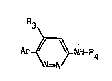Note: Descriptions are shown in the official language in which they were submitted.
Pyridazine derivative~ have been proposed a~
drugs for many years.
In a large number of case~, the~e are sub~tance~
which are active on the cardiova~cular sy~tem and have in
06 particular a hypoten~ive or vasodilative efect, in other
cases, an ~ntiinflammatory and analgesic action ha~ been
mentioned for pyridazine derivative~.
Finally, French patents 2 141 697, 2 610 997 and
2 510 998 di~clo~e pyridazine derivatives which are
variously ~ub~tituted on the pyridazine ring and all
carry, in the 3-po~ition, an amino sub~tituent of the
typ~
-NH-alkyl-N
y
in which X and Y independently are hydrogen or an alkyl
group or form~ with the nitrogen atom to which they are
bonded, a heterocycle such as morpholine.
All the~e compounds are active on the central
nervous sy tem a~ antidepres~ant~.
According to the pre~ent invention, it ha~ now
been found that by modifying the nature and~or position
26 of the substituents on the pyridazine ring, compound are
obtained which have lo~t their antidapre3sant activity
and ac~uired a valuable activity a~ M~-type muYcarinic
cholinergic ligand~
According to a fir t feature, the pre~ent inven-
tion relate~ to novel pyridazine derivative~ of thegeneral formula
R3
36 Ar_~ NH~R4 ( I~
s~
in which
- Ar is a group
R ~
Q5 R2
a pyridyl or a thienyl,
- R1 and Rz independently are each hydrogen, a halogen
atom, a hydroxyl group, a trifluoro~ethyl group, a C~-
C4 alkoxy group or a C1-C4 alkyl group;
- R3 iæ a C1-C4 alkyl group or a phenyl; and
- R4 is:
1~ - a group
R6
-Alk-N
R~
in which Alk i~ a C1-Ca alkylene group, R6 i~
hydrogen or a Cl-Cs alkyl group and R~ i8 a C1-C4
alkyl group, a benzyl or a C3-C7 cycloalkyl, or R6
and R~ form, with the nitrogen atom to which they are
bonded, a heterocycle selected from morpholine, thio-
morpholine, pyrrolidine, N-methylpi~erazine and
piperidine which is unsub~tituted or substituted by
one or more methyl groups, by a hydroxyl, by a phenyl
or by a benzyl;
- a group
-Alk'
R7
sq;~
in which Alk' is a Cl-c3 alkylene group and R7 i8 a
C1-C~ alkyl group; or
- a group
-A1~' ~
in which Alk' is a~ defined abov0 and substitute~ the
pyridine in the 2-, 3- or 4-position,
with the limitation that R1 and Rz are not simultane-
ously hydrogen when R4 i~ a group (CHz)2NR~Rs,
and to the salts of the compound~ of formula (I) with
mineral or organic acid~.
The compounds (I) according to the invention in
which Ar i~ a group
Rl~
R2
are preferred.
Among these, particularly preferred compounds are
those in which R~ is one of the following group~:
~(CH2)n ~ N or ~~CH2)m
~R6 N
R7
in which R~, R6 and R7 are as defined above for (I~, n is
between 1 and 6, and preferably equal to 2, 3 or 4 and m is between
1 and 3, preferably equal to 1 or 2.
When the compound (I) has an a~ym~etric carbon,
the 2 stereoi80mer~ form part of the invention.
According to ~ second feature 7 the pre3ent inven-
- 4 - ~~5
tion relates to a method of preparing the oompound~ o~
formula (I) which i5 repre~ented by the ~ollowing reac-
tion scheme:
05 IHO R3 ~H
coOC2Hs /CH - CH~
Ar - C-CH2-R3 ~ Ar - C C = O
~1 /
O O H5C20
1 2
R3 OH R3 H
NH2NH2 CH-CH C-C
~ Ar - C\ C = O ~Ar - C C = O
N - N N-N
H H
R3 H R3 H
POCl3 /C~c~ R4NH2 C_C\
- ~ Ar - C C-Cl - ~ Ar - C C-NH-R4
N-N N=N
S (I)
Heatin8 the ketone 1 with ethyl glyoxylate at a
temperature of between 80 and 140C gives the hydroxy-
ketoester 2. The crude reaction mixture is then taken up
in an inert ~olvent such as n-butanol, and hydrazlne
hydrate is added. Refluxing for 24 hour~, give~ the
hydroxypyridazinone ~, which, when heated in an acid
medium, yields the 2H-pyridazin-3-one ~ by dahydration.
Heating the latter with excess pho~phoru~ oxy-
chloride gives the 3-chlorop~ridazine 5. The reaction i~
;~$~
carried out without a solvent or in the pre~ence of an
inert ~olvent such as acetonitrile.
Finally, heating the chlorine derivative 5 with a
large exces~ of amine R4NHz at a temperature of between
05 100 and 150C~ in the presence of a ~mall amount of
ammonium chloride, yield~ the compound ~I). The reaction
i~ carried out without a sol~ent or in an inert solvent
such as n-butanol. The product (I) i~ i~olated by ex-
traction and purified by chromatography.
If desired, the resulting ba3e can be converted
to a ~alt by a known method and e~pecially by reaction
with an equimolecular amount of acid in a ~uitable ~ol-
vent.
The compounds (I) in which Ar i~ a hydroxyphenyl
or dihydroxyphenyl group are prepared from the corre~pon-
ding compounds (I) in which Ar i~ a methoxyphenyl or di-
methoxyphenyl ~roup by demethylation in an acid medium.
The following Example~ illustrate the preparation
of compounds of formula (I) without implying a limita-
tion.
EXAMPLE 1
3-[(N-Ethylpyrrolidin-2-yl)methylamino]-5-methyl-
6-phenylpyridazine (SR 96185)
(I) R1 = R2 = H R3 = C~3 R4 = -CH2 n
N
C2H5
A) 3-Chloro-5-methyl-6-phenylpyridazine
l Ethyl 2-hydroxy-3-methyl-4-phenyl-4-oxobutyrate
A mixture of 13.4 ~ of propiophenone and 15.3
of ethyl glyoxylate i8 heated at 135C for 5 hour3~
The resultin~ product is u~ed as ~uch for the
next operation.
- 6
2 5-Methyl-6-phenyl-2H-pyridazin-3-one
The crud0 product obtained above i~ di3~01ved in
150 ml of n-butanol, 9 44 ml of hydrazine hydrate are
then added and the mixture is refluxed for 24 hours.
05 Part of the n-butanol i~ di~tilled at ordinary
pressure in order to remove the water formed in the
reaction as an azeotrope. The mixture is then concen-
trated to dryness under vacuum. The residue i8 taken up
in a mixture of 100 ml of acetic acid and 10 ml of con-
centrated hydrochloric acid. The mixture i~ heated at
100C for 4 hours. The ~olution i8 poured into cold
water and left to crystallize.
The solid is filtered off and dried.
Weight: 11.6 g M p : 218C.
3. 3-Chloro-5-methyl-6-phenylpyridazine
50 ml of pho phorus oxychloride are added to 12 g
of the pyridazinone obtained above and the mixture i~
heated at 80C for 4 hour~.
The mixture is poured 510wly on to ice and ren-
dered alkaline with a 20% solution of sodium hydroxide.
The precipitate is filtered off, wa~hed copiously
with water and recry~tallized from i~opropanol.
9.9 g of the expected product are obtained.
M.p.: 122C.
B) SR 96185
A mixture of 2 g of the chlorine derivativ~
obtained above, 5 g of 2-aminomethyl-1-ethylpyrrolidine
and 0.5 g of ammonium chloride i8 heated at 130C ~or 3
hours under an inert atmo~phere. The reaction mixture i~
poured into water and extracted with ethyl acet~te. The
solution is dried and the solvent i~ evaporated off to
dryness. The re~idue i~ chromatographed on a 3ilica
column. Elution with an ethyl aoetate/methanol mixture
(95/5 vol/vol) give3 an oil, which cry~tallizes from a
small ~uantity of ethyl acetate.
~ 7 ~ 2~
Weight: 2 ~ M~p.: 92C.
EXAMPLE 2
3-(2-Diethylaminoethylamino)-5-methyl-6-(4-
05 methoxyphenyl)pyridazine dioxalate (SR 96194 A)
~C2H5
tI) Rl = -OCH3(4), R2 = H, R3 = CH3, R4 =-CH2CH2N
C2H5
A) 3-Chloro-5-methyl-6-(4-methoxyphenyl)pyridazine
1. Ethyl 2-hydroxy-3-methyl-4-(4-methoxyphenyl)-4-oxo-
butyrate
A mixture of 82 g of 4-methoxypropiophenone and
76.6 g of ethyl glyoxylate is heated at 135C for 15
hours.
The resulting product is u3ed as such for the
next operation.
2. 5-Methyl-6-(4-methoxyph0nyl)-2H-pyridazin-3-one
The crude product obtained ahove i~ di~olved in
250 ml of n-butanol, 38 g of hydrazine hydrate are then
added and the mixture is refluxed for 24 hours.
Part of the n-butanol is di~tilled at ordinary
pressure in order to remo~e the water formed in the
reaction as an azeotrope. The mixture i5 then concen-
trated to dryne~s under vacuum. The residue is taken up
in a mi~ture of~460 ml of acetic acid and 50 ml of con-
centrated hydrochloric acid. The mixture is heated at
100C for 4 hours. The ~olution i~ poured into cold
water and left to crystallize.
The solid i~ filtered off and dried.
Weight: 40 g M~p.: 216C.
3. 3-Chloro-5-methyl-6-(4-methoxyphenyl)pyridazine
2~0 ml of phosphorus oxychloride are added to
10 g of the pyridazinone obtained above and the mixture
Z~?5q~
is heated at 80C for 10 hours.
The mixture is poured ~lowly on to ice and ren-
dered alkaline with a 20% solution of sodium hydroxide.
The precipitate is filtered o~f, wa~hed copiou ly
05 with water and recrystallized from methanol.
9 g of the expected product are obtained.
M.p.: 132C.
B) SR 96194 A
A mixture of 2.3 g of the chlorine derivative
obtained above, 5 g o~ N,N-diethylethylenediamine and
0.6 g of ammonium chloride is heated at 135C for 4 hours
~nder an inert atmo~phere. The reaction mixture is
poured into water and extracted with et~yl acetate. The
solution is dried and the solvent is evaporated of to
dryness.
Wei~ht: 2.3 g.
n~Q~La~
The above base is dissolved in hot isopropanol~
and a solution of 2.2 equivalents of oxalic acid in the
minimum quantity of boiling i~opropanol is added. The
dioxalate precipitates on cooling. It is filtered off
and recrystallized rom ab~olute ethanol.
M.p.: 136C.
EXAMPLES 3 T0 80
A) The 3-chloropyridazines collated in Tables 1 and 2
below are obtained by followin~ the procedure indicated
in Example lA, but varying the starting ketone:
- 9 - ~
TABLE 1
5 6 R3
4 `~r C 1
05 R1 N N
3 2
R1 _R:~ Phy~3ical
con~tant~
.
C1(4) H CH3 M.p.: 178-180C
Cl(4) H CH~CH3 M.p.: 86C
Cl(2) Clt4) CH3 M.p.: 116C
C1(3) H CH3 M.p.: 95C
CF3(3) H CH3 M p.: 120~C
F(4) H CH3 M.p.: 153C
CH3(4~ H CH3 M.p.: 165-166C
Cl(4) H CH~CH2CH3 M.p.: 95C
OCH3 ( 4 ) H CH2CH2CH3 M . p .: 68-69 C
H H CH;3 M . p .: 123- 124 C
H H phenyl M . p .: 1 15 C
Cl(3) Cl(!ij CH3 M.p.: 104C
Cl(3) Cl(4~ CH3 M.p.: 167C
H H CHxClH2CHs M . p .: 60 C
OCU3 ( 2 ) _ CH3 M . p . 74 C
- 10 ~
TABLE 2
R3
05 Ar ~ Cl
Ar Rs Physical constant~
Pyrid-2-yl CH3 135C
Pyrid-3-yl CH3 142C
Thien-2-yl CH3 99C
B) The compounds (I) collated in Tab~ 8S 3 and 4 are ob-
tained from the chlorine derivative~ of Example~ lA, 2A
and 3A by ollowing the techni~ue of Example lB, varying
the amines used. These compounds are characterized by
their meltin~ ~oint (M.p.) or their optical rotation
20 ~a]~.
, , .
~s~
TABLE 3
6 R3
05 4 ~ ~i ~ NH-R4
Rl N=N
3 2
Ex SR ref. Rl R2 R3 R4 Salt or base
n n M.p. or [a]~
3 96181 H H(CHz3z-CH3 C~2 _~ Ba~e 94C
C2H5
4 96198 Cl(4) H CH3 .. .. Ba~e 90C
96222 Cl(2)Cl(4) CH3 .. .. Base 113C
6 46004A CFs(3) H CH3 ~, .. Dihydro-
chloride
0.5H~0 180C
tdOno)mpo~i-
7 96204A H H CH3 CH2~ ~ Dihydro-
N ~ 2h21ocide
8 96240A Cl(4) H CH3 ~, .. Dihydro-
. chloride
222-223C
9 46005A Cl(3) H CHa .. .. Hydro-
chloride
lHzO 182C
45991A Cl(43 H CHzCH3 " .. Dihydro-
chloride
. 21~-217C
- 12 -
ll 96220A H H CH3 CH2 ~ Dihydro-
_N ClHlOrlod9C
05 12 96205A H H CH3 (CH2)2 ~ Dihydro-
N chloride
. lHzO 133C
13 96239A Cl(4) H CH3 ,. .. Dihydro-
chloride
224C
14 46035A Cl(3) H CH3 CH2 n Dihydro-
N ClHlOrlde2C
C2H5
46079A Cl(4) H CH2CH3 .. Fu6m3arla65~ec
16 96193A oCH3(4) H CH3 CH2CH2-N 0 Dihydro-
V 2hl900cide
2~
17 96197A Cl(4) H CH3 .. .. Dihydro-
chloride
0.5HzO 247C
18 96223A Cl(2) Cl(4) CH3 .. .. Dihydro-
250C (de-
composition)
19 45964A CF3(3) H CH3 ., .. Dihydro-
chloride
2H20 183C
45944A Gl(3) H CH3 .. ,. cDhhloYrdrde
1.5HzO 212C
21 46179A Cl(3) Cl(5) CH3 ., " Dhhlyrride
lHzO 198C
- 13 - ~ S~3~
22 46197A Cl(4) H CHzCHaCH2-c~J2-N ~ Difumarate
~ 168-17~C
05 23 46222A F(4) H CHa " .. Dihydro-
chloride
217-219C
24 46223A CH3(4) H CH3 ,. ,. Fumarate
165-167C
46224A Cl(3~ Cl(4) CH3 ., ,. cDhhlYodride
~28-230C
26 46405A Cl(4~ H CH3CHz-CHz-N(iPr) 2 Dihydro-
lChlOride
157-160~C
27 46534A Cl(4) H n.CaH7 .. .. Oum5Har20t~
130C
28 46431A Cl(4) H CHaCH2-CH2-N ~ O Dihydro-
~ H 2h26or228Oec
29 46432A Cl(4) H CH3CH2-CH2-N 3 chloride
208-210C
~
46514A Cl(4) H CHaCHz-CH2-N Dihydro-
chloride
172-174C
31 46637ACl(4~ H n.C3H7 ll ll Dihydro-
chloride
lHzO 205C
32 46636A H H n.C3H7 ll ll Dihydro-
chloride
lHzO 207C
- 14 -
33 96224A Clt4) H C~l3 CH2CHz-N 1D3loxclate
C2H5
34 96230A Cl(2) Cl(4) CH3 " .. Dioxalate
05 lHzO 106C
96194A OCH3(4) H CH3 ., " Dio~alate
136 C
36 96266A Cl(3) H CH3 " ., Dioxalate
136C
~CH3
37 96232A Cl(4) H CH3 CH2CH2N Dihydro-
. CH3 c2h58or259ec
38 46010A Cl(4) H CH2CH3 " " Dihydro-
chloride
0.3H~0
. 223-22~C
39 46081A F(4) H CH3 " " Dihydro-
chloride
0 25H20
~0 . 233-235C
46082A CH3 (4) H CH3 ., " Dihydro-
chloride
. CH3 235-237C
41 45960A Cl(4) H CH3 (cH2)3-N Dihydro-
CH3 chloride
256-258C
42 46080A Cl(4) H CH~CH3 " " Dihydro-
chloride
0.5H20 202C
CH3
CH3 ~
43 46731A Cl(4) H CH3 CH2-CHz-N \ Fumarate
~ 213-215C
CH3 CH3
44 46730A H H n. C3H7 " " 2 5 Fumarate
193-195C
- 15 - ~ 3 ~
~45 96231A Cl(4) H CH3CHzCHz~H-CHa OlHXzala~e
05 /__~
46 46961A Cl(4) H CHa(CH233-N O Dihydro-
\_/ 2hQ-25i2deC
/CH3
47 45988A Cl(4) H CH3(cH2)4-N Dihydro-
\CH3 chloride
244-246C
48 45989ACl(4) H CHzCH3 ,l ,l Dihydro-
2HhlOride
214-216C
49 46377A H H n. C3H7( CHz~z~CH2C6H6 Dihydro-
Pr chloride
0.5H20
5Q 46532A H H n.CaH7CH2-CH2- 18~-184 C
N ~ 152C
\/
51 46728ACl(4) H CHa (CH2)z- Dihydro-
~ chlorido
-N -CH2C6H5 0.5H20
\_ 224-226C
52 9629QA H H CHaCH2-CH~-N S Diox~late
~ 181 C
.
2~ 5~.
- 16 -
53 96291 H H C~3 (C~z)z- Ba~e 98C
-N N-CH3
0~
54 46352A H H n.CaH7 CHz-CHz-N(iPr)z Fumarate
156-157C
46359A H H ,. (CHz)z-~CHzCH3 Fumarate
(~Hz)3CHa 113~114C
56 46378A H H .l (CHz)2 N(nBu)z Fumarate
57 46533A H H " (CHz)z-N(nPr)z Fumarate
109-110C
58 46180Cl(3) Cl(5) CH3 CH2 ~ Dihydro-
N chloride
C2H5 192C
59 46195Cl(4) H CzH~ CH2 / N Fumarate
-/ 180-182C
46196CH3(4) H CH3 CH2- ~ Ba~e 106C
C2H5
61 96268Cl(4~ H CH3 C(+2) ~ a~ 65
N (2% CHCl3)
C2H5
62 96269Cl(4) H CH3 CH2 ~ Yallow oil
C2H5 (2% CHCl3;
- 17 -
63 96272 H H n.C3H7(3 D;.hydro-
. 218C
64 46433 H H n.CaH7CH2- ~/ a22 = +68.6
C2H5 ~HCl3)
46434 H H n.C3H7( ~ a2l = _70o
C2H5 CHCl3)
66 46430 F(4) H CH3CH2 ~ Dhhlydrride
C2H5 206C
67 46514 Cl~4) H CH3 r-~ Dihydro-
CH2-CH2-N ~ chloride
\ ~ lH20 174C
B8 46636 H H n.C3H7 / ~ Dihydro-
. CH2-CH2-N ~ chloride
~ lH20 207C
69 46637 Cl(4) H n.C3H7CH2-CH2-N ~ chlorid~
~ lH20 205C
~7046 ~ U H ~ C~Hc~2~3 ~ He lf~=~r~te
2~5~
¦71 47186 H H C~H6 CH2- ~ Fumarate
C2~15
05 .
72 47226 H H CsH~ CH2-CH2- ~ Se~qui-
CH3 f1~1acate
73 96305OH(2) H CH3 CH2 ~ 0.3H20
C~Hs
74 96306ACl(4) H CH3 CH3 0 DchhlYdide
0 5Hz3
96307ACl(4) H CH3 CH2CH2N ~ 0179Hocate
76 96308A H H CH3 CH2CH2N ~ Hydro-
B chloride
. _ 196C
- 19 --
TABLE 4
R3
Ar ~ ~ NH-Alk-N
05 N_N R6
~x. SR ref. Ar R~ ~R6 Salt or base
n' Alk- ~ M.p.
7746457A Pyrid-2-yl CH3CHz-CHz-N(iPr)z Fumarate
1~ 7846578A Pyrid-3-yl CHa \ Fumarate
7946640A Pyrid-2-yl CH3CH2-CH2-N ~ 146C
8046641A Thien-2-yl CH3 .l .l Dihydro-
~ ~ 0.5HzO
The product~ acoording to the invention were
studied or their pharmacological propertie~ and in par
ticular for their affinity for the mu~carinic choliner~ic
receptors.
In vitro, the compounds (I) were te~ted according
to the technique de~cribed by Wat~on J.D. et al. (Life
Science, 1982, ~1, 2019-2029) for their activity on the
Ml-type receptor~, and according to the techni~ue de3-
cribed by Hammer R. et al. ~Nature, 1980, 2~, 90-92) and
Hulme E.C. et al. (Molecular Pharmacology~ 1978, 1~, 737-
3~ 750) for their activity on the M2-type receptor~
~d~ 5~
- 20 -
The compounds according to the inventlon ha~e a
good affinity for the Ml-type receptor~ and a marked
specificity for the Ml-kype central receptor~ campared
with the M~-type peripheral receptors.
05 By way of example, the compound SR 96181 ~howed a
50% inhibitory concentration, expre~ed in micromol, of
0.03 on the ligand or the Ml receptor~ and 0.35 on the
li~and for the Mz receptor~.
Likewi~e, the compound SR 96204 A showed 50% in-
hibitory concentrations of 0.75 and 50 on the ligand for
the Ml and M2 receptor~ re~pectively.
In vivo, the compounds according to the invention
were 3ubjected to the te~t for the rotation~ induced by
pirenzepine, de3cribed by Wormæ P. et al. (Psychopharma-
cology, 1987, ~3, 489-493), with the modification that
oral admini~tration of the product~ took place 4 hours
before instead of 30 minutes before the injection of
pirenzepine.
At a do~e of 3 mg per kg of body weight, the
product~ according to the invention stron~ly inhibit the
number of rotation~ induced by pirenzepine.
Thus, by way of example, the compound~ SR 96181
~nd SR 96204 A inhibit the rotation~ induced by piren-
zepine to the extent of 71% and 67% respectively.
Furthermore, the re~ult~ obtained with variou~
compound~ of formula (I3 will be found in Table 5.
Thi~ Table ~hows the re3ult3 obtained with the
only compound of French patent n~ 2 510 998 ~ub~tituted
by an alkyl group in the 5-po~ition and described in said
patent a~ an antidepre~ant.
Thi~ compound ha~ the formula
- 21 -
c~3
~ </ ~/- NH - Cll2Cll2N ~ 0
05
Compound A
TABLE 5
Product % inhibition of pirenzepine-induced
n rotations at a dose of 3 mg/kg per os
SR 96197 A -70% ~*
SR 96224 A -59% **
15 SR 96231 A -40% *~
SR 96232 A -40% **
SR 96223 A -74%
SR 96230 A -41% *~
SR 46964 A -3B% ~*
Compound A -20% *
Student t te~t
2 * P < ~.05
5 ** p c o Ol
At a dose of 3 mgfkg per 08, the compound~
accordin~ to the invention inhibit the number of rota-
tions induced by pirenzepine wi-th an inten~ity 2 to 3.5
times greater than the compound o the prior art.
Finally, the compounds accordin~ to the invention
showed no signs of toxicity at the dose~ at which they
are active.
Conse~uently, the compounds (I~ can be used as
drug~.
- 22 ~
The re~ult3 indicated make it pos~ible to con-
sider using the products according to the invention in
all cases where a cholinergic deficiency is Pvident,
especially for the treatment of memorY and cognitive
05 disorders and degenerative syndromes associ~ted with
senescence and with ~enile dementia.
According to another of it~ features, the pre~ent
patent application therefore relates to pharmaceutical
compositions in which at least one of the compounds of
formula (I) or one of their pharmaceutically acceptable salts is
present as the active ingredient.
In the pharmaceutical compo~itions of the pre~ent
invention for oral, sublingual, percutaneous or rectal
admini~tration, the active ingredients of formula (I)
above can be administered to humans in unit forms of
adminiætration, mixed with the conventional pharmaceuti-
cal excipients, especially for the treatment of senile
dementia. Appropriate unit forms of administration
include forms for oral administration, such as tablets,
gelatin capsules, powder~, granules and solutions or
suspensions to be taken orally, forms for sublingual and
buccal administr~tion, form~ for subcutaneous, intra-
muscular or intravenous administration and forms for
rectal admini~tration.
To obtain the desired effect, the dose of active
~principle can vary between 20 and 500 mg per day.
Each unit dose can contain from 5 to 200 mg of
active ingredient in combination with a pharmaceutioal
excipient. This unit dose can b~ administered 1 to 4
times a day.
I~ a solid compo~ition in the form of tablets iæ
prepared, the main active ingredient i~ mixed with a
pharmaceutical vehicle ~uch as gelatin, starch, lactose,
magne~ium stearate, talc, gum arabic or the like. The
tablets can be coated with sucrose or othsr appropriate
S~
- 23 -
substance~ or they can be treated 80 a~ ko have a pro-
longed or delayed activity and 80 a~ to release a pre-
determined a,~ount of active principle continuou~lY~
A preparation in the form o~ gelatin capsules i~
05 obtained by mixing the active ingredient with a diluent
and pouring the re~ulting mixture into 30ft or hard
gelatin capsule~.
WatPr-dispersible granule~ or powder3 can contain
the active ingredient mixed with disper~ant~ or wetting
agent~ or with su~pending agentc ~uch a~ polyvinylpyr-
rolidone, as well a~ with sweetener~ or ta~te corrector~.
Rectal administration i~ effected using ~upposi-
tories which are prepared with binder3 melting at the
rectal temperature, ~or example cacao butter or poly-
ethylene glycols.
Parenteral administration is effected ucingaqueou~ ~u~pen~ion~ otonic 3aline solution~ or ~terile
and inJectable ~olutions which contain pharmacologically
compatible disperAant~ and~or wetting agent~, example~
bein~ propylene glycol and butylene glycol.
The active principle can al~o be formulated as
microcapsule~, if appropriate with one or more excipientæ
or additive~.
A~ pharmaceutical preparation~, it is po~ible to
prepare ths following gelatin cap~ule~:
EXAMPLE 81
SR 96204 A 0.010 g
Lacto~e 0.050 g
Magne~ium stearate 0.005 g
The above ingredient~ are intimately mixed and the mix-
ture i~ poured into hard gelatin cap~ule~.
- 24 -
EXAMPLE 82
SR 96197 A 0.010 g
Lacto~e 0.050 g
Magnesium stearate 0.005 g
05
~0