Note: Descriptions are shown in the official language in which they were submitted.
20101'12
MANGANESE DIOXIDE CATHODE FOR A RECHARGEABLE ALKALINE
CELL, AND CELL CONTAINING THE SAME
FIELD OF THE INVENTION:
This invention relates to rechargeable ~lk:~line cells having m~n~anese dioxide
cathodes. Generally, the cells in keeping with the invention, and for which specific
examples are given, are alkaline m~nganese dioxide-zinc cells; however, other cell systems
5 are contemplated which are cells having alk~line electrolytes and m:~n~nese dioxide
cathodes, and having anodes such as hydrogen, iron, cadmium, mercury, lead, or bismuth.
Indeed, the present invention also is relevant to cells having lithium anodes, m~nganese
dioxide cathodes, and a non-aqueous electrolyte.
What the present invention particularly provides is a cell having an unconstrained
10 rechargeable alkaline m~nganese dioxide cathode. By "unconstrained", it is meant that
unlike many prior art patents, there is no additional wire screen or "cage" which is provided
to specifically mechanically constrain the cathode from growing due to the tendency of
m~nganese dioxide cathodes to swell during a discharge cycle. The cells in keeping with
the present invention may exhibit high drain rate capabilities, as well as long cycle life.
BACKGROUND OF THE INVENTION:
It must always be recognized that when cells having rechargeable m:mganese
dioxide cathodes, with zinc or other anodes as discussed above and noted hereafter, are
assembled in their fully charged state. That is to say, the first cycle to which any such cell
20 is subjected to, in use, is a discharge cycle, after which the cell is required to be re-charged
for subsequent use. This is, of course, in contradistinction to nickel cadmium (Ni-Cd) cells,
which must first be charged before they are capable of being used.
Because any cell in keeping with the present invention -- whether a bobbin cell, or
a coin or button cell -- is subjected to discharge when it is first put into use, and because
25 m:~ng~nese dioxide cathodes (especially in the presence of alkaline electrolyte) have a
tendency to swell during discharge, care must be taken to ensure that the cathode m~int~in~
B
20101~2
its integrity and does not disintegrate or substantially swell in such a manner as to disturb
the internal structure of the cell, thereby rendering it ineffective for further use. That, of
course, is what happens or may happen generally if primary alkaline m~ng~nese dioxide-
zinc cells are subjected to a charge cycle following discharge.
Generally, for cells in keeping with the present invention, the m~ng~nese dioxide
cathode -- which is specifically the subject of the present invention and which is discussed
in greater detail hereafter -- is provided together with an anode, a separator, and an
electrolyte, all in a suitable container, and sealed therein by a suitable closure. In general,
the electrolyte is 6N KOH to 12N KOH. The anolyte -- which is essentially electrolyte
which is used for formulating the anode -- is generally also 6N KOH to 12N KOH, but
may have zinc oxide dissolved in it in an attempt to reduce corrosion of the zinc metal of
the anode, and so as to provide an overcharge reserve for the cell.
The separator which is used between the cathode and the anode is generally a
cellulose, non-woven material, which may optionally have a fibre structure in it or
associated with it for reinforcement.
When the anode is zinc, it is generally a zinc powder mixed with a gelling agentwhich may be such as NaCMC. Corrosion inhibitors such as mercury, lead, cadmium,indium, gallium, and thallium, may also be included in the anode formulation, in an attempt
to reduce hydrogen gassing within the cell.
For a more complete understanding of the present invention, some discussion
follows with respect to the characteristics of a zinc anode -- as being the most typical anode
used in commercial cells embodying a m~ng;mese dioxide cathode -- and with respect to
certain characteristics of m~ng~nese dioxide when used as a cathode.
First, having regard to a zinc anode as discussed above, it is noted that during the
first few cycles of a rechargeable cell, a certain portion of the active zinc mass may become
inactive. Typically, a gelled cylindrical anode has a central current collector (the nail)
placed down its centre, particularly in such as cylindrical or bobbin-type rechargeable
m~ng~nese-zinc cells, and the anode may have in the order of 50%-70% by weight
(amalgamated) of zinc powder. Electrically conductivity within the gelled anode is
established throughout the contact of the individual metallic zinc particles within the anode
B
201Q142
(the so-called zinc chain). However, as discharge of the cell proceeds, the highly
conductive zinc particles are oxidized to become non-conductive ZnO or Zn(OH)2, each of
which is a solid. Later, the zinc oxide or zinc hydroxide may dissolve to form zincate ions.
However, after the electrolyte in the neighbourhood of the metallic zinc particles is locally
5 saturated with zincate the compounds no longer dissolve and the discharge reaction will
stop due to passivation of the anode. [This is particularly discussed in Falk and Salkind,
"Alkaline Storage Batteries", published by John Wiley & Sons, 1969, at pages 156-159.]
When the cell is recharged, zinc is replated in the anode, initially near the nail or
current collector, but the conductive zinc chain which originally existed can no longer be
10 completely be re-established without a significant overcharge of the cell. The addition of
conductive additives which do not participate in the discharge and charge reaction will
remedy this situation, and is contemplated in Unites States patent 5,164,274 issued
November 17, 1992, in the names of Kordesch, Sharma and Tomantschger.
As to the m~ng~nese dioxide, Falk and Salkind (above) at pages 180-182, describe15 the discharge reaction of m~ng~nese dioxide in alkaline solution. The discharge reaction
is quite complex, and may proceed in various steps. It is now generally accepted that the
mechanism proposed by Kozawa best describes the discharge of m~ng~nese dioxide
("Batteries", Volume 1, Manganese Dioxide -- edited by Kordesch -- chapter 3). The MnO2
discharge curve has a sloping characteristic, indicating a homogenous phase reaction. The
20 potential of the MnO2 changes continuously while protons origin~ting from the water of the
electrolyte are introduced into the ionic lattice of the m~ng~nese dioxide, according to the
equation;
MnOz + H2O + e' = MnOOH + OH' (Equation 1)
However, the MnO2 lattice expands, and at a certain point during the discharge, the
25 mechanism changes. After that time the discharge occurs in a heterogenous phase reaction,
according to the reaction;
MnOOH + H2O + e' = Mn(OH)2 + OH' (Equation 2)
This second reaction step involves the dissolution of MnOOH in the form of
[Mn(OH)4]', with electrochemical reduction on the graphite in the m~ng~nese dioxide
30 cathode to [Mn(OH)4]", and the precipitation of Mn(OH)2 from it.
2010142
Reference is made to a further copending application assigned to the assignee
hereof, in the name of Kordesch, Gsellman, and Tomantschger, which discusses a practical
approach to the problem of loss of capacity of m~ng~nese dioxide cathodes, beingapplication Serial No. 609,955, filed August 31, 1989. In that invention, it is proposed that
5 the m~ng~nese dioxide material be pre-conditioned to have an oxidation state at the time
that the cell is finally assembled and sealed between 1.70 and 1.90.
It should also be noted that MnO2 which has been discharged in its homogenous
phase may be recharged. However, any Mn3O4 that is formed during discharge is not
capable of being recharged. The Mn(OH)2 noted in equation 2 above, may be re-oxidized
10 to become Mn3O4. There is no evidence that MnOOH is to be found in a discharged
msln~;~nese dioxide cathode.
The discharge of MnO2 is also discussed in, for example Dzieciuch et al U.S. Patent
4,451,543 where it is suggested that MnO2 may be rechargeable to the two electron level.
There, it was found that MnO was reduced in a homogenous phase to MnOl 6, thereby
15 forming an alpha MnOOH (groutite) having a gamma structure. Beta MnO2 (chemical
m~ng;~nese dioxide -- CMD) was only reduced homogenously to about MnOI 96 or MnOI 98.
Boden et al, J. Electrochem. Soc. 114, at 415 (1967) confirm that the discharge of
EMD is a homogenous phase discharge, but they postulate an amorphous intermediate.
This was because the internal resistance was found to rapidly increase with MnO, 6, and that
20 it reached a ten-fold value of MnO2 at about MnOl 4.
Euler, in Electrochimica Acta 15, at 1233 (1970) studied commercial battery
electrodes, and revealed the influence of conductivity of the cathode mix and electrolyte
penetration. This is complicated, however, by the ability of MnO2 cathodes to recuperate
from an homogenous phase discharge. This suggests therefore, that there are potential
25 gradients within the m~ng~nese dioxide cathode, under load conditions; and this suggests,
therefore, that rechargeable MnO2 electrodes may have been locally over-discharged.
One further problem that develops generally in m~ng~nese dioxide cathodes is thepossible migration of the zincate from the anode to the cathode. Zincate ions can be
transported to the m~ng~nese dioxide cathode and there they form a mixed oxide,
30 hetaerolite (ZnO.Mn2O3). The hetaerolite irreversibly affects the behaviour of a manganese
B
20101~2
dioxide cathode. It has been particularly recognized by Kordesch et al in Electrochimica
Act 25 (1981) at 1495 to 1504 that the longevity of a rechargeable alkaline m~ng~nese
dioxide cathode in its homogenous discharge phase above about MnO, 55 was limited by the
mechanical failure of the electrode (cathode). It has been well shown that a m~ng~nese
5 dioxide cathode expands during discharge and contracts during charge. Kordesch et al have
shown that cycling an unconfined m~ng~nese dioxide cathode through four discharge-
charge cycles resulted in the thickness of the MnO2 electrode becoming more than double
its original thickness, and that the electrode failed due to the bulging and the mechanical
disintegration which occurred. This was notwith~t~nding the fact that a binder (in this case,
10 polysulfone) was employed.
Kordesch et al also demonstrated that if a similar electrode was confined by a
perforated disc, the confined electrode continued its cycling life well beyond the fourth
cycle; and that the change in dimension between the charged and the discharged electrode
was only about half of that which occurred in the unconfined electrode. It was
demonstrated that a mounting pressure of about 250-750 N/cm2 was required to increase
the cycle life from less than about 5 cycles -- shown, above, to be because of poor
conductivity and mechanical disintegration -- and to achieve at least a cycle life of 75
cycles. A peak of 92 cycles was found at 500 N/cm2. However, it was also found that at
higher mounting pressure the cycle life would drop because of the loss of pore volume
within the m~ng~nese dioxide cathode, thereby creating problems with respect to electrolyte
penetration within the cathode.
When the m~ng~nese dioxide cathode is present in the form of a sleeve or a disc,additional difficulties may arise. The internal resistance of the electrode may increase, and
the mechanical disintegration of the cathode may be particularly severe. Kordesch, in
"Batteries, Vol. 1" at pages 201 to 219 discusses these problems. Several prior art
references show attempts to preclude the expansion of a m~ng~ese dioxide cathode during
discharge and, indeed, to try to prevent its contraction during charge, including such matters
as the addition of a binder such as a cement (U.S. Patent 2,962,540); the addition of
graphitized textile fibres (U.S. Patent 2,977,401); the addition of latex binders (U.S. Patent
3,113,050); use of combination binders such as cement and steel wool (U.S. Patent
20101~2
3,335,031); and the use of supplementing binders as described in U.S. Patent 3,945,847.
None of those patents, however, could preclude the mechanical disintegration of the
cathode, ~pal~lllly due to the limited binding strength of the materials being used.
Kordesch and Gsellmann in U.S. Patent 4,384,029 teach cylindrical "bobbin" cells5 which may use mechanical enclosures such as tubes, springs, mechanical wedges, and
perforated cylinders, to preclude expansion of the cathode during discharge of those bobbin
cells. What this patent attempts to do is to create a constant volume cathode, which means
that the cathode must always be under a certain mounting pressure at all times. That patent
suggests that by increasing the mounting pressure, the number of usable cycles for the cell
10 will increase. By providing the metal cage, which is essentially rigid, the tendency of the
cathode to swell creates internal pressure within itself, which acts against the metal cage
and between the cage and the can, thereby counteracting the tendency to swell; and by
m~int~ining the cathode under pressure, it m~int~in~ a substantially constant volume during
discharge as well as charge.
A different approach, using combinations of binders with a mechanical retainer or
multiple mechanical retainers is disclosed in a further application assigned to the present
assignee, in the name of Kordesch, Gsellmann and Tomantschger, being application serial
no. 571,629, filed July 8, 1988.
What must be recognized is that, while it is shown that the use of means particularly
20 such as the cages of the two Kordesch et al patents noted immediately above provides for
a structure having up to several hundred cycles, there are several disadvantages to be
considered. Particularly, where cement or other non-conductive binders are used, they may
be present in the range of typically 5%-10% by volume of the cathode, and therefore the
amount of active ingredients that can be placed in the cathode is reduced. This results in
25 a decrease in the usable capacity of the cell, and it may also result in a decrease in the
conductivity of the cathode of the cell. On the other hand, if an insuff1cient amount of
binder content is used, typically the m~ng~nese dioxide cathode may tend to crumble and/or
crack, so that a coherent electrode is not achieved and its integrity is seriously affected.
B 6
2~10142
If mechanical structures such as cages or screens are employed, then there is a
significant increase in the material costs of the cell, as well as a significant increase in the
cost of assembly of the cell. Indeed, there may be a significant effect and complication
with respect to the use of high speed production equipment. Moreover, the use of a
5 mechanical component such as a perforated iron cage or plate may significantly increase
the probability of cell gassing within the cell.
Still further, the use of the mechanical cage or screen adjacent to the separator of
the cell may significantly affect the capability of the cell to operate in a high drain
condition. Any mechanical means which restricts the electrode interface between the
10 cathode and the anode will act to limit the current density achievable within the cell.
PURPOSES OF THIS INVENTION:
The present inventors have found that, quite unexpectedly, the mechanical integrity
of a cathode can be m~int~ined during cycling, without the necessity for any physical
15 confinement of the m~ng~nese dioxide cathode, and without the necessity of a binder to be
present in the cathode mix. That means, therefore, that the present invention provides a
cell having an unconstrained m~ng~nese dioxide cathode.
However, the present invention achieves its purpose of providing an unconstrained
cathode by a variety of ways. It may achieve that purpose by restricting the cathode from
20 significantly ch~nging its dimensions during discharge by, essentially, filling the entire
volume within the cell intended for the cathode and thereby leaving essentially no void
above the cathode between the top of the cathode and the closure of the cell. The invention
may also achieve its purpose by providing a cathode which may have admixed thereto a
small quantity of fibres, usually conductive fibres. In yet another approach, the cathode
25 may have admixed thereto a small amount of a metal-based additive which, surprisingly,
may be zinc, zinc oxide, or zinc stearate. Various examples will be discussed hereafter,
showing one or more of the above approaches.
2 7
L~
2010142
Indeed, the present invention has shown that, although there may be changes in the
dimensions of the cathode during cycling -- expansion during discharge, contraction during
charge, as noted above -- and that while the overall size of the cathode may slowly increase
as the number of cycles which the cell has been subjected increases, the present invention
S does provide a cathode for use in cell which exhibits at least equivalent operating
characteristics, or better operating characteristics, than any of the prior art cells which
employ binder materials or mechanical components as noted above.
It is determined that with an unconstrained cylindrical or sleeve-type cathode in a
cylindrical or bobbin cell, and a disc cathode in a coin or button cell, the cathode structure
10 may be subjected to a variety of forces as the cell is being cycled. Particularly during
discharge, the tendency of the cathode to swell is inhibited by the metal can or container
within which the cell is assembled. It appears that the tendency for the cathode of a bobbin
cell to expand inwardly towards the anode is significantly reduced, which may in part be
due to the sleeve geometry and due to the presence of the separator and the cylindrical
15 anode within it. [In a coin or button cell, the tendency for the cathode to expand upwardly
towards the anode appears likewise to be reduced.] What also appears to be the case as
that the tendency for the m~ng~nese dioxide cathode to swell may be substantially linear
in all directions; and since the cathode is significantly longer in length or higher than its
thickness or the width of its annulus, the expansion of the cathode during discharge will
20 for the most part be longitudinal -- that is the height of the cathode will increase
significantly more than the width or thickness of the annulus, and the amount of that
increase is in some way related to although it may be greater than the ratio of the initial
condition height and width of the cathode.
However, in keeping with the present invention, there is substantially little void
25 space within the cell can above the cathode and between it and the cell closure member,
so that to all intents and purposes the cathode is restricted from significantly ch~n~ing its
dimensions during discharge by interference at its outer periphery, and at its bottom, with
the cell can or container; and by interference at its inner periphery with the separator of the
cell; and by interference at its top with the closure member for the cell.
L~ 8
l~
20101~2
As noted above, the present invention contemplates that a further improvement to an unconstrained cathode will comprise the addition of a small amount of fibres to the
cathode mix, usually but not necessarily conductive fibres as discussed hereafter. In that
case, it is possible that there may be a slightly greater void space permitted within the cell;
5 and in any event, the mechanical disintegration of the cathode is substantially precluded.
Examples will be shown of cells having cycle life which has heretofore only been possible
by the use of mechanical structural components such as cages, or by the use of inorganic
binders such as cement, or by the use of organic binders such as PTFE; and of course the
advantages of cells of the present invention are particularly the provision of greater cell
10 capacity and the capability of the cell to operate under high discharge rate conditions -- as
well as decreased production costs.
As noted above, there is always the possibility of migration of zincate from theanode to the cathode in a m~ng~nese dioxide-zinc cell having an alkaline electrolyte. This
results in the formation of hetaerolite within the cathode, materially and irreversibly
15 affecting the behaviour of the MnO2 cathode, and its capability of being recharged.
Unexpectedly, the present invention has shown that by the addition of a metal-based
additive such as zinc, zinc oxide, or zinc stearate to the cathode formulation, the tendency
of the performance characteristics of the cell to fade due to the formulation of hetaerolite
within the cathode is significantly reduced.
BRIEF DESCRIPTION OF THE DRAWINGS:
The figures which accompany the following discussion are representations of typical
cells in keeping with the present invention. In this instance:
Figure 1 shows a typical m~ng~nese dioxide-zinc cell of the present invention in the
25 AA size; and
Figure 2 shows a typical construction of a coin or button of the present invention.
B g
~010142
DESCRIPTION OF THE PREFERRED EMBODIMENTS:
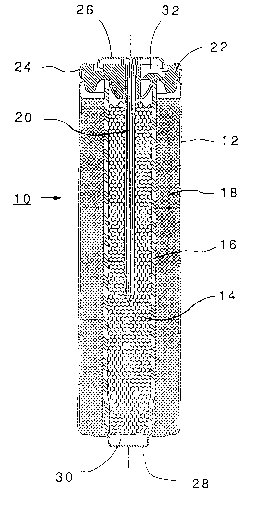
First, having regard to the figures, typical cell structures for a bobbin cell 10 and
coin or button cell 40 are shown. For ease of discussion, similar cell components are
shown having identical reference numerals. Each cell includes a container or can 12, which
5 may be nickel plated steel or any other convenient can of the sort generally used for the
manufacture of primary or secondary cells. Within the can 12 there is an anode 14, a
separator 16, and a cathode 18. Typically, the separator may be a single layer of a
cellulosic, non-woven material or it may be a dual layer having a separate fibrereinforcement and an ion permeable layer.
In the bobbin cell, there is extending downwardly into the anode 14 is a nail orcurrent collector 20, which pierces and extends through the cell closure 22, by which the
cell is sealed as by crimping such as at 24. Typically, the nail or current collector is made
of brass or bronze.
Each cell has a negative cap 26 associated with and in the electrical conductivity
15 with anode 14, either directly or, in the case of the bobbin cell 10, through the nail or
current collector 20. In a usual embodiment of a bobbin cell such as that shown in Figure
1, the positive terminal is formed such as by a pip 28 formed in the can 12; an insulative
washer or cup 30 is placed below the anode 14; and in the embodiment shown, the
separator 16 extends down into the insulative cup 30, which protects the anode from
20 coming into contact with the can 12.
It will also be noted in the embodiment of Figure 1 that the separator 16 extends up
to contact and interfere with the bottom surface of the closure member 22. A relief
membrane 32 is shown moulded into the closure member 22, and it is intended to burst at
a pre-determined pressure in the event of a significant build up of internal gas pressure
25 within the cell.
The coin or button cell 40 uses the can 12 as its positive t~rmin~l; and it is crimped
over the grommet 34 so as to insulate the positive and negative terminals of the cell from
each other.
B lo
2010142
What the present invention provides, therefore, is a rechargeable electrochemical cell
having a container 12, an anode 14, a separator 16, and a m~ng~nese dioxide cathode 18.
There is an ion conductive electrolyte present within the cell, providing the ion transfer
medium for current to flow between the cathode and the anode, and a closure member 22
or grommet 34 which is also a closure member. As noted, all of the internal components
are sealed within the container.
[As discussed, the usual embodiments of the present invention contemplate the use
of aqueous alkaline electrolyte. However, non-aqueous, non-alkaline electrolytes may be
used is some circumstances, but within the ambit of and other wise in keeping with the
teachings of the present invention -- for example, lithium cells.]
In keeping with one provision of the present invention, the cathode of a bobbin cell
is restricted from significantly ch~nging its dimensions during discharge by interference at
its outer periphery and its bottom with the internal surfaces of the container 12, at its inner
periphery by interference with the separator 16, and at its top by interference with the
underside of the closure member 22. The cathode of a coin or button cell is likewise
restricted by the container 12 and separator 16.
Usually, as noted, the anode may be zinc; but it may in certain circumstances bechosen from any one of the group consisting of zinc, hydrogen, iron, cadmium, mercury,
lead, bismuth, and lithium.
In general, bobbin cells according to the present invention are cylindrical, having
the cathode in the form of an annulus or a series of rings or pellets, and a cylindrical anode
axially placed within the cathode. Coin or button cells have both the cathode and anode
in the form of a disc or wafer.
It is usual, and will be shown in examples below, that the cathode may have certain
additives admixed to its formulation. In general, from about 4% to about 8% by weight
of the cathode is the alkaline electrolyte -- generally 6N KOH to 12N KOH.
Still further, in general the cathode will contain a small amount of graphite --usually in the amount of from about 5% to about 15% by weight of the cathode -- to
increase the electrical conductivity characteristics thereof.
20101~2
Moreover, the cathode may contain a small quantity of conductive carbon such as
carbon black or other equivalent conductive carbon materials, generally in the range of
from about 0.1% to about 10% or as much as 15% by weight of the cathode.
As noted above, a further formulation of the cathode according to the present
5 invention will provide for the addition of a small quantity of fibres to the cathode. In
general, those fibres are conductive, and they may be chosen from the group consisting of
carbon fibres, graphite fibres, carbon fibres plated with nickel, carbon fibres plated with
silver, graphite fibres plated with nickel, graphite fibres plated with silver, copper fibres
plated with nickel, and copper fibres plated with silver. The fibres (which are milled
10 carbon fibres and/or chopped carbon fibres) will generally have a length of from about 100
microns up to about 5 centimeters; and a typical fibres is CARBOFLEX [TM] provided by
Ashland Carbon Fibres of ~hl~n~l, Kentucky. The fibres, especially conductive fibres,
may typically be present in the cathode in the amount of from about 0.1% to about 5.0%
by weight thereof.
In keeping with the present invention, several processes for the addition of fibres
to the MnO2 cathode formulation are considered. In one instance, chemical grade MnO2
(CMD) may be precipitated in a carbon fibre slurry. In another instance, electrochemical
grade MnO2 (EMD) may be prepared in an acidic electrolyte [e.g. H2SO4.MnSO4] where
carbon fibres are suspended in the acidic electrolyte.
As noted above, yet a further embodiment of the present invention is for an
unconstrained cathode having as an admixture thereto a small quantity of metal-based
additive chosen from the group consisting of zinc, zinc oxide, and zinc stearate. Generally,
that metal-based additive may be present in the amount of from about 1.0% to about 5.0%
by weight of the cathode.
It is postulated that the presence of the metal-based additive within the cathode does,
itself, create a specific charge or potential gradient within the cathode. This tends to repel
the likelihood of zincate migration, and this in turn tends to inhibit the unwanted
developments of hetaerolite within the cathode. Thus, the unexpected consequence of the
B 12
20101~2
addition of the metal-based additive to the cathode is that, rather than effectively
"poisoning" the cathode, the metal-based additive acts rye repel the migration of the
polluting elements that would poison the cathode.
The present invention also provides a method of preparing a cathode mix for use
5 in a rechargeable ~lk~line electrochemical cell, where the cell is substantially as described
above. As noted, the cell will comprise internal components which include a cathode, an
anode, a separator, and an alkaline electrolyte; and those internal components are sealed
within the container by a closure member.
Further, as noted, the cathode mix will generally comprise m~ng~nese dioxide,
10 together with from about 4% to about 8% by weight thereof of the alkaline electrolyte --
usually 6N to 12N KOH; and optionally from about 5% to about 15% by weight thereof
of graphite; and optionally from about 0.1% to about 10.0% by weight thereof of
conductive carbon; and optionally from about 0.1% to about 5.0% by weight thereof of
conductive fibres which may be chosen from the group consisting of carbon fibres, graphite
15 fibres, carbon fibres plated with nickel or silver, graphite fibres plated with nickel or silver,
or copper fibres plated with nickel or silver; and optionally from about 1.0% to about 5.0%
by weight of the cathode of a metal-based additive which may be chosen from the group
consisting or zinc, zinc oxide, and zinc stearate.
The method according to the present invention comprises the steps of:
20 (a) mixing the m~ng~nese dioxide and any of the optional admix components to form
a uniform dry mix;
(b) adding the amount of alkaline electrolyte to be used in the cathode composition to
the uniform dry mix, and continuing the blend the mix;
(c) screening the mix to remove agglomerates therefrom, and continuing to blend and
25 screen until a uniform moist blended mix is achieved;
(d) compacting the blended mix;
(e) granulating the compacted blended mix;
(f) screening the granulated blended mix;
(g) forming cathode pellets from the screened blended mix; and
13
B
20101~2
(h) placing the pellets in the appropriate cell containers for use as cathodes in the cells
to be manufactured.
Typically, step (g) of forming the cathode pellets or annular sleeves is carried out
at pressures ranging from about 1,000 Newtons per square centimeter (N/cm2) to about
20,000 Newtons per square centimeter (N/cm2).
The method of the present invention may optionally be followed by a further stepof recompacting the cathode pellet(s), after it (they) has (have) been placed in the cell
container. The recompaction is generally carried out at the same pressure or within the
same pressure range noted above. One or several pellets may be used in a cathode for a
bobbin cell; Figure 1 suggests that three pellets may be used in the cell that is illustrated.
What now follows are a number of examples of various cells manufactured in
keeping with the present invention, whereby various formulations of unconstrained cathodes
have been provided and tested, with the results being given in each instance.
Example 1
In this case, a cathode was provided having a small additional amount of graphite
fibres and a small additional amount of zinc stearate included in the cathode formulation.
A standard anode was provided, and cells were tested, as noted:
The anode composition was as follows:
Zinc 54.4% [6%Hg ~m~lg~m~ted]
ZnO 10.0%
Gelling Agent 1.0%
Anolyte 34.6% [9N KOH with 5% ZnO dissolved in it]
The cathode composition was as follows:
MnO2 82.43%
Graphite 7.88%
Graphite fibre 1.00%
Carbon 0.47%
Electrolyte 6.54% [9N KOH]
Metal-based additive 1.68% [Ni(OH)2]
L~ 14
l~
20101~2
Test results showed that the cells according to the above formulations averaged 375
cycles at a discharge of 420 mAh/day. They were discharged into 24 ohms, and showed
a 14% depth of discharge of the cathode, with a 60% depth of discharge of the anode. The
cells ultimately had anode failure.
Example 2
Here, cells having the standard anode composition noted above were built, and the
additives in the cathode included graphite fibre and metallic zinc. The cathode formulation
was as follows:
MnO2 82.43%
Graphite 7.88%
Graphite Fibre 1.00%
Carbon 0.47%
Electrolyte 6.54% [9N KOH]
Metal-based additive 1.68% [Zn]
The cells were tested as above in Example 1, cycling at 420 mAh per day into 24
ohms. Once again, the cells were discharged to about 14% depth of discharge of the
cathode, and about 60% depth of discharge of the anode; they averaged 375 cycles; and
once again the cells failed in an anode failure.
Example 3
In this case, tests were made to determine the effect of the addition of ZnO to the
cathode formulation, and a slightly different anode composition was used, all as follows:
The anode composition was:
Zinc 60.0% [6% Hg ~m~lg~m~ted]
ZnO 5.0%
Gelling Agent 1.0%
Anolyte 33.5% [9N KOH with 5% ZnO dissolved in it]
B 15
~0101~2
The cathode compositions were as follows:
Test Control
MnO2 80.03% 83.03%
Graphite 9.00% 9.00%
Graphite Fibre 1.00% 1.00%
Carbon 0.47% 0.47%
Electrolyte 6.50% [9N KOH] 6.50% [9N KOH]
ZnO 3.00% 0
It will be noted that the control cells had no ZnO added to the cathode formulation;
10 and that the test cells had 3.00% ZnO added to the formulation with that much less MnO2
content.
The cells were cycled at 500 mAh per day into 10 ohms, and showed a 19% depth
of discharge of the cathode and a 67% depth of discharge of the anode. All cells failed in
anode failure; however, the control cells without the ZnO additive only had a cycle life of
15 35 cycles, whereas the test cells had a cycle life of 75 cycles.
Example 4
In this case, an anode composition as noted in Example 3 was used, and the cathode
had no fibre or other additives but was constructed in a manner so as to substantially fill
20 all of the space allotted to it within the container, with substantially no void space above
the cathode beneath the cell closure.
The cathode formulation was as follows:
MnO2 81.5%
Graphite 12.00%
25 Electrolyte 6.50% [9N KOH]
Here, the cells were cycled at 420 mAh per day into 24 ohms, and were calculatedto have a 45% depth of discharge of the cathode, and a 50% depth of discharge of the
anode. The cells were cycled for 400 cycles, and there was an apparent imminent cathode
failure when the tests were termin~te~l
~2 16
2010142
Example 5
This series of tests was carried out to determine the relative amounts of in-cell
gassing of cells made according to the present invention compared with cells having copper
cages, either uncoated or coated with graphite. In this series of tests, the cathode
5 formulation was identical to that of Example 4, noted above, and the anode composition
was as follows:
Zinc 65.5% [6% Hg ~m:~lg~m~ted]
Gelling Agent 1.0%
Anolyte 33.5% [9N KOH]
Two sets of control cells were made, one having copper cages, the other having the
same copper cages coated with graphite. The test cells were in keeping with the present
invention, and had unconstrained cathodes -- i.e., no cages.
The cells were subjected to 75 deep discharge cycles (or as noted), being discharged
in each instance to 0.9V into 3.9 Ohms. The cage cells exhibited identical electrical
15 performance, and the gassing performance of all cells was observed.
The following were the performances noted of the caged and the test cells with
unconstrained cathodes in keeping with this invention:
Control (Cage) Cells Test Cells
Initial capacity [Ah] 6.0 6.0
Cycle 10 [Ah] 3.3 3.3
Cycle 20 [Ah] 3.0 3.0
Cycle 30 [Ah] 1.0* 2.7
Failure Mode Short N/A
*Two of three cells shorted at this time.
The in-cell gassing was observed, and was noted to be the lowest in the test cells
in keeping with this invention; with the cage cells having coated cages being higher, and
the cage cells having uncoated cages showing the highest gassing activity.
The present invention has been described above and shown in a variety of
Examples. It has been noted that in its widest concept, the present invention provides an
unconstrained MnO2 cathode for use in rechargeable cells, and finds its widest application
in rechargeable cells having aqueous alkaline electrolytes. The invention is applicable to
2010142
bobbin cells and to coin or button cells; and in optional forms the cathode of the present
invention may have admixed to its formulation such items as fibres (usually conductive
fibres), graphite, conductive carbon, and a metal-based additive such as zinc, zinc oxide or
zinc stearate.
S The scope of the present invention is determined by the accompanying claims.
18
B