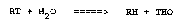Note: Descriptions are shown in the official language in which they were submitted.
2 ~ PM 89.025
APPLICATION FOR PATENT
INVENTORS: JOSEPH S. TANG, KELVIN N. WOOD and
BRAD C. HARKER
TITLE: SINGLE-WELL INTERNALLY CALIBRATED
METHOD FOR DETERMINING RESIDUAL OIL
SATURATION
Field of_the Invention
The present invention relates to a method of using a
pair of tracers to determine the in-situ residual oil
saturation surrounding a wellbore in subterranean
reservoirs. More specifically, the present invention
relates to the determination of the residual oil
saturation within a subterranean reservoir by injecting
into the reservoir at least one pair of chemical tracers
which are virtually identical except that one tracer
contains an isotope of hydrogen which is exchangeable with
water phase hydrogen atoms.
Backgro~und of the Invention
Typical oil reservoir formations are made up of rock
containing tiny, interconnected pore spaces which are
saturated with oil, water, and gas. Knowledge of the
concentrations of these fluids in the formation i~
critical for the efficient production of the oil. When
the formation is first drilled, it is necessary to know
the original oil saturation in order to plan the
exploitation of the field. Later in the life of the field
the amount of oil remaining in the formation will often
dictate the most efficient secondary and tertiary recovery
operations.
22658/20/1-1-1/900
`: : ` `'` "` '
2~LQ ~
Several methods are currently used to determine fluid
saturatlons of a formation. One techni~ue involves
coring, i.e., direct sampling of the formation rock and
fluids wherein a small portion of rock saturated with
fluids is removed and brought to the surface where its
fluid content can be analyzed. Coring, however, is
susceptible to several shortcomings. First, the small
sample may not be representative of the formation as a
whole since it only investigates the immediate vicinity of
the wellbore. Second, the coring process itself may
change the fluid saturations of the sample. Finally,
coring can usually only be done in newly drilled wells.
Another method of determining fluid saturations
involves logging techniques. This method also suffers
from the shortcoming of investigating a limited area which
is in the immediate vicinity of the wellbore. In
addition, logging techniques are often unable to
differentiate between properties of the rock and those of
its flui~s.
Another approach involves material balance
calculations based on production history. However, this
approach is susceptible to error because it requires a
knowledge of the initial fluid saturation of the formation
by some other independent means.
More modern method6 for determining fluid saturations
involve the injection and production of tracers. Many of
the techniques are ba8ed on chromatographic theory.
Typically, two tracers having different partition
coefficients are used. The tracers are
chromatographically retarded to different extents in the
formation. The degree to which the two tracers are
differentially retarded can be used to determine the
formation fluid saturations.
The two types of methods involving tracers are
single-well testing and interwell testing. Single-well
testing involves injection and production of the tracers
22658/20/1-1-1/900
, .
--3--
from the same well. Interwell testing requires injection
at one well and production from a communicating well.
A fundamental problem with single-well
chromatographic analysis is the "mirror image" effect.
The mirror image effect occurs where two or more tracers
having different partition coefficients are injected into
a formation. The tracers will separate as they are
injected into the formation because one tracer moves
faster than the other due to the difference in partition
coefficients. The degree of separation will be a function
of the residual oil saturation. However, when these
tracers are produced through the same well, the tracer
separation will usually disappear because the fastex
moving tracer will arrive back at the wellbore at
approximately the same time as the other tracer.
Several schemes were devised to avoid this problem.
In one technigue, the well is shut in for an extended
period of time after the injection of the tracers. This
technigue allows the tracers to drift, i.e. to move in the
formation under the influence of forces unrelated to the
injection or withdrawal of fluids at the well. When the
well is put on production, the tracers are somewhat
separated and a determination of fluid saturations i6 more
feasible. The problem with this technigue is that the
phenomenon i6 subtle and difficult to detect and measure.
~ nother way of avoiding the "mirror image" effect i8
to inject a nonreactive tracer along with a tracer
precursor. During the shut-in period, the preaursor is
allowed to react to form a tracer. The precursor and
corresponding tracer would have different partitioning
coefficients. During the injection phase, the precursor
and nonreactive tracer would move away from the well at
certain velocities determined by their partitioning
coefficients. During the production phase, the
nonreactive tracer would move back toward the wellbore at
the same rate that it went out, but the newly formed
tracer, which has a different partitioning coefficient
22658/20/1-1-1/900
:
~, . .
2 ~
from that of its precursor, would move at a rate different
from that of its precursor. The result would be a
separation at the wellbore of the two tracers. The
problem with this method is that it depends on chemical
reactions which are influenced b~ various factors, such as
formation t~mperature, salinity and acidity.
The mirror image effect can also be eliminated by
injecting into one well and producing from another
communicating well, i.e., interwell testing. S~ill,
single-well testing i generally less expensive than
interwell testing because interwell testing usually
involves re-completion of both the injector and producer.
Also, interwell testing may not be possible or practicable
unless at least two communicating wells are available in
reasonably close proximity to each other and in the
portion of the reservoir of interest. Thus, while
interwell testing could be effective, it would be more
desirable to measure residual oil saturation using a
single-well technique which is essentially free from the
defects outlined above.
Conventional single-well tracer techniques involving
tracer production profile matching, though fully proven
for sandstone formations, are unsatisfactory for
carbonates because current multiple-porosity reservoir
simulators are unable to handle the abnormal dispersion
and complex pore structures characteristic of carbonate
formations. There i8 still a need for a single-well
techni~ue which is more accurate than conventional
production profile matching and which allows residual oil
saturations to be calculated directly and inexpensively
from individual production samples instead of from
extensive production profiles.
Furthermore, present conventional techniques are not
applicable where there are serious losses of tracer
material. For example, such techniques cannot be applied
to gas lift wells where evaporation losses are
significant. There is also a need for a single-well
22658/20/1-1-1/900
-5- 2~
technigue which would include an internal calibration
standard so that accurate results could be obtained
without regard to any losses of tracer material to the
atmosphere or to other phases in the reservoir.
Conventional tracer separation techniques only work
for wells with an oil cut lower than about 5%. It would
also be desirable to have a technique which could be
accurately used in reservoirs having mobile oil.
SI~RY OF THE I NVENT I ON
The present invention relates to a process in which
fluid saturations of a hydrocarbon-containing formation
are determined by injecting a fluid containing at least
one pair of nearly identical tracers into the formation.
The tracers which constitute this pair have essentially
identical chemical and physical properties. The first
tracer used in the present invention is a chemical
compound with a labile, exchangeable hydrogen. The second
tracer is the same chemical compound as the first tracer
except that the labile hydrogen has been replaced by a
hydrogen isotope, i.e., deuterium or tritium. This second
tracer can be distinguished from the first tracer by the
presence of the hydrogen isotope.
In practicing the method of this invention, the pair
of tracers is injected into a well, allowed to soak, and
then produced back through the same well. While in the
reservoir, the second tracer exchanges its hydrogen
isotope for hydrogen in the water phase. The exchange can
only take place while the hydrogen isotope tracer is in
the water phase. The extent of exchange o the hydrogen
isotope for hydrogen will depend on the relative amounts
of water and oil phases in the reservoir.
In principle, the amount of oil and water phase in
the reservoir can be determined from the extent o the
hydrogen isotope/hydrogen exchange. However, determining
oil and water content from the isotope exchange rates
would require knowledge of loss and dispersion of the
22658/20/1-1-1/9oO
2 ~
isotopes in the reservoir. Use of the first tracer having
identical chemical and physical properties obviates the
need for precise knowledge of losses and dispersion
effects and serves as an internal standard for loss and
dispersion calibration.
The residual oil concentration can be directly
calculated for each produced reservoir sample by knowing
the relative amounts of injected and produced tracers,
their partition coefficient, the water phase hydrogen
isotope exchange rate, and the time that the tracers were
in the reservoir.
DETAILE~ ~SCRIPTION OF THE INVENTION
The chemical tracer used in this invention consists
of at least one pair of virtually identical chemicals.
More specifically, the chemical tracer consists of a first
tracer having a labile, exchangeable hydrogen and a second
tracer identical to the first tracer but having the labile
hydrogen replaced by a hydrogen isotope. The chemical
tracer can be any of the large class of compounds which
contain a labile, exchangeable hydrogen atom. Some
examples of such chemicals include, but are not limited
to, alcohols, carbonyl compounds with alpha hydrogens,
benzyl compounds, amines, and the like. The only
practical concern is the time it takes for a detectable
amount of exchange to occur. Generally, an exchange
half-life of from 3 to 15 days is acceptable. The
exchange half-life is defined as the time required for
half of the second tracer to exchange its hydrogen isotope
for hydrogen. Obviously, if the exchange rate is too fast
or too slow, it will not be possible to make a meaningful
calculation of the oil saturation. One skilled in the art
can readily determine if a tracer has an acceptable
exchange rate simply by performing routine lab work.
The preferred embodiment of this invention uses
tritium as the hydrogen isotope. Tritium is radioactive
whereas deuterium is not. Therefore, tritium can be
22658/20/1-1-1/900
2 ~ ~ ~
detected in much lower concentrations than can deuterium.
For simplicity, this disclosure will use the H-3 isotope
or tritium as the hydrogen isotope. It is understood,
however, that the invention is not intended to be limited
to the use of tritium as the hydrogen isotope.
In the preferred embodiment, a radioactive
counterpart of the chemical tracer is formed by replacing
the labile hydrogen with tritium. On contact with
formation water, the radioactive tracer will gradually
lose its tritium. The loss of tritium occurs through
exchange of the tritium for hydrogen found in the
reservoir water molecules and can be illustrated by the
following reaction:
RT + H2O =====> RH + THO
where, RT is the tritiated tracer, RH is the formerly
radioactive tracer which has lost its tritium through
exchange, and THO is the tritiated water which has
exchanged one of its ~,ydrogen atoms for the tritium.
It is to be noted that following the tritium/hydrogen
exchange, the formerly radioactive tracer, RH, becomes
identical to the non-radioactive tracer. In the method of
analysis to be described below it is necessary that the
amount of radioactive tracer injected be much lower than
the amount of non-radioactive tracer. The relative tracer
amounts should be chosen 80 that the amount of
non-radioactive tracer formed in the reservoir as a result
of the exchange reaction with the water hydrogen will be a
negligible fraction of the original non-radioactive
tracer. Preferably, the ratio of tracer to isotope should
be greater than about 1 litre to 100 milli Curies.
The non-radioactive tracer, which acts as an inert
carrier for the radioisotope, functions mainly as an
internal standard for loss and dispersion calibration.
The quantity of radioactive and non-radioactive components
is determined by the size of the formation zone to be
22658/20/1-1-1/900
,.. , . , .~ : : :
2~3 ~
tested, the nature of the reservoir, and the anticipated
losses and dispersion. The extent of the exchange in the
formation is governed by the length of the soak period and
the inherent exchange rate of the radioactive tracer
selected.
In the method of this invention, at least one pair of
tracers is injected into the reservoir as a slug, as a
series of slugs, or continuously. Several pairs of
tracers can be used simultaneously to improve the accuracy
of the measurement, and injection can be at a constant or
a variable rate. After injection, the well is shut in for
a period of time sufficient to allow lO to 90% of the
radioactive tracer to exchange its tritium for hydrogen.
Depending on the selection of tracer, this could be any
convenient period of time, typically 1 to 15 days. The
well is put back on production either at constant or at
variable rate. Produced reservoir samples are
periodically collected and analyzed for the presence of
both the radioactive and the non-radioactive tracers. The
residual oil concentration can be directly calculated for
each produced reservoir sample by knowing the amounts of
injected and produced tracers, the tracer water~oil
partition coefficient, the water phase tritium/hydrogen
exchange rate, and the elapsed time from tracer injection
until production.
In the absence of any tritium/hydrogen exchange, the
ratio of the non-radioactive chemical tracer to its
radioisotope in the produced fluid would equal the ratio
of the non-radioactive chemical tracer to its radioisotope
in the injected fluid. In equation form,
RHi/RTi = RHn/RTn (1)
where, RHi is the concentration of the non-radioactive
tracer injected, %
22658/20/1-1-1/goo
9 20~3 ~
RHn is the concentration of the non-radioactive
tracer in the produced fluid sample, %
RTi is the specific activity of the radioisotope
injected, dpm/cc
~Tn is the specific activity of the radioisotope
in the produced fluid sample, dpm/cc
By contrast, when exchange of tritium and hydrogen
does take place, the non-r~dioactive to radioactive
compound concentration ratios in the produced fluids and
the injected fluids will not remain equal. In that case,
the degree of exchange can be computed as follows:
1 - D = (RHi * RTn) / (RHn * RTi) (2)
where D is the degree of exchange, ranging from no
exchange (D - 0) to complete exchange of all the tritium
(D = 1).
The partitioning rate of the tracers between the oil
and water phases is normally much faster than the
tritium/hydrogen exchange rate. Since the exchange rate
is limiting, it is pos~ible to treat the partitioning step
as an equilibrium. The exchange reaction, which is the
rate limiting step, is a pseudo first order reaction and
is practically irreversible. The residual oil saturation
can thus be related to the two-phase exchange rate through
the following equation:
1 - D = exp~-Ke * t / (1 + BETA)~ (3)
where Ke is the tritium/hydrogen exchange rate
constant, 1/hr
t is the time available for exchange, hr
BETA is Kp * Sor / (1 - Sor) (4)
where Kp is the oil/water tracer
partition coefficient, and Sor is the
residual oil saturation.
22658/20/1-1-1/900
: : : . .
--10-- 2 ~ 1 r ~
In the above equations, "t" is the total time that
the tracers spend in the reservoir, or the sum of
injection time, soaking time, and production time at which
the sample is collected. The partition coefficient ("Kp")
of the tracer between the water and oil phases can be
accurately measured in the laboratory. The exchange rate
constant of hydrogen for tritium in the water phase can
also be accurately measured in the laboratory. The
residual oil saturation (Sor) can readily be solved by
combining equations 2, 3 and 4.
The principle of the invention and the best mode in
which it is contemplated to apply that principle have been
described. It is to be understood that the foregoing is
illustrative only and that other means and techniques can
be employed without departing from the true scope of the
invention defined in the following claims.
22658/20/1-1-1/900