Note: Descriptions are shown in the official language in which they were submitted.
PROCESS AND SYSTEM FOR RECOVERING OIL
FROM OIL BEARING SOIL SUCH AS OIL SH~LE A~D
TAR SANDS AND OIL PRODUCED B'f SUCH PROCESS
BACKGROUND OF THE INVENTION
This invention relates to processes and a
systems for recovering oil from oil bearing soil such
lS as oil shales and tar sands. In particular, it relates
to recovering of SUCll oil by processes that include
contacting oil bearing soil with liquid mediums.
The world population, especially in the indus-
trial countries, consumes enormous amounts of energy.
For example, in 1978 an equi~alent of about 13.4 billion
barrels of fuel oil was consumed in the United States
alone. About half of that amount of energy was actually
supplied by petroleum products. As the traditional
sources of oil are being exhausted in industrial countries
-- 1--
and as the price of the oil continues to be at a relative-
ly high level, there is a growing need for the development
of alternate sources of oil.
A virtually inexhaustible source of oil is
oil bearing soil such as oil shale and tar sands. Oil
recovered from oil shale and tar sands by conventional
processes, such as retorting, is not identical in com-
position to the conventional crude oil recovered from
the ground and for many applications such oil has to be
further treated by distillation, coking of residue and/or
hydrogenation to achieve the required composition. As
used herein "oil" means organic materials includiny
primarily hydrocarbons recovered from the ground, and
the term is meant to include conventional crude, pro-
cessed oil as well as oil recovered from soil even ifthe composition, impurities and the API number of such
oil are different from the conventional crude or from
the conventional processed oil.
The deposits of both tar sands and oil shale
have been found on all of the inhabited continents.
Although at present the precise amounts of oil available
in these deposits cannot be determined, it is estimated
that they would yield some 2 quadrillion barrels of
oil. The United States has large shale deposits in
several regions. The deposits in the Green River forma-
tion in Colorado, Utah and Wyoming are of particular
interest because they contain oil shale having the
highest oil concentration of any oil shale in the world.
One of the largest known deposits of tar sands extends
for several thousand square miles in the Athabasca
District of Alberta, Canada. Large oil shale or tar
sand deposits have also been found in Australia, Brazil,
Bulgaria, Burma, Congo, France, Germany, Great Britain,
Italy, Israel, New Zealand, South Africa, Spain, Sweden,
Switzerland, Thailand, U.S.S.R. and Yugoslavia.
Despite the fact that enormous amounts of oil
are present in the oil shale and tar sand deposits and
the fact that such deposits are located in many of the
most technologically advanced countries (including the
United States and Canada), the amounts of oil actually
obtained from these deposits are insignificant in the
total energy picture. The reason for is that oil is
trapped in the oil shale and tar sands and is extremely
difficult and expensive to recover. Specifically, in
the oil shale, the organic fraction (called kerogen) is
composed of carbon and hydrogen molecules cross-linked
together by sulfur and oxygen atoms to form macromolecules
with molecular weights of about 3000. These macromolecules
are embedded within a matrix of inorganic or mineral
materials. The problem in recovering oil from oil shale
is that it is extremely difficult to separate kerogen
from the inorganic matrix. Kerogen is insoluble in
most standard petroleum solvents and has to be heated
to relatively high temperatures in order to effect a
separation. At a temperature of about 204C (400F)
chemical bonds between and within the organic molecules
break down and the smaller molecules formed as a result
can be separated as a liquid or gaseous product from
the inorganic matrix.
It is also difficult to separate and recover
oil from tar sands because organic material is bound to
and between interstices of sand. Thus, heat or special
solvents are required to break the organic material
away from the sand.
Most of the commercial operations for recover-
ing oil from shale or tar sands have used the heating
(retorting) method for separating the oil. In fact,
the recovery of oil by heating oil shale or tar sands
goes back some 150 years. The efforts to improve on
the basic method have been expanded in a number of
countries, spurred by the oil embargo and the significant
rise in the price of oil. Despite the use of the finest
technical talents and the expenditure of enormous amounts
of money by giant industrial corporations, these efforts
have been unsuccessful in devising a commercially successful
process which can be used to make oil shale a significant
contributor of world's energy requirements. Some of
the retorting processes, developed thus far, are de-
scribed in a report entitled "An Assessment Of Oil Shale
Technology" published by The Congress of the United
States, Office of Technology (1980), and in an article
appearing in Chemical Engineering, September 7, 1981,
pages 47-51, and 63-71~ The magnitude of the efforts
that went into the unsuccessful development of a com-
mercially feasible and profitable process and system isillustrated by the withdrawal of Exxon Company, U.S.A.
from the oil shale venture after spend.ing millions of
dollars.
The reason for the failures of the retorting
processes is that they use enormous amounts of energy,
require huge capital expenditures and produce by-products
-- spent shale or sand -- which have to be treated in
order to support vegetation. More specifically, enormous
amounts of energy must be used to heat large amounts of
oil bearing soil to the required retorting temperatures
which generally range between about 500C (900F) and
about 800C (1500F). Large capital expenditures are
needed for building the equipment to effect heating of
the oil bearing soil, even if the heating is done in
situ. The spent (or processed) soil subjected to a
retorting process generally has an alkaline pH.
Processed shales retorted at temperatures of about
500C (900F) generally have pHs ranging fFom about 8
to 9 and those retorted at temperatures of 750C to
800C (1400 to 1500F) have pHs of 11 or 12. In order
V9~
to use such spent shales as growth meclia for plants,
their alkalinity must be reduced. The pH reductions
can be achieved by adding acids or acid-formers to the
shale; however, such treatment significantly raises the
overall costs of the recovery process. Additionally,
the spent shales have high concentrations of boron,
molybdenum, selenium, arsenic and Fluorine which can be
toxic to animals which feed on plants grown in such
soil. The retorting processes also result in relative-
ly low yields -- 30 to 40% -- in part because heating
shales to above about 500C transforms some of the
organic materials into char.
Another approach to recovering oil from oil
bearing soil including shale and tar sands has been to
extract oil from oil bearing soil with solvents. For
example, U.S. Patent No. 4,242,195 (Rudnick) discloses
extraction of organic constituents, such as hydrocarbons
and phenols from tar sands and oil shales. The Rudnick
patent discloses extraction using a solvent predominantly
comprising certain organic sulfoxides, organic sulfones,
or mixtures thereof. U.K. Patent No. 1,495,722 (Williams,
et al.) discloses extracting oil from oil shale using a
variety of organic solvents at elevated temperatures
(80C to 550C) and at elevated pressures (500 to 10,000
psi). Recently, McKay et al. have proposed a process
for recovery of organic components of oil shale which
involves penetrating the shale with a methanol-water
solution and then extracting the organic material by
refluxing it with a benzene/methanol mixture. This
experimental process is conducted at about 400C. See
"Nonretorting Method Recovers Shale Organics," Chemical
& Engineering News September 14, 1981.
The proposed extraction processes have not
been entirely successful because they employ orclanic
solvents which are generally quite expensive. Addi-
tionally, many of the processes also require, for com-
mercial production, high temperatures and high pressures.
The use of hlgh temperatures and/or pressures, in turn,
necessitates sophisticated and expensive equipment.
There is, therefore, a long felt and unsatis-
fied need for a commercially viable process and system
for recovering oil from oil bearing soil including oil
shale and tar sands, which process does not suffer from
the aforementioned disadvantages.
Thus, one object of the present invention is
to provide a process for recovering oil from oil
bearing soil including oil shale and tar sands, which
is inexpensive and creates minimal pollution and
disposal problems.
Another object of this invention is to provide
a process for extracting oil from oil bearing soil
including oil shale and tar sands that requires small
capital expenditures and significantly less energy to
operate than the retorting processes.
A further object of the present invention is
to provide a process for recovering oil from oil bearing
soil, such as oil shale or tar sands that does not re-
quire high temperatures and pressures for its operation.
Still another object of the present invention
is to provide a process and a system for recovering oil
from oil bearing soil, such as oil shale or tar sands,
which utilizes a liquid medium that is inexpensive.
A still further object of the present inven-
tion is to provide a process for recovering oil from
oil bearing soil, such as oil shale or tar sand, using
a liquid medium which can be recycled.
Still another object of the present invention
is to provide a process for the recovery of oil from
oil bearing soil such as oil shale and tar sands, which
--6--
2C~9~4
produces a spent soil that does not impair vegetation
and has a pH value close to neutral.
A still further object of the present invention
is to provide a process for the recovery of oil from
oil bearing soil, such as oil shale or tar sands, which
uses an inexpensive and easily available medium composed
primarily of water.
Still another object of the present invention
is to provide a high-yield process for recovering oil
from oil bearing soil such as oil shale or tar sands.
Other objects of the invention will become
apparent to those skilled in the art upon studying this
disclosure.
BRIEF DESCRIPTION OF THE I~ENTION
In accordance with one aspect of the present
invention, oil bearing soil, such as oil shale or tar
sands, is contacted in a contacting zone with a liquid
medium comprising water and a lipophilic solvent which
is miscible or soluble with water. The contacting
produces an emulsion which comprises the oil from the
oil bearing soil and the liquid medium. The inorganic
portion of the soil is dispersed in the emulsion and it
is separated from the emulsion by gravity or other
suitable means. The emulsion is then broken by an
emulsion breaking agent into two phases. The two
phases are allowed to separate into two layers. The
first layer comprises the oil and some liquid medium.
The second layer comprises the liquid medium and some
oil. The first layer is then recovered. The medium
from the second layer can be recycled into the contac-
ting zone. In the alternative, the inorganic portion
of the soil can be separated and removed after -the
emulsion is broken.
20~ 4~
In accordance with another aspect of the pre-
sent invention, oil bearing soil, such as oil shale or
tar sands, is contacted in a contacting zone with a
liquid medium comprising water, a lipophilic solvent
which is miscible or soluble with water, and a yield
improving agent comprising a soluble ionic salt or a
soluble ionic acid. Unexpectedly superior results are
obtained when isopropyl alcohol is used as the solvent
especially with a yield improving agent. Unexpectedly
good results are achieved when the yield improving
agent is ammonium sulfate especially if the amount of
ammonium sulfate is at or near the saturation point.
Unexpectedly good results are also achieved when the
solvent is acetone, and when the solvent is ethyl
acetate and the yield improving agent is sulfuric acid.
The inorganic portion is separated from the
emulsion. The emulsion is then broken by an emulsion
breaking agent and the resulting two phases are allowed
to separate from each other into two layers. If the
yield improving agent is at or near the saturation point
of the medium, the emulsion breaking agent can be addi-
tional solid ionic salt, preferably of the same type as
the yield improving agent. The inorganic portion can
also be separated after the emulsion is broken.
The first layer comprises the oil, minor amounts
of the medium and minor amounts (if any) of tlle inorganic
portion. The second layer comprises the medium, minor
amounts of the oil and minor amounts (if any) of the
inorganic portion. The first layer is then recovered
and the second layer can be recycled to the contacting
zone.
In accordance with a third aspect of the pres-
ent invention, tar sand is contacted with successive
liquid mediums. In the first step, tar sand is contacted
in a first contacting zone with a first liquid medium
comprising water, a solvent which is not miscible or
appreciably soluble with water, a surfactant and a non-
caustic alkali compound all intimately mixed to form a
phase emulsion. The first liquid medium and bitumen
released from the tar sand are separated from the sand,
followed by separation of the bitumen and solvent from
the aqueous portion of the separated liquid. The bitumen
is recovered and the solvent may be recycled. The
aqueous portion, containing the surfactant and non-
caustic alkali compound, may also be recycled.
The sand left from treatment in the firstcontacting zone is treated in a second contacting zone
with a second liquid medium comprising water, a
surfactant and a non-caustic alkali compound. The
lS second liquid medium and additional recovered bitumen
are separated from the sand. The bitumen is recovered
and the second liquid medium may be recycled. The
resulting sand may be contacted again with the second
liquid medium and this step of the process repeated one
or more times, each time recovering the bitumen and, if
desired, recycling the liquid medium.
Other aspects of the present invention will
become apparent to those skilled in the art upon studying
this specification and the appended claims.
BRIEF DESCRIPTION OF T~E DRAWINGS
FIG. 1 depicts a schematic flow diagram of an
embodiment of the process relating to the first two
aspect of the present invention.
FIG. 2 depicts a flow diagram of the process
and system of the preferred embodiments of the first
two aspects of the present invention.
FIG. 3 depicts a flow diagram of the process
and system of the preferred embodiment of the third
aspect of the present invention.
DETAILED DES~RIPTION OF THE
FIRST TWO ASPECTS OF THE INVENTION
In accordance with the first two aspects of
the present invention, it has been discovered that oil
can be recovered from oil bearing soil, such as oil
shale or tar sands, by contacting them with a hetero-
genous liquid medium. The medium comprises water and a
lipophilic solvent which is miscible or soluble with
water.
It is believed that upon contacting with the
oil bearing soil, the heterogenous liquid medium disrupts
the bonding within the kerogen-silicious system structure.
As a result, oil is freed and attracted to the molecules
of the medium. Since the medium contains water, the
release of the oil into the medium creates an emulsion.
The inorganic portion of the oil bearing soil,
dispersed throughout the emulsion, is separated by gravity
or other suitable means. The emulsion is then contacted
with an emulsion breaking agent which separates it into
two phases. The two phases are allowed to separate
from each other to form two layers. The first layer,
comprising the oil and some medium, is separated and
removed for shipment or further processing. The second
layer, comprising the medium and some oil, can be recycled
to the contacting zone.
The process and system of the first two aspects
of the present invention offer numerous advantages over
those of the prior art. One major advanta~e of the
process and system is that they are inexpensive when
compared with the prior art. The medium used in the
process is inexpensive because its primary ingredient
is water. Since the process can be operated at ambient
temperatures and pressures, the required equipment is
inexpensive. Therefore, the capital expenditures of
the process are significantly lower than those of the
--10--
2~914~.
prior art processes. The process is also inexpensive
to operate as it requires minimal amounts of energy.
Very little or no heat need be supplied to carry out
the process and the only other energy is supplied to
pump liquids and to effect mixing. The overall expense
of the process is also comparatively lower than that of
the prior art processes because the spent oil bearing
soil does not need to be treated to support vegetation.
Unlike spent oil bearing soil from many processes, the
spent oil bearing soil of the process is generally close
to neutral. As long as nutrients are added to the spent
oil bearing soils resulting from the process, they will
support vegetation. In fact, if the medium contains
phosphorus, nitrogen, potassium or other nutrients
required for plant growth, the spent oil bearing soils
contain residual nutrients thereon.
Unexpectedly superior results are achieved by
the process of the first two aspects of the present
invention when the solvent is isopropyl alcohol and
also when the liquid medium includes a yield improving
agent in addition to the solvent and water. Particularly
superior results are achieved when the solvent is isopro-
pyl alcohol and the yield improving agent is an ionic
salt such as ammonium sulfate, sodium chloride or sodium
nitrate. Much superior and better results are also
achieved when the solvent is n-butanol. Other higher
alcohols including iso-butyl alcohols, amyl alcohols,
and ethyl and methyl alcohol can be used.
Unexpectedly good results are achieved when
the solvent in the medium is acetone and especially if
a yield improving agent, comprising an ionic salt such
as ammonium sulfate or sodium chloride, is included in
the medium.
--11--
9~4
Also unexpectedly good results are achieved
when the solvent is ethyl acetate and the yield improv-
ing agent is sulfuric acid.
General Description of the Process of the First Two
Aspects of the Present Invention
The process of the first two aspects of the
present invention will now be described in connection
with the drawings. Referring now to the drawings,
FIG. 1 depicts the schematic of a process for recover-
ing oil from oil bearing soil carried out in accordancewith the present invention. As shown in FIG. 1, water
is first mixed with a lipophilic solvent and a yield
improving agent to form a liquid medium. As explained
above, the medium is a heterogenous liquid which includes
water and a lipophilic solvent which is miscible or
soluble in water. For best results the medium also
includes a yield improving agent. The amount of the
solvent should be sufficient to effect the desired recov-
ery of oil but should be low enough to keep the cost of
the raw materials used in the process at a minimum.
The optimum amounts used depend on the particular process
and materials; however, in general the amount of the
solvent is in the range from about 10 to about 50 volume
percent based on the volume of the solvent-water solution.
The amount of a yield improving agent depends on economics.
The cost of the additional agent must be weighed against
the improvement of the yield or the reduced contact
time to achieve the desired yield. Generally, if a
salt is used as the yield improving agent, economics
generally dictate the addition of the maximum amount of
the salt so that the salt is added to saturate or nearly
saturate the medium. The addition of the amount of the
salt that brings the concentration thereof to or near
3~4~
the saturation point is highly desirable for yet another
reason. Once the emulsion is formed, it can be broken
by adding additional pure salt to the emulsion which
contains enough salt to saturate the medium.
The medium is contacted with oil bearing soil,
such as oil shale or tar sand, in a contacting zone of
a reactor l0. The contacting is carried out at suffi-
ciently high volume ratio of medium to oil bearing soil
and for a sufficient time period to free from the oil
bearing soil and bring into the emulsion a desired pro-
portion of the oil contained in the soil. Generally,
the ratio of medium to oil bearing soil is in the range
from about l/l to about l00/l and preferably in the
range from about 2/l to about 20/l. The shale (or tar
lS sands) are preferably pulverized to achieve the optimum
surface to volume ratio. The incremental cost of smaller
sized shale or sand particles has to be balanced against
the incremental increase of the yield of the process
and shortened processing time.
Upon contacting, the medium disrupts the bond-
ing within the oil-kerogen-silicious system structure
and attracts the oil molecules. The oil molecules are
freed from the soil and they enter the medium. Since
the medium includes water a water-oil emulsion i.s
formed. It is believed that the yield improving agent
in the medium facilitates the disruption of the bonding
in the soil and helps to attract the oil molecules to
the medium.
The emulsion formed upon contacting has inor-
ganic portions of the oil bearing soil dispersed through-
out its volume. These portions are separated from the
emulsion by gravity, filtration or other suitable means
and the substantially particle-free emulsion is then
passed to a settling tank 15 where it is contacted with
an emulsion breaking agent.
~0~)9~ 44
The emulsion breaking agent causes the emulsion
to break into two distinct phases which are allowed to
separate into two layers. The first layer, which is
generally the upper layer, comprises the oil and minor
amounts of the medium. The second la~er, which is
generally the lower layer, comprises the medium and
minor amounts of the oil. Both the first and the
second layer may contain small amounts of inorganic
portions of the oil bearing soil that have not been
removed in the reactor 10.
The first layer is recoverecl and passed for
shipment or for further processing. The second layer
is passed through a filter 20 or another suitable means
to remove any particulate inorganic portions which are
present therein. The filtered part of the second layer
is then recycled to the reactor 10 after adjusting the
water-solvent salt concentration ratio (not shown).
The Materials Used In The Process of the First Two
Aspects of the Present Invention
Starting Materials
' The process of the first two aspects of the
present invention can be performed on any type of oil
bearing soil including oil shale, oil shale found in
the Green River basin in the United States, and any
type of tar sand, including those fro:n Alberta, Canada.
The oil shale and tar sands for use in this
process can be obtained from the deposits of any con-
ventional method including those used to obtain shale
and tar sands for retorting. The large size shale or
tar sand is preferably comminuted in order to increase
its surface to volume ratio and thereby expose more
surface to the licluid medium. The optimum size of the
particles is determined by balancing the costs of
comminuting these materials against the increased pro-
fits due to improved yields and more rapid processing.
-14-
The Solvents
As explained above, the medium includes a
solvent. The solvents suitable for the use in the med-
ium are lipophilic solvents which are miscible with
water including alcohols and ketones like acetone.
Partially soluble ethyl acetate is also suitable.
Unexpectedly good yields and processing times were
obtained when using solvents such as, isopropyl alco-
hol, acetone, n-butanol and ethyl acetate. However, by
far the best results were obtained using n-butanol and
isopropyl alcohol. N-butanol gave better extraction
results than isopropyl alcohol.
The Yield Improving Agents
The yield improving agents suitable for the
use in the medium include water-soluble, ionic salts
and water-soluble ionic acids. The preferred salt is
ammonium sulfate but other salts such as sodium nitrate,
potassium nitrate and sodium chloride can also be used.
It is often desirable to have a sufficient amount of
the salt in the medium to bring the concentration there-
of to or near the saturation point.
The Emulsion Breaking Agent
It is preferred that the emulsion formed in
the contacting step be broken by contacting it with a
solid salt of the type utilized in the medium. If the
medium is saturated or nearly saturated by such salt,
the additional salt disrupts the emulsion causing it to
separate into two distinct phases. Other emulsion break-
ing detergents or agents can also be used including
30 Triton X100 and Tween 80.
z~ 44
The Product Of The Present Invention
The oil recovered by the process of the present
invention is different in its composition than the oil
recovered by retorting processes and conventional extrac-
tion processes because the kerogen is disrupted in adifferent manner than in these processes.
DESCRIPTION OF THE PREFERRED EMBODIMENTS OF
THE FIRST TWO ASPECTS OF THE INVENTION
The first two aspects of the invention will
now be described in connection with preferred embodi-
ments depicted in FIG. 2. The preferred embodiments of
the invention depicted in FIG. 2 are described in con-
nection with a process for treating oil shale. It should
be understood, however, that other oil bearing soils,
such as tar sands and oil saturated sands, could be
treated in the same manner by the process of the first
two aspects of the present invention.
The First Preferred Embodiment
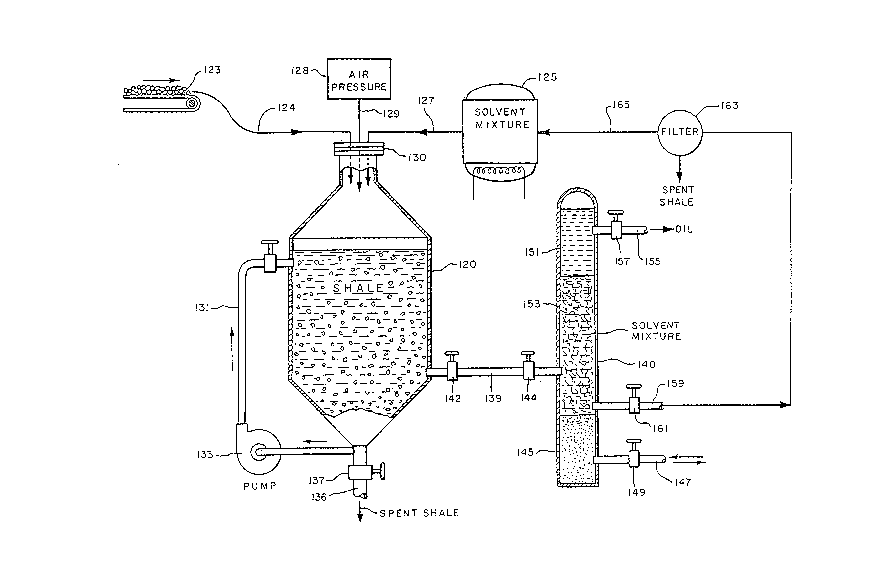
As shown in FIG. 2, oil shale is transported
to a reactor 120 by a conveyer belt 123 or by other
suitable means via a line 12~. A premixed li~lid medium
stored in a tank 125 is introduced into the reactor 120
via a line 127. The medium comprises about 15 to 30
and preferably 17 to 25 percent by volume of water-
isopropyl-alcohol solution of isopropyl alcohol, wa-ter
and enough ammonium sulfate to bring it to or near a
saturation point. Generally, that amount of ammonium
sulfate is between about 7.5 grams per 100 milliliters
of the solution to about 8.0 grams per 100 milliliters
of the solution. The amount of ammonium sulfate
depends upon the mineral contents of the water used.
The solvent is introduced at a temperature of about
60-80C in order to speed up the reaction and increase
the yield.
-16-
~0~ 4~
The pressure in the reactor 120 is kept in
the range from about lO kg/cm2 to about 70 kg/cm2 in
order to facilitate the extraction process. The pres-
sure in the reactor 120 is maintained at desired levels
by introducing therein air from a supply 128 via a line
129 through the gas seal 130.
In the reactor 120, oil shale and the medium
are contacted together until a desired proportion of
the oil is extracted from the oil shale. The contacting
times can vary depending on the size of the ecluipment,
specific type of shale and specific operating condition
but it is in the range from about 1/2 to about 6 hours
and most often up to 4 hours. The agitation of the
medium-oil shale mixture is provided by recycling it
via a line 131 using a pump 133. The contacting produces
an emulsion. The spent oil shale which is dispersed
throughout the emulsion is allowed to settle to the
bottom of the reactor 120 and it is removed therefrom
via a line 136 by opening a valve 137.
The emulsion, which is substantially particle
free, is then passed via a line 139 from the reactor 120
to a separation tank 140 by opening valves 142 and 144.
In the separation tank 140 the emulsion is
contacted with a bed 145 of solid ammonium sulfate
stored at the bottom of the tank 140. Ammonium sulfate
is introduced and withdrawn from the tank 140 via a
line 147 by opening a valve 149. As a result of the
contacting under agitation, the emulsion breaks and
separates into two distinct phases. The phases separate
from each other forming two layers. The upper layer
lS1 contains the oil, minor amounts of inorganic portion
of the oil shale (spent shale) and minor amounts of the
medium. The lower layer 153 contains the medium, minor
amounts of the oil and minor amounts of the inorganic
portion of the shale (spent shale).
2~
The upper layer is recovered via a line 155
by opening a valve 157 and passed to shipment or to
further processing. The lower layer is passed via a
line 159 through a valve 161 to a filter or a screen
163 to remove oil and shale particles therefrom.
The spent shale, removed from the filter or
screen 163 and from the bottom of the reactor 120, is
washed with water to recover therefrom isopropyl alcohol
and ammonium sulfate adhering thereto. Isopropyl alcohol
is recovered by distillation and the ammonium sulfate
recovered by evaporation of water in open cement tanks
exposed to solar heat or similar drying process. Residual
ammonium sulfate left behind after water wash is sufficient
to provide nutrition to vegetation that might be planted
on the spent shale. The preferred system for recovering
of the solvent and the yield improving agent from the
spent shale is conventional in other solvent recovery
systems and is not shown in the drawings.
The particle free medium is then passed via a
line 165 to the tank 125.
The Second Preferred Embodiment
This embodiment is operated in the same manner
and uses the same material and equipment as the first
preferred embodiment depicted in FIG. 2 and described
above, except that n-butanol was used as the solvent.
The concentration of n-butanol was the same as that of
isopropyl alcohol.
The yields in this embodiment were better
than those in the first preferred embodiment.
The Third Preferred Embodiment
This embodiment is operated in ~he same manner
and uses the same materials and equipment as the first
preferred embodiment depicted in FIG. 2 and described
-18-
above, except that acetone is used as the solvent in
the liquid medium instead of isopropyl alcohol. The
concentration of acetone is generally from about 5 to
30 volume percent by volume of acetone-water solution.
The yields of the third embodiment are less
superior than those of the first embodiment.
The Fourth Preferred Embodiment
This embodiment is operated in the same manner
and uses the same materials and equipment as the first
preferred embodiment depicted in FIG. 2 and described
above, except that the liquid medium and the emulsion
breaking agent are different. The medium is formed as
follows. A water solution containing about 5 to about
10 ml of sulfuric acid per 100 ml of the solution is
first made. Enough ethyl acetate is then added to form
a saturated ethyl acetate solution. Generally, 10 ml
of ethyl acetate is needed per each 100 ml of the solu-
tion.
Upon contacting of the medium of the fourth
preferred embodiment with oil shale, an emulsion similar
to those of the first, second, and third preferred embodi-
ments is formed. The emulsion is broken preferably by
adding sodium chloride. Generally about 5 to 7.5 grams
of sodium chloride needs to be added to each 100g of
the emulsion in order to break it.
Example 1
One hundred grams of pulveri~ed oil shale was
reacted in a flask with 200 ml of a medium consisting
of isopropyl alcohol, water and ammonium sulfate. The
amount of isopropyl alcohol was 17.5 volume percent of
the isopropyl alcohol-water solution. The quantity of
ammonium sulfate was 8 grams percent, which saturated
the medium with ammonium sulfate. The oil shale medium
--19-
- ~V~3~44
was agitated for about 45 minutes and heated in a water
bath at 75-~0C. Thereafter, the heat was removed and
the mixture allowed to stand to separate and to allow
the inorganic sediments (spent shale) to settle down.
The liquid portion was decanted or filtered and trans-
ferred to a cylindrical container in which 25 grams of
solid ammonium sulfate was placed at the bottom. Upon
slight agitation the emulsion breaks causing the oil
layer to separate out at the top. A small layer of
sediments aggregated below the oil layer and was
separated off by filtration. The remaining medium was
filtered and reused for the next batch after adjusting
the isopropyl alcohol concentration. The spent shale
at the bottom was washed with 10 ml of water and then
the mixture was filtered to recover the isopropyl
alcohol and ammonium sulfate. The oil was examined and
it showed the usual properties of a hydrocarbon mixture.
Example 2
The procedures of Example l were repeated
except that 10 grams of Bakersfield oil saturated sand
was used instead of pulverized oil shale. The results
were the same. The amount of oil initially recovered
was 4.3 ml. The oil was golden brown in color. ~7hen
the procedure was repeated on the same sand an addi-
tional 2.4 ml of oil was recovered.
Examples 3 and 4
The procedures of Examples 1 and 2 were re-
peated except ammonium sulfate was deleted from the
medium. The emulsion was formed much slower and the
amount of oil recovered was significantly less than in
Examples 1 and 2.
-20-
~V~
Example 5
Ten grams of Atabasca tar sand containing
heavy tar-like material was contacted with the 20 ml of
the medium of Example l. The mixture was stirred with
a spatula. After about 5-lO minutes the sand started
to become pale gray and after about 15-20 minutes it
was pale gray. The liquid above it had a color of a
soap water. The tar separated and stuck to the spatula.
Upon breaking of the emulsion in the manner described
in Example 1, a clear transparent oil was formed. The
amount of oil that was recovered was 2.2 ml. This
example shows that the process not only removes oil
from tar sand but also separates the tar from the oil.
The procedure was repeated two more times using the
same sand. An additional 1.6 and 0.9 ml of oil were
recovered, respectively.
Example 6
The same process as in Example 1 was used
except 17.5% isopropyl alcohol was replaced by 20%
acetone, the rest of the process including the addition
of ammonium sulfate remained the same. The results
were the same as in Example 1 except that it took
longer to form the emulsion and less oil was recovered.
Example 7
One hundred grams of powdered shale was reacted
with 200 ml of a medium consisting of S volume percent
of sulfuric acid and 10 volume percent of ethyl acetate
in water. The mixture was well agitated. Upon agitation
an emulsion was formed as in Example l. Eighty grams
of sodium chloride was added to break the emulsion to
separate the oil. The separation proceeded in the same
manner as in Example 1. The results were the same as
in Example 1 except that it took longer to form the
emulsion and less oil was recovered.
-21-
' 20V91~
Example 8
10 gms of tar sands was contacted with a satur-
ated solution of n-butanol (butyl alcohol) in water.
The concentration of n-butanol in the solution was about
12 volume percent.
Slightly more than 8 gms of ammonium sulfate
was then added to the mixture. The mixture was then
agitated. A black tar-like material separated from the
sand, leaving grayish clear sand at the bottom of the
container. Yellow, yellow orange to red colored oil
droplets separated from the sand and floated toward the
top. The mixture was then allowed to stand until it
separated into two layers. The top layer was decanted
into a separate container. The top layer was an emulsion
which included oil and some solution.
The top layer has the oil part with some of
the solvent and some water. The top layer was trans-
ferred to another container and there contacted with a
bed of solid ammonium sulphate, with mild agitation.
The emulsion broke resulting in a typical oily layer
containing some solvent. In this example especially
when no heat is employed, the amount of water in the
top oily layer emulsion is minimal, thus indicating a
better process than the other examples. The lower water
content in the oily layer and faster breaking of the
emulsion is of industrial significance in terms of faster
separation process.
Example 9
The procedure of Example 8 was followed except
that a 15% solution of n-butanol was used. Upon addition
of butanol to water there was a slight separation of
butanol on top of water. The solution was shaken before
contacting it with tar sand. Upon shaking, the solution
turned turbid.
-22-
~)9-~
The results were better than in Example 8.
Exam~le lO
The procedure of Example 8 was followed except
that a 17.5% solution of n-butanol was used. The slight
separation of n-butanol was observed as in ~xample 1.
The solution was shaken before contacting it with tar
sand. Upon shaking, the solution turned turbid.
The results were better than in Example 9.
Example ll
The top oil layer obtained in Examples 8, 9
and 10 after breaking of the emulsion by contacting
with solid ammonium sulphate was then studied for its
chemical content using a spectroscopic technique.
The oil extracted by the isopropyl alcohol-
water-salt technique was found to contain smaller
amounts of the oil and lighter fractions as compared to
the n-butanol-water-salt system where extraction was
more complete and better. In each instance the "oil"
layer was subjected first to a spectroscopic study
using Gas-Liquid Chromatography technique using several
types of GLC columns.
It was found that the solvent in each case,
namely ispopropyl alcohol in the first and n-butanol in
the second separated out in the first phase and would
hence be removed when this process is applied at larger
industrial levels.
Subsequent to the solvent phase and quite
clearly separated from it appeared the remaining
materials that had been extracted out in the oil.
Owing to the heterogenous nature of the materials
extracted in the first Gas-Liquid Chromatography
studies, one found distinct "manifolds" or groups of
-23-
2~
.
peaks of materials, with composition of molecules with
one carbon to twenty-six carbons (Cl to C26), with a
significant majority in the C15 to C22 range.
To determine whether the organic materials
were hydrocarbons, the samples were subjected to a Mass
Spectra analysis. The results confirmed that the
hydrocarbons entracted were between those with 8 to 26
carbon atoms; the majority of the hydrocarbons being
those with from 10 to 22 carbon atoms. The GLC-Mass
Spectra analysis further showed that the hydrocarbon
fractions include alk_nes, cycloalk_nes, alke_es and
alk~nes in the carbon number ranges cited above.
DETAILED DESCRIPTION OF TH~ T~IRD
ASPECT OF THE PRESE~T INVENTION
FIGURE 3 depicts a flow diagram for a pre-
ferred embodiment of the third aspect of the present
invention. In this third aspect, tar sands are con-
tacted in a first contacting zone (labeled mixing cham-
ber 1 in EIG. 3) with a first liquid medium (labeled
liquid medium A in FIG. 3). The first liquid medium
comprises an intimately mixed phase emulsion of 1) a
solvent which is not miscible or appreciably soluble
with water, and 2) an aqueous solution of water, a sur-
factant and a non-caustic alkali compound.
After the tar sands and first liquid medium
are mixed in the first contacting zone, the liquids and
solids are separated. The liquids include the first
liquid medium and a major portion of the originally
present bitumen. The solids left over are sand and the
remaining bitumen. The liquid is next separated in a
supernatant separator into a hydrocarbon stream con-
taining the solvent and the bitumen and an.aqueous stream
containing the water, surfactant and alkali compound.
This aqueous stream may be fed to a holding tank and
~n~l~4
recycled. The hydrocarbon stream is separated, recover-
ing and recycling the solvent and providiny the bitumen
product for further processing.
The solids removed from the first contacting
zone are mixed with a second liquid medium (labeled
liquid medium B in ~IG. 3) in a second contacting zone
(labeled mixing chamber 6 in FIG. 3). The second liquid
medium comprises water, a surfactant and a non-caustic
alkali compound. In the preferred embodiment, the second
liquid medium is the same as the aqueous solution used
in making the first liquid medium.
The solids removed from the second contacting
zone contain sand and may still include small amounts
of bitumen. If further recovery of this bitumen is
desired, the processes associated with the dash line
blocked portion of FIG. 3 may be repeated one or more
times. The steps in the blocked portion of the flow
diagram are identical to the contacting, mixing and
separating steps associated with the second contacting
zone, utilizing additional amounts of the second liquid
medium. Prior to disposal, the sand resulting from the
one or more mixings with the second liquid medium may
optionally be washed with water to remove any traces of
surfactants and alkali compounds remaining with the
sand.
The liquid separated from the sand in the
second contacting zone are subjected to the same treat-
ments as the liquid removed from the first contacting
zone. However, there is much less solvent associated
with the bitumen in the liquid recovered from the second
contacting zone as compared to the liquid from the first
contacting zone since in the preferred embodiment, the
second liquid medium contains no solvent.
2~gl4~
Inasmuch as the aqueous portion of the first
liquid medium has the same composition as the second
liquid medium in the preferred embodiment, the material
from the holding tank can be recycled to either the
S first or second contacting zone. Of course before being
introduced into the first contacting zone, the recycled
aqueous material must be mixed with solvent (either
from recycle or from a make-up source) to produce an
intimately mixed phase emulsion. The ratio of tar sand
to the liquid medium in each contacting step is preferably
between about 0.5 to about 2.0 liters of liquid medium
per kilogram of tar sand. Most preferred is a ratio of
about one liter of liquid medium per kilogram of tar
sand. Mixing and settling times need to be sufficient
for separating and recovering the bitumen Actual
times will depend on the equipment and size of the
containers used.
The aqueous component of the first liquid
medium and the second liquid medium of the preferred
embodiment of the third aspect of the present invention
comprise about .025% to about 0.5% surfactant, most
preferably about 0.1%. A preferred surfactant is
sodium lauryl sulphate. Other suitable surfactants
include Tween-80 (polysorbate-80), sodium tetradecyl
sulfate, diocyl calcium sulfosuccinate, sodium lauryl
sulfoacetate and sodium lauroyl sarcosinate.
The aqueous component of the first liquid
medium and the second liquid medium also comprise about
0.1% to about 5.0% non-caustic alkali compounds, most
preferably about 1.0%. A preferred alkali compound is
sodium bicarbonate. Other suitable alkali compounds
include sodium carbonate, potassium carbonate, potassium
bicarbonate and ammonium carbonate.
-26-
2~o~
The volume ratio of solvent to aqueous com-
ponent in the first liquid medium should be between
about 1:10 and about 4:6, most preferably about 1:4.
The preferred solvent is light naphtha-50. However,
other suitable solvents include commercial gasoline
(unleaded), well-head raw gasoline, kerosene, hexane,
cyclohexane, pentane, cyclopentane, Stoddard's solvent,
tetrachloroethylene, carbon tetrachloride, benzene,
petroleum ether, toluene and xylene.
It is essen~ial that the aqueous component
and solvent be intimately mixed together into a phase
emulsion before contacting the tar sands. It has been
found that contacting the tar sands with the intimately
mixed phase emulsion produces better results than
treating the sands first with an equivalent amount of
solvent followed by treatment with the aqueous component
of the first liquid medium.
A good example of a phase emulsion is an oil
and aqueous vinegar salad dressing. After being shaken
up, the materials stay in a phase emulsion for a short
time phase, but eventually separate into two layers.
Likewise, in the present invention, the solvent and the
aqueous component must be intimately mixed, then con-
tacted with the tar sands before they separate into two
layers.
It is speculated that the solvent acts as a
"trigger," allowing the surfactant and alkali compound
in the aqueous solution to begin extracting away the
bitumen from the sand. However, the solvent in the
relatively small quantities used herein is simply ab-
sorbed by the tar sands if the aqueous component is not
present at the same time.
When the process of this third aspect of the
present invention is carried out in a batch process,
the container used for the flrst contacting zone can be
2(~
used for each of the subsequent contacting zones, de-
canting off the liquid after each step and leaving the
solids in the container. In such batch processes it
has been found that three contacts with the second
liquid medium is sufficient to produce a clean sand,
recovering almost all of the bitumen.
As with the other aspects of the present
invention, the process of this aspect produces a high
yield from the tar sands at low temperature operation.
In the preferred embodiment, the process is practiced
at room temperature using cold tap water in the aqueous
component of the liquid mediums. This process has been
successfully practiced even using refrigerated water.
Various tar sands may successfully be utilized
in the process of the third aspect of the present inven-
tion. ~iater-wet tar sands such as the Athabasca tar
sands in Canada, oil-wet tar sands such as those in the
United States, and even Mc~ittrick diatomaceous tar
sands, have successfully been processed in accordance
with the third aspect of the present invention.
The following examples provide batch process
illustrations of the third aspect of the present inven-
tion as it has been practiced on various tar sands.
Example 12
Two kiloyrams of Athabasca tar sands contain-
ing approximately 12% bitumen were placed in an open
container. A first liquid medium was prepared as
follows: 0.1 grams of sodium lauryl sulfate and 1.0
gram of sodium bicarbonate per 100 ml. of aqueous
solution were first dissolved in cold tap water. On a
volume basis, 80% of the aqueous solution and 20% light
naphtha-50 were poured into a bottle to provide two
liters of first liquid medium. The bottle was approxi-
mately 2/3 full. The bottle was capped and vigorously
-28-
9~4~
shaken by hand for about two minutes. The liquid medium
in the bottle was intimately mixed, forming a phase
emulsion. The contents of the bottle were quickly poured
into the container with the tar sands. A slow mechanical
stirring device was used to stir the first liquid medium
and tar sands for about ten minutes. Other than the
use of cold tap water, all of the ingredients were used
at room temperature. No heat was applied to the
container.
The contents of the container were allowed to
stand for about five minutes. Then the top layer of
liquid, containing a major portion of the bitumen and
naphtha, was decanted into a second container.
Underneath the top layer was a layer of water with
suspended sand particles. On the bottom of the
container was a layer of sand.
Two liters of a second liquid medium com-
prising just the aqueous component of the first liquid
medium (namely, 0.1 grams of sodium lauryl sulfate and
1.0 gram of sodium bicarbonate per 100 ml of solution)
were next poured into the container. The slow mechanical
stirrer was used to mix the contents of the container
for about five minutes. After standin~ five minutes,
the container was again decanted, pouring off the top
layer of liquid into the second container already
containing the bitumen from the first contacting
operation.
Two liters of the second liquid medium were
again added to the container and mixed for five minutes.
After settling for five minutes, the liquid was decanted.
This step was repeated again with another two liters of
the second liquid medium, five minutes of mixing, five
minutes of settling, and decanting.
-29-
20~9~
Less and less bitumen was contained in each
of the top layers from the successive mixings. Also,
the middle layer of water with suspended particles was
smaller and smaller each time, so that after the last
mixing the liquid was decanted completely from the
sand. The remaining sand was visibly white with a few
small flecks of unextractable alsphaltines. Analysis
of the sand indicated that 93% of the bitumen originally
present had been removed.
The bitumen and naphtha formed a layer on top
of the aqueous phase in the second container. The
bitumen could be scooped out with a ladle. If left for
several days, the naphtha would evaporate, leaving a
soft pancake-like layer of bitumen.
ExamPle 13
One kilogram of Athabasca tar sand containing
8% bitumen by weight was placed in a container. A
first liquid was prepared using the same ingredients as
in the first liquid medium of Example 12, except that
one liter of liquid medium was prepared using a volume
ratio of 15% naphtha and 85% aqueous component. This
first liquid medium was agitated as in Example 12 to
form an intimately mixed phase emulsion, which was
immediately added to the container with the tar sands.
Thereafter, mixing, settling and decanting, as in Example
12, were carried out. Li~ewise, the second, third and
fourth contacting with the second liquid medium of
Example 12 was carried out, except only one liter~of
liquid medium (as compared to two liters in Example 12)
were used each time. After the fourth contacting, the
sand was visibly white and bitumen recovery appeared to
be the same as in Example 12.
-30-
2~
Example 14
Oil-wet Kentucky tar sand was mechanically
crushed to obtain a mixable tar sand material, one
kilogram of which was placed in a container. One liter
of the first liquid medium of Example 13 was intimately
mixed then poured into the container. Mixing, settling
and decanting were carried out as in Example 13, as
were three subsequent mixing, sett].ing and decanting
steps using the second liquid medium of Example 13.
After the fourth contacting, the sand was visibly white
and bitumen recovery appeared to be the same as in
Example 12.
Example 15
The process of Example 13 was carried out
using one kilogram of McKittrick diatomaceous tar
sands. After the fourth contacting, the sand was
visibly white and bitumen recovery appeared to be the
same as in Example 12.
In industrial operatings, it would be most
preferred to prepare the second liquid medium
containing the surfactant and non-caustic alkali
compound in quantity. The first liquid medium could
then be intimately mixed from the required amounts of
solvent and second liquid medium.
It is speculated that instead of using
10%-40% solvent in the first liquid medium ancl no
solvent in the liquid medium used for the second and
subsequent contactings, the solvent micJht be propor-
tionately reduced and one common liquid medium used in
each contacting. For example, instead of 20% solvent
in the first liquid medium, tar sands could be success-
ively contacted with four liquid mediums each contain-
ing 5% solvent intimately mixed with the aqueous
~9~ ~
component. However, at the present time, the process
using solvent in only the first liquid medium is pre-
ferred, subject to further experimentation.
The examples have illustrated the process of
the third aspect of the present invention using four
contacting and separating steps. The necessity of the
fourth step, and possibly the third step 2S well, may
be eliminated with better mixing and separating equip-
ment. The appropriate number of additional contacting
steps after the first two (possibly even more than the
two illustrated) is subject to the trade-off in addi-
tional cost versus diminishing recovery for each addi-
tional steps. At present, the preferred batch operation
uses four contacting and decanting steps in total.
Many changes and modifications may occur to
those skilled in the art upon studying this disclosure.
All such changes and modifications that fall within the
scope of the invention as defined by the appended claims
are intended to be included within its scope.
-32-