Note: Descriptions are shown in the official language in which they were submitted.
2~08~1~
TETRAHYDROBENZIMIDAZOLE DERIVATIVES
FIELD OF THE INVE~TION
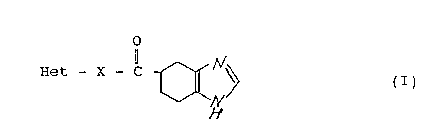
This invention relates to a tetrahydrobenzimidazole
derivative represented by formula (I) shown below or a
pharmaceutically acceptable salt thereof which are useful as
a 5-HT3-receptor antagonist.
o
Het - X - C ~ ~
wherein Het represents a heterocyclic group which may be
substituted with 1 to 3 substituents selected from the group
consisting of a lower alkyl group, a lower alkenyl group, a
lower alkynyl group, a cycloalkyl-lower alkyl group, an aralkyl
group, a lower alkoxy group, a nitro group, a hydroxyl group,
a lower alkoxycarbonyl group, and a halogen atom; and X
represents a single bond or -NH- which is bonded to the carbon
atom or nitrogen atom of the heterocyclic group.
BACKGROUND OF THE INVENTION
Conventionally known antagonists to 5-HT3-receptors include
azabicyclo compounds as disclosed in British Patents 2,125,398,
2,166,726, 2,166,727, and 2,126,728 (corresponding to JP-A-59-
36675 and JP-A-59-67284, the term "JP-A" as used herein means
an "unexamined published Japanese patent application"),
200~8~
tetrahydrocarbazole compounds as disclosed in sritish Patent
2,153,821 (corresponding to JP-A-60-214784), and azabicyclo
compounds as disclosed in EP 200,4~4 (corresponding to JP-A-
61-275276).
SUMMARY OF THE INVENTION
The inventors have conducted extensive in~estigations on
compounds showing antagonism against 5-HT3-receptors. As a
result, they have found that the compound represented by
formula (I) is a novel compound exhibiting high 5-HT3-receptor
antagonistic activity and thus reached the present invention.
The compounds according to the present invention are entirely
different in structure from any of the above-described known
5-HT3-receptor antagonists.
DETAILED DESCRIPTION OF THE INVENTION
In formula (I), the heterocyclic group as represented by
Het includes residues of monocyclic or condensed heterocyclic
~ings. Specific examples of the heterocyclic ring are
morpholine,
pyrrolidine, piperidine,~plperazlne,~pyrrole, furan, thiophene,
imidazole, oxazole, thiazole, pyrazole, isoxazole, isothiazole,
triazole, thladiazole, oxadiazole, pyridine, pyridazine,
pyrimidine, pyrazine, 4H-cyclopentathiazole, indole, isoindole,
~,3-dihydroindole (indoline), isoindoline, hydroxyindole,
indazole, indolizine, benzothiophene, benzofuran,
benzothiazole, benzimidazole, benzoxazole, ~,5,6,7-
tetrahydrobenzothiophene, 2,3-dihydrobenzimidazol-2-one,
-- 2
2 ~ 0 ~ 8 ~ ~
quinolina, iso~uinoline, 1,2,3,4~tetrahydroquinoline, 1,2,3,4-
t~trahydroisoquinoline, 1,4-benzoxazine, phenothiazine,
carbazole, ~-carboline, etc.
(at optional position(s))
The heterocyclic group may have a substituent(s)~, such as
a low~r alkyl group, a lower alkenyl group, a lower alkynyl
group, a cycloalkyl-lower alkyl group, an aralkyl g~oup, a
lower alkoxy group, a nitro group, a h~droxyl group, a lower
alkoxycarbonyl group, a halogen atom, etc.
Unless otherwise specified, the term "lower alkyl group" as
used herein means a straight chain or branched alkyl group
having ~rom 1 to 6 carbon atoms. Specific examples of the
lower alkyl group are methyl, ethyl, propyl, butyl, pentyl,
hexyl, isopropyl, isobutyl, t-butyl, isopentyl, t-pentyl,
isohexyl groups, etc.
Examples of the "lower alkenyl group" include vinyl,
allyl, l-propenyl, 2-butenyl, isopropenyl groups, etc.
Examples of the "lower aIkynyl group" include ethynyl,
2-propynyl groups, etc. Examples of the "cycloalkyl-lower
alkyl group" include cyclopropylmethYl, cyclopentylmethyl,
cyclohexylmethyl, cyclohexylethyl, cycloheptylmethyl groups,
etc. ~xamples of the "aralkyl group" include benzyl,
phenethyl groups, etc. Examples of the "lower alkoxy group"
include methoxy, ethoxy, propoxy, butoxy, pentyloxy,
hexyloxy, isopropoxy, isobutoxy, t-butoxy, isopentyloxy,
t-pentyloxy, isohexyloxy, 2-ethylbutoxy groups, etc.
Examples of the "lower alkoxycarbonyl group" include
methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl,
-- 3
2~8815
isopropoxycarbonyl, butoxycarbonyl, t-butoxycarbonyl,
pentyloxycarbonyl, hexyloxycarbonyl groups, etc.
The halogen atom includes chlorine, bromine, iodine, and
fluorine atoms.
Of the compounds represented by formula (I), preferred are
those wherein Het is represented by formula:
~R~
~ '
wherein R~ represents a hydrogen atom, a lower alkyl group, a
lower alkenyl group, a lower alkynyl group, a cycloalkyl-lower
alkyl group, or an aralkyl group; R2 represents a hydrogen
atom, a lower alkyl group, or an aralkyl group; and R3
represents a hydrogen atom, a lower alkoxy group, a nitro
group, a hydro~yl group, a lower alkoxycarbonyl group, or a
halogen atom;
and X represents a single bond,
and those wherein Het represents a nitrogen-containing
heterocyclic group; and X is a single bond connected to the
nitrogen atom of the nitrogen-containing heterocyclic ring.
Also included under the present invention are salts of some
of the compounds of formula (I). Examples of such salts
include salts with inorganic bases, e.g., sodium and potassium;
salts with oryanic bases, e.g., ethylamine, propylamine,
diethylamine, triethylamine, morpholine, piperidine, N-
- 4 -
2~0~
ethylpiperidine, diethsnol~mine, and cyclohexylamine; s~lts
with basic ~mino ~cids, e.g., lysine and ornithineS ammonium
salt; salts with mineral acids, e.g., hydrochloric ~cid,
sulfuric acid, phosphoric ~cid, ~nd hydrobromic acid; salts
with org~nic scids, e.g., ~cetic acid, oxalic ~cid7 succinic
acid, citric acid, aleic acid, malic acid, f~maric acid,
dibenzolytartaric acid, tartaric acid, and methanesulfonic
acid; snd salts with acidic amino acids, e.g., glutamic s~id
and ~spartic acid.
~ hile the compounds ~ccording to the present invention are
represented by formula (I'), the present invention further
includes ~automers of these compounds, i.e., compounds
represented by formula:
Het - X - C ~ ~
Furthermore, the compounds of ~he present invention ca~ry
asymme~ric carbon atoms in the molecule, and all the isomers
assigned to these asymmetric carbon atoms, such zs optically
active compounds, racemates, diastereomers, etc., are included
in the compounds according to the present invention.
Processes for preparing the compounds according to the
present invention are described below.
CA 02008815 1997-10-17
PROCESS 1 (Amidation):
HOOC _N\ ~
Het - X - H t ~H ~Het - X C~C¢N~
(II) (Ia) H
wherein ~et is as defined above; and X~ represents a single
bond connected to the nitrogen atom of the heterocyclic group,
or Xl represents -NH- connected to the czrbon atom of the
heterocyclic group.
The compound (Ia) of the present invention can be obtzined
by reacting an amine, an amide, or urea represented by formulz
(III) with 4,5,6,7-tetrahydrobenzimidazole-5-carboxylic acid
represented by formula (II) or a reactive derivative thereof.
The reaction is carried out by any of various known
processes for amide linkage formztion. Solvents to be used
are not particularly limited and include dioxane, diethyl
ether, tetrahydrofuran, chloroform, ethyl acetate, and
dimethylformamide.
The compound (II) is subjected to the reaction 25 being
eithe~ in the form of a free acid or in the form OI a reac~ive
deriv2tive thereof, e.g., an acid halide, an acid anhydride, an
acid 2zide, and various active esters ~enerally used in peptide
syntheses. In the former case, the amide linkage formztion
2~3~1~
can be effected by using any of commonly employed condensing
agents, for example, N,N-dicyclohexylcarbodiimide.
In some cases depending on the kind of the reactive
derivative of the compound (II), the reaction is preferably
carried out in the presence of a base, such as inorganic bases,
e.g., sodium hydrogencarbonate, potassium hydrogencarbonate,
sodium carbonate, and potassium carbonate; and organic bases,
e.g., triethylamine, diisopropylethylamine, dimethylaniline,
and pyridine.
The compound (III) is usually used as it is or, if desired,
after being conYerted to an alkali metal salt thereof.
The co..,~ound (III) is desirably used in an equimolar or
excessi~e amount with respect to the compound (Il) or a
reactive derivative thereof.
The reaction is possibly carried out at room temperature,
under cooling, or under heating as selected depending on the
kind of the amide linkage xeaction mode but usually at room
temperature or under cooling.
PROCESS 2:
Het-X -H I ~ ~ > Het_x2_
(II) (Ib)
.
200g8~
wherein He~ is as defined above; and XZ represents a single
bond connected to the carbon atom of the heterocyclic ring as
represented by Het.
The compound ~Ib) can be obtained by reacting a
heterocyclic compound represented by formula (IIIa) with a
carboxylic acid represented by formula (II) or a reactive
derivative thereof.
The reaction can be carried out by any of various known
pxocesses for synthesizing carbonyl compounds using a
carboxylic acid or a derivative thereof.
Where a carboxylic acid of formula (II) is employed, the
reaction with the compound of formula (IIIa) is a dehydrating
condensation reaction using polyphosphoric acid, for instance,
as a condensing agent. The reaction is carried out with or
without a solvent. Solvents which can be used are not limited
as long as inert to the reaction, but, usually, solvents having
an appropriate boiling point are selected taking the reaction
temperature into consideration. Examples of suitable solvents
are decalin, tetralin, diglyme, etc. The reaction is effected
at room temperature or preferably under heating.
Where an acid halide of the carboxylic acid of formula (II)
is employed, the reaction is a Friedel-Crafts reaction which
can be carried out by known processes or various modifications
thereof using a Lewis acid, e.g., aluminum chloride, ferric
chloride, stannic chloride, boron trifluoride e~hyl etherate,
2~08~1~
and titanium tetrachloride. Solvents inert to the reaction may
be employed preferably being selected depending on the kind of
the Lewis acid used. Examples of usable solvents are
acetonitrile and carbon disulfide. The reaction is performed
at room temperature or, usually, under heating.
Where an acid amide of the carboxylic acid of formula (II)
is employed, the reaction is a Vilsmeyer reaction, which is a
known reaction mode frequently employed for synthesis of
heterocyclic ca.rbonyl compounds. Reagents for converting the
acid amide to a Vilsmeyer complex include general halogenating
agents, e.g., phosphorus pentachloride and phosphorus
oxychloride. This reaction may be effected with or without a
solvent. In using a solvent, various kinds of solvents can be
employed as long as inert to the reaction. A suitable example
of solvents is 1,2-dichloroethane. The reaction is performed
at room temperature or under heating, and preferably under
heating.
PROCESS 3 ~N-Alkylation):
O O
Hetl - X.- C ~ ,9 ,~ Het2 _ X ~ 11 ~
(Id) ~ (Ic) ~/
tt ~
wherein X is as defined above; Hetl represents a heterocyclic
group having -NH- in the ring thereof; and Het2 represents a
heterocyclic group in which the -NH- moiety in Het~ is
2~0~
converted to -N-, wherein R4 represents a lower alkyl group, a
R4
lower alkenyl group, a lower alkynyl group, a cycloalkyl-lower
alkyl group, or an aralkyl group.
This reaction is an N-alkylation reaction. The terminology
~alkylation~ as used herein means introduction of a lower
alkyl, lower alkenyl, lower alkynyl, cycloalkyl-lower alkyl or
aralkyl group. Any of various known alkylation techniques is
applicable. For example, in case where the alkylation is
carried out by direct N-alkylation using an alkylating agent,
the reaction is conducted under cooling, at room temperature,
or under heating, and preferably under cooling or at room
temperature. Any solven~ inert to the reaction, e.g., dioxane
and dimethylformamide, may be employed. The reaction is
effected in the presence of a base or by using an alkali metal
salt of the compound (Id) at the amino group thereof. Examples
of suitable alkylating agents include alkyl halides and alkyl
sulfates. Examples o~ suitable bases include inorganic bases,
sodium hydride,
e.g.,~sodium hydrogencarbonate, potassium hydrogencarbonate,
sodium carbonate, and potassium carbonate; and organic bases,
e.g., triethylamine, diisopropylamine, dimethylaniline, and
pyridine.
The thus prepared compound of the present invention is
isolated and purified either in the free form or in the form of
a salt through usual chemical means, such as extraction,
-- 10 --
2~8~5
crystallization, recrystallization, and various chromatographic
techniques.
The compound as obtained in the form of a racemate can be
led to stereochemically pure isomers by using an appropriate
starting compound or by general resolution techniques (for
example, a method comprising once obtaining a diastereomer salt
~ibenzoylJ
with an ordinary optically active acid, e.g.,~tartaric acid,
followed by optical resolution).
The compounds according to the present invention and the
salts thereof specifically inhibit transient bradycardia
induced by serotonin in anesthetized rats as demonstrated by
Test Example 1 hereinafter described and are thus believed to
have antagonism against 5-HT3-receptor. Therefore, the
compounds of the present invention and the salts thereof are
considered to suppress vomiting induced by anticancer agents,
e.g., Cisplatin, or radiation and to be useful in the
prevention and treatment of migraine, cluster headache,
trigeminal neuralgia, anxiety, gastrointestinal disorders,
peptic ulcer, irritable bowel syndrome, etc.
A pharmaceutical composition containing at least one of the
compounds of the present invention or salts thereof as an
active ingredient is prepared in various dose forms, such as
tablets, powders, granules, capsules, pills, liquids,
injections, suppositories, ointments, pastes, and the like
using carriers, excipients and other additives conventionally
2~0~
used in pharmaceuticals. The preparation may be a~ninistered
or~lly, inclusive of sublingual administration, or
parenterally.
Carriers or excipients for pharmaceutical compositions
include solid or liquid non-toxic pharmaceutically acceptable
materials, e.g., lactose, magnesium stearate, staxch, talc,
gelatin, agar, pectin, gum arabic, olive oil, sesame oil, cocoa
butter, ethylene glycol, and the like.
A clinical dose of the compound of the present invention is
appropriately determined, taking into account conditions, body
weight, age, sex, etc. of the patient. It usually ranges from
0.1 to 10 mg/day for intravenous administration and from 0.5 to
50 mg/day for oral administration for adult in a single or
several divided doses.
The pharmacological effects of the compounds of the present
invention were confirmed by Test Examples.
TEST EXAMPLE 1
Antaqonism Aqainst 5-HT3-Receptor
Nine-week-old Wistar male rats were anesthetized by
intravenous in~ection of 1 g/kg of urethane, and blood pressure
and heart rate were measured under artificial respiration.
Transient reduction in heart rate and blood pressure induced by
intravenous administration of serotonin or 2-methylserotonin
which is a selective agonist of 5-HT3 was taken as an index of
2 ~
the reaction via 5-HT3 receptor [Bezold-Jarish reflex; Paintal,
A.S., Pysiolo. Rev., Vol. 53, p. 159 (1973)]
When the compound of the present invention or a salt
thereof was intravenously administered (0.03 to 3 ~g/kg) or
orally administered (l to 30 ~g/kg) 10 minutes or 60 minutes
before the administration of serotonin (or 2-methylserotonin),
respectively, the reduction in heart rate and blood pressure
induced by serotonin or 2-methylserotonine was dose-dependently
inhibited.
Inhibitory activity of the compound of the present
invention on serotonin-induced Bezold-Jarish (BJ) reflex in
rats is shown in Table 1 below.
TABLE 1
Example No. ofBJ Reflex Inhibitory Activity
Test Compound~EDs~; ~q/kq, i.v.)
2 0.29
4 0.044
9 0.80
36 0.063
TEST EXAMPLE 2
Inhibition on Anticancer Aqent-Induced Vomitinq
When male ferrets weighing from 1 to 1.5 kg subcutaneously
or orally received 0.01 to 0.3 mg/kg of the compound of the
present invention, vomiting induced by intraperitoneal
administration of 10 mg/kg of Cisplatin was inhibited.
- 13 -
2~8~
TEST EXAMPLE 3
Inhibition on Stress Defecation
Nine-week-old male Wistar rats were encased in a cage for
restricted stress, and the number of feces was measured.
Intravenous administration of the compound of the present
invention or a salt thereof (1 to 100 ~g/kg) dose-dependently
inhibited acceleration of defecation induced by restricted
stress.
TEST EXAMPLE 4
Toxicity
Acute toxicity of the compounds of the present invention in
male mice was from 100 to 150 mg/kg i.v. as determined by an
up-and-down method, indicating that the compounds are of low
toxicity.
The present invention is now illustrated in greater detail
with reference to Reference Examples, Examples, and Formulation
Examples, but it should be understood that the present
invention is not deemed to be limited thereto.
REFERENCE EXAMPLE 1
N ~ COOCH, ~l ~ COOCH,
~H2SO~ H2SO~
In 600 m~ of acetic acid was dissolved 40.0 g of methyl 5-
benzimidazole carboxylate sulfate in a 1 ~-volume autoclave,
- 14 -
2 ~
and ll g of 10% palladium-on-carbon was added to the solution
as a catalyst to conduct hydrogenation at 80~C at a pressure of
60 atm for 5 hours. The catalyst was separated by filtration,
and the mother liquor was concentrated under reduced pressure
to obtain 41.0 g of methyl 4,5,6,7-tetrahydrobenzimidazole-5-
carboxylate sulfate as an oil.
(b) ~r~o~ HCI ~'C OOH
H2 S 0~ , H2 S 0~
In a mixture of 350 m~ of water and 340 mQ of concentrated
hydrochloric acid was dissolved 41.0 g of the oily ester
sulfate as obtained in (a) above, and the mixture was stirred
at 100~C for 3 hours. After concentration, the resulting
crystal was washed with acetone to obtain 29.6 g (76.8~ based
on the benzimidazole ester) of 4,5,6,7-tetrahydrobenzimidazole-
5-carboxylic acid sulfate.
Physicochemical Properties:
Melting Point: 145-148~C
NMR (d6-DMS0): ~ 1.60-3.00 (7H, m), 8.84 (lH, s)
Mass Spectrum (EI): m/z 166 (M+, as a free compound)
(CI): m/z 167 (M++1, as a free compound)
- 15 -
REFERENCE EXAMPLE 2
o
HOOC (H5~ ~ C
To 0.30 g of 4,5,6,7-tetrahydrobenzimidazole-5-carboxylic
acid hydrochloride containing sodium chloride was added 5 mQ of
thionyl chloride, followed by stirring at 90~C for 2 hours.
The excess of thionyl chloride was removed by distillation
under reduced pressure. ~o the residue was added 10 m~ of
dichloromethane, and 2 mQ of diethylamine was added thereto at
5~C, followed by stirring at room temperature for 16 hours. To
the mixture was added 40 mQ of dichloromethane, and the mixture
was washed with a saturated aqueous solution of sodium
hydrogencarbonate and dried over anhydrous magnesium sulfate.
The solvent was removed by distillation under reduced pressure
to obtain 0.22 g o-f N,N-diethyl-4,5,6,7-
tetrahydrobenzimidazole-5-carboxamide.
Physicochemical Properties:
NMR (TMS, CDCR3) ~ 1.15 (t, 6H), 2.0-3.5 (m, 7H), 3.10 (q,
4H), 8.15 (s, lH), 9.50 (s, lH)
Mass Spectrum (FAB, Pos) m/z 222 (M~
To the above obtained compound was added 1 m~ of a 4N
solution of hydrogen chloride in ethyl acetate, and the solvent
was removed by dis-tillation under reduced pressure to obtain
- 16 -
20~1a
0.27 g of N,N-diethyl-4,5,6,7-tetrahydrobenzimida~ole-5-
carboxamide hydrochloride.
EXAMPLE 1
O
N ~ N
~ TCI , ~ ~> ~CCOO~I
CH, CH~ ~ ~OOCC~
In 0.7 m7 of thionyl chloride, 0.13 g of 4,5,6,7-
tetrahydrobenzimidazole-5-carboxylic acid hydrochloride
(containing sodium chloride) was refluxed for 30 minutes,
and volatile components were removed by distillation under
reduced pressure. The residue was added to a solution of
0.15 g of 3,3-dimethylindoline and 0.15 m~ of triethylamine in
2 m~ of dichloromethane under ice-cooling. After stirring the
mixture at room temperature overnight, 5 mQ of a sodium
carbonate aqueous solution was added thereto, and the mixture
was extracted with chloroform. The organic layer was dried,
and the solvent was removed by distillation under reduced
pressure. The residue was subjected to silica gel column
chromatography using chloroform/methanol as an eluent to obtain
0.11 g of 5-[(2,3-dihydro-3,3-dimethylindol-l-yl)carbonyl]-
4,5,6,7-tetrahydrobenzimidazole as an oily substance. The oily
substance was then treated with a solution of fumaric acid in
methanol~acetonitrile to obtain 0.09 g of 5-[(2/3-dihydro-3r3
2~0~81~
dimethylindol-1-yl ) carbonyl ] -4, 5, 6, 7-tetrahydrobenzimidazole
f umarate .
Physicochemical Properties:
Melting Point: 119-123~C
Elemental Analysis for Cl8H2lN3O ~ C4H404 ~ H2O ~ O ~ 3CH3CN:
Calcd. (96): C 61.44; H 6.36; N 10.46
Found (%): C 61.60; H 6.03; N 10.46
Mass Spectrum (EI): m/z 295 (M~, as a free compound)
In the same manner as in Example 1, the following compounds
were synthesized.
EXAMPLE 2
5- [ ( 2, 3-Dihydroindol-1-yl ) carbonyl ] -
4, 5, 6, 7-tetrahydrobenzimidazole fumarate
NJ~N~ ~ HC COOH
H HOOC CH
Phys i cochemi ca l Properti es:
Melting Point: 206-208~C (methanol/acetonitrile)
Elemental Analysis f or Cl6Hl7N3O ~ C4H404 . 0 ~ 3H2O:
Calcd. (%): C 61.78; H 5.60; N 10.81
Found (%~: C 61.92; H 5.53j N 10.68
Mass Spectrum (EI): m/z 267 (M+, as a free compound)
- 18 -
2~881~
EXAMPLE 3
5-[(2-Methyl-2,3-dihydroindol-1-yl)-
carbonyll-4,5,6,7-tetrahydrobenzimidazole
Physicochemical Properties:
Melting Point: 230-234~C (dec.) (recrystallized from
eth~l acetate/hexane)
Elemental Analysis for Cl7Hl9N3O:
Calcd. (%): C 72.57; H 6.81; N 14.93
Found (%): C 72.76; H 6.78; N 14.62
Mass Spectrum: m/z 281 (M+)
EXAMPLE 4
o
T C2Hs~ ~ N
CH, H
'~N HOOC
CH, H
A mixture of 0.27 g (1.05 mmol) of N,N-diethyl 4,5,6,7-
tetrahydrobenzimidazole-5-carboxamide hydrochloride, 0.16 m~
(1.25 mmol) of 1-methylindole, and 0.15 m~ (1.65 mmol) of
phosphorus oxychloride was heated at 80~C for 2 hours while
stirring. 30 ml of water were added thereto, and
-- 19 --
20~8~1~
the mixture was rendered basic with a lN sodium hydroxide
aqueous solution, followed by extracting with eth~1 acetate.
The ethyl acetate layer was dried over anhydrous magnesium
sulfate and filtered. The filtrate was distilled under reduced
pressure, and the residue was purified by silica gel column
chromatography (eluent: dichloromethane/methanol/aqueous
ammonia = 10:1:0.1 by volume) and preparative thin layer
c h r o m a t o g r a p h y ( d e v e 1 o p i n g s o 1 v e n t :
dichloromethane/methanol/aqueous ammonia = 10:1: 0.1 by volume)
~to obtain~
~20 mg of a foaming substance. To the product was added 10 mg
of fumaric acid to convert it to a fumarate. Recrystallization
from eihyl acetate/methanol (10:1 by volume) gave 10 mg of 5-
[(1-methylindol-3-yl)carbonyl]-4,5,6,7-tetrahydrobenzimidazole
fumarate.
Physicochemical Properties:
Melting Point: 97-102~C
Mass Spectrum (EI): m/z 279 (M+, as a free compound)
NMR (CDC~ (as a free compound):
1.90-3.00 (7H, m, CH2, CH), 3~80 (3H, s,
N-Me), 7.20 (2H, m, ArH), 7.50-8.00 (4H,
m, ArH), 8.30 (lH, m, NH)
- 20 -
2 ~
EXAMPLE 5
o
11
~ 'X2 S 0~ ~N-C ~ ~
H H HCl
In 53 mQ of acetonitrile were added 5.3 g of 4,5,6,7-
tetrahydrobenzimidazole-5-carboxylic acid sulfate and 2.9 mQ of
thionyl chloride, and the mixture was stirred at 53 to 55~C
for 1.5 hours. The mixture was distilled under reduced
pressure to remove 10 to 15 mQ of the solvent. ~f~er 15 mQ of
acetonitrile was added thereto, the mixture was further
distilled under reduced pressure to remove 10 to 15 m~ of the
solvent. The residual solution was added dropwise to a
solution of 14.2 g of pyrrolidine in 50 mQ of acetonitrile at
2~C or lower. After the addition, the temperature was returned
to room temperature, and the mixture was stirred for 1 hour,
followed by concentration under reduced pressure. To the
residue was added 30 m~ of a saturated sodium chloride aqueous
solution, and the mixture was extracted with chloroform
(50 mQ x 3). The chloroform layer was dried over anhydrous
magnesium sulfate, concentrated under reduced pressure, treated
with hydrochloric acid in ethanol, and recrystallized from
ethanol/ethyl ace~ate to obtain 4.25 g (82.9%) of N-[(~,5,6,7-
tetrahydrobenzimidazol-5-yl)carbonyl]pyrrolidine hydrochloride.
230881~
Physicochemical Properties:
Melting Point: 234-236~C
Elemental Analysis for Cl2HI8N3OCQ~0.2H2O:
Calcd. (%): C 55.57; H 7.15; N 16.20; C~ 13.67
~ound ~%): C 55.64; H 6.99; N 16.18; C~ 13.79
Mass Spectrum (EI): m/z 291 (M+, as a free compound)
In the same manner as in Example 5, the following compounds
were synthesized.
EXAMPLE 6
4-(4,5,6,7-Tetrahydrobenzimidazol-5-
ylcarbonyl)-2,3-dihydro-1,4-benzoxazine fumarate
COOH
~ ~ H HOOC~
Physicochemical Properties:
Melting Point: 176-178~C (methanol/acetonitrile)
Mass Spectrum (EI): m/z 283 (M~, as a free compound)
Elemental Analysis for Cl6Hl7N3O2.C4H4O4:
Calcd. (%): C 60.14; H 5.30; N 10.52
Found (%): C 59.95; H 5.28; N 10.55
EXAMPLE 7 ~~
HOOC ~ ~ >
HCI ~ COOH
HOOC
In 5 m~ of thionyl chloride was added 0.58 g (0.98 mmol) of
4,5,6,7-tetrahydrobenzimidazole-5-carboxylic acid hydrochloride
- 22 -
,
2~8~1~
~ ntaining sodium chloride),)
having a purity of 34.5% ~and the mixture was stirred at 90~C
for 4 hours. After cooling, the solution was distilled under
reduced pressure to remove thionyl chloride. To the residue
was added 10 m~ of dichloromethane, and 0.20 m~ (1.59 mmol) of
1,2,3,~-tetrahydroquinoline and 0.35 m~ (2.53 mmol) of
triethylamine were added thereto, followed by stirring at room
temperature for 48 hours. To the reaction mixture was added
40 m~ of dichloromethane, and the mixture was washed with a lN
sodium hydroxide aqueous solution and dried over anhydrous
magnesium sulfate. The solvent was removed by distillation
under reduced pressure, and the residue was subjected to silica
g e l c o l u m n c h r o m a t o g r a p h y u s i n g
dichloromethane/methanol/aqueous ammonia (10:1:0.1 by volume)
as an eluent to obtain 100 mg of a foaming substance, which was
then treated with 40 mg of fumaric acid in ethanol to be
converted to a fumarate. Recrystallization from ethyl
acetate/methanol (10:1 by volume) gave 90 mg (33.3%) of 1-
[(4,5,6,7-tetrahydrobenzimidazol-5-yl)carbonyl]-1,2,3,4-
tetrahydroquinoline fumarate.
Physicochemical Properties:
Melting Point: 98-100~C
Elemental Analysis for C~7HI9N3O.C4H4O4.2H2O:
Calcd. (%): C 58.19; H 6.27; N 9.69
Found (%): C 58.43; H 5.73; N 9.53
2~n8~
NMR (DMSO-d6) ~ ppm: 1~90 (4H, q, 7Hz, quinoline CH2x2),
2.00-3.00 (7H, m, benzimidazole CH2x3,
CH), 3.70 (2H, t, J=7Hz, CH2N), 6.60
(2H, s, fumaric acid CHx2), 7.16 (5H, m,
ArH, NH), 7.55 (lH, s, imidazole CH)
Mass Spectrum (EI): m/z 281 (M~, as a free compound)
EXAMPLE 8 o
HOOC ~ ~ ~ ~ N~
H H
HCI
To 5 m~ of thionyl chloride was added 0.58 g (0.98 mmol) of
4,5,6,7-tetrahydrobenzimidazole-5-carboxylic acid hydrochloride
~ (containing sodium chloride),~
having a purity of 34.5~followed by~stirring at 90~C for 4
hours. After cooling, the reaction mixture was distilled under
reduced pressure to remove thionyl chloride. To the residue
were added 10 m~ of dichloromethane, 0.20 m~ (1.57 mmol) of
1,2,3,4-tetrahydroisoquinoline, and 0.35 m~ (2.53 mmol) of
triethylamine, and the mixture was stirred at room temperature
for 48 hours. To the reaction mixture was added 40 m~ of
dichloromethane, and the mixture was washed with a lN sodium
hydroxide aqueous solution and dried over anhydrous magnesium
sulfate. The solvent was removed from the residue by
distillation under reduced pressure. The residue was suhjected
to silica gel column chromatography using
dichloromethane/methanol/aqueous ammonia (10:1:0.1 by volume)
- 24 -
~ag~s
as an eluent to obtain 0.15 g of a white foaming substance,
which was then recrystallized from diethyl e-ther/ethyl acetate
to obtain 40 mg (14.8%) of 2-[(4l5/6t7-tetrahydrobenzimida
5-yl)carbonyl]-1,2,3,4-tetrahydroisoquinoline.
Physicochemical Properties:
Melting Point: 128-130~C
NMR (DMSO-d6) ~ ppm: 2.00-3.00 (7H, m, CH2x3, CH,
benzimidazole), 3.00 (2H, t, J=5Hz,
CH2), 3.40 (2H, t, J=5Hz, -CH2-), 4.24
(2H, s, CH2N), 7.22 (6H, m, ~rH, NH)
Mass Spectrum (EI): m/z 281 (M')
EXAMPLE 9
HO~C~ ~ NCO N
H~SO~ >
H ~ H H
A mixture of 0.78 g of 4,5,6,7-tetrahydrobenzimidazole-5-
carboxylic acid sulfate and 3 m~ of thionyl chloride was heated
at 50~C for 20 minutes, and the excess of thionyl chloride was
removed by concentration under reduced pressure to obtain a
carboxylic acid chloride. A solution of the resulting
carboxylic acid chloride in 3 m~ of dimethylformamide was added
to a dimethylformamide solution (30 m~) of 1.61 g of 2-
hydroxybenzimidazole and 0.50 g of 60% oily sodium hydride
under ice-cooling, and the reaction mixture was stirred at room
temperature for 1 hour, followed by concentration under reduced
- 25 -
2~8~1~
pressure. The residue was made acidic with 0.SN hydrochloric
acid, and any insoluble matter was removed by filtration. The
filtrate was made basic with potassium carbonate, and the thus
formed crystal was collected by filtration, washed with water,
and stirred in acetone overnight. The resulting crystal was
collected by filtration to yield 0.20 g (24%) of 1-[(4,5,6,7-
tetrahydrobenzimidazol-5-yl)carbonyl]-2/3-dihydrobenzimidazol
2-one.
Physicochemical Properties:
Melting Point: 271-274~C (dec.)
Elemental Analysls for Cl5HI4N4O2Ø4H2O:
Calcd. (~): C 62.23; H 5.15; N 19.35
Found (~): C 62.41; H 5.02; N 19.06
Mass Spectrum (EI): m/z 282 ~M+)
In the same manner as in Example 9, the following compounds
were synthesized.
EXAMPLE 10
5-Methoxy-1-[(4,5,6,7-tetrahydrobenzimidazol-5-yl)-
carbonv11-2,3-dihvdrobenzimidazol-2-one fumarate
~ ~CO ~ ~ ~COOH
CH3 H N HOOC
Physicochemical Properties:
Melting Point: 215-218~C (dec.) (recrystallized from
methanol)
Mass Spectrum (EI): m/z 312 (M+, as a free compound)
NMR (DMSO-d6) ~ ppm: 1.57-2.34 (2H, m), 2.34-3.10 (4H, m),
3.76 (3H, s), 3.90-4.28 (lH, m), 6.58
- 26 -
2 ~
(2H, s), 6.3~-6.84 (2H, m), 7.62 (lH,
s), 7.89 (lEI, d, J=8Hz)
EXAMPLE 11
1-Methyl-3-[(4,5,6,7-tetrahydrobenzimidazol-5-yl)-
carbon~ll-2,3-dihydrobenzimidazol-2-one fumarate
~ HOOC~
Physicochemical Properties:
Melting Point: 145-147~C (recrystallized from me-thanol/
acetonitrile)
Mass Spectrum (EI): m/z 296 (M~, as a free compound)
Elemental Analysis for Cl6Hl6N4o2~c4H4o4-o-5H2o)
Calcd. (%): C 57.00; H 5.02; N 13.30
Found (%): C 56.91; H 5.06; N 13.31
EXAMPLE 12
~COOH ~ NH C~
~ H23O, H
In 10 me of 1,2-dichloroethane were heat-refluxed 1.32 g of
4,5,6,7-tetrahydrobenzimidazole-5-carboxylic acid sulfate and
1.78 g of thionyl chloride for 30 minutes. The excess of
thionyl chloride and the solvent were removed by distlllation
under reduced pressure, and the residue was dissolved in 4.0 m2
of dry dimethylformamide. The solution was added to a solution
of 2.7 g of 2-aminobenzothiazole in 10 m~ of dry
-- 27 --
2~08~1~
dimethylformamide under ice-cooling, followed by stirring at
room temperature for 1 hour. The solvent was removed by
distillation under reduced pressure, and the residue was
purified by silica gel column chromatography using a methylene
chloridetme~hanol mixed solvent as a developing solvent
followed by recrystallization from ethanol to obtain 0.8 g
(53.7%) of N-(2-benzothiazolyl)-4,5,6,7-tetrahydrobenzimidazol-
5-ylcarboxamide.
Physicochemical Properties:
Melting Point: 165-167~C
Elemental Analysis for C15H~4N4OSØ25H2O:
Calcd. (%): C 59.49; H 4.82; N 18.50; S 10.59
Found (%): C 59.30; H 4.73; N 18.49; S 10.68
Mass Spectrum (EI): m/z 298 (M~)
In the same manner as in Example 12, the following
compounds were synthesized.
EXAMPLE 13
N-(2-Benzimidazolyl)-4,5,6,7-tetrahydro-
benzimidazol-5-ylcarboxamide
~ H~
Physicochemical Properties:
Melting Point: 182-185~C
Elemental Analysis for Cl5Hl5N5O-0-6H2O:
Calcd. (%): C 61.67; H 5.58; N 23.97
- ~8 -
2~8~1~
Found (%): C 61.63; H 5.44; N 23.97
Mass Spectrum (EI): m/z 281 (M+)
EXAMPLE 14
N-(Quinolin-3-yl)-4,5,6,7-tetra-
hydrobenzimidazol-5-ylcarboxamide
~'~C~?
Physicochemical Properties:
Melting Point: 296-297~C
Elemental Analysis for CL7Hl6N4O 0 25H2O:
Calcd. (%): C 68.79; H 5.60; N 18.87
Found (%): C 68.69; H 5.66; N 18.75
Mass Spectrum (EI): m/z 292 (M+)
EXAMPLE 15
N-(5-Methyl-1,3,4-thiadiazol-2-yl)-
4,5,6,7-tetrahydrobenzimidazole-5 carboxamide
'N N ~
CX3J~S~ C~
H
Physicochemical Properties:
Melting Point: ~300~C
Elemental ~lalysis for CIlHl3N5os.o-2H2H:
Calcd. (%): C 49.50; H 5.06; N 26.24; S 12.01
Found (~): C 49.86; H 4.97; N 26.40; S 11.68
Mass Spectrum (EI): m/z 263 (M~)
- 29 -
2~0~81~
EXAMPLE 16
N-(9-Ethylcarbazol-3-yl)-4,5,6,7-
tetrahydrobenzimidazol-5-carboxamide
o
- ~ NHC
C2H5
Physicochemical Properties:
Melting Point: 168-170~C
Elemental Analysis for C22H22N4OØ5H2O:
Calcd. (%): C 71.91; H 6.31; N 15.25
Found (%): C 71.77; H 6.13; N 15.13
Mass Spectrum (EI): m/z 358 (Mi)
EXAMPLE 17
N-[(4j5,6,7-Tetrahydrobenzimidazol-
5-yl)carbonyllPhenothiazine hYdrochloride
N ~
O-C ~ ~ HCl
Physicochemical Properties:
Melting Point: 268-270~C
Elemental Analysis for C2oHI7N3OS.HC~Ø5H2O:
Calcd. (~): C 61.14; H 4.87; N 10.69; C~ 9.02
Found (~): C 61.15; H 4,64; N 10.60; C~ 8.59
Mass Spectrum (EI): m/z 347 (M+, as a free compound)
- 30 -
200~
EXAMPLE 18
N-(5,6-Dihydro-4H~cyclopentathiazol-2-yl)-
4,5,6,7-tetrahydrobenzimidazole-5-carboxamide
~ ~ N~
Physicochemical Properties:
Melting Point: 164-165~C
NMR (DMSO6) ~ ppm: 1.70-3.00 (13H), 7.426 (lH)
Mass Spectrum (EI): m/z 288 (M+), 255
EXAMPLE 19
N-(Pyrimidin-2-yl)-4,5,6,7-tetrahydro-
benzimidazol-5-carboxamide dihydrochloride
N O
~N~ ~r~C
2HCI
H
Physicochemical Properties:
Melting Point: 287-289~C
Elemental Analysis for C12Hl3N5O.2HC~.1.4H2O:
Calcd. (~): C 42.22; H 5.25; N 20.51; C~ 20.77
Found (%): C 42.35; H 5.00; N 20.69; C~ 20.45
Mass Spectrum (EI): m/z 243 (M~, as a free compound)
2~881~
EXAMPLE 20
N-tPyridin-3-yl)-4,5,6,7-tetrahydro-
benzimidazole-S-carboxamide dihydrochloride
o
~ '0[~9
Physicochemical Properties:
Melting Point: 285-287~C
Elemental Analysis for Cl3Hl4N4O.2HC~:
Calcd. (~): C 49.54; H 5.12; N 17.77
Found (%): C 49,74; H 5.26; N 17.53
Mass Spectrum (EI): m/z 242 (M+, as a free compound)
EXAMPLE 21
N-(3-Ethoxycarbonyl-4, 5, 6,7-tetrahydrobenzo[b]thiophen-2-
yl)-4,5,6,7-tetrahydrobenzimidazole-5-carboxamide
Il
NHC
COOC2H5 H
Physicochemical Properties:
Mel+~ing Point: 159-161~C
Elemental Analysis for ClgH23N3O3S:
Calcd. (%): C 61.10; H 6.21; N 11.25; S 8.59
Found (%): C 60.87; H 6.16; N 11.05; S 8.62
Mass Spectrum (EI): m/z 373 (M~)
- 32 -
2aos~l~
EXAMPLE 22
N-(Indazol-6-yl)-4,5,6,7-
tetrahydrobenzimidazole-5-carboxamide
Physicochemical Properties:
Melting Point: >300~C
Elemental Analysis for C15HI5N5O:
Calcd. (%): C 64.04; H 5.37; N 24.89
Found (%): C 63.79; H 5.42; N 24.75
Mass Spectrum (EI): m/z 281 (M )
EXAMPLE 23
> ~,~ fbc3 ~ou~
4 g of N-[(4,5,6,7-tetrahydrobenzimidazol-5-
yl)carbonyl]pyrrolidine hydrochloride obtained in Exampl~ 5
were added to 40 m~ of dichloroethane, and 2.74 g of indole and
4.4 m~ of phosphorus oxychloride were added thereto. The
mixture was stirred at 80 to 85~C for 7 hours and then at room
temperature overnight. To the mixture was added 40 m~ of a
cold potassium carbonate aqueous solution, followed by
extraction with chloroform. The extract was drled over
anhydrous magnesium sulfate, and the solvent was removed by
distillation under reduced pressure. The residue was subjected
2~088~
to column chromatography using chloroform/methanol as an eluent
to obtain 1.82 g oE 5-[(indol-3-y:L)carbonyl~4,5,6,7-
tetrahydrobenzimidazole as a foaming substance. In 1 m~ of
methanol were dissolved 0.16 g of the resulting product and
0.06 g of fumaric acid, and S m~ of acetonitrile was added to
the solution. The formed crystal was collected by filtxation
to obtain 0.13 g of 5-[(indol-3-yl)carbonyl]-4,5,6,7-
tetrahydrobenzimidazole fumarate.
Physicochemical Properties:
Melting Point: 153-154~C
Elemental Analysis for Cl6Hl5N3O.C4H4O4Ø15CH3CNØ65H2O:
Calcd. (%): C 61.07; H 5.24; N 11.05
Found (%): C 61.11; H 5.01; N 11.04
Mass Spectrum (FAB): m/z 266 (Mi+l, as a free compound)
In the same manner as in Example 23, the following
compounds were synthesized.
EXAMPLE 24
5-[(1,2-Dimethylindol-3-yl)carbonyl]-
4,5,6,7-tetrahydrobenzimidazole.3/4 fumarate
~[~ 3/4 ~D~CJJ'
C~
Physicochemical Properties:
Melting Point: 220-223~C
Elemental Analysis for Cl8HlgN3O.3/4C4H404:
Calcd. (%): C 66.30; H 5.83; N 11.05
-- 34 --
2~0881~
Found (~): C 66.50; H 5.83i N 11.13
Mass Spectrum (EI): m/z 293 (M+, as a free compound~
E~AMPLE 25
5-[(2-Methylindol-3-yl)carbonyl]-
4,5,6,7-tetrahydrobenzimidazole.~fumarate
~ J~ oo~
Physicochemical Properties:
Melting Point: 221-223~C
Elemental Analysis for C~7HI7N30.~csH4o4.o.25H2o
Calcd. (~): C 66.75; H 5.75; N 12.29
Found (%): C 66.73; H 5.75; N 12.29
Mass Spectrum (EI): m/z 279 (M~, as a free compound)
EXAMPLE 26
5-[(2-Benzylindol-3-yl)-carbonyl]-
4,5,6,7-tetrahydrobenzimidazole-fumarate
~ ~oo5
Physicochemical Properties:
Melting Point: 183-186~C
Elemental Analysis for C23H2lN30.c4H404-O-lH20:
Calcd. (%): C 68.52; H 5.37; N 8.88
Found (%): C 68.38; H 5.50; N 8.87
Mass Spectrum (EI): m/z 355 (M+, as a free compound)
- 35 -
~0~881~
EXAMPLE 27
5-[(s-Methoxyindol-3-yl)carbonyl]~
4,5,6,7-tetrahYdrobenzimidazole~3/4 fumarate
C~3~ oc~co
Physicochemical Properties:
Melting Point: 162-164~C
Elemental Analysis for Cl7H~7N3O2.3/4C4H404Ø2CH3CNØ85H2O:
Calcd. (%): C 60.36; H s.54; N 11.04
Found ~%): C 60.33; ~ 5.25; N 10.93
Mass Spectrum (EI): m/z 295 (M+, as a free compound)
EXAMPLE 28
5-[(5-Chloro-2-methylindol-3-yl)-car~onyl]-
4,5,6,7-tetrahydrobenzimidazole.fumarate
CGO~
Physicochemical Properties:
Melting Point: 212-213~C
Elemental Analysis for Cl7H16N3OC~C4H4O4:
Calcd. (%): C 58.68; H 4.67; N 9.78; C~ 8.25
Found (%): C 58.43; H 4.91; N 9.67; C~ 8.24
Mass Spectrum (FAB, Pos): m/z 314 (M++l, as a free compound)
2o~88l~
EXAMPLE 29
5-[(5~Nitroindol-3-yl)carbonyl]
4 5,6,7-tetrahvdrobenzimidazole
Physicochemical Properties:
Melting Point: 227-229~C
NMR (DMSO-d6) lOOM, ~: 2.00 (2H, m), 2.70 (4H, m), 3.63 (lH,
m), 7.44 (lH, s), 7.64 (lH, d,
J6.7=12HZ), 8-10 (lH, dd, J67-12Hz,
J46=4Hz), 8.72 (lH, s), 9.07 (lH, d,
J4.6=4Hz), 12-56 (lH, br)
Mass Spectrum (EI): m/z 310 (M+)
EXAMPLE 30
5-[(5-Methoxycarbonylindol-3-yl)-
carbonyll-4,5,6,7-tetrahYdrobenzimidazole
O o
~JJ~l~
~ H
Physicochemical Properties:
Melting Point: 205-209~C
NMR (DMSO-d6) lOOM ~:1.90-2.15 (2H, m), 2.83 (4H, br), 3.75
(lH, br), 7.56 (lH, d, J67=12Hz), 7.84
(lH~ dd~ J67=12Hz, J46=3Hz), 8.62 (lH,
- 37 -
2~8~1~
d~ J2NH=4Hz), 8-90 (2H, s), 12.60 (lH,
d, Jz~NH=4Hz)
Mass Spectrum (EI): m/z 323 (M~)
EXAMPLE 31
5-[(5-Hydroxyindol-3-yl)carbonyl]-
4,5,6,7-tetrah~drobenzimidazole.~fumarate
a
~ Y,2, H OGC
Physicochemical Properties:
Melting Point: 282-286~C
NMR (DMSO-d6) lOOM ~:1.90 (2H, br), 2.85 (4H, br), 3.74 (lH,
br), 6.76 (lH, s), 6.84 (lH, dd,
J67=12Hz, J4~6=4Hz), 7.41 (lH, d,
J67=12Hz), 7.74 (lH, d, J46=4Hz), 8-50
(lH, d, J2N~=SHz), 9.07 (lH, s), 11-95
(lH, d, J2.N~)
Mass Spectrum (EI): m/z 281 (Mi, as a free compound)
EXAMPLE 32
O ~ -~OOt ~
In 5 m~ of dry dimethylformamide was added 0.04 g of 60%
~ carbonyl])
oily sodium hydride, and 0.51 g of 5-~(i.ndol-3- ~ -4,5,6,7-
tetrahydro-lH-benzimidazole as obtained in Example 23 was
slowly added thereto at room temperature. Thirty minutes
- 38 -
2~88 1~
later, 0.07 g of benzyl bromide was slowly added thereto at
0~C, followed by stirring at room temperature overnight. To
the reaction mixture were added 20 m~ of water and 20 m~ of
chloroform for liquid-liquid separation. The organic layer was
washed with water and dried over anhydrous magnesium sulfate.
The solvent was removed by distillation, and the residue was
subjected to chxomatography using chloroform/methanol as an
eluent. The resulting foaming substance (0.12 g) was
recrystallized together with 0.04 g of fumaric acid from
ethanol/ethyl acetate to obtain 0.10 g of 5-[1-benzylindol-3-
yl)carbonyl]-4,5,6,7-tetrahydrobenzimidazole fumarate.
Physicochemical Properties:
Melting Point: 117-118~C
Elemental Analysis for C23H2,N3O.C4H4O4.0-75H2O:
Calcd. (%): C 66.86; H 5.51; N 8.66
Found (%): C 66.83; H 5.48; N 8.88
Mass Spectrum (EI): m/z 321 (M~, as a free compound)
In the same manner as in Example 32, the following
compounds were synthesized.
- 39 -
20~8~ 5
EXAMPLE 33
S-[(1-Cyclohexylmethylindol-3-yl)carbonyl]~
4,5,6,7-tetrahydrobenzimidazole.fumarate
~ O~C~
r~ GG~
~H~ ~
Physicochemical Properties:
Melting Point: 95-100~C (ethanol/ethyl acetate)
Elemental Analysis for C23H27N3O-C4H4O4Ø5AcOEt :
Calcd. (%): C 62.46; H 7.05; N 7.54
Found (%): C 62.59; H 6.69; N 7.19
Mass Spectrum (EI): m/z; 361 (M+, as a free compound)
EXAMPLE 34
5-[(1-Allylindol~3-yl)carbonyl]-
4,5,6,7-tetrahydrobenzimidazole-fumarate
O
ho~
11 ~c~
~l1L- CH=C~Il
Physicochemical Properties:
Melting Point: 144-145~C (methanol/ethyl acetate)
Elemental Analysis for ClgHl9N3O.C4H404Ø35AcOEtØ3H2O:
Cacld. (%): C 64.03; H 5.81; N 9.18
Found (~): C 64.00; H 5.74; N 9.17
- 40 -
20~881~
Mass Spectrum (EI): m/z 305 (M+, as a free compound)
EXAMPLE 35
5-[(1-n-Butylindol-3-yl)carbonyl]-
4,5,6,7-tetrahydrobenzimidazole-fumarate
o
~/C~ AC~
Physicochemical Properties:
Melting Point: 104-106~C (ethanol/acetonitrile)
Elemental Analysis for C20H23N3O-C4H4O4 ~-8H2~
Calcd. (%): C 63.78; H 6.38; N 9.30
Found (%): C 63.82; H 6.14; N 9.33
Mass~Speetrum (EI): m/z 321 (M~, as a free compound)
EXAMPLE 36
5-[[1-(2-Propynyl)indol-3-yl]carbonyl]-
4,5,6,7 tetrahydrobenzimidazole-fumarate
~C~
C~ ~_CH
Physicochemical Properties:
Melting Point: 130-131~C (ethanol/acetonitrile)
Elemental Analysis for Cl9HI7N3O.C6H4O4.1-3H2O:
Calcd. (%): C 62.38; H 5.37; N 9.49
Found (%): C 62.38; H 5.19; N 9.21
- 41 -
2 ~
Mass Spectrum (EI): m/z 303 (Mt, as a free compound)
EXAMPLE 37
' O ' O
~N~ H~S0~ S04 > ~l;~N~ HOOC~
~1 ~ H , ~I coo H
In 5 m~ of acetonitrile was suspended 0.53 g of 4,5,6,7-
tetrahydrobenzimidazole-5-carboxylic acid sulfate, and 0.29 m~
of thionyl chloride was added to the suspension. The
suspension was stirred at 55 to 60~C for 1 hour, and the
solvent was removed by distillation under reduced pressure. To
the residue was added 4.6 m~ of benzothiophene, and 0.4 g of
aluminum chloride was then added thereto. After stirring at
60~C for 3 hours, the reaction mixture was poured into a cold
potassium carbonate aqueous solution. The solution was
adjusted to a pH of 8 to 9 and extracted with chloroform. The
organic layer was dried over anhydrous magnesium sulfate, and
the solvent was removed by distillation. The residue was
purified by silica gel column chromatography using
chloroform/methanol as an eluent to obtain 5-[(benzothiophen-
3-yl)carbonyl]-4,5,6,7-tetrahydrobenzimidazole, The product
was treated with an equimolar amount of fumaric acid in a usual
- 42 -
200~
manner and recrystallized from ethanol/acetonitrile to obtain
0.04 g of 5-[(benzothiophen-3-yl)carbonyl]-4,5,6,7-
tetrahydrobenzimidazole fumarate.
Physicochemical Properties:
Melting Point: 135-137~C
Elemental Analysis for Cl6HI4N2OS.C4H404Ø3EtOHØ2H2O):
Cacld. (%): C 59.50; H 4.90; N 6.74; S 7.71
~ound (%): C 59.41; H 5.07; N 6.53; S 7.91
Mass Spectrum (EI): m/z 282 (M~, as a free compound)
EXAMPLE 38
il~~C~~ H~.SOu - > ~
A mixture of 2 g of polyphosphoric acid, 5 mQ of thiophene,
and 2.91 gof 4,5,6,7-tetrahydrobenzimidazole 5-carboxylic acid
sulfate was stirred at 100~C for 8 hours. After cooling, 20 m~
of cold water was added thereto, and the reaction mixture was
washed with toluene (20 m~ x 2). The aqueous layer was
adjusted to a pH of 8 to 9 with potassium carbonate and
extracted from chloroform. The organic layer was dried over
anhydrous magnesium sulfate, and the solvent was removed by
distillation. The residue was treated with a 4N solution of
hydrogen chloride in ethyl acetate and then recrystallized from
- 43 -
2~881~
methanol/acetonitrile to obtain 0.12 g of 5-[~thiophen-2-
yl)carbonyl]-4,5,6,7-tetrahydro~enzimidazole hydrochloride.
Physicochemical Properties:
Melting Point: 218-220~C
Elemental Analysis for C12Hl2N2OS.HCQ:
Calcd. (%): C 53.63; H 4.88; N 10.42; S 11.93
Found (%): C 53.25; H 4.98; N 10.62; S 11.70
Mass Spectrum (EI): m/z; 232 tM+, as a free compound)
E~PLE 39
~ o
C ~-C ~ ~ ~~ ~ C~3 ~ ~
To a solution of 0.50 g of N-~(4,5,6,7-
tetrahydrobenzimidazol-5-yl)carbonyl]pyrrolidinehydrochloride
and 0.39 g of 2-methylindolizine in 5 m~ of 1,2-dichloroethane
was added dropwise 0.90 g of phosphorus oxychloride. The
reaction mixture was refluxed at 85~C for one night. After
cooling to room temperature, 5 m~ of water was added thereto.
The organic layer was removed, and lO m~ of chloroform was
added to the aqueous layer. The solution was adjusted to a pH
of 9 with a 20% aqueous solution of sodium hydroxide and then
extracted with chloroform. The organic layer was dried over
anhydrous magnesium sulfate, and the solvent was removed
by distillation. The residue was purified by silica
- 44 -
2~08~1~
gel column chromatography using chloroformtmethanol as an
eluent, followed by recrystallization from ethanol to obtain
0.21 g of 5-[(2-methylindolizin-3-yl)carbonyl]-4,5,6,7-
tetrahydrobenzimidazole.
Physicochemical Properties:
Melting Point: 260-264~C
Elemental Analysis for Cl7H~7N3OØ15C2H5OHØ2H2O:
Calcd. (%): C 71.68; H 6.36; N 14.50
Found (%): C 71.71; H 6.16; N 14.46
Mass Spectrum (EI): m/z 279 (M+)
EXAMPLE 40
In the same manner as in Example 39, except for replacing
2-methylindolizine with pyrrole, 5-[(2-pyrrolyl)carbonyl]-
4,5,6,7-tetrahydrobenzimidazole of formula shown below was
synthesized.
~ '
Physicochemical Properties:
Melting Point: 225 - 226~C
Elemental Analysis for Cl2HI3N3O
Calcd. (%): C 66.96; H 6.09; N 19.52
Found (%): C 66.74; H 6.23; N 19.41
Mass Spectrum (EI): m/z 215 (Mf )
- 45 -
2~08~1~
EXAMPLE 41
o
L,~ ~ > ~ ~~~J
~ CH3
In a suspension of 7.0 g of N~[(4,5,6,7-
tetrahydrobenzimidazol-5-yl)carbonyl]pyrrolidinehydrochloride
and 5.4 g of N-methylindole in 70 m~ of ethylene chloride was
added 12.6 g of phosphorus oxychloride, and the mixture was
stirred at 80 to 85~C for 7 hours. After allowing the mixture
to cool, the mixture was cooled to 0 to 5~C, and 70 m~ of cold
water was slowly added to the reaction mixture while
maintaining the temperature of the mixture below room
temperature to thereby decompose the excess of phosphorus
oxychloride. The organlc layer was removed, and the aqueous
layer was adjusted to a pH of 9 with a 20% sodium hydroxide
aqueous solution under cooling, followed by extrac~ing from
chloroform. To the chloroform layer was added 70 m2 o~ water,
and 6N hydrochloric acid was added thereto under ice-cooling
while stirring to adjust to a pH of from 2.4 to 2.8. The
chloroform layer was removed. The aqueous layer was washed
with chloroform, and 40 m~ of methanol was added thereto. The
solution was made alkaline with a 20% sodium hydroxide aqueous
solution while cooling. The formed crystal was collected by
filtration and washed with a cold 1:1 (by volume) mixture of
- 46 -
20~8~1~
methanol and water to give 6.87 g (89.9%) of 5-[(1-methylindol-
3-yl)carbonyl]-4,5,6,7-tetrah~drobenzimidazole.
Physicochemical Properties:
Melting Point: 139-141~C
Mass Spectrum (EI): m/z 279 (Mf)
H-NMR (CDC~3 DMSO-d6):
1.80-2.32 (m, 2H), 2.56-3.04 (m, 4 H), 3.32-3.60 (m, lH),
3.90 (s, 3H), 7.12-7.20 (m, 3H), 7.40 (s, lH), 7.92 (s,
lH), 8~20-8.40 (m, lH)
Elemental Analysis for Cl7Hl7N3OØ2EtOHØ35H2O:
Calcd. (%): 70.88; H 6.46; N 14.25
Found (%): 70.83; H 6.50; N 14.23
EXAMPLE 42
o
1~ ~¦J~
c~3
5- [ ( l-Methylindol-3-yl ) carbonyl ] -4, 5, 6, 7 -
tetrahydrobenzimidazole was treated with a half molecular
amount of fumaric acid in ethanol in a known manner to obtain
5- [ ( l-methylindol-3-yl ) carbonyl ] -4, 5, 6, 7-
tetrahydrobenzimidazole ~fumarate.
Physicochemical Properties:
Melting Point: 224-225~C
Elemental Analysis for Cl9HI9N3O3:
Calcd. (%): C 67.64; H 5.68; N 12.45
-- 47 --
2008~1~
Found (%): C 67.56; H 5.66; N 12.35
Mass Spectrum (FAB): m/z 280 (M++1 , as a free compound)
EXAMPLE 43
Optical Resolution (1) of 5-[(1-Methylindol-3-yl
carbonyll-4,5,6,7-tetrahydrobenzimidazole
(a) In 60 m~ of methanol was added 5.87 g of 5-[(1-methylindol-
3-yl)carbonyl]-4,5,6,7-tetrahydrobenzimidazole obtained in
Example 41, and a solution of 7.52 g of (+)-dibenzoyltartaric
acid in 240 m~ of methanol was added thereto to once form a
clear solution. On leaving the solution to stand at room
temperature for one night, there were precipitated crystals,
which were collected by filtration and recrystallized three
times from dimethylformamide/water to obtain 2.30 g of (R)-(-)-
5 - [ ( 1 - ~ e t h y li n d o l - 3 - y l ) ca r b o n y l ] -4 , S , 6 , 7 -
tetrahydrobenzimidazole (+)-dibenzoyltartarate.
Physicochemical Properties:
[ ~ ~ 20 = + 30.6~ (c=1.10, dimethylformamide)
Melting Point: 169.0-170.0~C
Elemental Analysis for Cl7HI7N3O.Cl8Hl4O8.H2O:
Calcd. (%): C 64.12; H 5.07i N 6.41
Found (%): C 64.13; H 5.03; N 6.5~
Mass Spectrum (FAB): m/z ~80 (M+ + 1, as a free compound)
(b) In a 2N hydrochloric acid aqueous solution was added 2.2 g
of the compound obtained in (a) above, and the solution was
washed with ethyl acetate and then adjusted to a pH of about 9
with sodium carbonate. The aqueous layer was extracted wi~h
- 48 -
2~08~5
chloroform/methanol (4:1 by volume). The extract ~as dried
over anhydrous magnesium sulfate, and the solvent was removed
by distilla-tion to obtain 0.94 g of (R)-(-)-[(5-l~methylindol-
3-yl)carbonyl]-4,5,6,7-tetrahydrobenzimidazole as a foaming
substance.
[a]D~ = -16 ~ 5o ( c=l .13, methanol)
(c) (R)-(-~(1-methylindol-3-yl)carbonyl]-4,5,6,7-
tetrahydrobenzimidazole (0.56 g) obtained in (b) above was
treated with 0.21 g of fumaric acid in methanol/acetonitrile to
obtain 0.64 g of (R)-(-)-5-[(1-methylindol-3-yl)carbonyl]-
4,5,6,7-tetrahydrobenzimidazole fumarate.
E~] 2~=-28.1~ (C=1.22, methanol)
Melting Point: 150.5-151.5~C
Elemental Analysis for Cl7Hl7N30.C4H404Ø35CH3CNØ25H20:
Calcd. (%): C 62.91; H 5.49; N 11.33
Found (%): C 62.94; H 5.41; N 11.35
Mass Spectrum (EI): m/z 279 ( M~, as a free compound)
EXAMPLE 44
In ethanol/ethyl acetate was dissolved 100 mg of (R)-(-)-
5 - [ ( 1 -methyl - 3 -indolyl ) carbonyl ] -4, 5, 6, 7 -
tetrahydrobenzimidazole obtained in Example 43(b), and a
solution of hydrogen chloride in ethyl acetate was added
thereto. The thus formed crystal was collected and
recrystallized from ethanol to obtain 70 mg o~ (R)-(-)-5-[(1-
methylindol-3-yl)carbonyl]-4r5r6r7-tetrahydrobenzimidazole
hydrochloride.
-- 49 --
2 ~ r)
[CL]D = -42.9~ (c=1.02, methanol)
Melting Point: 215 - 230 ~ C
Elemental Analysis for Cl7HI7N3O.HC~:
Calcd. (%): C 64.66; H 5.75; N 13.31; C~ 11.23
Found (%): C 64.37; H 5.80; N 13.12; C~ 11.17
Mass Spectrum (EI): m/z 279 (M+, as a free compound)
EXAMPLE 45
Optical Resolution (2) of 5- [ (1-methylindol-3-
yl ) carbonyl l -4,5,6,7-tetrahydrobenzimidazole
(a) In the same manner as in Example 43(a), except for using
( - ) -dibenzoyltartaric acid, ( S ) - ( + ) -5- [ (1 methylindol-3-
yl ) carbonyl ] -4, 5, 6, 7 -tetrahydrobenzimidazole ( - ) -
dibenzoyltartarate was obtained.
[ ~ ~ D0 = _ 30.3 ~ ( c = 1.07, dimethyl f ormamide )
Melting Point: 168.5-169.5~C
Elemental Analysis f or C17HL7N3O ~ ClsH14~a ~ H2O
Calcd. (%): C 64.12; H 5.07; N 6.41
Found (gs): C 64.13; H 5.13; N 6.71
Mass Spectrum (FAB): m/z 280 (M+~1, as a free compound)
(b) In the san~e manner as in Example 43 (b), except for using
the salt as obtained in ( a ) above, ( S ) - ( + ) -5- [ ( l-methylindol-
3-yl ) carbonyl ] -4,5,6,7-tetrahydrobenzimidazole was obtained as
a foaming substance.
[, ~20 = +16.7~ (c=~.35, methanol)
( c ) In the same manner as in Example 43 ( c ), except f or using
( S ) - ( + ) -5- [ ( l-methylindol - 3 -yl ) carbonyl ] - 4, 5, 6, 7
-- 50 --
2 ~
tetrahydrobenzimidazole as obtained in (b) above, a crystal of
(S)-(+)-[(1-methylindol-3-yl)carbonyl]-4,5,6,7-
tetrahydrobenzimidazole fumarate was obtained.
[~]DO = +2g.3~ (c=1.14, methanol)
Melting Point: 151.0-152.0~C
Elemental Analysis for Cl7Hl7N30.C4H404.O.35CH3CNØ25H~O:
Calcd. (%): C 62.91; H 5.49; N 11.33
Found (%): C 62.96; H 5.39; N 11.37
Mass Spectrum (EI): m/z 279 (M~, as a free compound)
EXAMPL~ 46
~~ S ~ ~-H So ~ ~ ~ ~
~ ,5,6,7-Tetrahydrobenzimidazole-5-carboxylic acid sulfate
(1.32 g) was refluxed in 10 mQ of 1,2-dichloroethane
together with 1.78 g of thionyl chloride for 30 minutes, and
the excess of thionyl chloride and the solvent were removed by
distillation under reduced pressure. To the residue was added
10 m~ of 1,2-dichloroethane, and 1.6 mQ of indoline was added
dropwise thereto at 30~C or lower while sitrring, followed by
stirring at room temperature for 2 hours. The reaction mixture
was successively extracted once with 30 m~ of water and twice
with 20 mQ of water. The combined aqueous layer was adjusted
to a pH of 9 to 10 with a 10% sodium hydroxide aqueous solution
~Og(~l ~
and then extracted with methylene chloride. The combined
methylene chloride layer was washed with water and dried over
anhydrous magnesium sulfate. The solvent was removed by
distillation under reduced pressure. The residue was
recrystallized from ethyl acetate to obtain 1.1 g (82.7%) of 5-
[ ( 2, 3 -dihydroindol- 1 -yl ) carbonyl ] -4, 5, 6, 7 -
tetrahydrobenzimidazole.
Melting Point: 175-178~C
Mass Spectrum (EI): m/z 267 (Mt)
H-NMR (CDCQ3-DMSO-d6):
1.80-2.36 (m, 2H), 2.48-3.12 (m, 5H), 3.24 (t, 2H), 4.20
(t, 2H), 6.84-7.30 (m, 3H), 7.50 (s, lH), 8.20 (dd, lH)
Elemental Analysis for Cl6Hl7N30Ø25H20:
Calcd. (%): C 70.70; H 6.49; N 15.46
Found (%): C 70.79; H 6.37; N 15.19
EXAMPLE 47
5-[(2,3-Dihydroindol-l-yl)carbonyl]-4,5,6,7-tetrahydrobenz-
imidazole as obtained in Example 46 was treated with
hydrochloric acid in ethanol in a usual manner to obtain 5-
[ ( 2, 3-dihydroindol- 1 -yl ) carbonyl ] -4, 5, 6, 7 -
tetrahydrobenzimidazole hydrochloride of formula:
-- 52 --
2 0 ~
Physicochemical Properties:
Melting Point: >250~C
Elemental Analysis for Cl6HI8N3Oc~:
Calcd. (%): C 63.26; H 5.97; N 13.83; C~ 11.67
Found (%): C 63.15; H 5.97; N 13.80; C~ 11.78
Mass Spectrum (EI): m/z 267 (M+ , as a free compound)
EXAMPLE 48
Optical Resolution (1) of 5~[(2,3-Dihydro-
indol-1-yl)carbonyll-4,5,6,7-tetrahydrobenzimidazole
(a) 4 g of 5-[(2,3-dihydroindol-1-yl)carbonyl]-4,5,6,7-
tetrahydrobenzimidazole as obtained in Example 46 were
dissolved in 50 m~ of methanol, and a methanolic solution
(250 m~) of 2.70 g of (-)-dibenzoyltartaric acid was added
thereto. The thus formed crystal was collected by filtration,
and the crystal was recrystallized twice from
dimethylformamide/water to obtain 2.88 g of a (-)-
dibenzoyltartarate showing optical rotation of -34.0~ (20~C,
sodium D-line, c=0.63 g/d~, dimethylformamide).
Physicochemical Properties:
Melting Point: 163.5-165.0~C
Elemental Analysis for Cl6HI7N3~~clsHl4~a o 7DMF 2~2H20
Calcd. (%): C 60.59; H 5.66; N 7.22
Found (~): C 60.53; H 5.28; N 7.26
Mass Spectrum (EI): m/z 267 (Mt , as a free compound)
(b) The above prepared salt (2.65 g) was added to 2N
hydrochloric acid, and the solution was washed with ethyl
- 53 -
20~881 ~
acetate. The solution was then adjusted to a pH of 9 with
sodium carbonate. The aqueous layer was extxacted with
chloroform/methanol (4:1 by volume), and the extract was dried
over anhydrous magnesium sulfate. The solvent was removed by
distillation to obtain 0.95 g of a base showing optical
rotation of -6.3~ (20~C, sodium D-line, c=1.05 g/dQ, methanol)
as a foaming substance.
Physicochemical Properties:
Melting Point: 100-106~C
Elemental Analysis for Cl6HI7N3OØ2AcoEt.o-5H2o:
Calcd. (%): C 68.64; H 6.72; N 14.29
Found (%): C 68.62; H 6.53; N 14.30
Mass Spectrum (EI): m/z 267 (Mt)
(c) The above obtained foaming base was dissolved in
ethanol/ethyl acetate, and the solution was treated with a
solution of hydrogen chloride in ethyl acetate to obtain 0.94 g
of a crystal of a hydrochloride showing optical rotation of
+19.1~ (20~C, sodium D-line, c=1.06 g/d~, methanol).
Physicochemical Properties:
Melting Point: 241-244~C (dec.)
Elemental Analysis for C~6Hl7N3O.HCQ:
Calcd. (%): C 63.26; H 5.97; N 13.83; CQ 11.67
Found (%): C 63.18; H 6.04; N 13.78; C~ 11.45
Mass Spectrum (EI): m/z 267 (M~ , as a free compound)
- 54 --
2~8~1~
EXAMPLE 49
Optical Resolution (2) of 5-[(2,3-Dihydroindol-
1-yl)carbonyll-4,5,6,7-tetrahydrobenzimidazole
(a) In the same manner as in Example 48(a), except for using
(+)-dibenzoyltartaric acid, a crystal of a
(+)-dibenzoyltartarate showing optical rotation of +33 4~
(20~C, sodium D-line, c=0.60, dimethylformamide) was obtained.
Physicochemical Properties:
Melting Point: 165.0-166.5~C
Elemental Analysis for Cl6Hl7N3O-C~8Hl4O8-0~7DMF-1-85H2O:
Calcd. (%): C 61.13; H 5.61; N 7.28
Found (%): C 61.12; H 5.28; N 7.28
Mass Spectrum (EI): m/z 267 (M+ , as a free compound)
(b) In the same manner as in Example 48(b), except for using
the salt as obtained in (a) above, a base showing optical
rotation of +7.9~ (20~C, sodium D-line; c=1.06, methanol) was
obtained as a foaming substance.
Physicochemical Properties:
Melting Point: 98-103~C
Elemental Analysis for Cl6H~7N3OØ15AcOEtØ5H2O:
Calcd. (~): C 68.86; H 6.68; N 14.51
Found (%): C 68.65; H 6.66; N 14.45
Mass Spectrum (EI): m/z 267 (M+)
(c) In the same manner as in Example 48(c), except for using
the foaming base as obtained in (b) above, a crystal of a
- 55 -
2~8~ ~
hydrochloride having optical rotation of -19.2~ (20~C, sodium
D-line, c=1.07; methanOl) was obtained.
Physicochemical Properties:
Melting Point: 239-242~C (dec.)
Elemental Analysis for Cl6H~7N3O.HC~:
Calcd. (%): C 63.26; H 5.97; N 13.83; C~ 11.67
Found (%): C 63.07; H 5.99; N 13.76; C~ 11.58
Mass Spectrum (EI): m/z 267 (~ , as a free compound)
EXAMPLE 50
3 ~'Cl ,~ HooC~
In 40 ml of acetonitrile was suspended 5.00 g of
4,5,6,7-tetrahydrobenzimidazole--5-carboxylic acid sulfate, and
2.75 ml of thionyl chloride was added to the suspension. The
suspension was stirred at 55~C for 1 hour, and the solvent was
distilled off under reduced pressure. To the residue was added
20 ml of nitrobenzene and 1.80 ml of 2-methylbenzofurane, and
2.20 ml of tin tetrachloride was then added thereto. After
stirring a-t 85~C for one night, 40 ml of l M aqueous hydrochloric
acid solution and 40 ml of ethyl ether were added thereto.
The organic layer was removed, 40 ml of chloroform was added
and then the solution was adjusted to a pH of 9 with 10 ~ aqueous
solution of sodium hydroxide. The reaction solutlon was filtered
through celite and then extracted with chloroform containing
- 56 -
2~8~
lO % methanol. The organic layer was collect~d and the solvent
was distilled off. To the free base of objective product
obt~ined by treating the residue with silica gel column
chromatography using chloroform/methanDl was added calculated
amount of fumalic acid to convert it to a fumarate and
recrystallized from ethanol to obtain 0.14 g of
5-C(2-methylbenzofuran-3-yl)carbonyl~-4,5,6,7-tetrahydrobenz-
imidazole fumarate.
Physicochemical Properties:
Melting Point: 188-189~C
Elemental Analysis for C17H16N202-C4H4O4
Calcd. (%) C 63.63; 'H 5.09; N 7.07
Found ~) C 63.47; H 5.06; N 7.01
Mass Spectrum (EI): m/~ 230 (M , as a free compound)
EXAMPLE 51 2 ~ ~ 8 ~15
S-~(indolizin-3-yl~carbony~ ~4,$,6,7-~etr~hydrobenzimidazole
~3~tl~
In the same manner as in Example 39, except for repl~cing
2-methylindolirine with indolizine, above-mentioned compound
was obtained.
Physicochemical Properties:
Melting Points 210-212-C
Elemental Analysis for C~ N30-0.1 H20
Calcd. (~): C 71.94; ~ 5.74 N ~5.73
Found ~ts ~ 72.08;~H~.79 N 15.67
Mas- Spectrum (EI)s m/z 265 ~M )
EXAMPLE 52
5-~(1-methylindolizin-3-yl)
carbonyl7-4,5,6,7-tetrahydrobenzimidazole
c~3
In the same manner as in Example 39, except for replacin~
2-methylindolizine with 1-methylindolizine, above-mentioned
compound was obtained.
Physicochemical Propertie~.
Melting ~oint: 122-123CC
Elemental AnalysiS for C17Hl~N30~0.25 C~HloO-0.4 H20
Calcd. (~): C 70.87; H 6.71; N 13.77
~ound (~)s C 70.88 H 6.68; N 13.66
Mass Spectrum (EI)s mlz 279 (~ )
2~8~1~
FORMULATION EXAMP~E 1 ( ~ ~ )
Compound of Example 44 (hereinafter 0.2 mg
referred to Compound A)
Lactose 106.4 mg
Corn starch 48.0 mg
Hydroxypropyl cellulose 4.8 mg
Magnesium stearate 0.6 mg
___________________________________________________
Total: 160.0 mg/tablet
Compound A (200 mg), lactose (106.4 mg), and corn starch
(48 g) were uniformly mixed, and 48 m~ of a 10~ aqueous
solution of hydroxypropyl cellulose was added thereto. The
mixture was granulated by means of a granulator. To the
granules was added 0.6 g of magnesium stearate, and the mixture
was punched to obtain 1000 tablets each weighing 160 mg.
- 58 -
2~0~
FORMULATION EXAMPLE 2 ( ~D~cr~ )
Compound A 0.4 mg
Mannitol 770.0 mg
Corn starch 199.6 mg
Polyvinylpyrrolidone 30.0 mg
___________________________________________________
Total: 1000.0 mg
Compound A (0.4 g), mannitol (770 g), and corn starch
(199.6 g) were uniformly mixed, and 300 m~ of a 10~ aqueous
solution of polyvinylpyrrolidone was added thereto, followed by
granulation by means of a granulator to prepare 1 kg of
powders.
FORMULATION EXAMPLE 3 f~,ps~ )
Compound A0.2 mg
Corn Starch 198.8 mg
Calcium stearate 1.0 mg
___________________________________________________
Total: 200 mg
Compound A (0.2 g), corn starch (198.8 g), and calcium
stearate (1 g~ were uniformly mixed, and the mixture was
charged in No. 3 capsules by 200 mg to prepare 1000 capsules.
FORMULATION E~AMPLE 4 (~
Compound A 0.2 mg
Sucrose 8.0 mg
Pure water to make 5 mQ
Compound A (0.2 g) and sucrose (8 g) were dissolved in
distilled water to prepare S Q of a syrup.
- 59
3 1 ~
FORMULATION EXAMPLE 5 (o~ CZ~,~'S )
Compound A 0.3 mg
Sodium chloride 9 mg
Injectable distilled water to make 1.0 mQ
Compound A (300 mg) and sodium chloride (9 g) were
dissolved in injectable distilled water to prepare 1000 mQ of
a solution. The solution was filtered and charged in 1000
ampules by 1 mQ while displacing the atmosphere of the ampule
with nitrogen gas. The ampules were sterilized by autoclaving.
While the invention has been described in detail and with
reference to specific embodiments thereof, it will be-apparent
to one skilled in the art that various changes and
modifications can be made therein without departing from the
spirit and scope thereof.
- 60 -