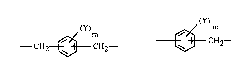Note: Descriptions are shown in the official language in which they were submitted.
2~859
I
K-17438/+/CGM 344
Novel polymers
The present invention relates to novcl polyamide-polyimide block copolymers (described
below as PAPI block copolymers).
PAPI block copolymers are known per se and are described, for example, in DE-A
2,342,464. Owing to their good electrical,thermal and/or mechanical properties, they are
suitable for use, for example, as matrix resins for the production of composite materials, as
coating resins in the electrical and electronics industries, as flexible laminating resins, as
spread adhesives or as compression-moulding materials. Conventional PAPI block
copolymers are insoluble or only slightly soluble in organic solvents, as is known, for
example from US-A 4,503,285. In order to utilize the good properties of the PAPI block
copolymers, it is therefore generally necessary to start from the readily soluble precursor,
the polyamide-polyamic acid block copolymers. This procedure is not satisfactory in
many respects. Firstly, the soluble precursors are, as a rule, only stable on storage to a
limited extent, since the amount of water formed by cyclization results in a degradation of
the molecular weight and/or the cyclization results in precipitation of the polymer. If, in
addition, the imidization is not carried out until processing, the resulting product can
exhibit defects, such as holes and/or bubbles, as a result of the water which escapes.
There is, therefore, a desire for soluble PAPI block copolymers which do not have these
disadvantages. Polymers of this type have already been described in JP-A 62/30,121 and
in F.P-A 260,709. The previously known compounds are characterized by the use oftetranuclear aromatic diamine units in the polyamide blocks and the polyimide blocks and
by the presence of polyamide-imide units in the polyimide blocks.
Soluble PAPI block copolymers of this type have advantages in processing and can be
stored virtually indefinitely in the form of solutions or solids. On the other hand, PAPI
block copolymers which are soluble in organic solvents have the disadvantage of being
attacked by such solvents. A soluble PAPI block copolymer which, after application from
solution, can be stabilized in a subsequent step against attack by solvents is, therefore,
desirable.
2~G~35~
- 2 -
It has now been found that this object can be achieved by the incorporation of
xylylenediamine units or aminobenzyhlmine units. The PAPI block copolymers according
to the invention are distinguished by a surprisingly high solubility in dipolar, aprotic
solvcnts and by a high reactivity during crosslinking by heat.
It has also becn found that the PAPI block copolymers according to the invention can be
dried at high temperatures approximately up to the glass transition point, which permits
rapid purification of the polymers without appreciable crosslinking. Surprisingly, the
difference between maximum possible drying temperature and curing temperature issmall, so that technical advantages in use also result.
The present invention relates to soluble polyamide-polyimide block copolymers having an
average molecular weight Mn of 1,000-50,000, particularly 5,000-40,000, containing
combinations of blocks of the formulae Ia together with IIb and/or IIc or of the forrnulae
Ib together with lIa and/or IIc or of the formulae Ic together with IIa and/or IIb
O Or 0 01
Il 11 11 11
--NH--C--Rl--C --Nl-l--R2--NH--C--Rl--C---NH--(la)
o O o
--N/ \R3~ ~N--R4--N~ ~R3~ ~N-- (Ib),
o o O o
o o
--NH--C--R5~ ~N--R6--NH C--R~j~N (Ic),
oo
o o
11 11
--R2---NH--C--Rl--C--NH--R2---- (IIa),
3 2~ 9
o o
--R4~N\ /R3\ ~N R4~ (llb),
O b
~ 0 11
R6 ---NH C--R5 /N R6 (IIc),
c
in which the indices a, b and c independently of one another are integers from 1 to 100,
is a radical of the formulae ~CnH2n~~ ~)m ~Q~m
~ , {~} or ~ Q ~ , n is an integer from 2 to
12, m is an integer from O to 4, Y is alkyl or halogen, Q is a direct bond or -CH2-,
-CH2-CH2-, -CH(CH3)-, -C(CH3)2-, -C(CF3)2-, -O-, -S-, -SO2- or -CO-, R2 is a radical of
the fonmulae ~CnH2n~~ ~ ' ~ ~
~3}Q~, ~Q~Q~Q~,
, ~ , ~ CH2~ , ~ Y)C~12--
or _ CH2~ cH2_, n, m, Y and Q have one of the meanings defined above, R3 iS
a radical of the fonnulae ~ "~Q~ X or ~, Q
has one of the meanings defined above, Rs iS a radical of the fonmula ~, and R4
and R6 independently of one another have one of the meanings defined for R2, with the
2~ i859
- 4 -
proviso that 25-100 mol% of all tlle radicals R2, R4 and R6, relative to the total quantity of
these radicals, have the formula _ cH2~ cll2_ and/or ~y) H2--
The term "soluble PAPI block copolymer" is, in general, to be understood as meaning a
copolymer which is soluble in dipohlr, aprotic solvents and from which solutionscontaining at least 5 % by weight, preferably at least 10 % by weight, of PAPI block
copolymer, relative to the solution, can be preyared.
The ratio of polyamide blocks to polyimide blocks or to polyamide-imide blocks in the
copolymers according to the invention is determined, in general, by the desired solubility
of these copolymers in dipolar, aprotic solvents. The proportions of each of these blocks
and the content of xylylenediamine units and/or aminobenzylamine units are so chosen
that the block copolymer is soluble in dipolar, aprotic solvents and, in the crosslinked
state, is virtually not attacked by these solvents.
The pre~erred ratio of amide groups to imide groups in the copolymers according to the
invention is 4:1 to 1:4.
The average molecular weights Mn of the polyamide blocks Ia or Ila or of the polyimide
blocks Ib or lIb or of the polyamide-imide blocks Ic or IIc are, in general, 300 to 20,000,
preferably 500 to 10,000.
As well as the combinations of two blocks mentioned above, the PAPI block copolymers
according to the invention can also contain three-block combinations of the formulae Ia, Ib
and IIc or Ia, Ic and IIb or Ib, Ic and IIa.
Block copolymers which consist essentially of the recurring structural units of the
formulae Ia and IIb or Ib and IIa are particularly preferred.
Block copolymers of this type which are very particularly preferred contain the
xylylenediamine units or the aminobenzylamine units or a combination of
xylylenediamine units and aminobenzylamine units only in the polyimide blocks Ib or IIb.
Crosslinked products formed from compounds of this type are distinguished by a
particularly high heat stability.
2~ s~
-s-
The index n is preferably 6 to 12 and the index m is prc~erably O or 1, especially 0.
An alkyl substituent Y in the iabove formulae can be branched or, preferably, linear. Linear
Cl-C6alkyl is preferred. Examples of these are methyl, ethyl, n-propyl, n-butyl, n-pentyl
and n-hexyl. Methyl is particul.lrly preferred.
As halogen, Y is preferably chlorine or bromine.
Examples of Rl in the above formulae are 1,3-phenylene, 1,4-phenylene, 2,4-tolylene,
1,5-naphthylene, 1,8-naphthylene, 2,6-naphtllylene, 4,4'-biphenylene,
~ ~ or __~ so
The preferred radical Rl is 1,3-phenylene.
Examples of Rl, R2, R4 or R6 as a group -CnH2n- are 1,2-ethylene, 1,2-propylene,1,3-propylene, 2,2-propylidene, 1,4-tetramethylene, 1,5-pentamethylene,
1,6-hexamethylene, 1,7-hept,amethylene, 1,8-octamethylene, 1,9-nonamethylene,
1,10-decamethylene, 1,12-dodecamethylene, 2,9-decamethylene or
2-methyl- 1 ,5-pentamethylene.
(Y)
m
As a group _ ~ , Rl, R2, R4 o} R6 can be 1,2-cyclohexylene, 1,3-cyclohexylene
or, preferably, 1,4-cyclohexylene.
Further examples of specific cycloaliphatic groups R2 and/or R4 and/or R6 are the
following
~CH2~ ~CH2~2 5
C2~15 C2H5
H3~-`CH2~ 3 H3C~CH2~13
CH3 CH3
6 2~ 359
-H2C~o" or ~/CI~--
Examples of specific araliphatic groups R2 and/or R4 and/or R6 are 1,3-xylylene,
I ~4-xylylene~ ~C}I2- ~L c~l2--
Examples of specific aromatic groups R2, R4 or R6 are 1,2-phenylene, 1,3-phenylene,
1,4-phenylene or one of the following groups
~ CH2~ ~ CH2~ --~ ~,
~0~ ~S02~ ~S02
~S ~3 ~C~ ~~C~
~Lol~~
~~ ~o~S
~o~o~,
~ ~ ¦ ~ O ~ ' ~ 0~ ~
Preferred radicals R2 and/or R4 and/or R6 are 1,3-phenylene, ~ so2~L
2~ S9
- 7 -
\~ S02~ , --CH2 ~- CH2-- --CH2~
and, especlally ~ . l~ CH2--.
and 0 c~12 - ~
~~
Rs IS preferably a radlcal of the formula ~
It has been found that the heat stability of the PAPI block copolymers according to the
invention and of the crosslinked products obtainable therefrom is particularly good if they
contain a high proportion of aromatic radicals. PAPI block copolymers containing mainly
aromatic or araliphatic radicals, for instance those enumerated above as examples of R1 to
R6, are therefore preferred.
A bridge member Q in the radicals Rl, R2, R3, R4 or R6 is preferably -CH2, -C(CH3)2-,
-O-, -SO2- or -CO-.
Several bridge members Q p}esent in a radical R2, R4 or R6 can be identical or different.
The tetravalent radical R3 is derived from a tetracarboxylic acid capable of forrning a
dianhydride.
Radicals of the formula
~ and ~J~ .
are particularly preferred in this regard.
The proportion of xylylenediamine radicals or aminobenzylamine radicals or both types of
these radicals in the compounds according to the invention is preferably 25 to 75 mol%,
relative to the proportion of all the diamine radicals. In general, these compounds are
distinguished by a particularly good solubility in dipolar, aprotic solvents.
Of these compounds, preferred compounds are those wherein, as well as the
Z~ 359
- X -
xylylenediamine radicals and/or the aminobenzylamine radicals, at least 25 mol% of the
radicals R2, R4 and, if present, R6 are derived from diaminodiphenyl sulfone. In general,
these compounds are distinguished by an even further improved solubility in dipolar,
aprotic solvents~
The invention thereforc preferably rclates to PAPI block copolymers as defined above,
wherein 25 to 75 mol%, in particul.lr 50 to 75 mol% of the radicals R2, R4 and, if present,
R6 are derived from xylylenediamine or from aminobenzylamine or from a combination of
xylylenediamine and aminobenzylamine, especially from 1,3-xylylenediamine, and at
least 25 mol% of the radicals R2, R4 and, if present, R6 are derived from diaminodiphenyl
sulfone, the proportion data relating to the total quantity of these radicals.
In particularly preferred PAPI block copolymers of the present invention virtually all of
the radicals R2, R4 and, if present, R6 which differ from xylylenediamine and/or from
aminobenzylamine are derived from diaminodiphenyl sulfone.
The preparation of the block copolymers according to the invention is effected in a manner
known per se and can, for example, be effected by one of the procedures described in
DE-A 2,342,464 by reacting preformed polyamide, polyamide-amic acid and polyamicacid blocks and subsequently cyclizing the polyamide-polyamic acid. Another method of
preparation comprises the reaction of polyamic acids of the formula IIIa and/or IIIb
o o -- o o --o o
~C~ ~C-NH-R4--NH C~ C OH HO-C~ C~
O R3 ,R3~ ~R3 ~O (IIla)
C C--OH HO--C C--NH-R4--NH---C C
O O _ O O _ O O
O O
~C~ 1l ~ 11 ~C - OH 1
O~ ~R5--C--NH R6- Nl--C--R5 ~C~
C C--NH- R6--NH- - C--R5 o (IIIb)
o x o c
o
with diamines of the formula IV
H2N-R2-NH2 (IV)
2~ 9
g
and with dicarboxylic acid chlorides of the formula V
Cl-OC-RI-CO-Cl (V)
and the subsequent cyclizatioll of the polyamide-polyamic acid block copolymers thus
obtained. In the formulae IIIa, IIIb, IV and V the symbols Rl, R2, R3, R4, Rs and R6 are as
defined above and x is an integer 2 O, preferably 2 1.
The preparation of the polyamic acids of ~he formulae IIIa or IIIb is also known per se and
is effected, for example, by reacting tetracarboxylic anhydrides of the formula VIa or
tricarboxylic anhydrides VIb or a corresponding tricarboxylic anhydride-chloride
o o o
Il ll 11
~ /R3/ \O (VIa), 110~ R5~ / (VIb)
Il 11 o 11
o o o
in which R3 and Rs are as defined above with a less than equivalent amount of a diamine
of the formula IV.
The starting materials of the formulae IV, V, VIa and VIb are known per se and are in
some cases available commercially.
The average molecular weights of the individual blocks can be adjusted to a desired value
by selecting suitable reaction conditions, for example by suitable selection of the molar
ratios of the reactants. This selection is known per se to those skilled in the art.
The polycondensation of the di-, tri- or tetra-carboxylic acid derivatives of the formulae
IIIa, IIIb, V, VIa or VIb with the diamines of the formula IV can be carried out in a
manner known per se, preferably in an anhydrous organic solvent and with the exclusion
of moisture, for example under nitrogen at temperatures between -20C and +50C,particularly about -15C to +10C.
Examples of suitable organic solvents are N,N-dimethylacetamide, N,N-diethylacetamide,
2~ 3S9
- 10-
N,N-dimethylformamide, N-methyl-2-pyrrolidone (NMP), N-acetyl-2-pyrrolidone,
N-methyl-~-caprolactam, N,N,N',N'-tetramethylurea, tetrahydrothiophene dioxide
(sulfolane) and dimethyl sulfoxide.
The reaction can also be carried out in mixtures of such solvents. On the other hand, it is
also possible to dilute these preferred solvent systems with other organic, aprotic solvents,
such as aromatic, cycloaliphatic or aliphatic hydrocarbons, if appropriate chlorinated
hydrocarbons, for example toluene, xylenes, cyclohexane, pentane, hexane, petroleum
ether, methylene dichloride, tetrahydrofuran, cyclohexanone and dioxane.
The polyamide blocks can also be prepared by interface polycondensation.
After the reaction is complete, the solvents can, if desired, be removed in a customary
manner, for example by distillation, if appropriate under reduced pressure. If desired, the
polyamide-polyamic acid block copolymers can be precipitated by methods known per se
by pouring the reaction solution into a precipitant, such as water or aliphatic
hydrocarbons, for example petroleum ether, but particularly methanol, isopropanol,
acetone, symmetrical ethers of mono-, di- or tri-ethylene glycol or acetonitrile, and can, if
desired, be dried.
The cyclization of the polyamide-polyamic acid block copolymers to give the
corresponding PAPI block copolymers is carried out by heating the polyamide-polyamic
acid block copolymers at temperatures between 50 and 300C, preferably without prior
isolation, i.e. without further treatment in the reaction solution described above, or treating
them with a dehydrating agent, on its own or mixed with a tertiary amine. Examples of
suitable dehydrating agents are acetic anhydride or propionic anhydride or a mixture of
acetic anhydride and triethylamine or pyridine. Processes of this type are described, for
example, in US-A 3,894,114, US-A 4,124,651 or US-A 4,503,285.
The compounds according to the invention can also be prepared by first synthesizing a
polyamide block or polyamide-imide block in a manner known per se and then reacting
the latter with a tetracarboxylic anhydride and a diamine to prepare the polyamic
acid-polyamide block copolymer, which is then cyclized.
The PAPI block copolymers according to the invention possess good solubility in aprotic,
dipolar solvents which is suitable for the problem mentioned initially. Very good
2~ S~
solubilities can be achieved, if appropriate with warming, in, for example,
N,N-dimethylacetamide, N,N-dimethylformamide, N,~-dimethylacetamide,
N-methyl-2-pyrrolidone, sulfolalle, dimethyl sulfoxide or r-butyrolactone.
It should be mentioned that a high concentration of the polymeric compounds is usually
desirable. The solutions thus prepared are stable on storage, in contrast with the
polyamide-polyamic acid precursor. The same also applies, of course, to the solid itself.
Solutions which are essentially free from by-products and residues of monomer can be
prepared in this manner. Processing is carried out extremely simply, because no
cyclization step has to be gone through and hence there is no elimination of water, which
results in undesirable effects and damage to the end product. When the PAPI block
copolymers according to the invention are used it is merely necessary to remove the
solvent and the preparation of products of a high quality and high heat stability which can
be used at high temperatures is possible.
In a subsequent stage or at the same time as the removal of the solvent, the copolymers
according to the invention can be crosslinked by heating. The temperatures for the
crosslinking step generally vary according to the copolymer used. As a rule the
copolymers are heated above their glass transition temperature (Tg value) or, in the case of
more than one T8 value, above their lowest T~ value, especially above their highest T8
value. Tg values can be determined, for example, by differential scanning calorimetry
(DSC), in which case the "onset value" [= point at which the prolonged base line cuts the
tangent to the experimental curve in the region of steepest increase] is used. The term
"glass transition temperature" relates to the values of the polymer in the particular
formulation. These can be values which are below the values for the pure polymer. Thus,
for example, polymers containing solvents and/or plasticizers can have T8 values which
are below the values for the pure polymer. As a rulet crosslinking is carried out at
temperatures between 250 and 350C.
The invention also relates, therefore, to a process for the preparation of crosslinked PAPI
block copolymers, which comprises heating the compounds containing blocks of theformulae Ia and IIb and/or IIc or of the formulae Ib and IIa and/or IIc or of the formulae Ic
and IIa and/or IIb defined above at temperatures above their glass transition point.
The invention aiso relates to the crosslinked products obtainable by this process.
~G~
- 12-
The process according to the invention makes it possible to prepare coatings having a
large layer thickness or moulded articles in an advantageous manner, since curing by heat
takes place essentially uniformly within the whole crosslinkable matelial.
The block copolymers accordhlg to the definition are distinguished by good processability,
and can be used for the manufacture of industrial products, such as fibres, fibre-reinforced
composite materials, laminated articles, cast articles, laminates, matrix resins,
honeycomb-core materials, lacquers, adhesives, foams, coating compositions, films,
compression-moulding powders, sintering powders and compression-moulded articles.
In particular, the block copolymers according to the invention can be used for the
production of coatings, films and flexible laminates or as adhesives, matrix resins or
compression-moulding materials.
For this purpose customary additives can be added to the block copolymers before Lhe
crosslinking stage, such as pigments, fillers, electrical conductors, for example carbon
black or metal particles, agents for increasing the abrasion resistance, lubricants or
reinforcing fibres, such as carbon, boron or glass fibres.
Laminated articles containing the block copolymers according to the invention can, if
desired, be provided with coating layers which improve the surface properties, for
example layers composed of phenolic resins or aluminium, and they are used, inter alia, in
aircraft construction.
Block copolymers according to the invention, preferably in the form of solutions, can also
be used as coating compositions and adhesives, if desired with the addition of pigments
such as titanium dioxide, customary fillers and foams, for coating and covering substrates
of a very wide range of types in any desired form, such as films, fibres, fibre nonwovens,
wires, lattice-like structures, fabrics or foams.
The following may be mentioned as suitable substrates: metals or alloys, such as cc.pper,
brass, aluminium, iron or steel; asbestos or glass fibre materials; polymers, such as
cellulose materials (cellulose esters or ethers or paper); perfluorinated hydrocarbon
polymers, for example polytetrafluoroethylene, polyolefins, polyesters, polyamides,
polyimides or polyurethanes.
13 ;2~ 35
The following examples illustrate the invention.
Synthesis Example 1:
Polyamic acid block: 44.13 g of 3,3',4,4'-biphenyltetracarboxylic dianhydride (0.15 mol)
and 250 g of N-methylpyrrolidone (NMP) are weighed out under nitrogen into a reaction
vessel with a double jacket, connection for protective gas, internal thermometer, dropping
funnel and stirrer. The rcaction vessel is evacuated three times and flushed with nitrogen.
After it has been cooled to -7C a suspension is formed. A solution of 15.57 g (0.0635
mol) of 4,4'-diaminodiphenyl sulfone and 8.52 g (0.0625 mol) of m-xylylenediamine in
156 g of NMP is added via the dropping funnel in the course of 80 minutes at a
temperature between -7 and -4C. The reaction mixture is then allowed to warrn up to
room temperature and is stirred for a further 2 hours.
Polyamide-polyamic acid block copolymer. The clear reaction mixture is cooled again
(-7C). 62.21 g (0.2500 mol) of 4,4'-diaminodiphenyl sulfone in 300 g of NMP are then
added dropwise in the course of 150 minutes via the dropping funnel. Next 45.68 g (0.225
mol) of isophthaloyl dichloride are added in portions at such a rate that the internal
temperature does not exceed 0C. About 150 minutes are required for this, and the
solution becomes increasingly viscous. Finally, the polymer solution is stirred at room
temperature for 50 minutes. A further 0.51 g (0.0025 mol) of isophthaloyl dichloride are
added and the mixture is stirred for 3 hours to complete the polycondensation. 34.00 g
(0.4725 mol) of butylene oxide are then added in the course of 20 minutes via a dropping
funnel (internal temperature 20-25C). This gives a solution of a polyamide-polyamic acid
block copolymer which has an intrinsic viscosity of 0.64 dl/g (0.5 % by weight solids
content in NMP/25C).
Cyclization: The polyamide-polyamic acid block copolymer solution now present is then
subjected to chemical cyclization to give the polyamide-poiyimide block copolymer. This
is effected by first adding 79.69 g (0.7875 mol) of triethylamine via a dropping funnel in
the course of 45 minutes and then adding 80.40 g (0.7875 mol) of acetic anhydride in the
course of 40 minutes at 20-25C. The mixture is then stirred for a further 6 hours at room
temperature. The intrinsic viscosity (0.5 % solids content, NMP, 25C) of a
polyamide-polyimide block copolymer prepared in this way is 0.74 dl/g.
One part of tl-e solution described above is diluted with NMP, precipitated in a 10-fold
- 14- 2~R~3~i9
amount of ethanol and dried in vacuo in a drying cabinet. The temperature is increased in
stages to 200C in the course of 72 hours and is kept at this temperature for 8 hours. After
this drying process, the polymer is soluble in NMP to the extent of more than 25 %. The
intrinsic viscosity (0.5 %, NMP, 25C) of the polymer is 0.60 dl/g.
Coatin: A 25 % by wei~ht solution of the polyamide-polyimide block copolymer in NMP
described above is applied to a copper foil by means of an applicator (height 200 llm). The
bulk of the solvent is removed by IR irradiation for 15 minutes. The polymer layer of a
test strip prepared in this way can be detached virtually completely by immersion for 30
minutes in NMP. Another test strip is additionally heated in vacuo (15 mbar) from room
temperature to 300C in the course of 30 minutes and is heated at this temperature for a
further 30 minutes. This gives a flexible Cu foil with a bubble-free coating. The foil
treated in this way is weighed, immersed in NMP for 30 minutes and weighed again. An
unchanged weight is found in this test, i.e. the coating is no longer attacked by NMP.
Similarly, the surface of the coating before and after the NMP treatment is unaltered.
Svnthesis Examples 2-12:
Examples 2-12 of soluble polyamide-polyimide block copolymers, shown in the following
table, are prepared as described under Example 1. The following abbreviations are used in
the table:
intrinsic viscosity, measured on a 0.5 % by weight solution of the polymer at
25C (in NMP),
TGA: thermogravimetric analysis (2 % loss in weight at the temperature shown
under N2, heating rate: 10C/minute),
BDTA: 3,3',4,4'-benzophenonetetracarboxylic dianhydride,
BPDA: 3,3,'4,4'-biphenyltetracarboxylic dianhydride,
IPC: isophthaloyl dichloride,
TMAC: trimellitic anhydride-chloride,
mXDA: m-xylylenediamine,
pXDA: p-xylylenediamine,
ABA: aminobenzylamine (mixture of m- and p-isomers),
mPDA: m-phenylenediarnine
mDDS: 3,3'-diaminodiphenyl sulfone,
pDDS: 4,4'-diaminodiphenyl sulfone.
2~ 359
,5
C~.S
r ~ s~ ~ * .
5 ~ 5 0 v ~ v v v v v
.c~ O
3 ~ O
o ~ ~ ~ o~ o o o _, o~ o o s~
O
.~ ~o
~i v~ Z ''~ ~ A ~
~l~ ~ ~ 0
.5 ~ ;~ o O O
F _ ~ . ~ ~ ~ ~ ~
C~ O O O O O O O O O O O O
c a ~ ~ ~ " '^ Q ~O Q O
~ ~ a- ~o. ~o. a- a-
~0 .~ P,o E o E o co c-.o
~ a ~ a ~ aa ,~,aa ~ a o ~ ~ a ~a ~ a ~
~ ~o ~oE o
u,~ x ~ a ~ aE x O ¢ O ¢ O
e ~ ~ ~ ~ ~ ~ O ~ 8 8 8 8 ~
o a ~ a `
D - E a O a o a ~ ~ ~ a ~ a ~ a ~ ~~ 80
a _ p.O ~o E o ~o P.o ~o o o
_ 5 o E o o E a c ~o
~: ~, o ~ v~ ~ v.¢ v~ o ¢ u~ ~ _ _ _~ o
~ ~ XE XE XE XE o. XE o XE æ ~ ¢ a O~
O b ~ ~a v~ a ~ a u. ~¢ ~ ~ ` `¢ ~a ~
m o~ O m o ~q o o E- ~ o ~ o P: O m o a~ o
I ... _
r- ~ ~ I~ ~O ~ ~ ~`~
- 16 - X~859
Example 13: This example shows the good mechanical properties of a flexible laminate
prepared with a PAPI block copolymer according to the invention.
A copper foil is coated ~i~h a 25 % solutioll in N-methylpyrrolidone of the block
copolymer according to Syntl-esis l xample 1, using an applicator (200 )lm). The coating is
dried with IR radiation (Heraeus model MBS 225/125) for 45 minutes. The coated foil is
then cut up into strips I cm long, and tl-e "flex-life" of the laminate is determined [=
number of flexings before the laminate breaks, determined by Universal-model 2 FDF
Flex Ductility Tester (manufacturer: Universal Manufacturing Co.) using a tensile weight
of 224 g and a 2 mm mandrel]. This gives an average value of 354 cycles for 5 test strips.