Note: Descriptions are shown in the official language in which they were submitted.
NN-0220A
1
TITLE
Catalyzed Reparter Deposition
FIELD OF THE INVENTION
This invention relates to assays and, more
particularly, to catalyzing reporter deposition via
an activated conjugate to amplify the detector signal
thereby improving detention and/or quantitation of an
analyte in a sample.
BACKGROUND OF THE INVENTION
The introduction of immunodiagnostic assays in
the 1960s and 1970s greatly increased the number of
analytes amenable to precise and accurate
measurement. Radioimmunoassays (RIAs) and
immunoradiometric (IRMA) assays utilize radioisotopic
labelling of either an antibody or a competing
antigen to measure an analyte. Detention systems
based on enzymes or fluorescent labels were developed
as an alternative to isotopic detection systems.
Enzyme based assays proved to be more sensitive,
faster, less dependent upon expensive, sophisticated
instrumentation.
The need for diagnostic assays having simpler
formats, increased sensitivity with less dependence
upon sophisticated and expensive instrumentation
prompted investigators to try to harness the
catalytic power of enzymes to develop these newer
assays.
D. L. Bates, Trends in Biotechnology, pages 204-
209, Vol. 5 No. 7 (1987), describes diagnostics which
use a method of enzyme amplification to develop more
1
0»2790 11:12 LUFONT LEGHL N0.010 005
2
sensitive and simple immunoassays. in this method a
second ~nzyme system is coupled to the primary enzyme
label, e.g., the primary enzyme can be linked
catalyticaily to an additional system such as a
substrate Cycle or an enzyme cascade. Thus, the essence
of enzyme amplification according to Bates is the
Coupling of catalytic processes wherein an enzyme is
modulated by the action of a second enzyme, either by
direct modification or by interaction with the product
of the Controlling enzyme.
v.s. patent ~,6s~,62~, is'ued to ~oellga~t on
May 26, 1987, describes application of an enzyme-linked
coagulation as$ay (ELCA) to develop an amplified
immunoassay using the clotting cascade to enhance
sensitivity ~f detection of $.mmune complexes. The
process involues clot formation due to thrombin
activated fibrin formation from insolubilized fibrinogen
and labeled solubilized fibrinogen. Amplification of
th~ amount of reportable ligand attached to solid phase
is obtained only by combinia~g use of clotting factor
conjugates with subsequent coagulation cascade
reactions, One of the disadvantages of this system is
that it can only be used to measure the presence of
materials which modulate the activity of one or more of
the blood clotting factors. Another disadvantage is
that the primary enzyme, thrombin, cannot be immobilized
or coupled to a reporter or a member of a specific
binding pair,
U.S. Patent 4,463,090, issued to Harris on Juiy 31,
1984, describes a cascade amplification immunoassay
requiring a combination of at least two sequential
Catalyses wherein a first enzyme activates a second
enzyme which in turn acts upon the substrate.
mother amplification system is described in U.S.
Patent 4,598,042, issued to Self on ,7uly 1, ~g86, and
' 2
ll:lc uuru~m ttUHt rvU.010 006
3
. Patent Application No. 2,059,x21 which was
published on Apxi1 23, 1981, which disclose an
immunoassay using an enzyme label to produce directly or
indirectly a aubstanoe that is capable of influencing a
oatalytic event without itself being consumed during the
Catalytic ev~nt. Mor~ specifically, a primary enzyme
system produces or remov~s a substance capable of
modulating a secondary enzyme system which results in
amplification. The enzyme systems use unconjuqated
enzymes to avoid the tendency to inactivate certain
enzymes on conjugation.
European Patent Application Publication I~o. 123,255
which was published on pctober 31, 1984, describes
another cascade amplification immunoassay wherein a
zymogen-derived°enzymd is coupled to a zymogen-to-enzyme
cascade reaction sequence to obtain multiple stages of
amplification in producing detectable masker material
used to quantify analyte amount.
European Patent l~pplication Publication No.
199, 799, published ~Yune 19, 1985, describes a specific
binding assay based on enzyme cascade amplification
wherein the label component employed in the detectant
reagent ie a participant in or a modulator of an enzym~
cascade reaction whenein a first enzyme acts on a first
substrate to product a second enzyme. The production of
the second enzym~ can be followed or the second enzyme
can act on a second substrate to produce a third enzyme.
Similarly, U.S. Patent 9,318,980, issued to
Boguslaski et al. on MasCh 9, 1982, de9Cribea a
hetcrogenous specific binding assay using a conjugate
formed of a specific binding substance coupled to the
reactant, i.e., an enzymatic reactant. The ability of
the reactant to participate in the monitoring reaction
to detect the presence of analyt~ is altered by the
presence of the ligand in the medium. Thus, the
3
t~J~ c r ~ ?~~ m : 1 s 1W rUN I LtLar-rL (J0. 010
4
conjugate in its free state is more active in th~
monitoring reaction than in its bound state.
A heterogenous specific binding assay using enzyme
amplification is described in Eritish Patent Application
'S No. 1,901,29? which was published on ,Tuly 30, 19'75 and
L1.S. Pat~nt 4,376,825, issued to Rubenstein et al. on
March 15, 1993. Amplification is achieved by bonding
the compound to be assayed or a counterfeit of it to an
enzyme. The re$ulting enzyms--bound-liqand competes with
Eras ligand for specific receptor sites. When the
~nzyme-bound ligand is displaced by the free ligand the
enzyme is than free to react with a large numb~r number
of substrate molecules and the concentration of the
remaining substrate or of the product oan be measured.
1S PCT International Publication No, t~~ 81/00'125 which was
published on March 19, 1981 describes a method of
det~rmining a substrata is a sample which comprises
converting the substrata to a product in a first stage
of a cyclic reaction sequence and converting the product
back to the substrate in a second reaction stags of the
CyCliC reaction sequence. At least one of the first and
second reaction stages is enxym~ catalysed.
PCT Application having Tnt~rnational Publication
Number PTO 84/02193, which was published on June 7, 1989,
describes a chromgenic support immunoassay wherein the
analyte is contacted with an enzyme--labeled antibody and
in which the signal generated by the reaction of the
enayme with its substrata is concentrated on an active
support.
European Patent Application Publication No.
181,?62, published on May 21, 1986, describes a method
to determine enzymatic activity in a liquid sample by
particle agglutination or inhibition of particle
agglutination. .
4
rl.~ c ~ ~ 7U 1 1 ' 1 V L~urulv i ~tuH.~ N0. 610 009
s %.
Substrato/cofactor cycling is another exempla of
amplification which is based on the cycling og a cofactor
or substrate which is gen~rated by the primary 0nayme
label. The primary enzyme converts the primary substrate
to an active ~orm which can be cycled by two enzymes of
the amplifier cycle. These two enzymes and provided in
high concentration and ar~ poised to turn over high
concentrations of substrata but era prevented from so
doing until the cycling substrate is formed. The product
of the primary enzym~ is a catalytic activator of the
amplifier cycle which r~sponds in proportion to th~
concentration of substrate and hence the concentration of
th~ ~nxym~ label.
in th~r early sixties, dowry et al., Journal of
Biological Chemiat~ry, pages 2746-2755, Vol. 236, No. 10
(October 1962), dascsibad the measurement of pyridine
nucleotides by enzymatic cycling in which the coenzyme
to be d~termined was made to amplify an enzymatic
dismutation between two substrates.
p, more complex substrate cycling system is
described in U.S. Patent 4,75,054, issued to Rabin at
al, on May 17, 1988. The Rabin system involves using a
sme~li anzymically inactive peptide fragment of an enzyme
as a label and conjugated with the complementary
fragment to form an enzyme which catalyzes a pra.mary
reaction whose product is, or leads to, an essential
coenzyme or prosthetic group for a second enzyme which
catalyzes a secondary reaction leading to a detectable
result indicating the pres~nce of analyte.
vary et al., Clinical Chemistry, pages 1696-1701,
Vol. 32 (1986) describes an amplification method suited
to nucleic acids. This is the strand displacement assay
which uses th~ unique ability of a polynucleotide to act
as a substrata label which can be released by a
3S phosphosyla'e.
5
CA 02013214 2000-04-OS
6
SUMMARY OF THE INVENTION
The present invention concerns a method to catalyze reporter
deposition to improve detection or quantitation of an analyte in a sample by
amplifying the detector signal which comprises immobilizing an analyte
dependent enzyme activation system which catalyzes deposition of reporter
by activating a conjugate consisting of a detectably labeled substrate
specific
for the enzyme system, said conjugate reacts with the analyte dependent
enzyme activation system to produce an activated conjugate which deposits
substantially wherever receptor for the activated conjugate is immobilized,
said receptor not being reactive with the analyte dependent enzyme activation
system.
In another embodiment the invention concerns an assay for detecting
or quantitating the presence or absence of an analyte in a sample using
catalyzed reporter deposition to amplify the reporter signal.
This invention also concerns novel compounds which can be used to
prepare novel HABA type conjugates.
Further aspects of the invention are as follows:
A conjugate comprising a detectably labeled phenol.
A method for the detection or quantitation of an analyte in an assay
which comprises using an analyte dependent enzyme activation system
comprising at least one enzyme to react with a conjugate consisting of a
detectably labeled substrate specific for the enzyme system to form an
activated conjugate which covalently deposits substantially wherever at least
one receptor for the activated conjugate is immobilized, said receptor not
being reactive with the analyte dependent enzyme activation system, wherein
deposited detectable labels either directly or indirectly generate a signal
which
can be detected or quantitated.
A method for producing an activated conjugate comprising reacting a
peroxidase enzyme with a detectably labeled phenol.
CA 02013214 2000-04-OS
6a
A method for the detection or quantitation of an analyte in an assay
which comprises using an analyte dependent enzyme activation system
comprising at east one enzyme to react with a conjugate consisting of a
detectably labeled substrate specific for the enzyme system to form an
activated conjugate which deposits substantially wherever at least one
receptor for the activated conjugate is immobilized, said receptor not being
reactive with the analyte dependent enzyme activation system, wherein
deposited detectable labels either directly or indirectly generate a signal
which
can be detected or quantitated.
An assay for detecting or quantitating the presence or absence of an
analyte in a sample which comprises
a) immobilizing the analyte;
b) reacting the product of step (a) with an analyte-dependent
enzyme activation system;
c) reacting the product of step (b) with a conjugate consisting of a
detectabty labeled substrate to form an activated conjugate which deposits
substantially wherever receptor for the activated conjugate is immobilized,
said receptor not being reactive with the analyte dependent enzyme activation
system; and
d) detecting or quantitating the presence or absence of the analyte
in the sample.
An assay for detecting or quantitating the presence or absence of an
analyte in a sample which comprises
a) reacting an analyte dependent enzyme activation system with a
conjugate consisting of a detectably labeled substrate to form an activated
conjugate which deposits substantially wherever receptor for the activated
conjugate is immobilized, said receptor not being reactive with the analyte
dependent enzyme activation system; and
b) detecting or quantitating the presence or absence of the analyte
in the sample.
CA 02013214 2000-04-OS
6b
A compound which is 6-(phenoxy-(4'-azo-2"-carboxyethylphenyl))-
hexanoyl-alkaline phosphatase.
BRIEF DESCRIPTION OF FIGURES
Figure 1 is a graph comparing results of an HSV antigen assay run with
and without catalyzed reporter deposition.
Figure 2 is a graph comparing results of an HIV p24 core antigen assay
using conjugate concentrations of 0.2, 0.4, and 0.8 NI/ml (Amp 1, 2, and 3,
respectively). "HRP" represents a non-amplified assay wherein the detector
antibody was directly labeled with HRP. "Biotin" indicates another non-
amplified assay wherein the detector antibody was conjugated to biotin and
detected with HRP labeled streptavidin.
Figure 3 is a graph of a mouse IgG assay run using an HRP ADEAS to
catalyze deposition of biotin-tyramine
which was det~cted with streptavidin-HRP (HRP~l~mp Hgp)
or with sir~ptavidin-AF (HRP-imp AP). The as'ay was
also run using only 1~F~P lab~led dctectox antibody or AP
lab~lod deteotor antibody.
Figure ~ prosonts two graphs comparing results
obtained from a Du Pont*8iV p24 antigen ELISA run with
and without using catalyzed reporter d~position to
amplify reporter signal.
~'igurs 5 is a graph comparing r~sults of a mouse
IgG assay without catalyzed reporter deposition (HIBP)
and with catalyzed reporter deposition (HRF~-~-Oal).
Figuxe 6 depicts a preparation of ethyl
2_(q._hydroxyphenylazo)ben~oat~-6valkaline phosphatase
(HEE-6-AP), the synth~sia of which is described in
Example 10.
~°igur~ '7 i~.lustrat~s the ~sterase catalyzed
conversion of IiEE to 2-(d°-hydraxyphenylazo)benzoic acid
(FiA9,~) /str~ptavidin complex.
~'he.term analyte dependent enzyme activation System
(SEAS) refers to an ~nxyme system wher~in (i) at least
ono enzymQ is coupled, in any manner known to those
skilled in the art, to a m~mber of a specific binding
pair, or (ii) the enzym~ need not be coupled to a member
of a specific binding pair when it is the analyte. Th~
enzym8, 8ither by itselg or in connection with a second
enzyme, catalyzes the formation of an activated
conjugate which then is deposited wherever a receptor
far the activated conjugate is immobilized,
The term amplification as used herein means
amplification of reporter signal due to d~position of a
Conjugate activated by an F~DEAS.
* trade mark
7
~~~~~ c n ~ =m 11 ~ ao L~VrUI~ i LtUNL NU. 010 H11
~~~.~~ ~!.~
S
Th~ term conjugate means a datactabiy labeled
substrate specific for th~ Id~EAS whether it ba a single
enzyme ,~pEAg or mufti-~nzyma ADEaS. Tha substrate must
have at least one component but is not limited to such.
For exempla, the substrat~ can consist of two
components. One component contains th~ binding site for
ths~ r~ceptor and is dat~ctably labeled. The oth:r
component is a constituent which prevents or interferes
with binding to the receptor until such tim~ as the
AREAS prim~s the conjugate as is discussed below.
Another example of a Conjug~,ta is biotin-tyramine
wherein tyramine is the substrate portion and biotin
aonetitut~s the detectable label as described below,
Conjugates are described in greater detail below as
well.
The term datectably labeled means that th~
substrate can ba coup.led to either a reporter or to an
unlabeled first member of a specific binding pair
provided that the reporter introduces a dilf~rant moiety
to th~ substrate as is discussed below. S9hen the
substrate is coupled to an unlabeled member of a
speoific binding pair, gollowing deposition, the
aubstrate~apecifiC binding partner complex is reacted
with the second member of the binding pair which is
coupled to g reporter. Alternately, the substrata-
specific binding partner complex can ba pre-reacted with
the detectably labeled other member of the specific
banding pair prior to deposition.
The term deposition means diraoted binding of an
activated conjugate to the receptox which results from
either the formation of a covalent bond or a specific
binding pair interaction as described below.
~'he term receptor means a site which will bind to
the activated conjugate either through the formation of
8
t~~ i G r ~ ?C~ 11 : 1 r LrUt'UN I LtUwL NU. 010 012
i. ~ ~ ~. t.~
9
a covalent bond or a specific binding pair interaction
as described b~low.
The term activated conjugate means that the
conjugate has been primed by the ApEAS to bind with the
r~ceptor.
One of the unique features of this invention is the
analyte dependent enzyme activation system which
catalyzes deposition of conjugate by converting th~
substrate portion of the eoajugate to an activated form
which is deposited wherever e~ specific receptor for the
activated conjugate is immobilized. ~ehe AREAS dose not
utilize enzyme cascade reactions or enzyme cycling to
eff~ct amplification. Rather, it uses either a single
enzymes or combination of enzym~s to activate the
35 conjuc~at~. Deposition of conjugate occurs only if the
analyta and analyte dependsnt enzyme activation system,
which can be the same if the analyte is an enzyme, for
exampl~ in the detection of an enzyme such as alkaline
phosphatase, or different, have been immobilized and a
r~ceptar, as described below, is immobiliz~d to bind the
activated conjugate. Thug, the At~EAS, conjugate, and
receptor are chosen to form an operational trio.
The following is one embodiment of a single enzyme
AREAS system applied to a forward sandwich immunoassay
format; the test sample containing the analyte is
reacted with an immobilized capture reagent, such as an
antibody, excess reagents are wash~d off; the
immobilized capture antibody-analyte complex is reacted
with an AD~AS, such as a second antibody specific ~or
the analyte which has been coups~d to an enzyme, e,c~,
horseradish peroxidase (HRpy, alkalia;e phosphatase (APy,
etc. The AREAS will bind only if the analyte has been
bound by the capture reagent. Otherwise the reagents
w~.ll be washed off. Coupling of the enzyme to a
specific binding partner does not affect the enzyme°s~
9
c~_,~~.~=m ~~~..~ Lmryvi LCUrt~ NU.bll1
F~ ~. e~3 t;, ~ ~~
1~
ability to react with the substrata portion of the
conjugate. MTh~n conjugat~ such a's biotin-tyramine or
HA~9A-tyramine e~nalog (~.g., N-(q~~o-hydroxyphenethyi)--6-
(phenoxy-(q~~azo-2'~-benzoic acid~)hexamide) is added to
the immobilized capture antibody-analyte-second
antibody-enzyme compl~x, the enzymes r~acts with the
substrate portion of the conjugate, e.g., with the
tyramine portion of the conjugate, convestinq it to an
active form which will bind to an immobilized receptor
which is either endogenous or eacogenous to the assay
syat~m. The amount of conjugate deposited will be a
function of immobilized AREAS. t~eposited conjugate such
as biotin-tyramine ar FIASA tyramine analog can then bs
detected by reacting with streptavidin-F3A8 and
orthoph8nylmnediamine. Th~ term FiABA-tyramine analog
means, g~nerally, unsubstituted or substituted FIAr3A,
coupled with or without a spacer, to a hydroxy-phenyl
containing compound such as tyraming. If the conjugate
la liuva~suelm-~.yramin~ cnen the peppglteCl Con~ugat~ can
b~ det$Cted dirgotly, or following reaction with a
labeled anti-fluoreacein antibody,
Thus, the AREAS is used to catalyze the deposition
of detectably labeled substrata (the Conjugate) to
generate additional signal. The AREAS is detected
directly as part of the overall signal when the enzyme
component of the AREAS is the same as the enzyme used ~as
the reporter. Figure 3 illustrates this situation as
wdll as the situation where an AD~p,S enzyme component
and reporter enzyme are different and thus, the AREAS
enzyme component is not detected directly as part of the
overall signal.
A multi-enzyme AREAS immunoassay fermat would
involve a similar approach. In the example above, the
ApEAS can be an antibody coupled to an enzyme such as
AP. In addition to the immobilized capture asntibody-
F~J~tli7t~ A1:1C LUh'I.INI LtL~NL NO.C~10 0lLj
~~.~~~i~.
13.
analyte-second antibody-Ap complex, a second enzyme such
as H~ could b~ immobilized on the support. Th~
conjugate can be a d~tectably labeled phenylphoaphate
which cannot. seact with Fill until it is
dephosphorylated. hP dephosphorylates the phenol which
th~n is fees to react with the immobilized HRP to gosm
an activated pheraolic con~u$ate which deposits wherever
receptors aze immobilized. After removing excess
reagent, deposited reporter is detected and c~uantitated.
Alternatively, HRP can be coupled to the second antibody
and AP can be immobilized on th~ surface of the support.
Th~ instant invention is surprising and unexpected
because amplification of reporter signal is obtained via
d~sposited activated conjugate without using cascade
13 mechanisms os enzyme cycling. The ADEAS reacts with the
conjugate to form an activated conjugate which will bind
with immobilized receptor specific for the activated
conjugate. The amounts of receptor and activated
conjugate are in excerss of the amount of ADEAS
immobilized.
The choice of an ADEAS is governed by the ability
of the enzyme or enzymes to convert a conjugate to an
activated form which will bind to an immobilized
receptor whether endogenous or exogenous. Accordingly,
a detailed knowledge of catalytic properties of each
specific enzyme is needed in order to properly design
the substsaC~ and receptor. Other important factors
include availability of the enzyme or enzymes, relative
ease or difficulty to couple it to the member of a
specific binding pair, stability of the enzyme or
enzymes as well as the stability of the conjugate and
the receptor. ~n some cases, an ADEAS can be purchased,
depending on the assay format.
Enzym~s suitable for use in an ADEAS include
hydrolases, lyases, oxidoreductases, transferases
11
tJ» C ! i 71:~ 1 1 : 1 7 L~Ur' U[V I LGUHL N~. 81
12
isomeras~s and liga$~a, There can b~ mentioned
peroxidase, glucose oxidase, phosphatase, esterase and
glycosides~. Specific examples include alkaline
phosphatase, lipase~s, beta-galactosidase, horseradish
p~roxidase, ;and porcin~ liver este~ras~,
Memb~ra of specific binding pairs suitable for uaa~
in practicing the invention can be of the immune or non-
immune type. Immune specific binding pairs axe
ex~ampiified by antigen/antibody systems or hapten/anti-
hapten systems. The antibody member, whether
polyclonal, monoclonal or an immunoreactive fragment
thereof, of the binding pair can be produced by
customary m~thods familiar to those e~cili~d in the art.
The terms immunoreactive antibody fragment or
13 immunoreactive fragment mean fragments which contain th~
binding region of the antibody. Such fragments may be
Fab-type fragments which a.re defined as fragments devoid
of the Fc portion, e.g., Fab, Fab' and ~'(ab')2
fragments, or may b~ ao-called "half-molecule" fragments
obtained by reductive olaavage of the disulfide bonds
connecting the heavy chain components of the inteect
antibody. If the antigen member of the specific binding
pair is not immunogenic, e.g,, a hapten. it can be
covalently coupled to a carrier protein to render it
immunogenic.
Non-immune binding pairs include systems wherein
the two components shar8 a natural affinity for each
other but are not antibodies. Exemplary non-immune
binding pair3 are biotin-avidin or biotin-streptavidin,
folic acid-folate binding protein, complementary prolbe
nucleic acids, etc. Also included are non-immune
binding pairs which form a covalent bond with each other
but are not antibodies, Exemplary covalent binding
pairs include sulfhydryl redctiv~ groups such as
maleimides and haloacetyi derivatives and amine reactive
12
k'1Ji G ( i 7th 1 1 : ck~ LUYkIlV I LtUHL r/G. 010 8
~el ~ ~~.. C,a
13
groups such as isothiocyanates, succinimidyl eaters and
sulfonyl halides, etC.
Suitable supports us~d in assays include synthetic
polymer supports, such as polystyrene, polypropyl~ne,
substituted polystyren~, e.g,, aminated or carboxylated
polystyren~; polyacrylamidesJ polyamides;
polyvinylchlorid~, etc.d glass beadsi agarose~
nitrocellulos~, etc.
l4nother important component of the invention is the
conjugate, i.~,, a detestably labeled substrate which
must be specitio for the ADEAS. As was stet~d above,
when the conjugate reacts with the AREAS, the enzyme ar
esnzymss catalyse formation of an activated conjugate
which binds wherever a receptpr is immobilized whether
exogonaus or endogenous, An immobilised exogenous
receptor means a receptor which does not originate
within the assay. Tt must be immobilized on the surface
of the support prior to adding the oon~ugate to the
reaction mixture. An endogenous rea~ptor means a
receptor which originates within the assay and does not
require immobilization prior to adding the conjugate
because the receptor is immobilized Within the assay
system,
For example, when an HRP 1~DEAS (H~ coupled to a
member of a specific binding pair) is reacted with
conjugate containing a phenolic substrate, an activated
phenolic substrate is produced, It is b~lieved thnt the
activated phenolic substrate binds to electron rich
moieties such as tyrosine and tryptophan present in the
proteins on the solid support. However, if a cliff~rent
conjugate is used, such as a labeled 3-methyl-2-
benzothiazolinone hydrazine (MBTH) which is discussed
below, a receptor, such as 3-(dimethylamino)benzoio acid
(DMAB), must b~ immobilized prior to addition of
oonjugato.
13
r~s~ c r ~ 7r~ 11: ~u LUPpNI LE(iAl.. N0. 010 01'7
14
Another embodiment involves reacting a conjugate
which becomes pho~phorylated by an Ad9EAS. Th~ activated
fphoaphorylatodD conjugated can then rgaet with sn
antibody sp~cific for the~aCtivated Conjugate,
yn moll another variation, an ~DHAS can be reacted
with a conjugate Consisting of a component which when
activated will bind to a receptor and which ie Coupled
to a component having a thiol reactive group such as a
maleimid~. The deposited mal~imide moiety can then be
d~tected by reacting with a sulfhydryl-containing
r~porter which Can be endogenous to the reporter, e.g.,
beta-galaCtoaidas~, or the sullhydryl groups can bo
added to reporters such as H12F or AP using thiolating
reagents such as N-'uGCinimidyl~S~acetylthioacetate
1~ (~~T~), g-aCetylmercaptosuccinic anhydride (SAMSA1, or
eaCCinimidyl-3-(acetylthio)-propionate (9ATP),
Alternatively, the ~eubetrato can b~ coupl~d to a
protected sulfhydryl containing group and this can be
used as the conjugate. After binding to the receptor,
this can be deprotected using conventional techniques
known to those skilled in th~a art. Detection can be '
effected using s reporter having a thiol reactive group
such as maleimide-HRP or iodoacetyl-gIIZP.
Another alternative is to use a conjug$te wherein
the substrate has two components as described above, a
datectably labeled first component which will band to
the receptor after the second compan~nt has been
activated or removed by the AD>;AS. An example of this
is a small organic molecule such as 2-(4'-hydroxy-
phenylazo)-benzoic acid (HA13A) which binds specifically
to avidity and streptavidin. The terms avidity and
streptavidin are used herein int~rchangeably. ~I~~ oan
ba detectabiy labeled using any of the reporters
described below, e.g " radioisotopes, enzymes, etc. Per
instance, alkaline phosphatase (AP) can be conjugated to
14
t9.~~ G l i 710 11 : G1 LK.It'U~V I l.tl7HL ~U. 0S 0 016
~3 '~ r.
ld .~ zc ~ a ~.3..
HABA, using techniques w~11 known to those skilled in
th~ art, with or without a spacer, to a functional group
on NASA. An exd~tpl~ of such a functional group is thd~
~°-hydroxyl moiety, Nloreovsr, NASA can ba modified to
5 possess a second component which prevents binding until
it has been removed by the AREAS. F'or exempla,
estsrification of HABA with ethanol produces a Hd~A
ethyl ester which doss not bind to straptavidin.
~etsctably label~d A ethyl asters will not bind to
10 strl~pt,avidin until the aster $soup has bean hydrolyzed
to the corresponding carboxylic acid. ~3ydrolysia can bs
off~cted using an enzyme such as an esterase, ~e.g.,
porcine liver esteraas. 3~hu8, detestably labeled HAHA
~stsrs such as 6-(phenoxy-(~'-azo-2°'-
15 carboxyethylph~nyl)-hexanoyl-alkaline phosphatase can be
depoeitsd using an AREAS having a suitable esterase
which will hydrolyzo the ester to paranit binding of
de~tsctnk~ly label~d HASA with streptavidin (i.~.,
exogenous receptor) which ha3 bean immobi~.ized on the
surfac~ of a support.
Compounds of the formula
0
II
a5 wher~~.n
Rl through R~ arm the same or difi'erent and
are selected from the group consisting of straight chain
i~
h~~cr~7y ll:ec UUt'UN1 LEG~sL N0~010 019
as
or branched alkyl groups having 1-~ carbon stoma, F, Cl,
Rr Os 3 j
2 is phenyl or naphthyl~
Y1 is 1«iy
x is P1, 0, ~; and
R~ can b~e H ar straight chain or branch~d
alkyl group having 1-9 carban atoms can be used to
synthesize t3ARA-type conjugates as d~scribed in thg
examples below. The term F3AEA-type conjugat~o m~ans A
derivatives: gi) which can b~ substituted or
unsubetituted and are coupled with a spaoe~r t~ a
reporter and (11) which contain an .SEAS activatabie
moiety that prevents the conjugate from binding t~
Stseptavidin until it has been activated or removed by
the ADEAS. The synthesis of such a compound is
illustrat~d in Figure 6 as w~11 in Example 10 below.
fibs approach described below can be modified by those
skilled in the art qen~rally to synthesise any of these
compounds using procedureB well known to those sk111ad
in the tart .
Other small organic molecule/receptor combinations
which are suitable to praot~.ce th~ invention include
haptens/antibodies, sugars and oligosaccharides/lectins,
biotin and dyes/avidin and/or streptavidin.
A8 is shown in 'Table 1, a number o! receptors are
available. The choice o~ a receptor will depend upon
the aonjugat~ seleoted.
The optimal concentration of conjugate is
determined according to the procedure explained in
Example 1. Optimal concentrations will vary depending
upon enzyme used in the ADEAS and substrate selected to
produce conjugate.
Conjugate can be synthesized using conventional
coupling and labeling techniques. substrate choice will
depend upon the ADEAS selected. To reiterate, detailed
~. 6
t~_~~ ~ ~ ~ . c.. i i . cc uurl4~ i LtUh-1~ NU. 1~11'J 020
~f'~9 r 6~ '[~?'~ '~
PJ~, ~ ~. C.
17
knowl~dg~ is required of the catalytic properties of
~ach specific en$yme in order to propegly d~sign a
useful synthetic substrate and, if necessary, a
receptor.
A wid~ variety of reporters .are available for
coupling to the substrate to produce tha conjugate or to
couple to a member of a specific binding pair. As ryas
discussed above reporter should introduce a different
moiety to th~ substrate. Reporters can be a radioactive
ieotopt~, such as, 125g~ enzymes, fluorogenic,
chemiluminescent, electrochemical or magnetic materials.
Interrit~lly labeled reporters (e. g., tritium ar other
such radionuclides) which do not introduce a differ~nt
moiety to the substrate are not contemplated for
practicing the invention.
Examples of reporter enzymes which can be used to
practice the indention include hydrolases, lyases,
oxidoreductasea, transferases, isomerases and ligases
some preferred examples are phosphatases, esterases,
glycosidases and peroxidases. There can be mentioned
peroxidase, glucose oxida,sa, phosphatase, esterase and
glycosidase. Specific examples include alkaline
phpsphvtase, lipases, beta-galactosidase, horseradish
peroxidase and porcine liver esterase. As was noted
above, if an enzyme is used as a reporter, it can be the
same as ox different from the enzyme or enzymes used in
the AREAS. ~'he instant invention can be used to
catalyze deposition of a radioisotopicaily labeled
conjugate or an enzyme-labeled conjugate, etc.
Another embodiment of the forward sandwich
immunoassay descrimed akrove would involv~c reactzng a
capture-antibody-analyte-second antibody complex with an
AREAS consisting of an anti--antibody coupled to an
enzyme such a9 HRP or AP. The anti-antibody would bind
an epitope on the second antibody.
17
t~» G I ~ Jt' 1 1 . Gs murur~ i ~tuHL NU, 010 021
18
Thin invention is net limited to sandwich
immunoassays. Tt is applicab~,~e to a widt~ v~ri~ty o!
assay lormata, !or example, nuc~.aic acid hybridisation
assays for both RD1A and ~~a~.
To lurth~r illustrate the invention, ~xamplaa ~!
singi~ and mufti-enzyme ~E~S', con~u~atas, receptors,
and race~ptor types ar~ present~d in Table 1 below.
18
~,5~~r~7U 11:24 DUPONT LEGAL N0.010 022
~~~~t
19
''°
°'
a~ ~ ~
a 'v ' ~4 ~N
19
11:24 PUPONT LEGHL N0.010 0
In th~ AP/~IRP mufti-enzyme ADEAS described above,
the conjugate must be dephosphorylated befors it wfli
react with HRpd and in the ~-gal/HRP mufti-enzyme ADE~1S,
the con~ugat~ moat be deglycosylated before it will
5 react with HRP.
=t should b~e clear to those skill~d in the art that
a larg8 numb~r of variations era possible and all these
variations fall within the scope of the invention.
The following examples are intended to illustrate
10 the invention. Unless otherwise indicated, 100 dal of
alI reagents were used. The one exception was that
200 ~,1 of blocking buffer was used,
15 prenar~tion of ~bni~ca~ and
pare-hydroxypheny7.propionyl biocytin (IiPPB) was
prepared by mixing a solution of p-hydroxyphenyl-
propionic acid-N-hydroxysuccinimide ester (50 mg [0,2
2D mMoi)/2 ml dimethyi sulfaxide) with biocytin (70.75 mg
[0.2 mMol,/2 ml 0.1 M NaHC03) overnight at room
temperature (RT). Biotin-tyramine (BT) was prepared by
mixing a solution of tyramine (40 mg (0.3 mMol~/1 ml
dimethyl sulfoxide) with biotin-N-hydroxysuccinimide
ester (100 mg [0.3 mMol)/1 ml dimethyl sulfoxide)
overnight at RT. The solutions of FiPFB and BT were used
as is. 'The Calculated conc~ntrntions were 26 mg/ml for
HPPB and 55 mg/ml for HT,
polystyrene EIA strips (N(JflG) were coe~ted witty
polyclonal anti-Herpes Simplex virus (HSV) antibody
(nako, Carpenteria, C~) in 0.1 M carbonate buffer pH 9,6
overnight at 4°C, and them block~d with Z~ bovine sexum
albumin (BSA) in carbonate buffer and then washed with
10 mM phosphate buffered saline, 0.05% Tween 20, pH 7.4
(pBST) , !~ dilution of HSV antigen in 1% BS,~, 10 mM
e3~~1~~~ 11:5 DLIPONT LEGHL N0.010 024
21
phosphate buffered salineo 0.05 Twe~n 20 pH 7.4 (BSA-
PBST), or buffer without antigen, was incubated for 1
hour at 37~C. The dilution waa sufficient to obtain the
optical densiti~s in the range reported in Tabie 1. It
was washed with PBST. The analyta dependent enzyme
activation syr~tem consisted of HRP coupled to anti-HSv
~HRP AOBAS) which was purchased from Dako. The HRP
ADBAS was added and incubated for 30 min, at RT and was
washed with PBST. Various conoentrations of HPPB or Bx
as set forth is Table 1 below, ware added in 50 mM tria-
HCl, 0.019 Hg~2, pH 5.0, for 15 min. at RT. After
washing with PBST, streptavidin-HRP was added and
incubated for 13 min, at RT to react with deposited
biotins. The plate was then washed with PBST. l~n HF3P
13 substrate, a-phenylenediamine (OPD), was added,
incubated for 30 min. at RT, and atopp~d with 4 N HZS04,
Optical d~naiti~s at 990 nm were recorded on a
miorotiter plate read~r.
ao
Result' are presented in Table 2. Column 1
presents the various concentrations in ul/ml of HPPB or
8T. Columns 2 and 3 present the optical densities
r~corded as a function of HPPS concentration. Columns A
25 and 5 present the results obtained using BT.
HPpB and BT were oonverted to activated forms by
HRP AbEAS. Catalyzed reporter deposition was achieved
without immobilizing a receptor.
In choo'ing the optimal concentration, one must
30 look at both the magnitude of signal amplification as
wall as the signal to noise ratio. With this in mind,
the optimal concentration of HPP$ was 20 H1/ml
(approximately 0.5 mg/ml), and that of $m, was about 0.3
~1/ml (approximately 16 ~g/ml).
21
~~y, ~ ": U 1 ~, : ~e UUF'UtV 1 LEGAL N0. 010
22
canc . IiF~P~
3 ar sa imps CorravG~TE PT cot~,~tl~~T~
c~l/mi~ xsv ~ut~~r xsV xu~f~r
cw/a Ag' cw/~ ~g>
*
p 0.079 0.031 0.079 0.031
20 1.155 0.181 0.700 0.165
10 0.904 0.140 __ _~
5 0.99 0.120 2,060 0.430
2.5 0.177 0.063 __- ~-
' 1.25 0.113 0.062 2.230 0.502
0.625 0.103 0.048 -~ _-
0.313 -- --_- 1.850 0.169
0.078 ~- -- . 0.263 0.051
0.020 -- - 0.090 0.040
* w/o ~,g ~ without tigen
an
AntioHS'V coated EIA etrips were prapar~d as
desCrib~d in ~xampia 1. ~ 1.:100 dilution of ~1SV anti~~n
wa~~ prepared and serially lour-fo7.d diluted. These
dilutians of HSV were incubated for 2 hours at 37'C with
the antl-xSV coated ET~1 stripe. Excess r~agant was
washed ofg with PBST. Th~ SEAS was the same as that
described in ~xampla 1 aboee. It was added to the anti-
xSV coated EIA strips Containing the anti~HaV_F?SV
complex and incubated !or 30 min. at ~tT and th~n w~.shed
with PSST. 20 ~1/ml o~ HPPB conjugate as determined in
Exempla 1 wao added in 50 mM tris-HC1, 0.0~.~ Ha02, p&I
6.0, and was incubated !ar 15 min. at RT and than washed
22
u~: ~ ~ : ~u ~ ~ : co uurutv i LtIaHL NU. 010 026
23
with PB9T. Dctposited biotin~9 were re$cted wi~Gh
9treptavidineHRP iSA-HRP) for 15 min. at R'T. It was
wash~d with PBST. The substrata, OPD, was added and
incubated 30 min, at AT, stopped with 4 N FizSO~, and the
absorbance at 490 nm was record~d on a microtiter plats
read~r.
I4on-amplified ~~~~ys were run in which (a) no H~P~H
and no SA-HIaP were used; (b) HPPE was used without SA-
HRP= ta) S.~r-HRP was used without HPPH.
The results shown in Figure 1 demoa~strate that
(a) catalyzed depositioa~ of reporter was obtained and
(b) both the conjugate and SA-HRP were needed for
detection b~cause the conjugate contained an uniabel~d
member of a ~pecifiC binding pair.
Results for the non-amplified assay (no I~PpB, no
SA-HRP) were plotted. The results for the other assays
were not plotted because the additional plots would
overlap with the non-amplified results already plotted.
Polystyrene EIA stripe (NUtdC) were coated with
rabbit anti-HZV p24 antibodie~ in 0.1 M carbonat~
buif8r, pH 9.6, overnight at 4°C, send th~n blocked with
2% BSA in carbonate buffer gollowed by washing with
PEST. HIV antigen was incubated for 2 hours at 37°C
(concentrations are indicated in E'igure 2). The plate
was then washed with PHST. A rabbit anti-H=V p24-HRP
analyte dependent enzyme activation system was then
incubat~d for 2 hours at 37°C, and washed with PBST.
various concentrations of BT conjugtate, (0.2, 0.9, and
23
b~~ ~ r~ ~u 11: a r DUFONT LEGr~L N0. 010 027
!a ~~ . _~,. -e~ %v .~. ~~~
24
0.9 ttl/ml) in 0.1 M borate buffer, 0.01 Fi2~~, pH 9.5,
were incubated for 15 min, at RT faliowed by washing
with FPS'. Then streptavidin-F3&tP was incubated for 15
min a ~t RT .
S ~g a comparison, a biotinylated anti-x=V p24
antit~ody was used, and detected with str~ptavidin-xRP.
OPD was added and incubated for 30 minutes, stopp~d with
9 N H~S~O~, and optical densities at 490 nm were record~d
on a microtit~r plate reader.
The results are shown in Figure 2 where Amp 1,
Amp 2, and Amp 3 refer to RT at concentrations of 0.2,
0.4, and 0.9 ~llmi respectively. Different lev~ls of
25 amplification w~sre achi~ved using catalyzed report~r
deposition depending on the concentration of conjugate:.
pigure 2 also presents results far a non-amplified
assay using a biotinylated antibody/SA-xRP detection
system (biotin) and a non-amplified assay wherein anti-
2o x~v p24 deteotor was directly labeled with aR~. ~h~
results obtained using the anti-xTV p24-xRP detector
were inferior compared to the significant increase in
detector signal obtained using catalyzed reporter
deposition.
25 Depending upon the concentration of conjugate,
signals a8 good, and greater, as those obtained with the
biotinylated antibody were obtained using th~ cataly$ed
reporter deposition method of the instant invention.
Best results were obtained using conjugate concentration
30 near the optimal amount s:s was determined in Rxa.mple 1.
g,~eDarat on ~n Pharao~!,;r~t .o Biotin. Tyr~gine
preparation of biotin-tyramin~: a solution of
35 biotin-N-hydroxysuccinimide, 170 mg (0.5 mMoles), and
2a
d~,~I,~L~ 11:2g DUFUNT LEG~iL N0.010 028
~~~.~-~~~"~~
tyramine (recrystallized i'rom water), 5~.5 mg (0.5
mMol~s), in 25 ml dim~thylformamide was treated with
10 ml of 1 M triethylammonium bicarbonate, pH 7.5, and
then heated at 50°C for 3 hours.
5 isolation: the solution was concentrated to dryness
on a rotary ~~raporator, and th~ residue was
recryatailixed from water, with a yield of 72~.
Ch,sraaterizationt the melting point was determined
to b~ 192193°C .
FXA'MPl.fl! S
~t i ~ aid ~.~~5~~,~ e~ g,'~~A Ma~9
Asia ~ ~aa~ r~at~,ly~~Pd Rer~or .er ~eposition
Polystyrene CIA stripe tNt7NC) were Coated with goat
anti-mouse zgG (Fa fragment specific) antibody (TCt~) in
0.1 M carbonate buffer pH 9.5, overnight at R'I. They
were then biock8d with 2~ eS~1 in carbonate buffer and
washed with PHST. Dilutions of mouse Ig~ in BSA-~P8ST
were incubat~d in the wells for 1 hour at 37°C followed
by washing with pBST. Conc~sntrations are set forth in
Figur~ 3. Goat anti-mouse Ig~-HIP (HRp A~~~1S) and goat
anti-mouse IgG-alkaline Pho9phatase d~ ~8~)
(8oehringer Mannheim) were diluted as recommended by the
manufacturer and incubated for 1 hour at 37°C. Assays
were run with and without oat~alyzed r~porter deposition.
The AP ADEPuS was not used to catalyze reporter
depo$ition in this experiment.
For catalyzed reporter deposition using the 1~RP
ADEAS, a 1 mg/ml stock solution of biotin tyramine (as
described in Example 9) in dimethyl sulfoxide was
prepar8d, and then added to a 0.1 M borate buffer,
pH g.5, 0.01% H202, at 10 ~t7./ml (10 ug/ml biotin
tyramine) and incubated for 15 min. at RT. "Ths plate
was then washed with PHST. Streptavidin-filtP (for HFtP-
Amp HItP), os streptawidin-Alkaline Phosphatase (for HFtP-
e, ,~ ~ r ~ ~n 1 i : ~a L~uruN i i_EGH~ IdO. 010 029
;,u ~. a~ ~ .> >.3
zs
Amp Alk Phos) were incubated for 1S mia, at RT and the
plate was washed with P9ST. Spectrophotometric
detection was achieved after the addition of oPD (for
HRF~), or p~nitrophenyl phosphat;r (for AP) for 15 min. at
R~'. Reactions wer~ stopped by the addition of A N HaSfJ~
(xRF~/OPD), or 1 N NaOH (Alk Phoa/pNph). Optical
densities, at 490 nm for ~iRF~/OHD and 405 nm for Alk
Phos/pidPP, were recorded on a microtiter plate reader.
The results are shown in figure 3. As is apparent,
one Can achieve signal amplification with a concomitant
lower detection limit by allowing the HRP t~ERS to
catalyze deposition of an activated ET conjugate
followed by detection with atreptavidir~ coupled to HRp
or AP. Thie exempla shows that if the reporter is an
enayme, it can be the same as, or different from, the
~nzyme used in the ADEA.9.
E, , 6
~~thhict'1 y~~lizPa, A H~o~~~irl~ ~ t~~r~n~r,.
Antibod~ /r Str~o ,vide n- RF p,steet t on~S a p~
The Du 8ont HIV p29 l~ntigen ELISA (catalog number
23 NEK OSO) was modified for catalyzed reporter deposition
as follows: SA-HRP was used at 1/4 the concentration
indicated in the directions. This was followed by a 15
min. RT incubation with biotin-tyramine, 10 ~tg/ml, in
0.1 M borate, 0.01% HaOZ px 8.5 buffer (as in
90 Example 5). Following washing with pBST, SR1-HRf at 1/16
the Concentration was incubated for 15 min. at RT.
Finally, OPD was added as per kit directions. Except
for e~ttending the standard concentrations down to 0.39
pg/ml, no other changes wer~ mad~.
26
ro ,~ : j a
a
i.r ~ .~ c~ a ~. ~Z
Z7
The results ar~ shown in ~°igure 4. This experiment
d~monatrated that one can amplify the signal generat~d
by a biotinylatad ~antibody/SA-H~ system using catalyz~d
reporter d~poaition. Because the concentration of SA-
HRP for both incubations was much lass than that for the
non-amplified away, it was clear that th~ incr~asad
ai~nal was attributabl~ to reporter depo,~ition and not
to a double 9A-HRP incubation.
~e~dt~rt s~ D~~~~ 1 ~ ~ en_an M~mhran~ 9
Nitrocellulose tSchlaichar & Schuall, 8A S5~ was
spott~d with HSV antigen, and than blocked with 1~ SSA,
1~ non-fat dry milk, in PBS buffer, overnight. xh~
membranes were incubated for 1 hour at R~ with the
analyta dependent enzyme activation sygtam described in
Example 1 above. mha membranes were than incubated with
biotin tyramina (from Exempla 1) at 2 y~lll0 ml 50 mM
tris-HC1, 0.01 H~02, pH ~.0 buffer for 15 min. at R°~,
which was followed by incubation with atreptavidin-
alkaline phosphatass !or 15 min. at R~°. Controls ware
sun where biotin tyramine was incubated without
streptavidin-alkaline phosphataaa, and straptavidin-
alkaline phosphatasa was incubated without biotin-
tyraminc. visualization of deposited alkaline
phosphatasa was lacilitat~d by the addition of SCIp/N~'r
(Kirkegaard & parry). BCTP is S-bromo-4-chloro-ir~doxyl
phosphate and NBT is 2,2'-di-ip-nitrophenyl)-5,5'-
diphenyl-3,3'-43,3'-dimethoxy-4,4'-diphenylene)-
ditetrazolium chloride, visualization of th~ bound
anti-HSv-HRP conjugate was facilitated by th~ addition
of diaminob~nzidine (D
trade mark
27
032?~90 11:30 DUPONT LE6r~L N0.010 031
as
Addition of DAB produced observable brown spotx
where HSV antigen wag spotted on the nitrocellulose
membrane. Rddition of BCIP/1VBT produced observable blue
spots wh~r~ HSV antig~n was spott~d when biotin tyramine
and streptavidin-alkaline phosphatas~ were incubated
with the membrane. This showed that alkalin~
phosphatase waa deposited due to HRP activation of the
biotin tyramine conjugate, followed by streptavidin-
alkaline phosphatase detection.
~~rQM~.~~~~8s and A~~f y '~on b~l ~''ILfSY'Bg~°~Tnm
Polystyrene EIA strips (~1t11~C) ware Coated with goat
anti-mouse ggG (fc lrag~rnent specific) antibody (3Cid) in
0.1 M carbonate bufger, pH 9.6, overnight at RT. They
w~ra than block~d with 2% BSa~ in carbonate buffer and
washed with PBST. Concentrations of mouse igG in sSA-
2p PBST, as set forth in ~°igure ~, were incubated for 1
hour at 37°C followed by washing with BST. Goat anti-
mouse IgG-HRP (AREAS) purchased from Boehringer Mannheim
was diluted as recommended by the manufacturer and
incubated fCr 1 hour at 37°C. The plate was then washed
23 with PBST. A 1 mg/ml stock solution of HT conjugate (as
describ~d in Hxample 4) in dimethyl sulfoxide was
prepared, and then added to a B.1 M borate, 0.01 Hz02,
pH 8.5 buffer at 10 ~tl/ml (10 ~tg/ml biotin tyramin~) ,
The mixture was added to the plate and incubmted for 15
30 min, at RT, and then washed with PBST. 8treptavidin-
beta galactosidase (B~thesda Research Labs) was added
and incubated for 15 min. at Rfi. The assay was also run
without catalyzed reporter deposition, i.e., without
adding HT. Colorimetric detection of thg non-amplified
33 assay was achieved after incubation with OPD (for HRp),
28
03i27i90 11:30 DUPONT LEGAL I~1Q.010 032
~' ~
2 9 !~ ~'. l.~
!or 15 min. at RT. Fluorescent detection of the
amplified assay was achieved after the addition of 4-
methylumbelliferyi beta-D-galaotoside (MUO) (for HRF-
beta Gal). Optical densities at 490 nm w~re recorded
far HRP/~PD on a microtitar plat~ reader. ~'luorescence
for HRP-b~ta Gal/MU~ was recorded on a fluoregc~nce
microtiter pieta reader (Dynatech Laboratories).
The results era shown in Figure 5. The fluorescent
signal was due to the quantitative deposition of
biotintyramine by the HRP ADHAS (allowed by incubation
with streptavidin beta-galactosidas~e.
Am~~al~~iCg~~ori ef a M~m~ran~ 10.a~~~
Fluoresceia~°tyramine (FT) was prepared as followsa
Solutions of 96.6 mg of 5-(and 6)-carboxyfiuorescein
succinimidyl ester in 0.3 ml dimethyl sulfoxide and 14.6
mg tyramirae in 0.3 ml dimethyl sulfoxide were pxepared.
Conjugation was achieved by mixing 0.25 mi of each
solution overnight at FtT. The solution was used as is.
Three nitrocellulose (Schl~icher & Sshuell, 19A S3)
strips were spotted wfth 1 ~tl of mouse gg0 at 10 wg/ml,
and serial two fold dilutons in PBS. The membranes were
blocked with 5% non-fat dry milk in PBST for 30 min, amd
then wash~d three times its ASST, A goat anti-mou~e IgG-
H~r conjugate (Hoehringer Mannehim) diluted 1/2000 in 1%
BSA-PBST was incubated for 30 min. at RT, and the
membrane were washed thre~ times with PBST. The third
membrane was incubated with FT at 20 ~g/m~. in 0.1 M
borate, 0.01% H20~, pEE 8.5 buffer for 15 min. at RT, and
washed three times in PBST, Then, the secoxid and third
membranes were incubated with an anti-fluorescein
3!f antibody (Chemicon) which was conjugated to HitP (by the
29
NJ~G~i7b 11:J1 LUh-'UN1 L~GHL N0.010 033
'' -;
a
3o r~.
SMCC method of gshibcawa, ~., et al., ~T. gmmunoa9aay, .~,
209-327, 19~3) diluted in 1~ HSA-PSST for 15 min at RT,
and washed three times in P~3T. Vieualixatian of all
three strips was facilitated by the addition of
diaminobenzidin~ for 5 mi.n.
Three spots could be assn on the ~isgt two strips
indicating a detection limit o~ 2.5 ~.g/ml, and that the
anti-fluoresCein-HRP con~ugat~e did not contribute to
additional signal. Six spots could been seen on the
third strip indicating a detection limit of 313 ng/ml.
Th~ us~ of the catalyzed reporter deposition
amplification method of the invention impsoved the
detection limit of the assay eight fold over that of the
non-amplified assay,
~,yntl2esis ef the C~;~~~ ~~~ ~~
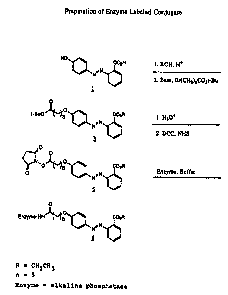
~uccin~ mi dY.l) -6- tnh~nox~,l~ ~ -axo-z °~-
~ar$Wxy~thyloh_~nYt'~xanoah.a" H~~-S-I~7i3~ and A13cm74ne~
h~yha~ase_..IAP
The following reaction scheme is illustrated in
Figure 6: Ethyl 2-(~~-hydroxyphenylazo)benzoate (HEE),
23 i.s prepared from 2-(4°-hydroxyphenylazo)-benzoic soil
(HABA, (~)), anhydrous ethanol and a catalytic amount of
acetyl chloride. The ethyl ester (HEE) is reacted with
t°butyl 6-iodohexanoate and sodium hydride using the
general procedure reposted by Castellanos et sl.,
Tetrahedron, pages 1691-1696, Vol. 37, (191), to
produce t-butyl-6-(phenoxy-(4~-azo-2"_
carboxyethylphenyl)hexsnoate (HEE-6-t--BU, (~)). The
tart-butyl ester is hydrolyzed by tr~atment with
txifluoroaceti.c acid using the general procedure
3S reported by Bryat: et al., ~?ournal of the American
t~_~ c r ~ ?t~ 11 ~ Jc UUf ~I[~~ I LtUHL ~V~J. 010
i
31
Chemical Society, pages 23x3-2355, Vol. 99, (1977), to
produce 6-(phenoxy-(4'-azo-2"-carboxyethylphcnyl)-
hexanoic acid (HH~-6-~I). (N-succinimidyl)-6-(phenoxy-
t4'-azo-2°-carboxyethylphenyl)hexanoate (HSE-6-N13S, (~))
is prepar~d =rom HEE-6-H, N-hydroxy9uccinimide and
dicyclohexylcarbodiimida in THF using the g~ngral
procedur~ reported by Aryan et al., Makromoleouiar
Chami~, pages 2375-2382, Vol. 186, (1985). The uHS
ester (~) is dissolved is a minimum volum~ of DMSO and
adda~d tc~ a buffered aqueous solution of alkaline
phosphatase (e. g. calf intestine) using the g~neral
procedure reported by p'Sullivan et al., Methods in
Enzymology, page9 147-166, Vol. 73, 11981) to 4ive the
con jugate gHEE~6-~,
E
SVnf~,~"~ at Of HABA-'f'vrami nr t~nn_imesrs~øw Iet W
from IN-Qnr.n~g1'~ m~ dy1 j -6- ( hi~~4 (~ ~-aZy a ~
~rboxvathyynY' ) hsYanoat~ (HEJ~~~,S r~~ ~
~ ,
The NHS ester (5) (1 mmol) described in Hxample 10
abov~, and tyramina (1 mmol) are dissolved in DNtF (5 ml,)
and stirred at RT for 48 hrs. The solution is
evaporated to dryness in vaGUO. The residue is
suspended in I~ZO (pFd 8.0, 50 mL) and porcin~ liver
esteras~ (100 mq) ie added. The pH of the solution is
maintained by adding 0.1 N Na~H as required. The
solution is evaporated to dryness after 24 hours, The
resulting H-T conjugat$ is isolated by chromatography
(silica gal, chloroform/methanol).
31
t~3~~7i~0 11:32 UUFONT LEGAL N0.010 935
32
L~ 12
c~m~zx~c~r,ion oz uer~~s,~~-q~'~ g M;p~9~ ~pC Aam~v
.LCaawmn rvav.sma u~~~y~.~,~~~~1 LnLaw~~a
~Iicrotiter plate strips (Nuno) are coated wi~.h a
miactura of goat anti-mouse igG (~'c fragment speoific)
antibody (ZCN) and atreptavidin (Scripps ~aboratori~s)
in 0.1M carbonate buffer pH 9.6 overnight at FtT. They
area then block~d With 2% ESA in PBS and wa3hed with
PEST. nilutions of mouse ggG (0-100 ng/mL) in sSA-p~ST
are incubated in the wells for 1 hr, at 37°C followed by
washing with BEST. A preparation of goat anti-mouse
YgG°PLE (PhE.:~DEAS) (0.75 mg/mh) is prepared by the
general method deecrib~sd by Hashida et al,, journal of
Appli~d Eiochemiatry vol. 6, pages 56-63, 1984 and
diluted 1:100-102000 with phosphat~ buffer (0.1 ~R, pH
8.0, 0.24 ESA) and is incubated for i hr. at 37°C and
washed with DEBT. ~tEE°6-AP (1 mg/mL) (prepar~d as
described in Example 10) is added to the microtiter
plate woll and incubated for at least 15 min. at 37°C.
. The plate is then wa9hed with PBST. SpeGtrophotometric
detection is achieved after the addition og
p-nitrophenyl phosphate. Heactiona are stopped by the
addition of 1 N NaOH. Optioal densitie3 at 405 nm are
r~corded on the miorotiter plate reader. hmplification
of detector signal in this example results from
catalyzed reporter-enzyme deposition, i.e., PLE
Catalyzes deposition of HEE-6-AFB whesc the receptor,
streptavidin, has been immobilised on the microtiter
plate surface.
32
03~27i90 X1:33 DUPONT LEGI~I. N0.01~ 036
1~~~.P~~ a t~
33
D~mon~ rat icon o~ ~h~ ~n~a~m~ l~od~1 ~t.~d g~ ndina o~ ~
locked finder
R suspension of porcin~ liv~s esterase was added
t10 uL, 2850 t1/mL, Cat . I~o. F3128, Sigma Chemical, 5t .
Louis, M~) to a solution of ethyl 2-t4'-hydroxy-
phenylazo)benzoate tHEE) 10.25 mM) and atreptanvidin t0.2
m~/mL) in phoaphat~ buffer t0.1 M, pH 8.0, 2m~). A
second solution identioal to the gisat was prepared
which contained no streptavidin. fhe absorbanc~ of the
two solutions w~a measured at 500 nm sa a function of
time. Figure 7 shows that th~ absorbents of the
solution which contained no atreptavidin decreased over
time indicatingr hydroly'is of HEM to 2-t~9°-hydxoxy-
phenylazo)benzoic acid iFIAB~). The absorbents of the
solution whieh contained streptavidin inor~ased over
time indicating the formation o~ the HAS~:streptavidira
complex, which is known to have a strong absorbents at
50A nm.
33