Note: Descriptions are shown in the official language in which they were submitted.
3~
5-17328/+
Microbicidal comeositions
The present invention relates to microbicidal mixtures having a syner-
gistically increased activity against plant diseases and to methods for
the use of such mixtures, especially in cereals.
The invention relates especially to the control and prevention of
diseases in cereal cultivation.
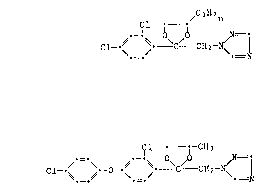
A mixture that has proved unexpectedly favourable is a combination of the
active ingredient component I)7 1-[2-(2,4-dichlorophenyl)-4-propyl-1,3-
dioxolan-2~ylmethyl]-lH-1t2,4-triazole (= propiconazole) of formula I
Cl - / n
Cl~ C - CH2-N\ i (I),
or a salt thereof, with the active ingredient component II), 1-~2~[2-
chloro-4-(4-chlorophenoxy)-phenyl]-4-methyl-1,3-dioxolan-2-ylmethyl}-
lH-1,2,4-triazole of formula II
~ CH3
C1~ 0~ \C/~ C~lz~
or a salt thereof.
The compound of formula I is descrlbed in GB 1 522 657.
- ,
: : : : ~ : .: . . .
.; . .
- 2 - ~ 3~4
The compound of formula II is described as a fungicidal active substance
in GB Patent Application No. 2 098 607. The activity of these two
triazole derivatives is founded especially on the inhibition of
ergosterol biosynthesis in ~he development cycle of phytopathogenic
fungi.
The mentioned salts of compounds I and II can be prepared by reacting the
respective base with acids.
Of the acids that are suitable, the following may be mentioned:
hydrohalic acids, such as hydrofluoric acid, hydrochloric acid, hydro-
bromic acid or hydriodic acid, as well as sulfuric acid, phosphoric acid,
nitric acid and organic acids, such as acetic acid, trifluoroacetic acid,
trichloroacetic acid, propionic acid, glycolic acid, thiocyanic acid,
lactic acid, succinic acid, citric acid, benzoic acid, cinnamic acid,
oxalic acid, formic acid, benzenesulfonic acid, ~-toluenesulfonic acid,
methanesulfonic acid, salicylic acid, p-aminosalicylic acid, 2-phenoxy-
benzoic acid, 2-acetoxybenzoic acid or 1,2-naphthalenedisulfonic acid.
The term "salts" also includes metal complexes of the basic components I
or II. These complexes consist of the fundamental organic molecule and an
inorganic or organic metal salt, for example the halides, nitrates, sul-
fates, phosphates, acetates, trifluoroacetates, trichloroacetates, pro-
pionates, tartrates, sulfonates, salicylates, benzoates etc. of elements
of the second main group, such as calcium and magnesium, and of the third
and fourth main groups, such as aluminium, tin or lead, and of the first
to eighth subsidiary groups, such as chromium, manganese, iron, cobalt,
nickel, copper, zinc etc.. The subsidiary group elements of the 4th
period are preferred. ~he metals may be in any one of their various
valencies. The metal complexes may be mono- vr poly-nuclear, that is to
say they may contain one or more organic molecular components as lig~ands.
Surprisingly, however, it has been found that the combination of active
ingredients I and II results in a quite unexpectedly substantial increase
in activity against seed-, air- and soil-born0 fungi. The increase in
.
.
.
~w~
-- 3 --
activity achieved by the combination according to the invention is
decisively greater than the activity to be expected by adding together
the activities of the two components individually.
The present inventicn relates not only to mixtures of components I
and II, but also to the use of the individual components I and II,
formulated as microbicidal compositions, in direct succession.
Favourable mixing ratios of the two active ingredients are: I:II = from10:1 to 1:10, especially from 5:1 to 1:5, and more especially from 4:1 to
1:4. Other mixing ratios are: I:II = 2:3, 1:3, 1:2, 2:5, 1:1, 5:2, 3:1,
2:1, 3:2 and 2.8:1. The mixing range I:II of from 3:1 to 3:2 is espe-
cially preferred. In this range there is an especially favourable inter-
action of the two active ingredients at total rates of application of
from 100 to 200 g/ha.
The combination of the active components I and II according to the
present invention has a beneficial systemic, protective and curative
action as well as a residual action in the control of seed- and soil-
borne plant diseases. The combinations according to the invention destroy
microorganisms in and on the plant and protect developing plants from
attack by microorganisms.
Compound I, propiconazole, is a commercially known fungicide for
controlling various cereal diseases, such as Erysiphe spp., Puccinia
spp., Pyrenophora spp. (= Helminthosporium spp.), Rhynchosporium spp. and
others.
Compound II is a broad spectrum fungicide for controlling powdery m-ildew
species (Erysiphe spp.), rust diseases (Puccinia spp., Hemiloia etc.),
leaf spot infestation (Septoria spp., Pyrenophora spp.) and others.
Particularly lasting success is achieved in the control of black fungi
such as Alternaria spp. and Cladosporium spp., in addition to rust and
leaf spot.
', :
:
'
- 4 ~
The combined use of the two compounds in cereals, whether in the form of
a ready-made mixture or a tank mix, provides an unexpectedly enduring
increase in activity in comparison with the rates of application
necessary if using the compounds individually, for example to control
Septoria spp. on leaves or ears. The same applies to the prevention of
attack by Puccinia recondita, Pyrenophora spp. and Fusarium spp.. The
extremely extensive prevention of these cereal diseases results in higher
yields per hectare with distinctly improved crop quality.
The two-component mixture of I and II according to the invention achieves
not only preventive protection, but especially also curative protection
in all cases where the disease has already attacked the plant and the
first signs of its spread are becoming visible. This result is observed
especially in the case of rust attack (Puccinia recondita), which even at
this stage can still be fully controlled. This advantageous proper~y is
important in all agricultural production systems that are based on
forecasts of attack based upon weather, temperature and épide~ics. Such a
mixture provides the user with a very flexible instrument for preventing
the spread of the disease even if attack has reached an advanced stag~.
The mixtures of the invention are active against phytopathogenic fungi
belonging to the following classes: Ascomycetes (for example the genera
Mycosphaerella, Pyrenophora); Basidiomycetes (for example the genera
Tilletia, Rhizoctonia); Fungi imperfecti (for example the genera
Fusarium, Septoria, Phoma, Alternariaj. Other species that are difficult
to control but that can be surprisingly well controlled by the mixture
according to the invention are Cercospora, Cercosporidium, Ascochyta,
Ramularia, ~enturia, Guignardia and Colletotrichum. The combinations
according to the invention are efEective especially in the treatment of
leaves and ears, but they are also suitable for the direct treatment of
seeds or other parts of the plant (fruit, tubers, grains). They ara wall
tolerated by plants and are ecologically harmless.
.
For its application, the mixture according to the invention is normally
used together with adjuvants conventionally employed in the art of
formulaeion. The aFtive componeDts of eormulae I and 11 are eormulated in
::
. ~ .
- .
'
-- 5 --
known manner, for example, into emulsifiable concentrates, coatable
pastes. directly sprayable or dilutable solutions, dilute emulsions,
wettable powders, soluble powders, dusts, granulates and also encapsula-
tions in e.g. polymer substances. The methods of application, such as
spraying, atomising, dusting, scattering, coating or pouring and the form
of the composition are chosen in accordance with the intended objectives
and the prevailing circumstances. Favourable rates of application are
generally from 50 g to 700 g of total active ingredient per hectare, the
propor~ion of I (propiconazole) ranging from 25 g to 400 g a.i./ha and
the proportion of II ranging approximately from 25 g to 300 g a.i./ha,
for example from 75 to 150 g of I + 40 to 125 g of II, for example I:II =
125 g:50 g or I:II = 75 g:75 g.
The target crops within the scope of this invention, are, for example,
the following species of plants: cereals (wheat, barley, rye, oats, rice,
sorghum, mai~e and related crops); beet (sugar beet and fodder beet);
leguminous plants (beans, lentils, soybeans, peas); oil plants (rape,
mustard, poppy, groundnuts, olives, sunflowers); cucumber plants
(cucumbers, marrows, melons); fibre plants Icotton, flax); vegetables
(cabbages, spinach, carrots, onions, garlic, tomatoss, potatoes,
paprika); ornamentals (tulips, daffodils, dahlias, chrysanthemums and
other flowers); tea, coffee, cocoa and mango plants, spice plants and
their seeds; pomes (apples, pears), drupes (cherries, plums, peaches,
nectarines); vines; turf.
,
A preferred method of applying the mixture of the invention comprises
spraying or wetting the plant material witll a liquid formulation or
treating the plant material with a solid formulation of the active
ingredient.
The active ingredients of formulae I and II in accordance with the inven-
tiOIl are applied in the form of compositions and can be applied, if
desired, together with further carriers, suractants or other applica-
tion-promoting adjuvants customarily employed in the art oE formulation.
.
,
:, . :
i3~
-- 6 --
Suitable carriers and adjuvants can be solid or liquid and correspond to
the substances ordinarily employed in the art of formulation, e.g.
natural or regenerated mineral substances, solvents, dispersants, wetting
agents, tackifiers, thickeners, binders or fertilisers.
A preferred method of applying a mixture of active ingredients of
formulas I and II or of an (agro)chemical composition containing these
active ingredients, is foliar application or application to the ears, if
the plant is a cereal. The number of applications and the rate of
application depend on the risk of attack by the corresponding pathogen
(species of fungus). The actlve ingredient mixturs can, however, also
penetrate the plant through the roots via the soil (systemic action) if
the locus of the plant is impregnated with a liquid formulation, or if
the substances are applied in solid form to the soil, or example in
granular form (soil application). The mixture of the compounds of
formulae I and II can, according to an especially preferred method, be
applied (coating) to seeds, tubers, fruit or other plant material to be
protected either by impregnating the material with a liquid formulation
of the active ingredients or coating it with a solid formulation. In
special cases, further types of application are also possible, for
example selectlve treatment of the plant stems or buds.
The com,oounds of formulae I and II are used in unmodified form or,
preferably, together with the adjuvants conventionally employed in the
art of formulation, and are therefore formulated in known manner e.g.
into emulsifiable concentrates, coatable pastes, directly sprayable or
dilutable solutions, dilute emulsions, wettable powders, soluble powders,
dusts, granulates, and also encapsulations in e.g. polymer substances. As
with the nature of the compositions, the methods of application1 such as
spraying, atomising, dusting, scattering, coating or pouring, are chosen
in accordance with the intended objectives and the prevailing circum-
stances.
The formulations, i.e. the compositions, preparations or mixtures
containing the active ingredients of formulae I and II and, where
appropriate, a solid os liquid adjuvant, are prepared in known manner,
: .
3~
-- 7 --
e.g. by homogeneously mixing and/or grinding the active ingredients with
extenders~ e.g. solvents, solid carriers and, where appropriate,
surface-active compounds (surfactants).
Suitable solvents are: aromatic hydrocarbons, preferably the fractions
containing 8 to 12 carbon atoms, e.g. xylene mixtures or substituted
naphthalenes, phthalates such as dibutyl phthalate or dioctyl phthalate,
aliphatic hydrocarbons such as cyclohexane or paraffins, alcohols and
glycols and their ethers and esters~ such as ethylene glycol monomethyl
ether, ketones such as cyclohexanone, strongly polar solvents such as
N-methyl-2-pyrrolidone, dimethyl sulfoxide or dimethylformamide, as well
as vegetable oils or epoxidised vegetable oils, or soybean oil; or water.
The solid carriers used e.g. for dusts and dispersible powders are
calcite, talcum, kaolin, montmorillonite or attapulgite, highly dispersed
silicic acid or absorbent polymers. Suitable granulated adsorptive
carriers are pumice, broken brick, sepiolite or bentonite; and suitable
nonsorbent carriers are, for example, calcite or dolomite.
Vepending on the nature of the active ingredients of Eormulae I and II to
be foroulated, suitable surface-active compounds are non-ionic, cationic
and/or anionic surfactants having good emulsifying, dispersing and
wetting properties. The term "surfactants" will also be understood as
comprising mixtures of surfactants.
Surfactants customary in the art of formulation are described, inter
alia, in the following publications:
"McCutcheon's Detergents and Emulsifiers Annual" MC Publlshing Corp.,
Ridgewood New Jersey, 1980, Sisley and ~ood, "Encyclopedia of Surface
Active Agents", Chcmical Publishing Co., Inc. New Yor~, 1980.
The agrochemical formulations generally contain from 0.1 to 95 % total
active ingredient, from 99.9 to 5 % of a solid or liquid adjuvant, and
Erom 0 to 25 %, especially from 0.1 to 25 %, of a surfactant.
2~ 33 0
-- 8 --
Whereas commercial products will preferably be formulated as
concentrates, the end user will normally employ dilute formulations.
The present invention relates to such (agro)chemical compositions.
Ths following Examples serve to illustrate the invention, "active
ingredient" indicating a mixture of "propiconazole" I and compound II in
a particular mixing ratio ranging from 10:1 to 1:10.
Wettable powders a) b) c)
active ingredient ~I:II =
o:l, 5:2, 1:3) 25 % 50 % 75 %
sodium lignosulfonate 5 % 5 %
sodium laurylsulfate 3 % - 5 %
sodium diisobutylnaphthalene-
sulfonate - 6 % lO %
octylphenol polyethylene glycol
ether ( 7-8 moles of ethylene oxide) - 2 %
highly dispersed silicic acid 5 % 10 % 10 %
kaolin 62 % 27 %
The active ingredient is thoroughly mixed with the adjuvants and the
mixture is thoroughly ground in a~suitable mill, affording wettable
powders which can be diluted with water to g:ive suspensions of the
desired concentration.
Emulsifiable con_entrate
active ingredient (I:II = 4:1) 10 %
octylphenol polyethylene glycol
ether (4-5 moles of ethylene oxide) 3 %
calcium dodecylbenzenesulfonate 3 %
castor oil polyglycol ether
(35 moles of ethylene oxide)4 %
cyclohexanone 30 %
xylene mixture 50 Yo
:
_ 9 _
Emulsions of any required concentration can be obtained from this
concentrate by dilution with water.
Dusts a) b)
active ingredient (I:II ~ 3:2 and 1:1) 5 % 8 %
talcum 95 %
kaolin - 92 %
Ready-for-use dusts are obtained by mixing the active ingredient with the
carrier and grinding the mixture in a suitable mill.
Extruder ~ranulate
active ingredient (I:II = 5:2) 10 %
sodium lignosulfonate 2 %
carboxymethylcellulose 1 %
kaolin 87 %
The active ingredient is mixed and ground with the adjuvants, and the
mixture is subsequently moistened with water. The mixture is extruded and
then dried in a stream of air.
Coated granulate
active ingredient (I:II = 3:2) 3 %
polyethylene glycol (MW 200)3 %
kaolin 94 %
(MW - molecular weight)
The finely ground active ingredient is uniformly applied, in a mixert to
the kaolin moistened with polyethylene glycol. Non-dusty coated
granulates are obtained in this manner~
:.
.
- 10- ~ 33~
Biological Examples
Example 1: Action against Puccinia graminis on wheat
a) Residual protective action
Wheat plants are sprayed 6 days after sowing with a spray mixture (6 ppm
active ingredient) prepared from a wettable powder formulation of the two
active ingredients (I:II - 5:2). After 24 hours, the treated plants are
infected with a uredospore suspension of the fungus. The infec~ed plants
are incubated for 48 hours at 95-100 % relative humidity and about 20C
and then stood in a greenhouse at about 22C. Evaluation of rust pustule
development is made 12 days after infection.
b) Systemic action
A spray mixture (2 ppm active ingredient based on the volume of soil)
prepared from a wettable powder formulation of the active ingredient is
poured onto wheat plants 5 days after sowing. After 48 hours, the treated
plants are infected with a uredospore suspension of the fungus. The
infected plants are then incubated for 48 hours at 95-100 % relative
humidity and about 20C, and then stood in a greenhouse at about 22C.
Evaulation of rust pustule development is made 12 days after infection.
The spread of the disease was prevented completely (= 100 % action) in
both test a) and test b).
Example 2: Action against Erysiphe graminis on barley
a) Residual protective action
Barley plants about 8 cm in height are sprayed with a spray mixture (6
ppm active ingredient) prepared from a wettable powder formulation of the
two active ingredients (I:II = 3:1). The treated plants are dusted with
conidia of the fungus after 3 to 4 hours. The inEected barley plants are
stood in a greenhouse at about 22C and the fungus attack is evaluated
aEter 10 days.
2~ 3~1~
-- 11 --
b) Systemic action
A spray mixture (2 ppm active ingredient based on the volume of the soil)
prepared from a wettable powder formulation of the active ingredient is
poured onto barley plants about 8 cnn in height. Care was taken that the
spray mixture did not come into contact with the parts of the plants
above the soil. The treated plants are dusted 48 hours later with conidia
of the fungus. The infected barley plants are stood in a greenhouse at
about 22C and evaluation of fungus attack ls made after 10 days.
The spread of the disease had been prevented completely (= 100 % action)
in both test a) and test b).
Example 3: Action against Helminthosporium sativum in wheat
Winter wheat infected naturally with Helminthosporium sativum is dressed
on a mixer roller with the test fungicide, a concentration of 60 ppm a.i.
(based on the weight of the seeds) being applied.
The active ingredient is a 1:3 mixture of components I and II.
The infected and treated wheat ls sown in October in the open with a
seeder in plots 2 m long containing 3 seed rows, with 3 replicates.
The test plants are cultivated under normal field conditions until the
attack is evaluated.
To determine the activity of the active ingredient, the emerged plants
are evaluated. Less than 5 % of the plants were infected.
Example 4: Act~ a~ainst Fusarium in rye
Rye of the Tetrahell variety, naturally inEected with Fusarium nivale, is
dressed on a mixer roller with the test fungicide, the following concen-
trations being used: 20 or 6 ppm a.i. (based on the weight of the seeds).
The a.i. is a 1:4 mixture of components I and II.
The infected and treated rye is sown in October in the open with a seeder
in plots 3 m long containing 6 seed rows. 3 replicates per concentration.
.. ' - .
.:
- :,
3~
- 12 -
The test plants are cultivated under normal field conditions (preferably
in a region with unbroken snow cover during the winter months) until the
attack is evaluated.
In order to evaluate the phytotoxicity, in autumn the seed emergence and
in spring the crop density/number of plants per unit area is evaluated.
To determine the active ingredient activity, the percentage number of
plants attacked by Fusarium is counted in the spring, directly after
the snow has melted. The number of infected plants in the present case
was less than 5 %. The emerged plants had a healthy appearance.
xample 5: Action a~ainst Septoria nodorum on wheat
Wheat plants are sprayed at the 3-leaf stage with a spray mixture (60 ppm
a.i.) prepared from a wettable powder formulatlon of the active
ingredients (2.8:1~.
24 hours later, the treated plants are infected with a conidia suspension
of the fungus. The plants are then incubated for 2 days at 90-100 %
relative humidity and stood in a greenhouse for a further 10 days at
20-24C. The fungus attack is assessed 13 days after infection. Less than
1 % of the wheat plants exhibited any attack.