melting point of
135C, a boiling trichloroethylene-insoluble content of 0% by
weight and a boiling n-pentane-soluble content of 0.2% by
weight.
F.x~m~le 16
72932-62
200831~
Example 12 was repeated except that the polymerization
was carried out under the total pressure of 7 kg/cm2 G at
50C for 1 hours using 1 mg atom in terms of Al atom of (iso-
C4Hg)2Al-O-Al(iso-C4H9)2 as the organoaluminum oxy-compound
[B-2] and 0.5 mg atom in terms of Al atom of the
organoaluminum oxy-compound [B-l] to obtain 35.1 g of
isotactic polypropylene having [~] of 2.2 dl/g, Mw/Mn of 2.1,
a melting point of 132C, a boiling trichloroethylene-
insoluble content of 0% by weight and a boiling n-pentane-
0 soluble content of 0.3% by weight
ComD~r~tive F.x~m~l e 3
Example 16 was repeated except that the organoaluminum
oxy-compound [B-2] was not used to obtain 2.4 g of isotactic
polypropylene having [~] of 1.5 dl/g and a melting point of
130C.
F.X~Dl e 17
A thoroughly nitrogen-purged 1 liter glass autoclave was
charged with 323 ml of toluene and 22 ml of 1-octene, and the
system was then elevated in temperature to 70C while blowing
ethylene gas thereinto. Successively, to the autoclave were
added 0.4 mg atom in terms of Al atom of ~iso-C4Hg)2Al-(O-
Al)2-OAl(iso-C4Hg)2 as the organoaluminum oxy-compound [B-2],
I
iso-C4Hg
D
. ~
66
20~8315
0.2 mg atom in terms of Al atom of the above-mentioned
organoaluminum oxy-compound [B-1] and 3 x 10-3 mmol of
ethylenebis(indenyl)hafnium dichloride to initiate
polymerization. The polymerization was carried out at 70C
for 30 minutes while continuously feeding ethylene gas to the
polymerization system to obtain 12.3 g of an ethylene/1-
octene copolymer having Mw/Mn of 2.49, MFR2 f 7.2 g/10 min,
MFRlo/MFR2 of 9.3 and a density of 0.853 g/cm3.
.