Note: Descriptions are shown in the official language in which they were submitted.
2 0 ~
TITLE
PYRIDINECARBOXYLIC ACID AMIDE DERIVATIVES AND
PHARMACEUTICAL COMPOSITIONS COMPRISING SAME
FIELD OF THE INVENTION
The present invention relates to new
pyridinecarboxylic acid amide derivatives, a process for
preparing the same and pharmaceutical compositions
comprising said derivatives.
The pyridinecarboxylic acid amide derivatives and
their physiologically acceptable salts of the invention
possess an activity of increasing blood flow of vertebral,
common carotid and femoral arteries and a hypotensive
activity, which are effective in the therapy and prevention
of disturbances of cerebral or peripheral circulation,
ischemic heart diseases and hypertensions.
BACKGROUND OF THE INVENTION
Nicotinic acid amide derivatives useful as a
therapeutic agent for cardiovascular diseases are disclosed
in Japanese Pa.ent Kokai No. 62-286968. Nitrate ester
derivatives useful as vasodilator are also disclosed in
Japanese Patent Kokai No. 62-205052. However, they are not
satisfactory in efficacy as therapeutic agent for
cardiovascular diseases. Thus there is a continuing need
for new compounds with more inproved pharmacological
2 ~
activities than known nicotinic acid amide derlvatives.
The present invention results from efforts to
develop new compounds possessing a high pharmacological
activity, being readily available on an industrial scale and
being satisfactory in practical use.
DISCLOSURE OF THE INVENTION
According to the invention, there are provided
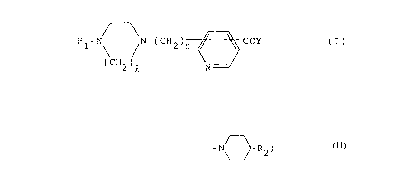
pyridinecarboxylic acid amide compounds of formula (I)
Rl-N /N-(CH2) ~ COY (I)
(CH2)Q N
wherein Rl is hydrogen; Cl-C6 alkyl; C3-C6 cycloalkyl or
diphenylmethyl;
Y is -NH(CH2~n~R2 or -N ~ R2;
R2 is OH or -ONO2;
Q is 2 or 3; m is 0 or 1; and n is 2 to 8; and
physiologically acceptable acid addition salts thereof.
Examples of Rl in formula (I) include hydrogen;
Cl-C6 alkyl such as methyl, ethyl, n-propyl, i-propyl, n-
butyl, i-butyl, tert-butyl, hexyl; C3-C6 cycloalkyl such as
cyclopentyl and cyclohexyl; and diphenylmethyl.
Representative examples of the compounds according
to the invention are as follows:
1) N-(2-Hydroxyethyl)-6-(4-methyl-1-piperazinyl)-
-- 3 --
nicotinamide,
2) N-(2-Hydroxyethyl)-6-(1-piperazinyl)nicotinamide,
3) N-(2-Hydroxyethyl)-6-(4-diphenylmethyl-1-piperazinyl)-
nicotinamide,
4) N-(2-Hydroxyethyl)-6-(4-ethyl-1-piperazinyl)-
nicotinamide,
5) N-(2-Hydroxyethyl)-6-(4-cyclopentyl-1-piperazinyl)-
nicotinamide,
6) N-(2-Hydroxyethyl)-6-(4-methyl-1-homopiperazinyl)-
nicotinamide,
7) N-(2-Hydroxyethyl)-2-(4-methyl-1-piperazinyl)-
nicotinamide,
8) N-(2-Hydroxyethyl)-6-(4-methyl-1-piperazinylmethyl)-
nicotinamide,
9) N-(2-Hydroxyethyl)-6-(4-diphenylmethyl-1-piperazinyl-
methyl)nicotinamide,
10) N-(3-Hydroxypropyl)-6-(4-methyl-1-piperazinyl)-
nicotinamide,
11) N-(2-Nitroxyethyl)-6-(4-methyl-1-piperazinyl)-
nicotinamide,
12) N-(2-Nitroxyethyl)-6-(4-methyl-1-piperazinyl)-
nicotinamide dihydrochloride,
13) N-(2-Nitroxyethyl)-6-(4-ethyl-1-piperazinyl)-
nicotinamide dihydrochloride,
14) N-(2-Nitroxyethyl)-6-(4-methyl-1-homopiperazinyl)-
nicotinamide dihydrochloride,
~2 ~
- 4 -
15) N-(2-Nitroxyethyl)-6-(4-methyl-1-piperazinylmethyl)-
nicotinamide,
16) N-(2-Nitroxyethyl)-6-(4-diphenylmethyl-l-piperazinyl-
methyl)nicotinamide,
17) N-(2-Nitroxyethyl)-2-(4-methyl-1-piperazinyl)-
nicotinamide,
18) N-(2-Nitroxyethyl)-2-(4-methyl-1-piperazinyl)-
nicotinamide dihydrochloride,
19) N-(2-Nitroxyethyl)-6-(4-diphenylmethyl-1-piperazinyl)-
nicotinamide,
20) N-(2-Nitroxyethyl)-6-(4-diphenylmethyl-1-piperazinyl)-
nicotinamide dihydrochloride,
21) N-(3-Nitroxypropyl)-6-(4-methyl-1-piperazinyl)-
nicotinamide,
22) N-(3-Nitroxypropyl)-6-(4-methyl-1-piperazinyl)-
nicotinamide dihydrochloride,
23) N-(4-Hydroxybutyl)-6-(4-methyl-1-piperazinyl)-
nicotinamide,
24) N-(4-Nitroxybutyl)-6-(4-methyl-1-piperazinyl)-
nicotinamide,
25) N-(5-Hydroxypentyl)-6-(4-methyl-l-piperazinyl)-
nicotinamide,
26) N-(6-Hydroxyhexyl)-6-(4-methyl-1-piperazinyl)-
nicotinamide,
27) N-(8-Hydroxyoctyl)-6-(4-methyl-1-piperazinyl)-
nicotinamide,
'~?.3~ 3
28) 4-HydrQxy~ 6-(4-methyl-1-piperazinyl)nicotinyl~-
piperidine,
29) N-~5-Nitroxypentyl)-6-(4-methyl-1-piperazinyl)-
nicotinamide,
30) N-(6-Nitroxyhexyl)-6-(4-methyl-1-piperazinyl)-
nicotinamide,
31) N-(8-Nitroxyoctyl)-6-(4-methyl-1-piperazinyl)-
nicotinamide, and
32) 1-~6-(4-methyl-1-piperazinyl)nicotinyl~-4-
nitroxypiperidine.
The compounds of the invention can be prepared by
reacting a compound of formula (II)
Rl-N\ / N-(CH2)m ~ C~2R3 (II)
(CH2)Q N
wherein R1, Q and m are as defined above and R3 is hydrogen
or Cl-C6 alkyl, with an amino compound of formula (III)
NH2-(CH2)n 2 (III)
wherein R2 and n are as defined above and optionally
subjecting the resulting reaction product where R2 is OH to
esterification with nitric acid to give a compound of
formula (I)
(CH2)Q ~ COY (I)
2 ~
wherein R1, Y, R and m are as defined above, or if
necessary, converting the compound thus obtained to a
physiologically acceptable acid addition salt.
Alternatively, the compounds of formula (I)
wherein Y is -NH(CH2)n-R2 (R2 is OH or -ON02 and n is 5-8)
and Y is -N 3 R2 (R2 is OH or -ON02) can be prepared by
reacting a compound of formula (IV)
X- ( CH2 )m\~cox
~ ~ (IV)
wherein X is halogen and m is O or 1 with a compound of the
formula NH2(CH2)nOH (n is 5-8) or HN 3 0H in an organic
solvent to form a compound of the formula
X- ( CH2 )m~CONH( CH2 )nOH
or
X-.(CH2)m ~ 3
wherein X, m and n are as defined above and further
condensing said compound with a compound of the formula
~ 3
Rl-N NH wherein Rl is as defined above in the presence of
an acid-binding agent to give a compound of formula (I)
wherein Y is -NH(CH2)n-OH or -N ~ OH, or esterifying the
resulting compound to form a compound of formula (I) wherein
Y is -NH(CH2)n-ONO2 or -N ~ ONO2.
In case of using a compound of formula (II)
wherein R3 is Cl-C6 alkyl, the reaction between a compound
of formula (II) and a compound of formula (III) is effected
using an excess amount of the compound of formula (III) with
or without an organic solvent in the presence or absence of
a catalyst such as 2-hydroxypyridine. The reaction is
accomplished by stirring at a temperature between ordinary
temperature and 150~C for a period in the range from several
tens minutes to several tens hours. Purification and
isolation of the desired compounds are carried out by a
conventional method. Thus a purified condensation product
is obtained by extracting the reaction product with an
organic solvent such as diethyl ether, ethyl acetate or
dichloromethane, distilling off the extraction solvent from
the extract and subjecting the residue to recrystallization
or chromatography.
In cases where R2 in the condensation product
obtained is OH, a compound of formula (I) wherein R2 is ONO2
can be produced by dissolving or suspending said
condensation product in an organic solvent, adding fuming
nitric acid or a mixture of fuming nitric acid and acetic
2 ~ t'.~
-- 8
anhydride to the solution or suspension under ice-cooling
and stirring the resulting mixture for 1-4 hours to form a
nitrate ester.
In case of using a compound of formula (II)
wherein R3 is hydrogen, the compound (II) and an amino
alcohol or its nitrate ester of formula (III) are subjected
to condensation reaction in an organic solvent in the
presence or absence of an appropriate amidating agent to
form a compound of formula (I).
The reaction solvents used in these reactions
include an aliphatic hydrocarbon such as n-hexane or
petroleum ether; an aromatic hydrocarbon such as benzene,
toluene or xylene; an alicyclic compound such as
cyclohexane; a halogenated hydrocarbon such as carbon
tetrachloride, chloroform, dichloroethane or
trichloroethane; an aliphatic ketone such as acetone or
methyl ethyl ketone; acetonitrile; N,N-dimethylformamide;
dimethylsulfoxide or the like. Purification and isolation
of the desired compounds are also carried out by a
conventional method. Thus, a purified desired condensation
product is obtained by distilling off the solvent after
completion of the reaction, pouring the residue into an
aqueous solution of sodium hydrogen carbonate, extracting
the resulting mass with an organic solvent such as dlethyl
ether, ethyl acetate or dichloromethane, distilling off the
extraction solvent from the extract and subjecting the
residue to recrystallization or chromatography.
The compounds of formula (I) thus produced can be
converted to acid addition salts thereof by a conventional
method. The acid addition salts include acid addition salts
of the compounds with an inorganic acid such as
hydrochloric, sulfuric, phosphoric, hydrobromic or nitric
acids, and acid addition salts of the compounds with an
organic acid such as acetic, propionic, succinic, butyric,
malic, citric, fumaric or tartaric acids.
The compound of formula ~II) can be produced by
condensing a compound of formula (V)
X~(CH2)m ~ ~C~2R3 (V)
wherein X is halogen and R3 is hydrogen or C1-C6 alkyl with
a compound of formula (VI)
/~
R1-N\ NH (VI)
(CH2)Q
wherein Rl is hydrogen, C1-C6 alkyl, C3-C6 cycloalkyl or
diphenylmethyl and Q is 2 or 3 in the presence of an acid-
binding agent. In cases where Rl in a compound obtained by
the condensation reaction is hydrogen, if necessary, the
compound and a compound of the formula R'l-X wherein R'l is
Cl-C6 alkyl and X is halogen may be reacted in an organic
-- 10 -
solvent in the presence of an acid-binding agent to give a
compound of formula (II) wherein R1 is Cl-C6 alkyl. A
compound of formula (II) wherein Rl is hydrogen can be
converted to a compound of formula (II) wherein R1 is methyl
by reaction with a mixture of formaldehyde and formic acid.
The reaction can be accomplished under the reaction
conditions described in Organic Synthesis Vol. 3, pages 723-
725.
As clearly seen from the results of a
pharmacological test shown below, the compounds of formula
(I) of the invention exhibit marked blood flow-increasing
and hypotensive actions in warm-blooded animals and can be
used for the therapy or prevention of diseases in the
cardiovascular system. Diseases in the cardiovascular
system include disturbances of cerebral or peripheral
circulation, ischemic heart diseases and hypertensions.
Thus, the invention further relates to
pharmaceutical compositions for use in the therapy or
prevention of the above-mentioned diseases, which comprise
as an active ingredient a compound of formula (I) or a
physiologically acceptable acid addition salt thereof.
The pharmaceutical compositions of the invention
can orally or parenterally be administered in the suitable
dosage forms. They can be administered alone, but are
generally administered with a pharmaceutical carrier
selected on the basis of the chosen route of administration
and standard pharmaceutical practice. The dosage forms
include tablets, capsules, suppositories, troches, syrups,
creams, ointments, pasters, cataplasms, granules, powders,
injections, suspensions and the like. Bi- or multi-layered
tablets can also be prepared in combination Witl.l other
drugs. Furthermore, tablets with conventional coating
applied, for example, sugar-coated tablets, tablets with
enteric coating or film-coated tablets can also be prepared.
In forming solid dosage forms there can be used
additives such as lactose, white sugar, crystalline
cellulose, corn starch, calcium phosphate, sorbitol,
glycine, carboxymethyl cellulose, gum arabic,
polyvinylpyrrolidone, hydroxypropyl cellulose, glycerin,
polyethylene glycol, stearic acid, magnesium stearate and
talc.
In forming semi-solid dosage forms, vegetable or
synthetic waxes or fats and the like are used.
In forming liquid dosage forms, there can be
employed additives such as an aqueous sodium chloride
solution, sorbitol, glycerin, olive oil, almond oil,
propylene glycol and ethyl alcohol.
The content of the active ingredient in the above
dosage forms is in the range between 0.1 and 100% by weight,
suitably between 1 and 50% by weight for oral administration
and between 0.1 and 10% by weight for injection.
The dosage administered will, of course, vary
- 12 -
depending upon the mode and route of administration, age,
sex and weight of the patient, nature and extent of symptoms
and the like. Usually a daily dosage of active ingredient
can be about 1 to 1000 mg per kg of body weight~
The invention is further illustrated by the
following non-limitative examples.
Example 1
Preparation of N-(2-hydroxyethyl)-6-(4-methyl-1-
piperazinyl)nicotinamide (compound 1)
A mixture of 3.29 g of methyl 6-(4-methyl-1-
piperazinyl)nicotinate, 2.02 g of 2-aminoethanol and 1.00 g
of 2-hydroxypyridine was heated to 120-130~C and stirred for
7 hours.
The resulting mixture was purified by column
chromatography (silica gel; chloroform : methanol = 5 : 1)
to give 3.02 g of N-(2-hydroxyethyl)-6-(4-methyl-1-
piperazinyl)nicotinamide (yield 94%).
Examples 2-10
The same procedure as in Example 1 was repeated
but replacing methyl 6-(4-methyl-1-piperazinyl)nicotinate by
methyl 6-(1-piperazinyl)nicotinate, methyl 6-(4-
diphenylmethyl-l-piperazinyl)nicotinate, methyl 6-(4-ethyl-
1-piperazinyl)nicotinate, methyl 6-(4-cyclopentyl-1-
piperazinyl)nicotinate, methyl 6-(4-methyl-1-
2 ~ 3
-- 13 -
homopiperazinyl)nicotinate, methyl 2-(4-methyl-1-
piperazinyl)nicotinate, methyl 6-(4-methyl-l-
piperazinyl)nicotinate, and methyl 6-(4-diphenylmethyl-1-
piperazinylmethyl)nicotinate, respectively to give N-(2-
hydroxyethyl)-6-(1-piperazinyl)nicotinate (compound 2), N-
(2-hydroxyethyl)-6-(4-diphenylmethyl-1-piperazinyl)-
nicotinamide (compound 3), N-(2-hydroxyethyl)-6-(4-ethyl-l-
piperazinyl)nicotinamide (compound 4), N-(2-hydroxyethyl)-6-
(4-cyclopentyl-1-piperazinyl)nicotinamide (compound 5), N-
(2-hydroxyethyl)-6-(4-methyl-1-homopiperazinyl)nicotinamide
(compound 6), N-(2-hydroxyethyl)-2-(4-methyl-l-
piperazinyl)nicotinamide (compound 7), N-(2-hydroxyethyl)-6-
(4-methyl-l-piperazinylmethyl)nicotinamide (compound 8) and
N-(2-hydroxyethyl)-6-(4-diphenylmethyl-1-piperazinylmethyl)-
nicotinamide (compound 9), respectively. Following the same
procedure, replacement of 2-aminoethanol used in Example l
by 3-aminopropanol gave N-(3-hydroxypropyl)-6-(4-methyl-1-
piperazinyl)nicotinamide (compound lO).
Examples 11-12
Preparation of N-(2-hydroxyethyl)-6-(4-methyl-l-
piperazinyl)nicotinamide (compound 11) and its
dihydrochloride (compound 12)
To an ice-cooled solution of ll.00 g of the
compound (obtained in Example 1) in 20 ml of methylene
chloride was added dropwise 5 ml of fuming nitric acid while
2~3~ 3
- 14 -
stirring below 0~C. The stirring was continued for 2 hours.
The reaction mixture was poured into an aqueous sodium
hydrogen carbonate solution and extracted with methylene
chloride. The extract was washed twice with water and once
with a saturated aqueous saline soluticn, dried over
anhydrous magnesium sulfate and concentrated to afford N-(2-
nitroxyethyl)-6-(4-methyl-1-piperazinyl)nicotinamide. The
product was dissolved in ethanol and to the solution was
added under ice-cooling hydrogen chloride-saturated ethanol.
The hydrochloride thus formed was recrystallized from
ethanol to give 0.54 g of N-(2-nitroxyethyl)-6-(4-methyl-1-
piperazinyl)nico-tinamide dihydrochloride (yield 34%).
Examples 13-16
The same procedures as above were repeated but
replacing compound 1 by compounds 4, 6, 8 and 9,
respectively to prepare N-(2-nitroxyethyl)-6-(4-ethyl-1-
piperazinyl)-nicotinamide dihydrochloride (compound 13), N-
(2-nitroxyethyl)-6-(4-methyl-1-homopiperazinyl)nicotinamide
dihydrochloride (compound 14), N-(2-nitroxyethyl)-6-(4-
methyl-l-piperazinylmethyl)nicotinamide (compound 15) and N-
(2-nitroxyethyl)-6-(4-diphenylmethyl-1-piperazinylmethyl)-
nicotinamide (compound 16), respectively.
Examples 17-18
Preparation of N-(2-nitroxyethyl)-2-(4-methyl-1-
piperazinyl)nicotinamide (compound 17) and lts
dihydrochloride (compound 18)
To a solution of 1.27 g of compound 7 prepared in
Examples 2-10 in 13 ml of acetonitrile was added dropwise a
mixture of 2.0 g of fuming nitric acid and 1.4 g of acetic
anhydride while maintaining the temperature below -lO~C.
The mixture was stirred for 4 hours, then poured into an
aqueous sodium hydrogen carbonate solution and extracted
with methylene chloride. The extract was washed twice with
water and once with a saturated a~ueous saline solution,
dried over anhydrous magnesium sulfate and concentrated to
give N-(2-nitroxyethyl)-2-(4-methyl-1-piperazinyl)-
nicotinamide (compound 17). The product was dissolved in
ethanol, and to the solution was added under ice-cooling
hydrogen chloride-saturated ethanol. The hydrochloride thus
formed was recrystallized from ethanol to give 1.32 g of N-
(2-nitroxyethyl)-2-(4-methyl-1-piperazinyl)nicotinamide
dihydrochloride (compound 18)(yield 66%).
Examples 19-22
The sa~e procedures as above were repeated but
replacing compound 7 by compounds 3 and 10, respectively to
prepare N-(2-nitroxyethyl)-6-(4-diphenylmethyl-1-
piperazinyl)nicotinamide (compound 19) and its
dihydrochloride (compound 20), N-(3-nitroxypropyl)-6-(4-
methyl-1-piperazinyl)nicotinamide (compound 21) and its
~ a ~
- 16 -
dihydrochloride (compound 22).
Example 23
Preparation of N-(4-hydroxybutyl)-6-(4-methyl-1-
piperazinyl)nicotlnamide (compound 23)
The same procedure as in Example 1 was repeated
but replacing 2-aminoethanol by 4-aminobutanol to afford N-
(4-hydroxybutyl)-6-(4-methyl-1-piperazinyl)nicotinamide.
Example 24
The compound obtained in Example 23 was esterified
in the same way as in Example 17 to give N-(4-nitroxybutyl)-
6-(4-methyl-1-piperazinyl)nicotinamide (compound 24).
Example 25
Preparation of N-(5-hydroxypentyl)-6-(4-methyl-1-
piperazinyl)nicotinamide (compound 25)
To 7.88 g of 6-chloronicotinic acid were added 7.3
ml of thionyl chloride and a few drops of DMF. The mixture
was heated under reflux for 2 hours, and then the excess of
thionyl chloride was distilled off to give crystals of 6-
chloronicotinoyl chloride. A solution of the crystals in 60
ml of tetrahydrofuran was added dropwise to a solution of
15.47 g of 5-aminopentanol in 200 ml of tetrahydrofuran at a
temperature below 0~C. The mixture was stirred overnight
while gradually raising the temperature to room temperature.
'~ O ~ 3
The solvent was distilled off and to the residue was added
water followed by extraction with ethyl acetate. Then, the
extract was washed successively with water and a saturated
brine solution, dried over magnesium sulfate. The solvent
was distilled off and the residue was recrystallized from
ethyl acetate to give 11.24 g of N-(5-hydroxypentyl)-6-
chloronicotinamide (yield 93%).
To a solution of 4.13 g of the amide obtained
above, 8.51 g of 1-methylpiperazine and 1.72 g of
diisopropylamine in 80 ml of p-xylene was added a catalytic
amount of NaI, and the mixture was heated to 120-1305C and
stirred for 4 hours. The reaction mixture was poured into
water and extracted with chloroform. The extract was washed
successively with water and a saturated brine solution, then
dried over anhydrous magnesium sulfate and concentrated.
The concentrate was purified by column chromatography
(silica gel, chloroform : methanol = 4 : 1) to give 4.65 g
of N-(5-hydroxypentyl)-6-(4-methyl-1-piperazinyl)-
nicotinamide (yield 89~).
Examples 26-28
The same procedure as in Example 25 was repeated
but replacing 5-aminopentanol by 6-aminohexanol, 8-
aminooctanol and 4-hydroxypiperidine, respectively to give
N-(6-hydroxyhexyl)-6-(4-methyl-1-piperazinyl)nicotinamide
(compound 26), N-(8-hydroxyoctyl)-6-(4-methyl-1-
2~1143
- 18 -
piperazinyl)nicotinamide (compound 27) and 4-hydroxy-1-~6-
(4-methyl-1-piperazinyl)nicotinyl)piperidine (compound 28),
respectively.
Examples 29-32
The compounds produced in Examples 26-28 were
esterified in the same manner as in Example 17 to prepare N-
(5-nitroxypentyl)~6-(4-methyl-1-piperazinyl)nicotinamide
(compound 29), N-(6-nitroxyhexyl)-6-(4-methyl-1-
piperazi.nyl)nicotinamide (compound 30), N-(8-nitroxyoctyl)-
6-(4-methyl-1-piperazinyl)nicotinamide (compound 31) and 1-
~6-(4-methyl-1-piperazinyl)nicotinyl)-4-nitroxypiperidine
(compound 32), respectively.
Table 1 shows compounds 1-32 prepared as above for
chemical structure, yield and physical properties.
Table 1
Com- m.p. (~C)
StructurC PN~ (recrys~ I R ~'~ax (Gm) IH - N M R
A 112-114(KBr)3414.3362,1608. (CDCQ 3 ) ô8.59(1H,d.J-2.4Hz),7.89(1H.dd~J-9.0,2.4Hz),
CH3 N N~CONH (CH ) OH 1 94% (Ethy1 ac~ 1501.1241 6.51(1H,d.l-9.QHz), 3.19(2H,t,J-4.4Hz).3.13-3.49(6H.m).
N =/ 2 2 tate) 2 . 49 (4H . t . J -S . OHz), 2 . 34 (3H . s)
~ ~ (KBr)3265.2920.2855. (D~ds ) o'8.59(1H.d.J-2.2Hz), 8.26-8.24(1H.m).7.94(1H.
HN N ~ CONH(CH2) 2 OH 2 69% 140-142 2830.1640.1600.1500 dd.J-8.8.2.2Hz).6.18(1H.d.J-8.8H2), 4.82-4.58(1H.m).3.60-
\~ N=/ 3.20(8H.m).2.82-2.18(4H.n )
C6 H5 A 190-192(KBr)3328.2850.1632. (DMS~dr ) ô8.58(1H.s).8.26-8.16(1H.m).7.94(1H.d.J-8.9Hzl
~N N ~ CONH(CH) OH 3 189~ Ptnni~r;l~ 1612.1501.1245 1.52-1.13(10H.m),6.11(1H.d.J-8.9Hz).4.70(1H.t.J-5.3Hz),
C6 H5 \ / N=/ 2 2 4.32(1H,s),3.66-3.40(6H.m).3.77-3.24(2H,m~.2.46-2.32(4H,m)
A ~ (l(Br)3312.3210.2928. (CDCQ s ) o8.59(1H.d.J-2.4Hz), 7.89(1H,dd.J-9Ø2.4Hz).
C H N N~/ \) CONH(CH2) 2 OH 4 95% 125-126 2836.1630.1608.1539. 6.90-6.18(1H,m).6.58(1H,d.J-9.OHz).3.65(2H.t.J-S.lHz),
~~
2 5 ~ N=/ (Ethyl aoe- 1299,118 3.11-3.51(2H,m).3.65(4H,t.J-i.lHz).2.53(4H.t,J-i.lHz).
tate) 2 . 46 (2H . q . J -I . 2Hz), 1.13 (3H . t . J-l . 2Hz)
A 171-174(KBr)3380.2910,2810, (CDCQ s ) o8.57(1H.d.J-2.4Hz).7.89(1H.dd.J-9Ø2.4Hz).
N N ~ CONH(CH2) 2 OH 5 72% (Aoetonitril~ 1641.1602.1537;1496. 6.60(1H.d.J-9.OHz).6.60-6.46(1H.m).3.82(2H.t.J-S.lHz~.
~/ ~/ N=/ tate) 1241 3.15-3.5$(611,c).2.78-2.43(51l.G).1.99-1.32(10H.m)
~--~ (Fil'")2944.2196.1113. (CDCQ s ) o8.78(1H.d.J-2.4Hz).l.99(lH.dd.J-9Ø2.4Hz).
CH3N N ~ CONH(CH2) 2 OH 6 94~6 Oily 1605.1431,1368.182 6.46(1R.d.l-9.OHz).3.91-3.83(2H,m),3.86(3H.s).3.10(2H.
W N=/ product t.J-6.4Hz).2.1$-2.66(2H.m).2.61-2.52(2H,m).2.38(3H.s).
2.10-1. 96(2H.m)
,~ - (KBr)3316,3260.1644. (CDCQ s ) o9.14-9.02(1H.m).8.34(1H.dd.J-4.9.2.0Hz).8.28
CONH (CH2 ) 2 OH 7 73% 123-l~S 1544.1421 (lH.dd.J-1.1.2.0).1.08(1H.dd.J-1.1,4.9Hz),3.48(2H,t,J-4.9
.N--\ A (Ethyl aoe- Hz).3.70-3.58(2H.m).3.25(4H.t.J-4.9Hz).2.60(4H.t.J-4.9Hz).
N~JNCH3 . tat~) 2.36(3H.s)
~able 1 (Continued)
Com- m.p. (~C)
Structure pound Yield (Solvent ~ I R (cm~l) 'H - N M R
No. zatlon) m~x
A , ~ilm)3290,30l5,2930~ (CDCQ a ) o 8.94(1H,d.J-2.2Hz), 8.10(1H,dd,J~8.1.2.2Hz).
CH3 N N--CH2 ~ CONII(CH ) 011 8 84% ~llY 2800,1640,1595.1545 7.49tlH,d,J-8.1Hz),7.28-7.02(1H,m),3.83(2H,t,J-4.9Hz),
N 2 2 product 3.70(2H,s),3.64(2H.t.J-4.9Hz),2.67-2.39(8K,m),2.31(3l1,s;
C 11 A ~i'm)3280~3080,2940~ (CDCQ 3 ) o 8.93(1H,d,J-2.2Hz),8.05(1H.dd,J-8.3,2.2Hz),
6 5 ~N N-CH2 ~ CONII(CH ) OH 9 95~6 Olly 2810,1650,1600,1490 7.52-7.10(10H,m),6.51(1H,d,J-8.3Hz3,6.32-6.23(1H,m~.4.23
C 11 \ / N=J 2 2 product (lH,s),3.82(2H,t,J-S.OHz).3.67(2H,s),3.62(2H.t,J-S.OHz~.
6 5 2.62-2.34(8H,z)
t~ 123-125 (KBr)3310,2938,2846, (CDCQ3 ) o8.56(1H.d,J 2.4Hz),7.90(1H,dd,J-9.0,2.4Hz~,
CH3 N N ~ CONH(CH2)30H 10 8896 (Ethyl ae 1623,1604.1545 6.68-6.l0(1H,m),6.61(1H,d,J-9.OHz),3.80-3.53(9H.m).2.50 ~ ~
\ / N=/ tate) (4H,t,J-S.OHz) 2.34(3H.s).1.86-1.11(2H,m) ~:3
A 95-97 (KBr)3280,2930,2790, (CCCQ 3 ) o8.57(1H,d,J-1.9Hz), 7.88(1H,dd,J-9.3.1.9Hz),
CH3 N N ~ -CONH(CH) ONO2 11 70% 1635,1605.1540,1500. 6.62(1H.d.J-9.3Hz),6.49-6.25(1H,m),4.65(2H.t,J-S.lHz),3.Q8
\ / N=/ 2 2 (llexane~) 1275 (2H,dt,J-5.0,5.0),3.88(4H,t,J-S.lHz),2.51(4H,t,J-S.lHz), ,~
ace one 2.35(3H,s)
A ~ 143-145 (KBr)3244,2926,1667, (CD3 OD) or8.56(1H,d,J~2.2Hz), 8.46(1H,dd,J-9.5,2.2Hz),
CH3 N N~ = ~CONH (CH2)2 ON02 12 34% (Dec.) 1647,1609,1545,1219 I.SO(lH,d,J-9.SHz),4.68(2H,t,J-5.4Hz),4.68-4.47(2H,m),
~211CQ (Ethanoll 3.85-3.10(7H,m),3.48-3.28(2H,m),3.00(3H,s)
A ~ 163-164 (K3r)3292,2782, 1676, (CD3 OD) o 8.55(1H,d,J-2.2Hz), 8.41(1H,dd,J-9.5,2.2Hz),
C2H5N N~ =~ CoNH(cll2)2oNo2 13 39% (Dec.) 1657,1641,1541,1282 7.47(1H,d,J-9.SHz),4.61(2H,t,J-5.4Hz),4.61-4.47(2H,m),
~ 2H CQ (Ethanol) 3.88-3.60(7H.m),3.42-3.18(6H,m),1.43(3H,t,!-1.3Hz)
CH N N ~ CONHtCH ) ON02 132-134 (KBr)3244,2948,2100, (CD3 OD) or8.51(1H,d,J-2.4Hz), 8.43~1H,dd,J-9.5,2.4Hz),
3 , / 2 2 14 129~ (Dec.) 1610,1637,1608.1541. 7.46(1H,d.J-9.SHz),~.68(2H,t,J-5.2Rz),4.36-4.05(2H,m),
N=' ~2HCQ (Ethan~l) 1281,761 4.00-3.60(1H,m),3.57-3.36(2H,m),2.98(3H,s),2.63-2.32(2H,m)
Table 1 (Continued)
Com- m.p. (~C)
Structure pound Yield(Solvent ~ R :~ (cm ') 'H - N M R
No. zatlon) rax
r~ Oily (Film)3300.2945,2800. (CDCQ 3 ) o8.94(1H,d,J-2.2Hz) 8.09(1H.dd.J-8.3.2.2i{z).7.53
C~13 N N C112 ~3CONH (CH ) ONO lS 12~ product 1640.1600.1545.1280 (lH,d.J-8.3Hz).6.79-6.68(1H.G) 4.68(2H.t.J-S.lHz).3.84(2H.dt,
N 2 2 2 . J-5.6.5.111z).3.72(2H.s).2.67-2.42~8H.m).2.32(3H.s)
C6 H5 ~ 111-112 ((Br)3060.3020.2930. (CDCQ 3 ) ô8.93(1H.d.J-2.2Hz), 8.07(1H.dd.J-8.4.2.2Hz).
~N N--Cll2~CONH(CH ) ONO 16 52% 2805.1635.1595.1275 7.59-l.11(12H.m).6.83-6.70(1H.m).4.66(2H.t.J-5.0Hz).4.25(1H.
C6 H5 \ / N=/ - 2 2 2 (Dec.) s).3.89-3.71(4H.r).2.69-2.33(8H.m)
~C H ONO Oily (Fil~n)3248,2942,28S0, (CCCQ 3 ) o9.41-9.26(1H.m~.8.41(1H.dd.J-4.9.2.011z).8.33(1H.
ONH (C 2 ) 2 2 17 70% 2810.. 1636.1219 dd J-l 8.2 OHz) 7 13(1H dd J-7 8.4 9Hz).4 69(2H.t.J-4 9Hz).
N=~ / \ product 3.90-3 77(2H.m) 3 23(4H t.i-4.9Hz); 2.61(4H.t.J-4.9Hzj.2.38. ~ C~
N~JN C H3 (3H.s)
~CONH (CH ) ONO 120-122 (KBr)3422,1662.1634. ~C3~ OD) o8.3o(lH~dd~J-5.J~2~oHz)~8.22(lH~dd~ .6~2.oHz)~
N=~< A 2 2 2 18 66% 1620,1386,1281 7 31(1H ddjJ-1 6-5 9HZ) 4 753(5H j3 4j_3 30(2H,~) 3 00(3H s)
N\JNCH3 ~2HCQ (Dec.)
C6 H5 ~ ~ (XBr)3020.29$5.2915. (CDCQ 3 ) o8.85(1H.d.J-2.2Hz), 7 86(1H.dd.J-9Ø2.2Hz),
~N N4~ CONII(CII ) ONO 19 75% 116-119 2845.2805.1635.1600. 7.53-7.16(10H.m).6.57(1H.d.J-9.OHz).6.49-6.35(1H.m).4.63(2H.
C6 H5 \ / N=/ 2 2 2 1495.1275 t.J-S.lHz).4.31(1H.s).3.7~(2H.dt.J-S.l,S.lHz),3.72-3.59(4H.
s) . 2 . 64-2 . 47 (4H. ~)
C6 H5 ~ ~ 120-121 (KBr)3120.2574.1636. ~CD3 OD) o8.55(1H.d.J-2.2Hz).8.49(1H.dd.J-8.3.2.2Hz).
~N N4~ CONH(CII ) ONO2 20 72% (Dec.) 1605.1280 7.93-1.82(4H.~).7.57-1.37(7H.m).S.62(1H.s), 4.67(2H.t.J-S.l
C6 H5 \J N=/ ~2HCQ (Ethy1 aoe- 11z).4.48-4.02(4H.m).3.73(2H.t.J-S.lHz).3.58-3.43(41l.m)
alod~l)
Table 1 (Cor~tinued)
Com- m.p. (~C)
Structure pound Yield (rOecryerslta~r- I R (cm l) IH - N M R
~ zation)
A 108-110 ~KBr)3262.2946.2848. (CDCQ 3 ) ~8.55(111,d,J-2.411z),7.89(11i,dd.J-9.2.2.411z),
CX3N N~CONH (CH ) ONO 21 90% 1625.1603.1283 6.63(1H.d.J-9.211z), 6.22-6.09(1H.m).4.57(211,t.J-6.011z).
N=/ 2 3 2 (DeC.) 3.68(211.t.J-5.311z).3.63-3.49~211.m).2.51(411.t.J-5.311z).
2.35(311.s) . 2.16-2.5(211.m)
/--\ 139-141 (KBr)3294.2868.2684. (CD3 OD) ~8.55(1H.d.J-2.411z).8.46(111.dd.J-9.5.2.4Hz).
CH N N~ --CONH (CH )ONO 22 7S% (Dec.l 1663.1645.1618.1545. 7.53(1H.d.J-9.SHz), 4.6l-4.45(411.m).3.89-3.60(411.m).3.56-
3 ~ ~ 2 3 2 ~thyl alco 1279 3.32(511.m).3.01(3H.s).2.12-1.94(211.m)
2 H C Q alcohol)
A (KBr)3325-2940-1630- (CDCQ 3 ) ~8.56(111.d.J-2.511z),7.91(111,dd,J-9.0,2,511z), C~
CH3 N N~CONH (CH2 ) 4 OH2349% Amorphous 1610.1500.1260 6.61(111.d.J-9.Ollz).6.54(111.m).3.71(211.t.J-5.811z).3.65(411.
\~ N=/ t.J-5.411z).3.47(2H.m).2.50(4R.t.J-5.011z).2.34~311.s).1.68
(4H . m)
A Colorless (KBr)3300-1640 1600- (CDCQ 3 ) ~8.53(1il,d,J-2.3Hz),7.89(11i,dd,J-9.3,2,3Hz), s:~
CH3 N N~3CONH (CH )ONO 24 57% crystal 1500.1250 6.62(1H.d.J-9.3Hz).6.13(1H.m).4.51(2H.t.J-5.911z).3.67(4ll.t,
\ / N 2 4 2 77-80 J-S.lHz).3.48(211.m).2.50(4H.t.J-5.2Hz).2.38(3H.s).1.97-
!. 62(4H,s)
A (KBr)3400-2950 1640- (CDCQ 3 ) ~8.55(1H.d.J-2.511z).7.90(1H.dd.J-9.2.2.511z).
CH3 N N4~CoNH (CH2 ) 5OH 25 89% Amorph~us 1600.1500.1260 -6.62(1H.d.J-8.7Hz).6.20(111.m).3.66(6R.m).3.44(21i.m).2.50
~/ N=/ (4H.t.J-5.3Hz).2.34(3H.s).1.78-1.45(611.m)
A . (KBr)3300.2950.1615. (CDCQ 3 ) ~8.54(1H.s).7.89(1H.d.J-9.01iz),6.60(1R.d.J-
CH3 N N~CONH (CH2 ) 6 OH2680% Amorphous 1605.1500.1260 9.011z).6.25(111.m).3.61(611.m).3.39(211.m).2.48(411.t.J-4.911z),
~/ N =/ 2 . 33 (3H . s) .1. 65-1. 29 (8H . r~)
Table 1 (Continued)
Com-d yl ~ (SolvPent(focr) _l
Structure No. rec~ys)talli- I R ~' a (cm ) IH - N M R
A (KBr)3300.2gSO. (Ci)CQ 3 ) ~8.55(1H.d.J-2.911z).7.91(111.dd.J-8.6.2.911z),
CH3 N N~CONH (CH2 ) 8 OH 21 71% Amorphous 1630.1610.1500. 6.62(1H.d,J-8.611z).6.11(1H.m).3.61(6H.m).3.40(2H.m).2.50
~/ N=J 1250 (4H.t.J-5.1Hz).2.32(311.s),1.66-1.12(1211.m)
cOlorless (KBr)3500~3350~ (Ci~CQ 3 ) ~8.27(1H.d.J-2.3Hz),7.61(111.dd.J-9.l.2.3Hz),
CH3 N N~CON/~OH 28 82%rlY40-143 1610,1440,1260, 6.62(11i.d.J-9.7Hz).3.96(111.m).3.63(411.t.J-5.711z).3.31(2il,
\~ N=/ \ J 1100 m).2.51(411.t.J-5.71iz),2.48(311,s),2.15-1.43(611,m)
Colorless (Kgr)3300,1630- (CiXQ 3 ) ~8.55(1H.d.J-1.9llz).7.90(111.dd.J-8.8.2.411z).
CH3 N N~CONH (CH ) ONO 2968~6 100-103 1610-1290.12506.63(111.d.J-8.8ilz),6.01(111.m).4.46(211.t.J-6.311z),3.67(4H.
\ / N=J 2 5 2 t,J-4.gHz),3.47(2H,m).2.50(411,t,J-5.311z),2.33(311 s~ 1.85-
1.40(611,m) ~ ~
A Colorless (KBr)3300,1620. (Ci)CQ 3 ) ~8.53(1H.d.J-2.9Hz).7.89(1H,dd.J-8.6.2.9Hz). ~,
CH3N N~CONH (CH ) ONO 3060~6 ~7-69 1600.1280.12506.62(11i.d.J-8.6Hz).5.97(1H.m).4.43(2H.t.J-5.71iz).3.67(411. j;a.
\ / N=/ 2 6 2 t.J-5.lliz).3.44(211.m),2.50(411,t,J-5.71iz),2.33(31J.s),1.80- ,~
1.30(8il,~)
colorleSS (Kgr)3400.1630, (CiXQ 3 ) ~8.53(1H.d.J-2.511z).7.92(111.dd.J-9.1.2.9Hz).
CH3N N~CONH (CH ) ONO 3176% 70-72 1600.12406.64(111,d,J-9.0Hz).6.00(1H.m).4.43(211.t.J-6.3Hz).3.66(41i.
\ ~ N =/ 2 8 2 t . J -5 . 7Hz), 3 . 41(211, m) . 2 . 50 (4H. t . J-5 . 7Hz) . 2 . 35 (3il . s) .1. 80-
- 1.20(12H.m)
Colorless (KBr)3400.1620. (Ci)CQ 3 ) ~i8.26(1H.d.J-2.9Hz).7.61(11l.dd.J-9.7.2.911z).
CH3N N~CON~oNo2 32 67%Cr~35-t9a6l 1600,1440.1240. 6.63(1H.d.J-9.7Hz).5.20(111.m).3.90(211.m),3.64(411,t,J-
\ / N=/ \ / 810 5.711z),3.60(21{.m).2.51(4H.t.J-5.7Hz),2.35(311,s),2.05(2H.m),
1 . 35(211,m)
20~1143
- 24 -
Blood flow-increasing and hypotensive actions were
evaluated for the representative compounds of the invention
by the method as described below.
(Experimental method)
-- Blood flow was measured unbloodily by means of an
electromagnetic blood flow meter for right vertebral artery,
right common carotid artery and left femoral artery of the
pentobarbital-anesthesized dog. Mean blood pressure was
measured from a cannula in a femoral artery with a blood
pressure transducer. The test compound was solved in saline
and was intravenously administered at a dose of 1 mg/kg.
Results of the test were expressed in terms of the
percentage of post-administration change from the value
prior to administration of a test compound.
Results of the measurement are shown in Table 2
below.
2 ~ ~2~ 3
Table 2
Percent ~%) Increase in Blood Flow Percent (%)
Compound Vertebral Common Femoral Decrease in
carotid Mean Blood
No.artery arteryarteryPressure
2 + 10 + 41 - -
12 +137 -~ 55 +80 -10
13 +158 + 50 +95 -16
14 + 69 + 18 +22 - 9
10 18 + 56 + 9 +46 -10
+ 36 + 11 + 2 - 2
22 +140 + 30 +97 -12
2~ + 80 + 50 - -17
29 tlO6 +113 - 25
15 30 + 97 +100 - -37
Useful pharmaceutical dosage-forms for
administration of the compounds of thls invention are
illustrated below.
Tablets (per tablet)
N-(2-Nitroxyethyl)-6-(4-methyl-1-piperazinyl)-
nicotinamide 10 mg
Lactose 67 mg
Crystalline cellulose 15 mg
Corn starch 7 mg
Magnesium stearate 1 mg
100 mg
The components were uniformly blended to prepare
powders for direct tableting. The powders were formed by
- 26 -
means of a rotary tableting machine to tablets 6 mm in
diameter each weighing 100 mg.
Granules (per pack)
N-(2-Nitroxyethyl)-6-(4-methyl-1-
piperazinyl)nicotinamide10 mg-
Lactose 90 mg
Corn starch 50 mg
Crystalline cellulose50 mg-
Hydroxypropyl cellulose10 mg~
Ethanol 90 mg
Component A was uniformly blended, to which was
added solution B. The mixture was kneaded. The kneaded
mass was graded by the extrusion granulating method and then
dried in a drier at 50~C. The dried granules were screened
1~ to a mesh range between 297 um and 1460 ~m to prepare
granules. One pack weighed 200 mg.
Syrups
N-(2-Nitroxyethyl)-6-(4-methyl-1-
piperazinyl)nicotinamide1.000 g
White sugar 30 000 g
D-Sorbitol 70 w/v% 25.000 g
Ethyl paraoxybenzoate 0.030 g
Propyl paraoxybenzoate 0.025 g
Flavors 0.200 g
Glycerin 0.150 g
96% Ethanol 0.500 g
Distilled water q.s.
Total to 100 ml
2 ~ 4 3
- 27 -
White sugar, D-sorbitol, methyl paraoxybenzoate,
propyl paraoxybenzoate and the active ingredient were
dissolved in 6Q g of warm water. After cooling, a solution
of the flavors in the glycerin and the ethanol was added.
5 To the resulting mixture was added the water to 100 ml.
Injections
N-~2-Nitroxyethyl)-6-(4-methyl-l-
piperazinyl)nicotinamide 1 mg
Sodium chloride 10 mg
Distilled water q.s.
Total to 1.0 ml
Sodium chloride and the active ingredient were
dissolved in distilled water to a total volume of 1.0 ml.
Suppositories
N-(2-Nitroxyethyl)-6-~4-methyl-1-
piperazinyl)nicotinamide 2 g
Polyethylene glycol 4000 20 g
Glycerin 78 g
Total to 100 g
The active ingredient was dissolved in the
glycerin. To the solution was added polyethylene glycol
4000 and the mixture was dissolved under heat. The solution
was poured into a suppository mold to prepare suppositories
each weighing 1.5 g.