Note: Descriptions are shown in the official language in which they were submitted.
2 ~
-- 1
RAN 4019/107
The present invention relates to amino acid
derivaeives. In particular, it i5 concerned with amino
acid derivatives of the general formula
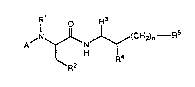
~N~ (CH2)n--F
wherein Rl signifie6 hydrogen or methyl, R
signifies ethyl, propyl, isopropyl, imidazol-2-yl,
imidazol-4-yl, pyrazol-3-yl, thiazol-~-yl, thien-2-yl,
ethoxycarbonyl, t-butylcarbonylmethyl. benzyloxy-
carbonylmethyl or t-butoxy, R signifie6 isobutyl,
cyclohexylmethyl or benzyl, R signifies hyd~oxy or
amino, R signifies alkyl, haloalkyl, cycloalkyl,
alkenyl, aryl or heteroaryl, n signifies 0, 1, 2, 3,
4, 5 or 6 and A signifies one of the groups
R6 ~ (a, and -y-z (b)
0
in which the dotted line can signify an additional
bond, R signifies phenyl, substituted phenyl,
benzyl or naphthyl and R ignifies hydrogen,
alkoxycarbonylalkyl, alkylcarbonylalkyl, cycloalkyl-
carbonylalkyl, heterocycloalkylcarbonylalkyl, aryl-
carbonylalkyl, aminocarbonylalkyl, substituted amino-
Kbr/20.2.90
7 ~
carbonylalkyl, aminoalkylcarbonylalkyl, substituted
aminoalkylcarbonylalkyl, aminoalkylsulphonylalkyl,
substituted aminoalkyl6ulphonylalkyl, alkoxycarbonyl-
hydroxyalkyl, alkylcarbonylhydroxyalkyl, cycloalkyl-
carbonylhydroxyalkyl, heterocycloalkylcarbonylhydroxy-
alkyl, arylcarbonylhydroxyalkyl, aminocarbonylhydroxy-
alkyl, substituted aminocarbonylhydroxyalkyl,
dialkoxypho~pho~oxyalkyl, diphenyloxyphosphoroxyalkyl,-
arylalkyl, alkoxycarbonylamino, arylalkoxycarbonyl-
amino, alkylthioalkyl, alkylsulphinylalkyl, alkyl-
sulphonylalkyl, arylthioalkyl, arylsulphinylalkyl,
arylsulphonylalkyl, arylalkylthioalkyl, arylalkyl-
sulphinylalkyl or arylalkylsulphonylalkyl, Y signifies
the bivalent residue of optionally N- and/or
a-methylated phenylglycine, cyclohexylglycine,
phenylalanine, cyclohexylalanine, 4-fluorophenyl-
alanine, 4-chlorophenylalanine, tyrosine, 0-methyl-
tyrosine, a-naphthylalanine or homophenylal~nine
linked with Z at the N-terminal and Z signifiPs
hydrogen or acyl, with the p~ovisos that
ti) R does not signify alkyl, cycloalkyl or
aryl where n signifies 2, 3, 4, 5 or 6,
(ii) the carbon atom of alkyl which is attached
to the CH2 g~oup is branched where n
signifies 1 and R5 signifies alkyl,
(iii) the carbon atom of R5 attached to the
carbon atom ca~rying the ubstituent R
has no methylene group in the a-position
where n signifies 0 and R5 signifies alkyl,
(iv) R does not signify alkyl where R2
signifies imidazol-4-yl, R4 signifies
hydroxy and Y signifies phenylalanine and
(v) R does not signify alkoxycarbonylamino or
arylalkoxycarbonylamino where R signifies
phenyl, benzyl or a-naphthyl,
in the form of optically pure diastereomers, mixturas of
diastereomers, diaste~eomeric racemates or mixtures of
diaste~eomeric ~acemates as well as pharmaceutically
usable salts of these compounds.
These compounds are novel and a~e distinguished by
valuable pharmacodynamic properties.
Objects of the present invention ace the compounds of
formula I and thei~ pharmaceutically usable salts per se
and for use as therapeutically active substances, the
manufacture o~ these compounds, medicaments containing
these and the manufacture of such medicaments, as well as
the use of compounds of formula I and their pharmaceu-
tically usable salts in the control or prevention of
illnesses or in the improvement of health, especially in
the control or prevention of high blood pressure and
cardiac insufficiency.
The following definitions of the general terms used in
the present description apply irrespective of whether the
terms in question appea~ alone or in combination.
The tsrm ~'alkyl" used in the present description
signifies straight-chain and branched, saturated hydro-
carbon residues with 1-8, preferably 1-4, carbon atoms
such as methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, sec.-butyl, t-butyl, pentyl, hexyl and the like.
The term "alkoxy" signifies alkyl ethec groups in which
the term "alkyl" has the above significance, such as
methoxy, ethoxy, propoxy, isopropoxy, butoxy, isobutoxy,
sec.-butoxy, t-butoxy and the like. The ~erm "cycloalkyl"
signifies saturated, cyclic hydrocarbon residues wi~h 3-8,
preferably 3-6, carbon atoms such as cyclopropyl, cyclo-
butyl, cyclopentyl, cyclohexyl and the like. The term
"heterocycloalkyl" relates in the same manner to
7~
saturated, 3-8-membered, preferably 5- or 6-membered, cyclic
hydrocarbon residues in which one or two methylene groups
is/are replaced by one or two oxygen, sulphur or optionally
alkyl-, phenylalkyl-, alkanoyl- or alkanoyloxy-substituted
nitrogen atoms, such as piperidinyl, eyrazinyl, N-benzyl-
pyrazinyl, morpholinyl, N-methylpiperidinyl, N-benzylmor-
pholinyl and the like. The term "haloalkyl" signifies alkyl
in which a hydrogen atom is replaced by halogen. The term
"alkenyl~ relates to straight-chain and branched unsaturated
hydrocarbon residues with 2-8, preferably 2-4, carbon atoms
such as vinyl, allyl, 2-butenyl, 3-butenyl, 3-pentenyl and
the like. The term "halogen" relates to the four halogens
fluorine, chlorine, bromine and iodine. The term "alkanoyl"
signifies the acid residue of a straight-chain or branched
alkanoic acid with 1-8, preferably 1-4, carbon atoms such as
formyl, acetyl, propionyl, butyryl, valeryl, isovaleryl and
the like. The term "aryl" denotes a mono- or bicyclic
aromatic hydrocarbon residue with 6-14 carbon atoms which is
optionally mono- or multiply-substituted by alkyl, alkoxy,
20 alkanoyloxy, amino, alkylamino, dialkylamino, alkylcarbonyl-
amino, hydroxy, halogen, trifluoromethyl or nitro, such as
phenyl, - or ~-naphthyl, indenyl, anthryl or phen~
anthryl and the like. The term "arylalkyl" denotes
straight-chain or branched alkyl groups in which one or
2~ more hydrogen atoms is/are reelaced by aryl groups, such as
benzyl, diehenylmethyl, trityl, a- or ~-naphthylmeth~l,
2-phenylethyl, 3-phenyl-2-propyl, 4-phenyl-3-butyl,
2-~a- or ~-naphthyl)ethyl, 3-a-naphthyl-2-propyl,
4--naphthyl-3-butyl and the like, whereby the aromatic
30 residue can in each case be mono- or multiply-substituted as
indicated above. The term ~substituted phenyl~ denotes
phenyl which is optionally mono- or multiply-substituted
by alkyl, alkoxy, alkoxyalkoxy, alkanoyl, alkanoyloxy,
hydroxy, halogen or trifluoromethyl, such as
35 4-hydroxyphenyl, 4-methoxyphenyl, 4-methylphenyl,
2~3~
4-chlorophenyl, 4-ethoxyethoxyphenyl and the like. The
term ~'heteroaryl~ denotes a mono- or bicyclic aromatic
hydrocarbon cesidue in which one or more carbon atoms
is/are replaced by one or two nitrogen atoms and/or an
oxygen or sulphur atom and which is optionally substituted
on a nitrogen atom by alkyl, phenyl or phenylalkyl and/or
on one or more carbon atoms by alkyl, phenyl, phenylalkyl,
halogen, hydroxy, alkoxy, phenylalkoxy or oxo and which
can be partially saturated, such as pyrrolyl, furyl,
thienyl, imidazolyl, pyrazolyl, oxazolyl, thiazolyl,
pyridyl, pyrazinyl, pyrimidinyl, indolyl, quinolyl,
isoquinolyl or quinoxalinyl, e.g. 2- or 3-py~rolyl,
phenylpyrrolyl, e.g. 4- or ~-phe~yl-2-pyrrolyl, 2-furyl,
2-thienyl, 2-imidazolyl, 2-, 3- or 4-pyridyl, 2-, 3- or
5-indolyl, substituted 2-indolyl, fGr example l-methyl-,
5-methyl-, 5-methoxy-, 5-benzyloxy-, S-chloro or
4,5-dimethyl-2-indolyl, 1-benzyl-2-indolyl, 1-benzyl-3-
-indolyl, ~,5,6,7-tetrahydro-2-indolyl, 2-, 3- or
4-quinolyl, 4-hydroxy-2-quinolyl, 1-, 3- or 4-isoquinolyl,
l-oxo-1,2-dihydro-3-isoquinolyl, 2-quinoxalinyl, 2-benzo-
furanyl, 2-benzoxazolyl, 2-benzthiazolyl and the li~e.
The term l'substituted amino" signifies an amino group
which is mono- or di-substituted by alkyl, arylalkyl,
alkanoyl, alkoxycarbonyl or arylalkoxycacbonyl or
disubstituted by C3-C6-alkylene which is optionally
interrupted by an oxygen, sulphur or optionally alkyl-~
phenylalkyl-, alkanoyl- or alkanoyloxy-substituted
nitrogen atom. The term "acyl" relates to the acyl group
o~ a carboxylic acid, of a half ester of carbonic acid, of
an optionally N-substituted carbamic or thiocarbamic acid,
of an optionally N-substituted oxalamide, of a sulphonic
acid or of an optionally N-substituted amidosulphonic
acid, especially those with the partial formulae R -C0-,
Ra-0-C0-, (Rb)(Rb)N-C0-, (R )(R )N-CS-,
(R )(R )N-C0-C0-, R -S02- or (R )(Rb)N-S02-,
2 ~ 7 ~
in which Ra signifies an unsubstituted or substitutea,
saturated or unsaturated, aliphatic, cycloaliphatic,
cycloaliphatic-aliphatic hydrocarbon residue with up to
18, preferably 10, carbon atoms which is optionally
functionali2ed with amino, monoalkylamino, dialkylamino,
alkanoylamino or alkanoyloxyamino, an unsubstituted or
substituted aromatic, heteroaromatic, aromatic-aliphatic
or heteroaromatic-aliphatic hydrocarbon residue with up to
18, preferably 10, carbon atoms or an unsubstituted or
substituted, saturated 5- or 6-membered heterocyclic
residue and R signifies hydrogen or has the
significance of R . The term "acyl" also relates to the
monovalent residue of an optionally acylated amino acid or
of an optionally acylated dipeptide attached via the
carboxyl group.
An unsubstituted or substituted, saturated or
unsaturated, aliphatic, cycloaliphatic or cycloaliphatic-
-aliphatic hydrocarbon residue R or R is, for
example, unsubstituted or substituted alkyl, alkenyl,
alkynyl, mono-, bi- or tricycloalkyl, monocycloalkenyl,
bicycloalkenyl, cycloalkylalkyl, cycloalkylalkenyl or
cycloalkenylalkyl. "Substituted alkyl" signifies an alkyl
residue in which one or more hydrogen toms can be
substituted by hydroxy, alkoxy, aryloxy, alkanoyloxy,
halogen, hydroxysulphonyloxy, carboxy. alkoxycarbon~l,
carbamoyl, alkylcarbamoyl, dialkylcarbamoyl, cyano,
phosphono, esterified phosphono, amino or oxo, whereby the
substituents are present in the l-position of the alkyl
residue only when this is a~tached to the carbonyl group
in the partial formula ~ -C0-.
Examples of substituted alkyl are 2-hydroxyethyl,
methoxymethyl, 2-methoxyethyl, phenoxymethyl, ~- or
~-naphtho~ymethyl, acetoxymethyl, Z-acetoxyethyl, chloro-
methyl, bromomethyl, 2-chloro- or 2-bromoethyl, hydroxy-
20~3~
sulphonyloxymethyl, 2-hydroxysulphonyloxyethyl, carboxy-
methyl, ~-carboxyethyl, methoxycarbonylmethyl, 2-methoxy-
carbonylethyl, ethoxycarbonylmethyl, 2-ethoxycarbonyl-
ethyl, carbamoylmethyl, 2-carbamoylethyl, methylcarbamoyl-
methyl, dimethylcarbamoylmethyl, cyanomethyl, 2-cyano-
ethyl, 2-oxopropyl, 2-oxobutyl, hydroxycarboxymethyl,
l-hydroxy-2-carboxyethyl, hydroxyethoxycarbonylethyl,
hydrox~methoxycarbonylethyl, acetoxymethoxycarbonylmethyl,
1,2-dihydroxy-2-carboxyethyl, 1,2-dihydroxy-2-ethoxy-
carbonylethyl, l,2-dihydroxy-2-methoxycarbonylethyl,
1,2-diacetoxy-2-ethoxyca~bonylethyl, 1,2-diacetoxy-2-
-methoxycarbonylethyl, l-~-naphthoxy-3-carboxyp~opyl,
1--naphthoxy-2-ethoxyca~bonylethyl, 1-a-naphthoxy-3- -
-t-butoxycarbonylpropyl, l-a-naphthoxy-2-benzyloxy-
carbonylethyl, l-a-naphthoxy-3-carbamoylpropyl,
-naphthoxycyanomethyl, l-a-naphthoxy-3-cyanopropyl,
l--naphthoxy-4-dimethylaminobutyl or 1-a-naphthoxy-3-
-oxobutyl.
The term "alkenyl" relates to an unsaturated hydro-
carbon residue such as has been defined above, whereby the
double bond can be present in the l-position of the
alkenyl residue only when this is attàched to the carbonyl
group in the partial formula R -C0-. The alkenyl
residues can be substituted by the same substi~uents as
the alkyl residues.
The term "alkynyl" relates to hydrocarbon residues
with 2-8, preferably 2-4, carbon atoms which contain a
triple bond, such as ethynyl, l-propynyl or 2-propynyl.
The term "bicycloalkyl" relates to bicyclic saturated
hydrocarbon residues with 5-10, preferably 6-9, caebon
35 atoms such as bicyclo[3.1.0]hex-1-yl, bicyclor3.1.0]hex-2-
-yl, bicyclor3.1.0]hex-3-yl, bicyclor4.1.0]hept-1-yl.
bicyclor4.1.0]hept-4-yl, bicyclor2.2.1]hept-2-yl, bicyclo-
r 3.2.1]oct-2-yl, bicyclo r 3.3.0]oct-3-yl, bicyclo r 3.3.1]-
non-9-yl, a- or ~-decahydronaphthyl and the like.
2~3~
-- 8
The term "tricycloalkyl" relates to a tricyclic
saturated hydrocarbon residue with 8-10 carbon atoms such
as l-adamantyl.
The term "cycloalkenyl~ relates to an unsaturated
cyclic hydrocacbon residue with 3-8, p.eferably 3-6,
carbon atoms such as l-cyclohexenyl, 1,4-cyclohexadienyl
and the like.
The term "bicycloalkenyl" relates to a bicyclic
unsaturated hydrocarbon residue with 5-10, preferably 7-10
carbon atoms ~uch as 5-norbornen-2-yl, bicyclot2.2.2]-
octen-2-yl, hexahydro-4,7-methanoind-1-en-6-yl and the
like.
Cyclopropylmethyl, cyclobutylmethyl, cyclopentyl-
methyl, cyclohexylmethyl and the like are examples of
cycloalkylalkyl. Cyclohexylvinyl and cyclohexylallyl and
the like can be named as examples of cycloalkylalkenyl.
l-Cyclohexenylmethyl, l,~-cyclohexadienylmethyl and the
like are examples of cycloalkenylalkyl.
The mentioned cycloaliphatic and cycloaliphatic-
-aliphatic residues can be substituted by the same
substituents as alkyl.
An optionally substitu~ed aromatic or aromatic-
-aliphatic hydrocarbon residue is, for example,
unsubstituted or substituted aryl, arylalkyl or aryl-
alkenyl. Styryl, 3-phenylallyl, 2-(a-naphthyl)vinyl,
2-(B-naphthyl)vinyl and the like are examples of aryl-
alkenyl.
In a heteroaromatic or heteroaromatic-aliphatic hydro-
carbon residue the heterocycle is mono-, bi- or tricyclic
and contains one or two nitrogen atoms and/or an oxygen or
2 ~ 7 ~
sulphur atom and is linked ~ith the group -C0-, -0-C0-,
>N-C0-. >N-CS-, >N-C0-C0-, -S02 or >N-502- with one of
its ring carbon atoms. Examples of such heteroaromatic
hydrocarbon residues are pyrrolyl, furyl, thienyl,
imidazolyl, pyrazolyl, oxazolyl, thiazolyl, pyridyl,
pyrazinyl, pyrimidinyl, indolyl, quinolyl, isoquinolyl,
quinoxalinyl, B-carbolinyl or a benz-fused, cyclopenta-,
cyclohexa- or cyclohepta-fused derivative of these
residues. The heteroaromatic residue can be substituted sn
a nitrogen atom by alkyl, phenyl or phenylalkyl, e.g.
benzyl, and/or on one or more ca~bon a~oms by alkyl,
phenyl, phenylalkyl, halogen, hydroxy, alkoxy, phenyl-
alkoxy or oxo and can be partially saturated. Examples ofsuch heteroaromatic residues are 2- or 3-pyrrolyl, phenyl-
pyrrolyl, e.g. 4- or 5-phenyl-2-pyrrolyl, 2-~uryl,
2-thienyl, 2-imidazolyl, 2-, 3- or 4-pyridyl, 2-, 3- or
5-indolyl, substituted 2-indolyl, for example l-methyl-,
5-methyl-, 5-methoxy-, 5-benzyloxy-, 5-chloro- or
4,5-dimethyl-2-indolyl, 1-benzyl-2-indolyl, 1-benzyl-3-
-indolyl, 4,5,6,7-tetrahydro-~-indolyl, cycloheptarb]-5-
-pyrrolyl, 2-, 3- or 4-quinolyl, 4-hydroxy-2-quinolyl, 1-,
3- or 4-isoquinolyl, 1-oxo-1,2-dihydro-3-isoquinolyl,
2-quinoxalinyl, 2-benzofuranyl, 2-~enzoxazolyl, 2-benz-
thiazolyl, benz[e]indol-2-yl, ~-carbolin-3-yl and the like.
Examples of heteroaromatic-alipha~ic hydrocarbon
residues are 2- or 3-pyrrolylmethyl, 2-, 3- or 4-pyridyl-
methyl, Z-(2-, 3- or 4-pyridyl)ethyl, 4-imidazolylmethyl,
2-(4-imidazolyl)ethyl, 2-indolylmethyl, 3-indolylmethyl,
2-(3-indolyl)ethyl, 2-quinolylmethyl and the like.
A saturated 5- or 6-membered heterocyclic residue has
at least one carbon atom, 1-3 nitrogen atoms and
optionally an oxygen or sulphur atom as the ring members
and is linked with the group -C0- or -0-C0-, >N-C0-,
>N-CS-, >N-C0-C0-, -S02- or >N-S02- wi~h one of its
2 ~ ~ 3 ~
-- 10 --
ring carbon atoms. The heterocycle can be substituted on
one of its carbon atoms or on a ring nitrogen atom by
alkyl, e.g. met~yl or ethyl, phenyl or phenylalkyl, e.g.
benzyl, or on one of its carbon atoms by hydroxy or oxo
and/or can be benz-fused on two adjacent carbon atoms.
Examples of such residues are pyrrolidin-3-yl, 4-hydroxy-
pyrrolidin--2-yl, 5-oxo~yrrolidin-2-yl, piperidin-2-yl,
piperidin-3-yl, 1-methyleiperidin-2-yl, l-methylpiperidin-
-3-yl, 1-~ethylpiperidin-4-yl, morpholin-Z-yl, morpholin-
-3-yl, thiomorpholin-~-yl, thiomorpholin-3-yl,
1,~-dimethylpiperazin-2-yl, 2-indolinyl, 3-indolinyl,
1,2,3,4-tetrahydroquinol-2-, -3- or -4-yl, 1,2,3,9-tetra-
hydroisoquinol-l-, -3- or -4-yl, 1-oxo-1,2,3,~-tetrahydro-
isoquinol-3-yl and the like.
As residues of an amino acid attached via the carboxyl
group there come into consideration natural a-amino
acids having the L-configuration, homologues of such amino
acids, e.g. in which the amino acid side-chain is
lengthened or shortened by one or two methylene groups
and/or a methyl group i6 replaced by hydrogen, substituted
aromatic a-amino acid~, e.g. substituted phenylalanine
or phenylglycine in which the substituent can be alkyl,
e.g. methyl, halogen, e.g. fluorine, chlorine, bromine or
iodine, hydroxy, alkoxy, e.g. methoxy, alkanoyloxy, e.g.
acetoxy, amino, alkylamino, e.g. methylamino, dialkyl-
amino, e.g. dimathylamino, alkanoyla~ino, e.g. acetylamino
or pivaloylamino, alkoxycarbonylamino, e.g. t-butoxy-
carbonylamino, arylmethoxycarbonylamino, e.g. benzyloxy-
carbonylamino, and/or nitro and can be present singly oc
multiply, benz-fused phenylalanine or phenylglycine such
as a-naphthylalanine or hydrogenated phenylalanine or
phenylglycine such as cyclohexylalanine or cyclohexyl-
glycine, a 5- or 6-membered cyclic benz-fused a-amino
acid, e.g. indoline-2-carboxylic acid or 1,2,3,~-tetra-
hydroisoquinoline-3-carboxylic acid, a natural or
~ 3
-- 11 --
homologous -amino acid in which a carboxy group in the
side-chain i6 present in esterified or amidated form, e.g.
as an alkyl ester group such as methoxycarbonyl or
t-butoxycarbonyl or as a carbamoyl group, as an alkyl-
carbamoyl group such as methylcarbamoyl or as a dialkyl-
carbamoyl group such as dimethylcarbamoyl, in which an
amino group of the side-chain is present in acylated form,
e.g. as an alkanoylamino group such as acetylamino or
pivaloylamino, as an alkoxycarbonylamino group such as
t-butoxycarbonylamino or as an arylmethoxycarbonylamino
group such as benzyloxycarbonylamino, or in ~hich a
hydroxy group of the side-chain is present in etherified
or esterified form, e.g. as an alkoxy group ~uch as
methoxy, as an arylalkoxy group such as benzyloxy or as a
lower alkanoyloxy group such as acetoxy, or epimers of
such amino acids, i.e. with the unnatural D-configuration.
Examples of such amino acids are glycine, alanine, valine,
norvaline, leucine, isoleucins, norleucine, serine,
homoserine, threonine, methionine, cysteine, proline,
trans-3- and trans-4-hydroxyproline, phenylalanine,
tyrosine, 4-nitrophenylalanine, 4-aminophenylalanine,
4-chlorophenylalanine, ~-phenyl~erine, phenylglycine,
-naphthylalanine, cyclohexylalanine, cyclohexylglycine,
tryptophane, indoline-2-carboxylic acid, ~,2,3,4-te~ra-
25 hydroisoquinoline-3-carboxylic acid, aspartic acid,
asparagine, aminomalonic acid, aminomalonic acid mono-
amide, glutamic acid, glutamic acid mono-t-butyl ester,
glutamine, N-dimethylglutamine, histidine, arginine,
lysine, N-t-butoxycarbonyllysine, ~-hydroxylysine,
30 ornithine, N-pivaloylornithine, ~,y-diaminobutyric
acid or ,~-diaminopropionic acid and the like. The
residue of the amino acid attached via the carboxyl group
can be substituted N-terminally by alkyl, e.g. me~hyl or
ethyl, in order to increase the stability of the compound
35 of formula I against enzymatic degradation.
The residue of a dipeptide attached via the carboxyl
group consists of two of the above-mentioned amino acids,
The term "acylated amino acid" or "acylated dipeptide"
relates to one of the above-mentioned amino acids or to a
dipeptide from two of the above-mentioned amino acids
which is N-terminally substituted by the acyl residue of a
carboxylic acid, of a half ester of carbonic acid, of an
optionally N-substituted caIbamic or thiocarbamic acid, of
an optionally N-substituted oxalamide, of a sulphonic acid
or of an optionally N-substituted amidosulphonic acid.
The term "pharmaceu~ically usable salts" embraces
salts with inorganic or organic acids such as hydrochloric
acid, hydrobromic acid, nitric acid, sulphuric acid,
phosphoric acid, citric acid, formic acid, maleic acid,
acetic acid, succinic acid, tartaric acid, methane-
sulphonic acid, p-toluenesulphonic acid and the like. Such
salts can be manufactured readily by any person skilled in
the art having regard to the state of the art and taking
into consideration the nature of the compound to be
converted into a salt.
The compounds of formula I have at least three
asymmetric carbon atoms and aee therefore present in ~he
form of optically pure dias~ereomers, mixtures of
diastereomers, diastereomeric racemates or mixtures of
diastereomeric racemates. The pEesent invention embraces
all forms. Mixtures of diastereomers, diastereomeric
racemates or mixtures of diastereomeric racemates can be
separated according to usual methods, e.g. by column
chromatography, thin-layer chromatography, HPLC and the
like.
Those compounds of formula I in which Rl signifies
hydrogen are preferred. R2 preferably signifies
2al3~
imidazol-2-yl, imidazol-4-yl or thiazol-9-yl, particularly
imidazol-4-yl. Further, those compounds of formula I in
which R3 signifies cyclohexylmethyl are preferred. R4
preferably signifies hydroxy. Those compounds ~f formula I
in which R5 signifies alkyl, preferably 3-pentyl, halo-
alkyl, preferably fluoroalkyl, cycloalkyl, preferably
cyclohexyl, or alkenyl, preferably 3-pentenyl, are also
preferredO The preferred significance of n is 0 or 1,
particularly 1. The compounds of formula I in which ~
signifies group (a) are also preferred. R preferably
signifies phenyl or substituted phenyl, particularly
phenyl. The preferred significance of R is alkyl-
carbonylalkyl, aminoalkylcarbonylalkyl, substitutedaminoalkylca~bonylalkyl, aminoalkylsulphonylalkyl,
substituted aminoalkylsulphonylalkyl or alkylsulphonyl-
alkyl, preferably alkylca~bonylalkyl or alkylsulphonyl-
alkyl, particularly Cl-C4-alkylcarbonylmethyl or
Cl-C4-alkylsulphonylmethyl. Where A signifies group
(b), then there are preferred those compounds of formula I
in which Y signifies the bivalent residue of phenylalanine
linked with Z at the N-terminal. Z pIeferably signifies
the group R -0-C0- or the residue of an a-amino acid,
preferably proline, acylated by this group, wherein R
signifies an optionally substituted, saturated aliph~tic
hydrocarbon residue with up to 10 carbon atoms or an
optionally subs~ituted heteroaromatic hydrocarbon residue
with up to 18 carbon atoms, particularly a saturated,
aliphatic hydrocarbon residue with up to 6 carbon atoms.
From the above it follows that there are particularly
preferred those compounds of formula I in which R
signifies hydrogen, R signifies imidazol-4-yl, R
signifies cyclohexylmethyl, R signifies hydroxy, R
signifies 3-pentyl, ~luoroalkyl, cyclohexyl or 3-pentenyl,
n signifies 1, R signifies phenyl, R signifies
Cl-C4-alkylcarbonylmethyl or Cl-C4-alkylsulphonyl-
~ 3~7~
- 14 -
methyl, Y signifies the bivalent residue of phenylalanine
linked with Z at the N-terminal and Z signifies the group
R -0-C0- or the residue of proline which is acylated by
thi~ group. wherein R signifies a saturated, aliphatic
hydrocarbon residue with up to 6 carbon atoms.
Quite specially preferred compounds of formula I are:
t-Butyl (R)-2-r[(S~-a-t[(S)-l-rt(lS,2S,4R)-l-(cyclo-
hexylmethyl)-~-hydroxy-4-ethyl-5-hexenyl]carbamoyl]-Z-
-imidazol-4-ylethyl]carbamoyl]phenethyl]carbamoyl]-1-
-pyrrolidinecarboxylate,
t-butyl (R)-2-t~(S)-a- r [ (S)-l-[[(lS,2S)-l-(cyclo-
hexylmethyl)-2-hydroxy-4-e~hylhexyl]carbamoyl]-2-imidizol-
-4-ylethyl]carbamoyl]phenethyl]carbamoyl~-1-pyrrolidine-
carboxylate,
(S)-N-[(lS,2S,4R)-l-(cyclohexylmethyl)-4-ethyl-2-
-hydroxy-5-hexenyl]-a-[(R)-a-(3,3-dimethyl-2-oxobutyl)-
hydrocinnamamido]imidazole-4-propionamide.
(S) -a- [ (S) -~- ~ ( t-butylsulphonyl)methyl]hydrocinnam-
amido~-N- r (lS,ZS.4R)-l-(cyclohexylmethyl)-4-ethyl-2-
-hydroxy-5-hexenyl]imidazole-5-propionamide,
t-butyl r (S)-a-[[(S)-l-[[(lS,2R or 2S)-3-cyclohexyl-
-l-(cyclohexylmethyl)-2-hydroxypropyl]carbamoyl]]-2-
-imidazol-4-ylethyl]carbamoyl]phenethyl]carbamate,
(2R or S,35)-3-(Boc-D-Pro-Phe-His-NH)-1,~-dicyclo-
hexyl-2-butanol and
(S)-N-[(lS,2S)-l-(cyclohexylmethyl)-4-ethyl-4-fluoro-
-2-hydroxyhexyl]-a-[(R)-a-(3,3-dimethyl-Z-oxo~utyl)-
hydrocinnamamido]imidazole-4-propionamide.
The compounds of formula I in the form of optically
pure diastereomers, mixtures of diastereomers, diastereo-
meric racemates or mixtures of diastereomeric racemates as
well as pharmaceutically usable salts thereof can be
manufactured by
2~3~7~J
- 15 -
a) for the manufacture of a compound of formula I in
which R signifies hyd~oxy and the remaining symbols
have the significance given above, reacting a compound of
the genecal formula
H~ ~ N ~ (CH2)n~ R5 II
R2 H OH
wherein R , R , R , R and n have the
significance given above,
with an acylating agent yielding the group
~ (a) or -Y-Z (b)
wherein R . R7. Y, Z and the dotted line have the
significance given above.
or
b) for the manufacture of a compound of formula I in
which R signifies hydroxy and the remaining symbols
have the significance given above. reacting a compound of
the ge~neral formula
R3
H2N ~ (CH2)n- R~ III
0~
wherein R , R and n have the significance given
above,
with a compound of the general formula
1 ' o
A ~ IV
~R2
wherein R , R and A have the significance given
above,
or an activated derivative thereof, or
c) for the manufacture of a compound of formula I in
which A signifies group (b), Z signifies the monovalent
residue of an optionally acylated amino acid or of an
optionally acylated dipeptide attached via the carboxyl
group and R signifies hydroxy and the remaining symbols
have the significance given above, reacting a compound of
formula T in which Z signifies hydrogen and the remaining
symbols have the significance given above with an
optionally acylated amino acid or an optionally acylated
dipeptide, or
d) for the manufacture of a compound of formula I in
which A ~ontains a free amino group and/or R4 signifies
amino and/or R signifies imidazol-2-yl, imidazol-4-yl
or pyrazol-3-yl, cleaving off the N-protecting group(s)
from a corresponding compound of formula I in which A
contains a N-pro~ected amino group and/or from a compound
of the general formula
y~
Rl 5
A~N~(CH2)n--R V
R41
~21
10 wherein R41 signifies hydroxy or N-protected amino
and R signifies ethyl, propyl, isopropyl, thiaz~l-
-4-yl, thien-Z-yl, ethoxycarbonyl, t-butylcarbonyl-
me~hyl, benzyloxycarbonylmethyl, t-butoxy or
optionally N-protected imidazol--2-yl, imidazol-4-yl or
pyrazol-3-yl.and the cemaining symbols have the
significance given above, with the proviso that a~
least one of R and R contains a N-protecting
group,
and
e) if desired, separating a mixture of dias~ereomeric
racemates into the diastereomeric racemates or optically
pure diastereomers, and/or
f) if desired, separating a mixture of diastereomers into
the optically pure diastereomers, and/or
g) if desired, convarting a compound obtained into a
pharmaceutically usable salt.
The acylation of a compound of formula II is effected
acco~ding to methods known per se. Especially suitable
acylating agents are activated acid derivatives such as
esters, mixed esteLs, acid halides and acid anhydrides or
mixed acid anhydrides. The reaction is carried ou~ in an
organic solvent or solvent mixture which is inert under
the reaction conditions at a temperature between about 0C
2 ~
- 18 -
and room temperature. As solvents there come into
consideration especially aromatic hydrocarbons such as
benzene, toluene or xylene, chlorinated hydrocarbons such
as methylene chloride or chloroform, ethers such as
diethyl ether, tetrahydrofuran or dioxan, and the like.
Where the acylating agent is a peptide, the reaction is
effected under reaction conditions which are usual in
peptide chemistry, i.e. preferably in the presence of a
condensation agent suc~ as HBTU (O-benzotriazolyl-
-N,N,N',N'-tetramethyluronium hexafluorophosphate), BOP
(benzotriazol-l-yloxy-bis-(dimethylamino)phosphonium hexa-
fluorophosphate), BOPC (bis(2-oxo-2-oxozolidinyl)phosphine
chloride), HOBT (N-hydro~ybenzotriazole), DBU (l,~-diaza-
bicyclo[5.4.0]undec-7-ene), DCC (dicyclohexylcarbodi-
imide), EDC (N-ethyl-N'(3-dimethylaminopropyl)carbodiimide
hydrochloride), Hunig base (ethyldiisopropylamine), and
the like. The reaction is conveniently carried out in an
organic solven~ or solvent mixture which is inert under
the reaction conditions at a temperature between about 0
and 50~C, preferably at about room temperature. As
solvents there come into consideration especially
dimethylformamide, methylene chloride, acetonitrile,
tetrahydrofuran, and the like.
The reaction of a compound of formula III wi~h a
compound of formula IV is also effected according to
methods which are known per se in peptide chemistry, i.e.
under the same conditions as given above for the reaction
of a compound of formula II with a peptide. Examples of
suitable activated derivatives of a compound of formula IV
are acid halides, acid anhydrides, mixed anhydrides,
esters, mixed esters~ and the like.
The reaction of a compound of formula I in which Z
si~nifies hydrogen with an optionally ac~lated amino acid
or an optionally acylated dipep~ide in accordance with
~V~3~,7J'
-- 19 --
process variant c) is likewise effected according to
methods which are known per se in peptide chemistry, i.e.
under the conditions given above for the reaction of a
compound of formula II with a peptide.
The cleavage of the N-protecting group(s) in
accordance with process variant d) is also effected
according to methods known per se depending on the nature
of the N protecting group to be cleaved o~f. However, the
cleavage is conveniently effected by acidic or basic
hydrolysis. For the acidic hydrolysis there i8
advantageously used a solution of a mineral acid such as
hydrochloric acid, hydrobromic acid, trifluoroace~ic acid,
sulphuric acid, phosphoric acid and the like in an inert
solvent or solvent mixture. Suitable solvents are alcohols
such as methanol or ethanol, ethers such as tetrahydro-
furan or dioxan, chlorinated hydrocarbons such as
methylene chloride, and the like. For the basic hydrolysis
there can be used alkali metal hydroxides and carbonates
such as potassium or sodium hydroxide or potassium or
sodium carbonate, organic amines such as piperidine, and
the li~e. Inert organic solvents such as have been named
above for the acidic hydrolysis can be added as
solubilize~s. The reaction temperature for the acidic and
basic hydrolysis can be varied in a range from about O9C
to the reflux temperature, with the reac~ion preferably
being carried out between about 0C and room temperature.
The ~-butoxycarbonyl residue is convenien~ly cleaved off
with trifluoroacetic acid or formic acid in the presence
or absence of an inert solvent. The Fmoc protecting group
is conveniently cleaved off with piperidine at about room
temperature. The benzyloxycarbonyl group can be cleaved
off in a known manner by acidic hydrolysis as described
above or hydrogenolytically.
~ 3
- 20 ~
The starting materials of formula II are novel and are
also an object of the present invention. These compounds
can be prepared by reacting a compound of formula III with
optionally N-methylated histidine, leucine, norleucine,
norvaline, thiazolylalanine, thienylalanine, aspartic acid
ethyl ester, glutamic acid t-butyl ester, glutamic acid
benzyl ester or t-butoxyserine. This reaction is also
effected according to methods which are known in peptide
chemistry, i.e. under the reaction conditions which are
described above for the reaction of a compound of
formula II with a dipeptide.
The starting materials of formula III are also novel
and are an object of the present invention. They can be
prepared, for example, by cleaving off the amino
protecting group and, where applicable, simultaneously
also the 0-protecting group in a compound of the general
formula
B-HN~(CH2)n--R51 o~ ~(cH2)n--Rs
OH ~o
V I V I I
wherein B signifies an amino protec~ing group,
preferably t-butoxycarbonyl or benzyloxycarbonyl, and
~5l signifies alkyl, cycloalkyl, alkenyl, aryl or
heteroaryl and R and R have the significance
given above.
The cleavage of the W-protecting group and, where
applicable, 0-protecting group is also effected according
to methods known per se, for example in an organic
solvent or solvent mixture which is inert under
2 ~ 7 ~
- 21 -
the reaction conditions at a temperature between about O~C
and room temperature with an acid such as hydrochloric
acid, trifluoroacetic acid and the like. Suitable solvents
are ethers such as tetrahydrofuran or dioxan, alcohols
such as methanol or chlorinated hydrocarbons such as
methylene chloride and the like. Under these reaction
conditions the oxazolidine ring in a compound of
formula VII is - as already mentioned - simultaneously
cleaved.
The starting materials of formula IV are known or can
be obtained in analogy to the preparation of the known
compounds.
The compounds of formulae VI and VII are also novel
and are an object of the present invention. The compounds
of formula VI can be prepared, for example, by reduction
of the corresponding keto compounds of the general formula
R3
B-HN ~ (CH2)n - R51 VIII
wherein B, R3 and R51 have the significance given
above.
The reduction of a keto compound of formula VIII is
also effected according to methods known per se, for
30 example with a complex metal hydride such as sodium
borohydride and the like in an organic solvent or solvent
mixture which is inert under the reaction conditions at a
temperature between about 0C and about room temperature.
The compounds of formula VII in which R signifies
alkyl, cycloalkyl, alkenyl, aryl or heteroaryl can be
2~ 7~
prepared, for example, by reac~ing a compound of
formula VI with 2,2-dimethoxypropane in the presence of
p-toluenesulphonic acid.
The compounds of formula VII in which R signifies
haloalkyl can be p~epared, for example, by subjecting a
compound of focmula VII in which R signifies aikenyl to
a reductive or oxidative ozonolysis. The compound of the
general formula
R3 /R8~ R10
$~ 1R9~0H I X
whe~ein Ra, R9 and R each independently
signify hydrogen or alkyl and m signifies 0, 1, Z, 3,
4, 5, 6 o~ 7, with the proviso that the group
~ R9m ~ R10 has a maximum of B carbon atoms,
OH
and B, R and n have the above significance,
which is obtained in the reductive ozonolysis can be
converted with a halogenating agent into a compound of
formula VII in which R5 signifies primary or secondary
haloalkyl. The compound of the general formula
- 23 -
~(CHZ)n--(IC~COCI I X
wherein B, R , R , R9, n and m have the
significance given above,
which is obtained in the oxidative ozonolysis can be
converted by reaction with, for example, diazsmethane into
the corresponding methyl ester of the general formula
~ (CH2)n ~ ~ ~ COOCH3 XI
~0
wherein B, R3, R , R , n and m have the
significance given above,
which can be converted by reaction with a compound of the
genecal fo~mula
~-Mg_Rll XII
wherein R 1 signifies alkyl and W signifies
chlorine, bromine or iodine, preferably bromine,
in a Grignard reaction into a compound of the general
formula
20~3~7~
- 24 -
~(CH~n--(~OH Xl 11
wherein B, R3, R~, R9, Rll, n and m have the
significance given above, with the proviso that the
group
R8\ R11
I ~ has a maximum of 8 carbon atoms.
This reaction is also effected according to methods known
per se, for example in an inert solvent which is inert
under the reaction conditions. such as an ether, at a
temperature between about 0C and 50~C, preferably at room
temperature-
Reaction of a compound of formula XIII with a
halogenating agent yields a compound of formula VII in
which R signifies tertiary haloalkyl.
The starting materials of formula V are also novel and
are an object of the present invention. Those in which
R signifies N-p~otected imidazol-2-yl, imidazol-4-yl
or pyrazol-3-yl can be prepared, for example, by reacting
a compound of formula III with a compound of fo~muia IV,
but in which R signifies N-protected imidazol-2-yl,
imidazol-4-yl or pyrazol-3-yl 01 an activated derivative
- Z5 -
thereof. The reaction is effected according to methods
which are known per se in peptide chemistry, i.e. under
the same conditions as have been given above for the
reaction of a compound of formula III with a compound of
formula lV.
Those compounds of formula V in which R signifies
N-protected amino can be ~repared, for example, by
reacting a compound of the general formula
R3
1 (CH2)n - R5
H2N ~ XIV
R42
wherein R4 signifie6 N-protected amino and R ,
R and n have the significance given above,
with a compound of formula IV. This reaction is also
effected under the conditions given above for the reaction
of a compound of formula III with a compound of formula IV.
The compounds of formula XlV are also novel and are an
object of the pcesent invention. Those compounds of
formula XIV in which R signifies alkyl, cycloalkyl,
alkenyl, aryl or heteroaryl can be prepared, for example,
by reacting a compound of focmula VIII with hydroxylamine
to give a compound of the general formula
R3
1 ~(CH2~n--R51
B-HN ~ XV
OH
- ~ v ~
wherein B, R3, R51 and n have the signif icance
given above,
reducing this to the corresponding amino compound of the
general formula
B-HN~ (CH2)n~ RS1 XV I
NH2
wherein B, R , R and n have ~he significance
given above,
and protecting the amino group with the formation of a
compound of the general formula
B-HN ~ (CH2)n - R51 XVII
R42
wherein B R3 R42 R51 a d h th
signiicance given above.
The co~Lesponding compound of formula ~IV is obtained by
cleaving off the amino protecting group B und`er the
conditions given for process variant d).
The compounds of formula XIV in which R signifies
haloalkyl can be prepared from those compounds o~
formula XVII in which R signifies alkenyl in an
analogous manner to that described above for the
preparation of the compounds of formula VII in which R
signifies haloalkyl.
The compounds of focmulae VlII, IX, X, XI, Xll, XIII,
XV, XVl, XVII, XVIII, XIX, XX, XXI, XXIl and XXlII are
known or can be obtained in analogy to the preparation of
the known compounds.
The various processes for the preparation of the
compounds of focmulae III, V. VI and VII starting from
compounds of formula XVIII are compiled in Scheme I
hereinafter. In this Scheme R52 signi~ies haloalkyl.
With respect to the precise reaction conditions as well as
further preparative processes, reference is made to the
experimental section.
2 0 ~ .3
8~NlCOOAI~
-- 28 --
Scheme I
B HN~(CH2)n R5t
vlll o
U~NJ~U~n--R51 RJ B N~
XV N~ 6~N~C~i)n~ V~
~I N1~2 \ /~1
cH2)n--(7 ~( X
HN~CH~n ~ R n~ IC~W.--(~COOCH~
--(R)--( R3 \ \ ¦ Xll
R ~
2 5 B N~ H2Jn--(~)--C~OCHl \ j
~I~/N~CH2)~--RS2 R3 ~ CH2)~--Rs2
XX ~42XX~ --(R ~C \
30\ ~ ~ /
~l~ H~)n RSH2N~(CH2h--RS
R 2 OH
3 5 x,v \/ "~
R O R~
~H J~
- 29 -
The compounds of formula I and their pharmaceutically
usable salts have an inhibitory activity on the natural
enzyme renin. The latter passes from the kidneys into the
blood and there brings about the cleavage of angio-
tensinogen with the formation of the decapeptide angio-
tensin I which is then cleaved in the lungs, the kidneys
and other organs to the octapeptide angiotensin II. Angio-
tensin II increases the blood pressure not only directlyby arterial constriction, but also indirectly by the
liberation of the sodium ion-retaining hormone aldosterone
from the adrenal gland, with which is associated an
increase in the extracellular fluid volume. This increase
is attributed to the action of angiotensin Il itself or ts
the heptapeptide angiotensin III which is formed therefrom
as a cleavage product. Inhibition of the enzymatic
activity of renin brings about a decrease in the formation
of angiotensin I and as a consequence thereof the
formation of a smaller amount of angiotensin II. The
reduced concentration of this active peptide hormone is
the actual reason for the blood pressure-lowering activity
of renin inhibitors.
The activity of renin inhibitors can be demonstrated
experimentally by means of the in vitro test described
hereinafter:
In vitro test with Pure human renin
The test is carried out in Eppendorf test tubes. The
incubation mixture consists of ~1) lO0 ~1 of hu~an renin
in buffer ~ ~O.lM sodium phosphate solution, pH 7.g,
containing 0.1~ bovine serum albumin, 0.1% sodium azide
and l mM ethylenediaminetetraacetic acid), suf~icient for
a renin activity of 2-3 ng of angiotensin I/ml/hr.; (2)
145 ~l of buffer ~; (3) 30 ~l of lO ~M human tetra-
decapep~ide renin substrate (hTD) in lO mM hydrochloric
acid: (4) 15 ~1 of dimethyl sulphoxide with or without
2~3~7~
_ 30 -
inhibitor and (5) ~0 ~1 o~ a 0.03 molar solution of
hyd~oxyquinoline sulphate in water.
The samples are incubated for three hours at 37C or
4C in triplicate. 2 x lO0 ~1 samples per experimental
test tube are used in order to measure the production of
angiotensin I via RIA (~tandard radioimmunoassay: clinical
assay solid phase kit). Cross reactivities of the antibody
used in the RIA are: angiotensin 1 100%; angiotensin II
0.0013%; hTD (angiotensin I-Val-Ile-His-Ser-OH) 0.09%. The
production of angiotensin I is determined by the
difference between the experiment at 37C and that at 4C.
The following controls are carlied out:
(a) lncubation of hTD sam~l0s without renin and without
inhibitor at 37C and 4C. The difference between these
two values gives the base value o~ angiotensin I
produ~tion.
(b) lncubation of hTD samples with renin, but without
inhibitor at 37C and 4C. The difference between these
values gives the maximal value of angiotensin I production.
In each sample the base value o~ the angiotensin I
production is subtracted from the angiotensin I production
which is det-ermined. The difference between the maximal
value and the base value gives the value of the maximal
substrate hydrolysis (= 100%) by renin~
The results are given as IC50 values which denote
that concentration of the inhibitor at which the enzymatic
activity is inhibited by 50%. The IC50 values are
determined from a linear regression curve from a logit-log
plot.
~ 3~3
- 31 -
The results obtained in this test are compiled in the
following Table:
Table
Compound IC50 values in ~mol/lt.
10 A 0.0002
B 0.001
C 0.0042
D 0.001
E 0.0026
15 F 0.001
G 0.005
A = t-Butyl (R)-2-rr(S)-a-[r(S)-l-[ r (lS,2S,4R)-l-(cyclo-
hexylmethyl)-2-hydroxy-4-ethyl-5-hexenyl]carbamoyl]-2-
-imidazol-4-ylethyl]carbamoyl]phenethyl]carbamoyl]-1-
-pyrrolidinecarboxylate,
B = t-Butyl (R)-2-[r(S)-a-r~(S)-l-[[~lS,2S)-l-(cyclo-
hexylmethyl)-2-hydroxy-4-ethylhexyl]carbamoyl]-2-imidazol-
-4-ylethyl]carbamoyl]phenethyl]carbamoyl3-1-pyrrolidine-
carboxylate,
C - (S)-N-r(lS,2S,4E~-l-(Cyclohexylmethyl)-4~ethyl-2-
-hydroxy-5-hexenyl]-a-r(R)-a-(3,3-dimethyl-2-oxobutyl)-
hydrocinnamamido]imidazole-4-propionamide,
D = (S)-a- r (s)-a-r (t-bu~ylsulphonyl)methyl]hydrocinnam- -
amido]-N-r(lS,2S,4R)-l-(cyclohexylmethyl)--4-ethyl-2-
-hydroxy-5-hexenyl]imidazole-5-propionamide,
E = t-Butyl r(S)-a-r[(S)-l-rt(lS,2R or 2S)-3-cyclohexyl-
-l-(cyclohexylmethyl)-2-hydroxypropyl]carbamoyl3-2-
-imidazol-4-ylethylJcarbamoyl]~henethyl]carbamate,
2 ~ 7
F = (2R or S,3S)-3-(Boc-D-Pro-Phe-His-NH)-1,4-dicyclo-
hexyl-2-butanol and
G = (S)-N-t(lS,2S)-l-(Cyclohexylmethyl)-4-ethyl-4-fluoro-
-2-hydroxyhexyl~-a-[(R)-a-(3,3-dimethyl-2-oxobutyl)-
hydrocinnamamido]imidazole-4-propionamide.
The compounds of formula I as well as their pharmaceu-
tically usable salts can be used as medicaments, e.g. in
the form of pharmaceutical preparations. The pharma-
ceutical preparations can be administered enterally such
as orally, e.g. in the form of tablets, coated tablets,
dragees, hard and soft gelatine capsules, solutions,
emulsions or sus~ensions, nasally, e.g. in the form o
nasal sprays, or rectally, e.g. in the form of
suppositories. However, the ad~inistration can also be
effected parenterally such as intramuscularly or
intravenously, e.g. in the form of injection solutions.
For the manufacture of tablets, coated tablets,
dragees and hard gelatine capsules the compounds of
formula I as well as theic pharmaceutically usable salts
can be prosessed with pharmaceutically inert, inorganic or
organic excipients. Lactose, maize starch or derivatives
th~reof, talc, stearic acid or its salts etc can be used
e.g. as such excipients for tablets, dragees and hard
gelatine capsules.
Suitable excipients for soft gelatine capsules are
e.g. vegetable oils, waxes, fats, semi-solid and liquid
polyols etc.
Suitable excipients for the manufacture of solutions
and syrups are e.g. water, polyols, saccharose, invert
sugar, glucose etc.
~J~3~7~
- 33 -
Suitable excipients for injection solutions are e.g.
water, alcohols, polyols, glycerol, vegetable oils etc.
Suitable excipients for suppositocies are e.g. natural
or hardened oils, waxes, fats, semi-liquid or liquid
polyols etc.
Moreover, the pharmaceutical preparations can contain
preserving agents, solubilizers, viscosity-increasing
substances, stabilizing agents, wetting agents,
emulsifying agents, sweetening agents, colouring agents,
flavouring agents, salts for varying the osmotic pressure,
buffers, coating agents or antioxidants. They can also
contain still other therapeutically valuable substances.
ln accordance with the invention ~he compounds of
general formula I as well as their pha~maceutically usable
salts can be used in the control or prevention of high
blood pcessure and cardiac insufLiciency. The dosage can
vary within wide limits and will, of course, be fitted to
the individual requirements in each particular case. In
general, in the case of oral administration there should
suf~ice a daily dofiage o~ about 3 mg to about 3 g,
preferably about 10 mg to about 1 g, e.g. approximately
300 mg per person, divided in preferably 1-3 unit doses,
which can e.g. be of the same amount, whereby, however,
the upper limit just given can also be exceeded when this
is found to be indicated. Usually, children receive half
of the adult dosage.
The following Examples are intended to illustrate the
presen~ invention, but are not intended to be limiting in
any manner. All temperatures are given in degrees Celsius.
The following abbreviations are used:
7 ~
- 34 -
Boc = t-butoxycarbonyl
Fmoc = 9-fluorenylmethoxycarbonyl
H-His OH = L-histidine
H-Phe-OH = L-phenylalanine
H-D-Pro-OH = D-proline
H-Phe-His-OH = N-[(S)-2-amino-3-phenylpropyl]-
-L-hi 6 tidine
(Phe-His-NH? = L-phenylalaninyl-L-histidinamido
(Fmoc)2His-OH = N--N-im-di-Fmoc-L-histidine
Exam~le l
To a solution of 227 mg (0.5 mmol) of (2)-a-amino-N-
-~(lS,2R or S)-l-(cyclohexylmethyl)-7-hydroxy-3-phenyl-
propyl~imidazole-4-propionamide dihydrochloride in 2 ml of
dimethylformamide while stirring and passing through argon
at 0 there are added in ~he given sequence 151 mg of
N-methylmorpholine, 1~6 mg sf Boc-Phe-OH and 208 mg of
HBTU. Thereafter, the reaction mixture i~ allowed to warm
20 to room temperature and the pH is adjusted to 8.5 by the
dropwise addition of N-methylmorpholine. After stirring a~
room temperature for 2 hours the reaction mixture ls
poured into saturated sodium bicarbonate solution and
stirred at room temperature overnight. Thereafter, the
25 mixture is extracted with ethyl acetate and the organic
ex~ract is washed with water, dried over magnesium
sulphate and evaporated under reduced pressure. The
residual brown oil is purified by chromatography on silica
gel with a 95:5 mixture of ethyl acetate and methanol as
30 the eluting agent, whereby there are obtained 128 mg (41%)
of t-butyl [(S)-a-[t(S)-l-[[(lS,2R or S)-l-(cyclohexyl-
methyl)-2-hydroxy-3-phenyl]carbamoyl]-2-imidazol-4-yl-
ethyl]carbamoyl]phenethyl]carbamate as a white solid which
melts at 91-93 after recrystalli7ation from ether, MS:
35 632 (M+H) .
2~ 3~
- 35 -
The (2)-a-amino-N-[(lS,2R or S)-l-(cyclohexyl-
methyl)-2-hydroxy-3-phenylpropyl]imidazole-4-propionamide
dihydrochloride used as the starting material was prepared
as follows:
A suspension of 1.45 g (0.06 gram atom) of magnesium
shavings in 15 ml of abs. ether is treated dropwise while
sticring and passing through argon with a solution of
10.26 g (0.06 mmol) of benzyl bromide in 50 ml of ether in
such a manner that the reaction mixture boils slightly
under reflux (35 minutes). ~fter completion o~ the
addition the reaction mixture is heated to reflux for
3 hours until all of the magnesium shavings have reac~ed.
Thereafter, the reaction mixture is cooled to -60 and
treated dropwise within 30 minutes with a solution of
4.82 g (19 mmol) of 2-t-butoxycarbonylamino-3(S)-cyclo-
hexylpropylaldehyde [prepared according to the method
described by J. Boger et al. in J. Med. Chem., 28, ~779
(1985)]. ThereafteL, the reaction mixture is allowed to
warm to room temperature and is poured into ~aturated
ammonium chloride solution cooled to 0~. The aqueous phase
i5 extracted several times with ether and the combined
ether extcacts are washed with saturated ammonium chloride
solution, dried over magnesium sulphate and evaporated
under reduced pressure. The oily residue is purified by
chromatography on silica gel with a 4:1 mixture o
methylene chloride and ethyl acetate-as the eluting agent.
Crystallization from hexane yields Z.295 g (31.5%) of
t-butyl [~lS,2R or S)-l-(cyclohexylmethyl)-Z-
-hydroxy-3~phenylpropyl]carbamate as a white solid,
melting point 91~.
500 mg (1.4 mmol) of t-butyl [(lS,2R or S)-l-(cyclo-
hexylmethyl)-2-hydroxy-3-phenylpropyl]carbamate are left
to stand at room temperature for 2 houcs in 5 ml o 4.6N
hydrochloric acid in methanol. ~hereater, the solvent is
2 ~ 1 3 ~ 7 ~
evaporated under reduced pressure. Crystallization of the
residue from hexane yields 369 mg (93%) of (R or S)-a-
-~(S)-l-amino-2-cyclohexylethyl]-2-phenylethanol hydro-
chloride as a white solid, melting point 137-14Z.
In an analogous manner to that described in the first
paragcaph o~ this Example, by reacting 1.42 g (5 mmol) o~
(R or S)-a-r(5)-l-amino-2-cyclohexylethyl]-2-phenyl-
ethanol hydrochloride with 1-(t-butoxycarbonyl)-N-(t-
-butoxycarbonyl)-L-histidine there were obtained 2.11 g
(72%) o~ t-butyl [~S)-2-[1-(t-butoxyca~bonyl)imidazol-4-
-yl ] - 1- [ r ( lS,2R or S)-l-(cyclohexylmethyl)-2-hydroxy-3-
-phenylpropyl]carbamoyl]ethyl]carbamate as colourless
crystals, melting point 98-105 (~rom hexane), MS: 585
(MIH) .
1 g (1.7 mmol) of ~-butyl ~(S)-2-rl-(t-butoxy-
carbonyl)imidazol-4-yl]-1-rr(lS,2R or S)-l-(cyclohexyl-
methyl)-2-hyd~oxy-3-phenylpropyl]carbamoyl]ethyl]carbamate
is left to stand at room temperature for 6 hours under
argon in 5 ml o~ 4.8N hydrochloric acid in dioxan.
Evaporation o~ the reaction mixture to dryness yields
(2)-a-amino-N-[(15,2R or S)-l-(cyclohexylmethyl)-2-
-hydroxy-3-phenylpropyl]imidazole-4-propionamide dihydro-
chloride which is used in the next step without further
purification.
xample 2
ln an analogous manner to that described in Example 1,
by reacting ~S)-a-amino-N- r (lS,2R or S)-3-cyclohexyl-1-
-(cylcohexylmethyl)-2-hydroxypropyl]imidazole-4-propion-
amide dihydrochloride with ~R)--(pivaloylmethyl)hydro-
cinnamic acid (see EPA 0,184,550) there was obtained
(S)-N-[(lS,2R or S)-3-cyclohexyl-1-(cyclohexylmethyl)-2-
-hydroxypropyl]--~(R)-a-(3,3-dimethyl-2-oxobutyl)-
2~ '7fjJ
hydrocinnamamido~imidazole-4-propionamide in 40~ yield as
a white solid. melting point 164-165 (fcom ethyl
acetate/isopropyl ether), MS: 621 (M~H) .
The (S)-a-amino-N-[(lS,2R or S)-3-cyclohexyl-1-
-~cyclohexylmethyl)-2-hydroxypropyl3imidazole-4-propion-
amide dihydrochloride used as the starting material was
prepared as follows:
A soluti~n of ~.7 g (13.5 mmol~ o~ t-butyl [(lS,ZR or
S)-l-(cyclohexylmethyl)-2-hydroxy-3-phenylpropyl]carbama~e
in 100 ml of methanol is hyd~ogenated in the presence of
5 g o rhodium on aluminium oxide (5~ a~ room temperature
and under a pressure of 350 kPa. Thereafter, the catalyst
is filtered off and the filtrate is evaporated under
reduced pressure, whereby there are obtained 4.7 g (98%)
of t-butyl [(lS,2R or S)-3-cyclohexyl-1--(cyclohexyl-
methyl)-2-hydroxypropyl]carbamate as a white foam which is
used in the next step without further puci~ication, MS:
298 (M-isobutylidene) 2Z6 (M-cyclohexylethanol).
A solution of 4.0 g (11 mmol) of t-butyl [(15,2R or
S)-3-cyclohexyl-1-(cyclohexylmethyl3-2-hydroxypropyl]-
carbamate in 10 ml of ethyl aceta~e and Z0 ml of 2.5N
hydrochloric acid in ethyl acetate is left to stand at
room temperature for 2 hours while passing through argon
and thereafter the solvent i6 evaporated under reduced
pressure. Recrystallization of the crystalline residue
obtained from ether/hexane yie~ds 2.42 g (76%) of (2R or
S)-3-amino-1,4-dicyclohexyl-2-butanol hydrochloride as
colourless crystals, melting point 188-190.
In an analogous mannec to that described in Example 1,
by reacting 2 g (7 mmol) o~ (2R or S)-3-amino-1,4-dicyclo-
hexyl-2-butanol hydrochloride with l-(t-butoxycacbonyl)-N-
-(t-butoxycarbonyl)-L-histidine there were obtained 0.80 g
- 38 -
(19.4%) of t-butyl 4-r(S)-2-(1-t-butoxyormamido)-Z-
-[t(lS.2R or S)-3-cyclohexyl-l-(cyclohexylmethyl)-2-
-hydroxypropyl]carbamoyl]ethyl]imidazole-l-carboxylate as
a colourless liquid and 3.0 g (72.6%) of the corresponding
diastereomer mixture as a colourless foam. The t-butyl
4-[(S)-2-(1-t-butoxyformamido)-2-t[(lS,2R or S)-3-cyclo-
hexyl-l-(cyclohexylmethyl)-2~hydroxypropyl]carbamoyl~-
ethyl]imidazole-l-carboxylate was used in t~e next step
without furthe~ purification.
In an analogous manner to that described in Example 1,
by cleavage of the Boc protecting group there was obtained
(S)--a-amino-N-r(lS,2R or S)-3-cyclohexyl-1-(cylcohexyl-
methyl)-Z-hydroxypropyl]imidazole-4-propionamide dihydro-
chloLide in 73% yield as a white solid, melting point
196-196 (dec., from ethyl acetate).
Example 3
ln an analogous manner to that described in Example 1,
by reacting (S)-a-amino-N-r(lS,2R or S)-3-cyclohexyl-1-
-(cylcohexylmethyl)-2-hydcoxypropyl]imidazole-4-propion-
amide hydrochloride with Boc-Phe-OH there wa~ obtained
t-butyl r(S)-a-rr(S)-l-[r(lS,2R or S)-3-cyclohexyl-1-
-(cyclohexylmethyl)-2-hydroxypropyl]carbamoyl]-2-imidazole-
-4-ylethyl]carbamoyl3phenethyl]carbamate in 49~ yield as a
white solid, melting point 183-lB4 (from hexane), MS:
638 (M~-H) .
Exam~_e 4
In an analogous manner to that described in Example 1,
by cleaving off the Boc erotecting group from t-butyl
[(S)-a-[t(5)-1-tt(lS,2R or S)-3-cyclohexyl-1-(cyclo-
hexylmethyl)-2-hydroxyp~opyl]carbamoyl]-2-imidazol-4-yl-
ethyl]carbamoyl]phenethyl~carbama~e there was obtained (2R
2 0 ~
- 39 -
or 2S,3S)-1,4-dicyclohexyl-3-(Phe-His-NH)-2-butanol in ~6%
yield as a white solid, melting point 193-195 (dec.,
from ether), MS: 538 (~H) .
Example 5
In an analogous manner to that described in Example 1,
by reacting ~2R or 2S,3S)-1,4-dicyclohexyl-3-(Phe-His-NH)-
-2-butanol with Boc-D-Pro-OH there was obtained (2R or
S,3S)-3-(80c-D-Pro-Phe-His-NH)-1,4-dicyclohexyl-2-butanol
in 40~ yield as a white solid, melting point 131-133
(from ethyl acetate/hexane), MS: 735 lM~H) .
ExamPle 6
In an analogous manner to that described in Example 1,
by reacting (S)-a-amino-N-[(lS,2R or S)-l-(cyclohexyl-
methyl)-2-hydroxyphenethyl]imidazole-4-propionamide with
(R)-a-(pivaloylmethyl)hydrocinnamic acid there was
obtained (S)-N-[(aS.~R or S)--(cyclohexylmethylj-A-
-hydroxyphenethyl]-- r (R)-a-(3,3-dimethyl-2-oxobutyl)-
hydrocinnamamido]imidazole-4-propionamide in 22% yield as
a white solid, melting point 95 (dec., from ethyl
acetate/hexane), MS: 601 (M~H) .
The (S)--amino-N-[(lS,2R or S)-l-(cyclohexyl-
methyl)-2-hydroxyphenethyl]imidazole-4-propionamide used
as the starting material was prepared as follows in an
analogous manner to that described in Example 1:
Reaction of 2-t-butoxycarbonylamino-3(S)-cyclohexyl-
propylaldehyde with phenylmagnesium bromide in a Grignard
reaction yields t-butyl r (aS)-a-(cyclohexylmethyl)-~-
-hydroxyphenethyl]carbamate in 34% yield as a white solid,
melting point 108 (~rom hexane), MS: 260 (M-t-butoxy),
226 (M-benzyl alcohol). Cleavage o~ the Boc protecting
- 40 -
group yields a-~(S)-l-amino-2-cyclohexylethyl]benzyl
alcohol hydrochlocide in ~7% yield as a white solid,
melting point 172 (from hexane). MS: 234 (M+H) , which
can be convected by LeactiOn with l-(t-butoxycarbonyl)-N-
-(t-butoxycarbonyl)-L-histidine in 55% yield into t-butyl
~(S)-2-[1-(t-butoxycarbonyl)imidazol-4-yl]-1-[[(aS,~R or
S)-a-(cyclohexylmethyl)-B-hydroxyphenethyl]carbamoyl]-
ethyl]carbamate, melting point 97~ (from hexane), MS: 571(M~H) , from which by cleavage of the Boc protecti~g
group there is obtained (S)-a-amino-N-r(15,2R or S)-l-
-(cyclohexylmethyl)-2-hydroxyphenethyl]imidazole-4-propion-
amide as an amorphous solid in 74% yield, melting poin~
197-199 (from hexane), MS: 371 (M~H) .
ExamPle 7
In an analogous manner to that described in Example 1,
by reacting (S)-a-amino-N-[(2-cyclohexyl-1-(cyclohexyl-
methyl)-2-hydroxyethyl]imidazole-4-propionamide hydro-
chloride (2:5) with (R)-a-(pivaloylmethyl)hyd~ocinnamic
acid there is obtained (S)-N-[(lS,2R or S)-2-cyclohexyl-1-
-(cyclohexylmethyl)-2-hydroxyethyl]-a[(R)--(3,3-
-dimethyl--2-oxobutyl)hydrocinnamamido]imidazole-4-propion-
amide in 20% yield as a white solid, melting point 97
(from hexane), MS: 607 (M~H) O
The (S)-a-amino-N-[(2-cyclohexyl-1-~cyclohexyl-
methyl)-2-hydroxyethyl]imidazole-4-propionamide hydro-
chloride used as the starting material was prepared as
follows:
Catalytic hydrogenation of t-butyl [(aS)-a-(cyclo-
hexylmethyl)-B-hydroxyphenethyl]carbamate in an analogous
manner to that described in Example 2 yields t-butyl
[(lS,2R or S~-2-cyclohexyl-1-(cyclohexylmethyl)-2-hydroxy-
ethyl]carbamate in 93% yield as a colourless foam, MS: Z66
2 ~ 7 ~
- 41 -
(M-t-butoxy), 226 (M-cyclohexylmethanol), which by
cleavage of the Boc protecting group with hydrochloric
acid in dioxan in an analogous manner to that described in
Example 1 yields (lR or S,2S)-2-amino-1,3-dicyclohexyl-1-
-propanol hydrochloride in 90~ yield as a white solid,
melting point 212 (from hexane). Reaction of this
compound with l-(t-butoxycarbonyl)-N-(t-butoxycarbonyl)-L-
-histidine in an analogous manner to that described in
Example 1 yields t-butyl [(S)-2 [l-(t-butoxycarbonyl)-
imidazol-4-yl]-1-t[(lS,2R or S)-2-cyclohexyl-1-(cyclo-
hexylmethyl)ethyl]carbamoyl]ethyl]carbamate in 70% yield
as a colourless foam, MS: 577 (M~H) , from which by
again cleaving of~ the Boc protecting group there can be
prepared (S)-a-amino-N-~(2-cyclohexyl-~-(cyclohexyl-
methyl)-2-hydroxyethyl~imidazole-4-propionamide hydro-
chloride (2:5) in 72% yield as a white solid, melting
point 175 (dec., from hexane), MS: 358 (M-H20).
Example 8
In an analogous manner to that described in Example 1,
by reacting (S)-a-amino-N-[(2-cyclohexyl-1-(cyclohexyl-
methyl)-2-hydroxyethyljimidazole-4-propionamide hydro-
chloride (2:5) with Boc-Phe-OH there was obtained t-butyl
[(S~-a- r [ (S)-1-[~2-cyclohexyl-1-(cyclohexylmethyl)-2-
-hydroxyethyl~carbamoylJ-2-imidazol-4-ylethyl~carbamoyl~-
phenethyl]carbamate in 20~ yield as a ~hite solid, melting
point 124-127 (from ethyl acetate/hexane), MS: 624
(MIH) .
The (S)-a-amino-N-[(2-cyclohexyl-1-(cyclohexyl-
methyl)-2-hydroxyethyl]imidazole-4-propionamide hydro-
chloride (2:5) used as the starting material was preparedas follows in an analogous manner to that described in
Example 1:
Reaction of Z-t-butoxycarbonylamino-3(S)-cyclohexyl-
propylaldehyde with cyclohexylmagnesium bromide in a
Grignard reaction yields in 59~ yield t-butyl ~(2-cyclo-
hexyl-l-(cyclohexylmethyl)-2-hydroxyethyl]carbamate as a
mixture of diastereomers in the form of a colourless oil,
MS: 284 (M-isobutylidene), 226 (~-cyclohexylmethanol),
which, after cleavage of the Boc protecting group with
hydrochloric acid in dioxan, yields a mixture of
diastereomers of 2-amino-1,3-dicyclohexyl-1-propanol
hydrochloride in 57% yield, melting point 191-198 (from
hexane) MS: 240 (M~H) , 156 (M-cyclohexane), 126
(M-cyclohexylmethanol). By reaction with l-(t-butoxy-
carbonyl)-N-(t-butoxycarbonyl)-L-histidine there i5
obtained in 48% yield t-butyl r ( s ) - 1- [ 1- ( t-butoxy-
carbonyl)imidazol-4-yl]-1-r[2-cyclohexyl-1-(cyclohexyl-
methyl)ethyl]carbamoyl]eth~l]carbamate as a white foam,
MS: 577 (M~H) . Cleavage of the Boc protecting group
~rom the just-named compound yields (S)-a-amino-N-[(2-
-cyclohexyl-l-(cyclohexylmethyl)-2-hydroxyethyl]imidazole-
-4-propionamide hydrochloride (2:5) in 72~ yield as a
white solid, melting point 175 (dec., from hexane), MS:
377 (M~H) .
Example__9
740 mg (1 mmol) of (S)-N-r(lS,2S)-l-(cyclohexyl-
methyl)-4-et-hyl-4-fluoro-2-hydroxyhexyl]--[(R)-a-
-(3,3 dimethyl-2-oxobutyl)hydrocinnamamido]-1-(t-butoxy-
carbonyl)imidazole-4-propionamide are dissolved in 10 ml
of methanol, treated with 10 mg of potassium carbonate and
stirred at room temperature for 1.5 hours. Thereafter, the
reaction mixture is evaporated and the residue is
chromatographed on 50 g of silica gel with a 1000:50:1
mixture o~ chloroiorm, ethanol and ammonia as ~e eluting
agent, whereby thece are obtained 57 mg (9~) of (R)-N-
-[(15,2S)-l-(cyclohexylmethyl)-4-ethyl-4-fluoro-~-hydroxy-
2 ~ 7 ~
- 43 -
hexyl]-a-r(R)-a-(3,3-dimethyl-2-oxobutyl)hydrocinnam-
amido~imidazole-4-propionamide as an oil and 317 mg (50%)
of the epimeric compound (S)-N-[(lS,2S)-l-(cyclohexyl-
methyl)-4-ethyl-4-fluoro-2-hydroxyhexyl]-a-r(R)-a-
-(3,3-dimethyl-2-oxobutyl)hydrocinnamamido]imidazole-4-
-propionamide as a foam, MS (both): 627 (M+H) .
The (S)-N- r (lS,2S)-l-(cyclohexylmethyl)-4-ethyl-4-
-fluoro-2-hydroxyhexyl]-~-[(R)-a-~3,3-dimethyl-2-oxo-
butyl)hydrocinnamamido]-l-(t-butoxycarbonyl)imidazole-4-
-propionamide used as the starting material was erepared
as follows:
0.95 g (2.9 mmol) of t-butyl [(lS,2S)-l-(cyclohexyl-
methyl)-2-hydroxy-3-(methoxycarbonyl)propyl]carbamate,
prepared according to the method described in
EPA 0,165,226, in 20 ml of dimethoxypropane and 50 mg
(0.2 mmol) of p-toluenesulphonic acid are stirred at room
temperature for Z4 hours. Subsequently, the reaction
mixture is extracted three times with 150 ml of ether each
time and the ether extracts are washed with sodium
bicarbonate solution and saturated sodium chloride
solution. After drying and evaporating the ether extracts
the residue is chromatographed on 80 g o~ silica gel with
a 9:1 mixture o~ toluene and ethyl acetate as the eluting
agent, whereby there are obtained 900 mg (84%) of t-bu~yl
(4S,5S)-l-(cyclohexylmethyl)-5- r (methoxycarbonyl)methyl]-
-2,2-dimethyl-3-oxazolidinecarboxylate, MS: 370 (M+H) .
850 mg (2.3 mmol) of t-butyl (4S,5S)-l-(cyclohe~yl-
methyl)-5-t(methoxycarbonyl)methyl]-2,2-dimethyl-3-
-oxazolidinecarboxylate in 15 ml of te~rahydrofuran are
added dropwise at room temperature to a Grignard solution
prepared ~rom 55~ mg (23 mgram atom) of magnesium sha~ings
and 1.72 ml (23 mmol) oi ethyl bromide in 15 ml of tetra-
hydrofuran. The reaction mixture is subsequently stirred
2 ~
- 44 -
at room tempecature for a further 1.5 hours, then poured
into a mixture of ice and ammonium chloride solution and
extracted three times with 150 ml of ether each time. The
ether extracts are washed with 70 ml of water, combined,
dried and evaporated. Chromatography of the residual oil
(6B0 mg) are chromatographed on 30 g of silica gel with a
95 5 mixture of toluene and ethyl acetate containing 1%
triethylamine as the eluting agent, whereby there are
obtained 600 mg (66~) of t-butyl (4S,5S)-4-(cyclohexyl-
methyl)-5-(2-hydroxy-2-ethylbutyl)-2,2-dimethyl-5-
-oxazolidinecarboxylate as an oil, MS: 398 (M~H) .
A solution of 5~0 mg (1.45 mmol) of t-butyl (4S,5S)-4-
-(cyclohexylmethyl)-5-(2-hydroxy-2-ethylbutyl)-Z,2-
-dimethyl-5-oxa~olidinecarboxylate in 2 ml of methylene
chloride is added dropwise at -78 under argon to a
solution of 0.53 ml (4.38 mmol) o~ diethylaminosulphur
trifluoride in 1 ml o methylene chloride and the reaction
mixture is subsequently stirred at this temperature for
5 hours. Thereafter, the reaction mixture is partitioned
between methylene chloride and water and the organic phase
is washed once with water, dried over magnesium sulphate
and evaporated. The residue (560 mg of oil) is chromato-
graphed on 70 g of silica gel with a 98:z mixture of
toluene and ethyl acetate as the eluting agent, whereby
there are obtained 500 mg of an oil which is used directly
in the next step.
500 mg of the above oil are stirred for 45 minutes at
room tempera~ure under argon in a solution of 2.5 ml of 4M
chlorotrimethylsilane in methylene chloride and 7.5 ml of
4M phenol in methylene chloride. Thereafter, the reaction
mixture is poured on to ice and extracted twice with
150 ml of methylene chloride. The organic extracts are
washed with 70 ml of water and 70 ml o~ saturated sodium
chloride &olution, combined, dried over magnesium
sulphate, filtered and evaporated. Chromatography of the
residual oil (1.66 g~ on 70 g of silica gel with a
20:~:0.1 mixture of methylene chloride, methanol and
ammonia as the eluting agent yields 177 mg of (aS,~S)-B-
-amino-a-(2-ethyl-2-fluorobutyl)cyclohexylpropanol as a
white solid, MS: 260 (M~H) .
167 mg (0.64 mmol) of (aS,~S)-B-amino-a-(2-ethyl-
-2-fluorobutyl)cyclohexylpropanol and 345 mg (0.71 mmol)
of l-(t-butoxycarbonyl)-N-r(R)-a-(3,3-dimethyl-2-oxo-
-butyl)-hydrocinnamoyl]-L-histidine in 10 ml oi dime~hyl-
formamide a~e treated in succession with 0.1 ml
(0.71 mmol) of triethylamine, 116 mq (0.71 mmol) of HOBT
and 289 mg (0.71 mmol) of HBTU and the reaction mixture is
stirred at oom temperatuce for 3 hours. Thereafter, it is
poured on to ice and 70 ml of 2N sodium bicarbonate
solu~ion and extracted three times with 150 ml of ethyl
acetate each time. The organic extracts are washed once
with ice and 70 ml of saturated ammonium chloride solution
and once each time with 70 ml of 2~ sodium bicarbonate
solution and 70 ml of saturated sodium chloride solution,
dried over magnesium sulphate, filtered and evapocated,
whereby there are obtained 740 mg of crude (S)-N-[~lS,2S)-
-l-(cyclohexylmethyl)-4-ethyl-4-fluoro-2-hydroxyhexyl]-a-
-[(R)-a-(3,3-dimethyl-~-oxobutyl)hydrocinnamamido]-1-
-(t~butoxycarbonyl)imidazole-4-propionamide which i6 used
directly in the next step without further purification.
The l-(t-butoxycarbonyl)-N- r (R)-a- (3, 3-dime~hyl-2-
-oxobutyl~-hydrocinnamoyl~-L-histidine which is also used
as a starting material was prepared as follows:
A suspension of 3.~ g (12 mmol) of (R)-a-(pivaloyl-
methyl)hydrocinnamic acid and 2.66 g (11 mmol) of
L-histidine methyl ester dihydcochloride in 340 ml of
dimethylformamide is treated at room temperature under a
~ J,
- A6 -
nitrogen atmosphere with 3.45 g (34 mmol) of triethylamine
and 4.58 g (12 mmol) of HBTU. The reaction mixture is
stirred at room temperature for 5 hours and subsequently
evaporated in a high vacuum. The residue is dissolved in
500 ml of ethyl acetate and washed in succession with
100 ml of water, three times with 100 ml of saturated
sodium bicarbonate olution each time and with 100 ml of
saturated sodium chloride solution. The organic phase is
dried oveL sodium sulphate, evaporated under reduced
pressure and the yellowish crude product obtained is
chromatographed on silica gel with a 95:5 mixture of
methylene chloride and methanol containing 0.1% ammonia.
In this manner there are obtained 3.6 g of N-r(R)-a-
-(3,3-dimethyl-2-oxobutyl)hydrocinnamoyl]-L-histidine
methyl ester as a coloucless foam, MS: ~99 (M) .
A solution of 3.56 g (8.9 mmol) of N-[(R)-a-(3,3-
-dimethyl-2-oxobutyl)hydrocinnamoyl~-L-histidine methyl
ester and 9.36 ml of lN sodium hydroxide solution in 50 ml
of methanol is stirred at room temperature for 15 hours
and thereafter evaporated under reduced pressure in the
cold. The residue is dissolved in 70 ml of dioxan and
25 30 ml o~ water, a solution of 2.95 (13.5 mmol~ of di-t-
-butyl dicarbonate is added dropwise the~eto at room
temperature and the mixture is thereafter sti~red at room
temperature for 15 hours. For the working-up, the reaction
solution is concentrated to about 1/3 of its volume under
reduced pressure and then diluted with 200 ml of ethyl
acetate. After the addition of 50 ml of ice-water the
reaction mixtule is adjusted to pH 2.5 and the aqueous
phase is saturated with solid sodium chloride. The aqueous
phase is extracted twice with ethyl acetate and the
combined ethyl acetate phases are dried over ~odium
sulphate and evaporated. The crude product obtained is
chromatographed on silica gel with a 95:5 mixture of
methylene chloride and methanol containing 0.1% acetic
2a~3~
- 47 -
acid, whereby there are obtained 3.5 g of l-t~-butoxy-
carbonyl)-N-[(R)-a-(3,3-dimethyl-2-oxobutyl)-hydro-
cinnamoyl]-L-histidine as a colourless powder, MS: 486
(MIH) ,
Exam~le 10
200 mg (0.3 mmol) of benzyl ~(S or R)-1-r(S)-2-cyclo-
hexyl-l-~(S)-a-r(R)-a-(3,3-dimethyl-2-oxobutyl)hydro-
cinnamamido]imidazol-4-propionamido]ethyl]-2-ethylbutyl]-
carbamate are hydrogenated for 8 hours at room temperature
and atmosphe~ic p~essure in the presence of 10 mg of 10
palladium on charcoal in 30 ml of methanol. ~ter
completion of the hydrogen uptake the catalyst is filtered
off and the filtrat,e i5 evaporated under reduced pressure,
whereby there are obtained 163 mg (100%) o~ (S)-N-[(lS,2S
or R)-2-amino-1-(cyclohexylmethyl)-3-ethylpentyl]-a-
-[(R)--(3,3-dimethyl-2-oxobutyl)hydrocinnamamido]-
imidazole-4-propionamide in the form of a solid, MS: 594
(M~H) .
In an analogous manner to that described above, by
catalytically hydrogenating 50 mg o4 benzyl [(R or S)-l-
-[(S)-Z-cyclohexyl-l- r (s)-- r (R)-a-(3,3-dimethyl-2-oxo-
butyl)hydrocinnamamido]imidazol-4-propionamido]ethyl]-2-
-ethylbutyl]carbamate in the presence of 5 mg of 10~
palladium on charcoal in 1~ ml of methanol there are
obtained 40 mg (98%) of (S)-N-r(lS,2R or 2S)-2-amino-1-
-(cyclohexylmethyl)-3-ethylpentyl]-a-r(R)-a-(3,3-
-dimethyl-2-oxobutyl)hydrocinnamamido]imidazole-4-proeion-
amide as a foam, MS: 594 (M~H) .
The benzyl r(S oc R)-l-[(S)-2-cyclohexyl-1-r(S)-a-
-[(R)-a-(3,3-dimethyl-2-oxobutyl)hydrocinnamamido]-
imidazol-4-propionamido]ethyl]-2-ethylbutyl]carbamate and
benzyl [(R or S)-l-[(S)-2-cyclohexyl-1-(S)-a-[(R)-a-
2~3~i
- 4a -
-(3,3-dimethyl-2-oxobutyl)hydr~cinnamamido]imidazole-4-
-propionamido]ethyl]-2-ethylbutyl]carbamate used as the
stacting materials were prepared as follows:
2.28 g (8 mmol) of t-butyl [(S)-l-(cyclohexylmethyl)-
-l-(methoxycarbonyl)methyl]cacbamate ~see J. Bogec et al.,
J. Med. Chem. 28, 1779 (1985~] in 30 ml of tetrahydrofuran
are added dropwise at room temperature to a Grignard
solution prepared from 0.61 g ~25 mgram atom) of magnesium
shavings and 3.12 ml (25 mmol) of 3-bromopentane in 35 ml
of tetrahydrouran and the rea~tion mixture is stirred at
room temperature overnight. Su~sequently, the mixture is
treated dropwise with abou~ 40 ml of saturated ammonium
chloride solution while cooling in an ice bath and
extracted three times with 300 ml of ather each time. The
o~ganic extracts are washed with 100 ml of water and
lO0 ml of saturated sodium chloride solution, combined,
dried and evaporated. Chromatogcaphy of the residue
(2.95 g) on 250 g of silica gel with a 9:1 mixture of
hexane and ether as the eluting agent yields 670 mg (26%)
of t-butyl [(S)-l-(cyclohexylmethyl)-3-ethyl-2-oxopentyl]-
carbamate as a yellow oil, MS: 336 (M~-H) .
A mlxture of 550 mg (1.66 mmol~ 4f t-butyl r(S)-l-
-(cyclohexylme~hyl)-3-ethyl-2-oxopentyl]carbamate, 3 ml of
pyridine, 1.15 g (1.66 mmol) of hydroxylamine hydro-
chloride and l.01 g of 4-dimethylaminopyridine is heated
3 to 100 for 5 hours undec argon. Thereafter, the reaction
mixture is evaporated to dryne~s in a high vacuum and the
residue is extracted three ti~es with 130 ml of ether each
time. The organic extcacts are washed in succession with
70 ml of 2N sodium bica~bonate solution, 20% copper
sulphate solution and water, dried and evaporated under
reduced pressure. The residue remaining (540 mg) in lO0 ml
of 3.5N methanolic ammonia is stirred under a hydrogen
atmosphere for 2 days in the presence of l g of Raney-
~3~
- 49 -
-nickel. Thereafter, the catalyst is filtered off and the
filtrate is evaporated under reduced pressure. Chromato-
graphy of the cesidue on 50 g of silica gel using a 95:5mixture of chloroform and ethanol as the eluting agent
yields 260 mg (48~) of t-butyl r(lS,2RS)-2-amino-1-(cyclo-
hexylmethyl)-3-ethylpentyl]carbama~e as an oil, MS: 327
(M~H)
~ mixture of 260 mg ~0.8 mmol) of t-butyl [(lS,2RS)-2-
-amino-l-(cyclohexylmethyl)-3-ethylpentyl]carbamate,
0.22 ml (1.60 mmol) of triethylamine, 240 mg ~0.9~ mmol)
of N-(benzyloxycarbonyloxy)succinimide and 15 ml of
methylene chloride is stirred at room temperature for
2 hours. Thereafter, the reaction mixture is evaporated
under reduced pressure and the residue is poured on to ice
and extracted twice with 150 ml of ether each time. The
organic ex~racts are washed with 2N sodium carbonate
solution and water, combined, dried and evaporated.
Chromatography of the residue on 50 g of silica gel with a
97:3 mixture of toluene and ethyl acetate as the eluting
agent yields the two epimeric compounds 2-benzyl l-t-butyl
r(lS,2S or R)-l-(cyclohexylmethyl)-2-(1-ethylpropyl)-
ethylene]dicarbamate (2~0 mg) and 2-benzyl l-t-butyl
~(lS,2R or S)-l-(cyclohexylmethyl)-2-(1-ethylpropyl)-
ethylene]dica~bamate (80 mg), both as an oil, MS (both):
461 (M~H) .
In an analogous manner to that described in Example 9,
by reacting 2-benzyl l-t-butyl r(lS,2S or R)-l-(cyclo-
hexylmethyl)-2-tl-ethylpropyl)ethylene]dicarbamate and
Z-benzyl l-t-butyl [~lS,2R or S)-l-(cyclohexylmethyl)-2-
-(l-ethylpropyl)ethylene]dicarbamate with l-(t-butoxy-
carbonyl)-N-[(R)-a-(3,3-dimethyl-2-oxobutyl)hydro-
cinnamoyl~-L-histidine and subsequently cleaving off the
Boc protecting group in the imidazole ring with potassium
carbonate in methanol there are obtained the two epimeric
2 ~
- 50 -
compounds benzyl [(S or R)-l-[(S)--2-cyclohexyl-1-t(S)-a-
-r(R~-a-(3,3-dimethyl-2-oxobutyl)hydrocinnamamido~-
imidazol-4-propionamido]ethyl]-2-ethylbutyl]carbamate and
benzyl t(R or S)-l-[(S)-2-cyclohexyl-1-r(S)-a-t(R)-a-
-(3,3-dimethyl-2-oxobutyl)hydrocinnamamido]imidazole-4-
-propionamido]ethyl]-2-ethylbutyl]carbamate, both as a
foam, MS (both): 728 (MtH) .
Example 11
In an analogous manner to that described her.einafter
for the preparation of the starting material, 750 mg
(1.19 mmol) of (S)-~-amino-N-t(lS,ZRS)-l-(cyclohexyl-
methyl)-2-hydroxy-4,4-dimethylpentyl]imidazole-4-propion-
amide and 337 mg (1.36 mmol) of (R)-a-(pivaloylmethyl)-
hydrocinnamic acid were reacted with one another in 20 ml
of dimethylfocmamide and the reaction mixture was worked-
-up. Chromatography of the residue on 65 g of silica gel
with a 200:1~:1 mixture of methylene chloride, methanol
and ammonia yields 420 mg (59%) of (S)-N-t(lS,2RS)-l-
-(cyclohexylmethyl)-2-hydroxy-4,4-dimethylpentyl]-a-
-[(R)-a-(3,3-dimethyl-2-oxobutyl)hydrocinnamamido]-
imidazole-4-propionamide as an amorphous powder, MS: 595
(M~H) .
The (S)-a-amino-N- r (lS,2RS)-l-(cyclohexylmethyl)-2-
-hydroxy-4,4-dimethylpentyl]imidazole-4-propionamide used
as the starting material was prepared as follows:
1.64 g (67 mgram atom) of magnesium shavings in ether
are treated dropwise with 8.85 ml (70 mmol) of l-bromo-
-2,2-dimethylpropane in 80 ml of ether in such a manner
that the reaction mixture boils slightly under reflux.
After completion of the addition the reaction mixture is
cooled to -60 and treated within 30 minutes with a
solution of 5.1 g (20 mmol) of 2-t-butoxycarbonylamino-
2~3.~
- 5~ -
-3(S)-cyclohexylpropylaldehyde in 60 ml of ether.
Subsequently, the reaction mixture is stirred at coom
temperature overnight, then cooled to 5 in an ice bath
and treated dropwise with 25 ml of saturated ammonium
chloride solution. The reaction mixture i8 thereafter
extracted three times with 300 ml of ether and the ether
extracts are washed with saturated ammonium chloride
solution and water. The oil (6.62 g) remaining after
drying and evaporation is chromatographed on 500 g of
silica gel with firstly a 98:2 and subsequently a 95:5
mixture of methylene chloride and ethyl acetate as the
eluting agent, whereby there are obtained 4.11 g (63~) of
t-butyl [(lS,2RS)-l-(cyclohexylmethyl)-2-hydroxy-4,4-
-dimethylpentyl]carbamate as an oil, MS: 32~ (M~H) .
4.Ll g (12.5 mmol) of t-butyl [(lS,2RS)-l-(cyclohexyl-
methyl)-2-hydroxy-4,4-dimethylpentyl]carbamate are left to
stand at room temperature for 4 hours in 1.58M hydro-
chloric acid in dioxan. Thereafter, the reaction mixture
is evaporated to dryness under reduced pressure, whereby
there are obtained 1.24 g (38%) of (aRs,~S)-B-amino-a-
-(2,2-dimethylpropyl)cyclohexylpropanol hydrochloride
which is u~ed in the next step without further purifica-
tion.
A mixture of 1.24 g (4.7 mmol) of (aRs,~S)-~-amino-
-a-(2,2-dimethylpropyl)cyclohexylpropanol hyd~ochloride,
3.24 g (5.4 mmol) o (Fmoc)2His-OH, 1.78 ml (14.1 mmol)
- of 4-ethylmorpholine, 1.6B g (10.8 mmol) of 87% HOBT,
1.244 g (6.49 mmol) of EDC and 50 ml of dimethylformamide
is left to stand a~ room temperature overnight. The
reaction mixture is thereafter concentrated at below 40
in a high vacuum and extracted three times with 5DO ml of
ethyl acetate. The organic extracts are washed in
succession with 120 ml of 2N sodium bicarbonate solution,
lZO ml of saturated ammonium chloride solution, 120 2N
sodium bicarbonate solution and 120 ml of saturated sodium
chloride solution. The residue (4.7 g) remaining after
drying and evaporation is dissolved in 100 ml of methylene
chloride and filtered. The filtrate is treated with 2 ml
of pyridine and stirred at room temperature for 2 hours.
Thereafter, the reaction mixture is evaporated and the
residue is chromatographed on silica gel, whereby there
are obtained 750 mg (44%) of (S)-a-amino-N-[(lS,2RS)-l-
-(cyclohexylmethyl)-2-hydroxy-4,4-dimethylpentyl]imidazole-
-4-propionamide as a thin-layer chromatographically
uniform p~oduct and are used directly in the next step.
ExamDle I2
In an analogous manner to that described in
Example 11, by reacting (S)-a-amino-N-[(lS,2RS)-l-
-(cyclohexylmethyl)-2-hydroxy-3,3-dimethylbutyl]imidazole-
-4-propionamide with (R)-a-(pivaloylmethyl)hydrocinnamic
acid there was obtained N-r(lS,2RS)-l-(cyclohexylmethyl)-
-2-hydroxy-3,3-dimethylbutyl]-a- r (K)-a- (3,3-dimethyl-
-2-oxobutyl)hydrocinnamamido]imidazole-4-pLopionamide as a
foam, MS: 581 (M~H) .
ln an analogous manner, by reaction with N-isobutoxy-
carbonyl-L-phsnylalanine in place of (R)-a- ~pivaloyl-
methyl)hydrocinnamic acid there was obtained isobutyl
[(S)--[[(S)-l-[[(lS,2RS)-l-(cyclohexylmethyl)-2-
-hydroxy-3,3-dimethylbutyl]carbamoyl]-2-imidazol-4-yl-
ethyl]carbamoyl~phenethyl]carbamate a~ a foam, MS: S98
(M~H) .
The (S)-a-amino-N-[(lS,2RS)-l-(cyclohexylme~hyl)-2-
-hydroxy-3,3-di~ethylbutyl]imidazole-4-propionamide used
as the starting material was prepared as ~ollows in
analogy to Example 11:
- 53 -
.
Reaction of 2-t-butoxycarbonylamino-3(S)-cyclohexyl-
propylaldehyde with t-butyllithium in hexane in place of
the reaction with a corcesponding Grignard compound yields
t-butyl t(lS,2RS)-l-(cyclohexylmethyl)-2-hydroXy-3,3-
-dimethylbutyl]carbamate as an oil, MS: 314 (M+H) ,
which by cleavage of the Boc protecting group with hydro-
chloric acid in dioxan can be converted into (aRS,~S)-B-
-amino-a-(2,2-dimethylethyl)cyclophexylpropanol hydro-
chloride, MS: 214 (M+H) . Reaction of this compound with
(Fmoc)2His-OH and cleavage of the ~moc protecting groups
with piperidine yields (S)-a-amino-N-r(lS,2RS)-l-(cyclo-
hexylmethyl)-2-hydroxy-3,3-dimethylbutyl]imidazole-4-
15- -propionamide which is used in the next s~ep withou~
further purification.
Example 13
In an analogous manner to that described in
Example 11, by reacting (S)-~-t(S)-l-amino-2-cyclohexyl-
ethyl]-2-benzimidazolehexanol with l-(t-butoxycarbonyl)-N-
-~(R)--(3,3-dime~hyl-2-oxobutyl)hydrocinnamoyl]-L-
-histidine and cleaving off the Boc protecting group with
potassium carbonate in methanol there was obtained (S)-N-
-[(15,2S)-7-(2-benzimidaæolyl)-1-(cyclohexylmethyl)-Z-
-hydroxyheptyl]-a-r(R)-a-(3,3-dimethyl-2-oxobutyl)-
hydrocinnamamido]imidazole-4-propionamide in the form of a
beige coloured amorphous solid, MS: 711 (M+~) .
The (S)-~-[(S)-l-amino-2-cyclohexyle~hyl]-2-benz-
imidazolehexanol used as the starting material was
prepared as follows:
A solu~ion of 40.7 ml (0.3 mol) of 6-bromo-1-hexene in
270 ml of ether is added dropwise within 75 minutes under
argon to 7.4 g ~0.3 gram atom) of magnesium shavîngs in
60 ml o~ ether in such a manner that the reaction mixture
~0:~3/~ ~
- 54 -
boils slightly. After completion of the addition the
reaction mixture is heated to rèflux fo~ a further
45 minutes, thereaEter cooled to -60~ and treated within
1 hour with 20 g (0.080 mol) of 2-t-butoxycarbonylamino-
-3tS)-cYclohexylpropylaldehyde in 270 ml of ether at -60
to -70. ~fter completion of the addition the reaction
mixture is stirred for 18 hours without cooling, there-
after again cooled to 5 and t~eated with 400 ml ofsaturated ammonium chloride solution. The two phases are
separated and the organic phase is dried over magnesium
sulphate and evaporated. The residue is chromatographed on
4Z0 g of silica gel with methylene chloride and increasing
amounts of ethyl acetate as the eluting agent, whereby
there are obtained 19.3 g (71~) of t-butyl r(lS,2S:R=4~
-l-(cyclohexylmethyl)-2-hydroxy-7-octenyl]carbamate as an
oil, MS: 284 (M-C4H7)-
18.5 g (54.5 mmol) of t-butyl ~(lS,2S:R=4:1)-1-(cyclo-
hexylmethyl)-2-hydroxy-7--octenyl]carbamate are dissolved
in 133 ml o~ 2,2-dimethoxypropane and treated with 2.55 g
(13 mmol) of p-toluenesulphonic acid monohydrate. There-
after, the reaction mixture is stirred at room temperature
for 4 hour~, subsequently poured into a mixture of ice-
-water and sodium bicarbonate solu~ion and extracted with
ethyl acetate. The organic ~xtracts are dried over
magnesium sulphate and evaporated, whereby there are
obtained 20.2 g of a yellow oil which is chromatographed
30 on 200 g of silica gel with a 9:1 ~ixture of methylene
chloride and hexane as the eluting agen~. There are thus
obtained 19.0 g ~92%) of t-butyl (4S,5S:R=4:1]-4-(cyclo-
hexylmethyl)-5-(5-hexenyl)-2,2-dime~hyl-3-oxazolidine-
carboxylate as a yellowish oil, MS: 3h4 (M-CH3).
2.5 ml (25 mmol) of borane-dimethyl sulphite complex
are added dropwise within 5 minutes to 19.0 g (50.1 mmol)
of t-butyl (4S,5S:R=4:1]-4-(cyclohexylmethyl~-5-(5-
~a~3~
- 55 -
-hexenyl)-Z,2-dimethyl-3-oxazolidinecarboxylate in 100 ml
of hexane. Thereafter, the reaction mixture is stirred at
0 for 10 minutes and subsequently a~ room temperature for
3.5 hours. Thereafter, it is again cooled and there are
added dropwise thereto firstly at 0-50 within 10 minutes
16.8 ml of ethanol and subsequently again within
10 minutes 8.5 ml of 2N sodium hydroxide solution. ~fter
stirring at 5 for 5 minutes 12.7 ml of 30% hydrogen
peroxide solution are added dropwise within 20 minutes at
a maximum temperature of 8. Thereafter, the reaction
mixture is heated to reflux for 2 hours, cooled, poured
into 500 ml of water and extracted with methylene
chloride. The methylene chloride extracts are dried over
magnesium sulphate and evapora~ed, and the residue is
chromatog~aphed on 240 g of silica gel, whereby there is
used as the eluting agent firstly a 98:2 mixture and then
a 95:5 mixture of methylene chloride and isopropanol. ln
this manner ~here are obtained 12.8 g (64%) of t-butyl
(4S,5S)-4-(cyclohexylmethyl)-5-(6-hydroxyhexyl~-2,Z-
-dimethyl-3-oxazolidinecarboxylate as a mixture of
isomers. By crystallization from ether/hexane there are
obtained 7.2 g (36%) of t-butyl (4S.5S)-4-(cyclohexyl-
methyl)-5-(6-hydroxyhexyl)-2,2-dimethyl-3-oxazolidine-
carboxylate as a pure isomeric compound, melting point 64
(~rom diethyl ether/hexane).
4.0 g (10.1 mmol) of t-bu~yl (4S,5S)-4-(cyclohexyl-
methyl)-5-(6-hydroxyhexyl)-2,2-dimethyl-3-oxazolidine-
carboxylate 2re dissolved in 60 ml of benzene and
thereafter treated with 7.95 g (50 mmol) of powdered
potassium permanganate, 34 ml of water and 1.4 g of
tricaprylmethylammonium chloride in 10 ml o benzene.
Thereafter, the reaction mixture is cooled to 5 and
treated with 6.7 ml of glacial acetic acid. Then, the
reaction mixture is stirred intensively at room
temperature for 3 hours, subsequently poured into 400 ml
2 0 ~
- 56 -
of ice-water, decolorized by the addition of sodium
pyrosulphite and extracted with methylene chloride. The
methylene chloride extracts are dried over magnesium
sulphate and evaporated. The residue obtained is chromato-
graphed on 60 g of silica gel with a mixture of methylene
chloride and isopropanol as the eluting agent, whereby
there are obtained 3.4 g (82%) of (4S,5S)-3-(t-butoxy-
cacbonyl)-4-(cyclohexylmethyl)-2,2-dimethyl-5-oxazolidine-
hexanoic acid as a colourle~s, crystallizing oil which is
used directly in the next step.
1.2 g (2.9 mmol) of (4S,5S)-3-(t-butoxycarbonyl)-4-
-(cyclohexylmethyl)-2,2-dimethyl-5-oxazolidinehexanoic
1 acid are dissolved in 5 ml of tetrahydrofuran and treated
with 0.44 ml (3.1 mmol) of triethylamine. Therea~ter, the
reaction mixture is cooled tn -20 and treated within
5 minutes with a solution of 0.42 ml (0.31 mmol) of
isobutyl chloroformate in 1 ml of tetrahydrofuran. After
stirring at -20 ~or 1 hour a solution of 0.63 g
(5.8 mmol) of 1,2-phenylenediamine in 5 ml of tetrahydro-
furan is added dropwise at -20 within 5 minutes. There-
after, the reaction mixture is stirred at -20 for
30 minutes and then at room temperature for 3.5 hours.
Then, the reaction mixture is poured into ice-water and
~xtracted with methylene chloride, and the methylene
chloride extracts are dried over magnesium sulphate and
evaporated. The residue obtained is chromatographed on
Z0 g of silica gel with a mixture of methylene chloride
and isopropanol as the eluting agent, whereby there are
obtained 1.4 g (96%) of t-butyl (4S,5S)-5-(5-[(o-amino-
phenyl)carbamoyl]pentyl]-4-(cyclohexylmethyl)-2,2-dimethyl-
-3-oxazolidinecarboxylate as an oil, MS: 501 (M~H) .
1.4 g (2.79 mmol) of t-butyl (4S,5S)-5-(5-[(o-amino-
phenyl)carbamoyl]pentyl]-4-(cyclohexylmethyl)-2,2-dimethyl-
-3-oxazolidinecarboxylate are dissolved in 25 ml of
2 ~
- 57 -
methanol, cooled to 0 and treated with 8.5 ml of 3.88M
hydrochloric acid in methanol. Subsequently, the reaction
mixture is stirred at room temperature for 24 hours. It is
then poured into ice-water, made alkaline with lN sodium
hydroxide solution and extracted three times with a 4:1
mixture of methylene chloride and isopropanol. The organic
extracts are dcied over magnesium sulphate and evaporated.
and the re5idue is heated to reflux for 1 hour in a
mixture of 15 ml of methanol and 1.5 ml of conc. aqueous
hydrochloric acid. The reaction mixture is then again
worked-up as described above and the crude product
obtained is heated to reflux or 2 hours in 20 ml of
aqueous 3N hydrochloric acid. The 760 mg of crude product
obtained after a third working-up as described above are
chromatographed on 8 g of silica gel with methylene
chloride and increasing amounts of isopropanol and aqueous
concentrated ammonia as the eluting agen~, whereby there
are obtained 346 mg (36%) of (S)-~-[(S)-l-amino-2-cyclo-
hexylethyl]-2-benzimidazolehexanol as an oil, MS: 344
(M~H)
ExamPle 14
0.35 g (1.33 mmol) of Boc-Phe-OEl, 0.28 g of EDC and
O.Zl g of HOBT are dissolved in 7.5 ml of methylene
chloride and trea~ed at -10 wi~h a solution of 0.50 g
(1.33 mmol) of (S)-a~amino-N-r(lS,2S,4R)-l-(cyclohexyl-
methyl)-4-ethyl-Z-hydroxy-5-hexenyl]imidazole-4-propion-
amide in 7.5 ml of methylene chloride. Thereafter, ~he
reaction mixture is stirred at -10 for 2 hours and
subsequently at room temperature for 18 hours. For the
working-up, the reaction solution is poured into 2N sodium
bicarbonate solution and extracted with methylene
chloride. The methylene chloride extracts are dried over
potassium carbonate and evaporated, and the residue is
chromatographed on 10 g of silica gel with methylene
- 58 -
chloride and increasing amounts o~ isopropanol as the
eluting agent. In this manner there is obtained 0.73 g
(88~) of t-butyl t(S)-a-[r(S)-l-[[(lS,ZS,4R)-l-cyclo-
hexylmethyl)-2-hydroxy-4-ethyl-5-hexenyl]carbamoyl]-z-
-imidazol-4-ylethylcarbamoyl]phenethyl]carbamate as an
amorphous white solid, MS: 624 (M+H) .
ln an analogous manner to that described above, by
reacting (S~-a-amino-N-r~lS,2S,4S)-l-cyclohexylmethyl)-
-4-ethyl-2-hydroxy-5-hexenyl~imidazole-4-propionamide
~here is obtained t-butyl r (s)-- [ [ (S)-l- r [ (lS,2S,4S)-l-
-~cyclohexylmethyl)-2-hydroxy-4-ethyl-5-hexenyl]carbamoyl]-
-2 imidazol-4-ylethyl]cacbamoyl]-Z-imidazol-4-ylethyl]-
carbamoyl]phenethyl]carbamate as an amorphous white solid,
MS: 624 (M~H) .
The (S)-a-amino-N-~(lS,2S,~R)-l-(cyclohexylmethyl)-
-4-ethyl-2-hydroxy-5-hexenyl]imidazole-4-propionamide and
(S)-a-amino-N- r (lS,2S,4S)-l-cyclohexylmethyl)-2-hydroxy-
-4-ethyl-5-hexenyl]imidazole-4-propionamide used as the
starting materials were prepared as follows:
A solution of 44.9 g (0.276 mmol) o~ 3-(bromomethyl)-
-l-pentene, prepared according to the method described by
C. Jennings-White and R. G. Almquist in Tetrahedron
Letters, 2533 (198Z), in 225 ml of ether is added dropwise
within 90 minutes to 6.72 g (0.28 gram atom) of magnesium
shavings in 75 ml of ether under argon in fiuch a manner
~hat the reaction mixture boils slightly. After completion
of the addition the reaction mixture is heated ~o reflux
for 3.5 hours, then cooled to -60 and ~reated dropwise
within 50 minutes with a solution of 22.6 g (0.075 mol) of
2-~-butoxycarbonylamino-3(S)-cyclohexylpropylaldehyde in
2Z5 ml of ether. whereby ~he temperature amounts to -60
to -70. After completion of the additisn the cooling bath
is removed and the reaction mixture is stirred at room
20~ 3~7~
- 59 -
te~perature for 18 hours. Then, it is cooled to 5 and
there are added thereto while stirring 100 ml of a
saturated ammonium chloride solution, whereby the
temperature rises to 20. The two phases are separated,
the organic phase is dried over magnesium sulphate and
concentrated, and the residue is chromatographed on 1 kg
of silica gel with methylene chloride containing 0 to 20%
ethyl acetate as the eluting agent. There are thus
obtained 9.8 g (38.5~) of (aS,~S)-B-t-butoxycarbonyl-
amino-a- r (R)-2-ethyl-3-butenyl]-cyclohexylpropanol,
2.7 g (11%3 of (aS,~S)-t-butoxycarbonylamino-a-t(S)-2-
-ethyl-3-butenyl]cyclohexyl~ropanol as well as 10.0 g
(39%) of a ~ixture of the two named diastereomers in the
~orm of oils which are used in the next step without
further purification.
3.5 g (10.3 mmol) of (aS,~S)-~-t-butoxycarbonyl-
amino-a-[(R)-2-ethyl-3-butenyl]cyclohexylpropanol are
stirred at room temperature for 90 minutes in a mixture of
25 ml of methylene chloride and 15 ml o~ gO% trifluoro-
acetic acid. Therea~ter, the reaction mixture is poured
into water, made alkaline and ex~racted with methylene
chloride. The methylene chloride extracts are dried over
magnesium sulphate and evaporated, and the cesidue is
chromatographed on 2~ g of silica gel with an 80:20:1
mixture of methylene chloride, isopropanol and ammonia as
the eluting agent, whereby there are obtained 2.2 g (89%)
of (2S,3S,5R)-2-amino-5-ethyl-1-cyclohexyl-6-hepten-3-ol
as a colourless oil, MS: 14Z (M-C7H13).
In an analogous manner, by reacting (nS,~S~-B-t-
-butoxycarbonylamino-a-r(S)-2-ethyl-3-butenyl]cyclohexyl-
propanol with trifluoroacetic acid there is obtained
(2S,3S,5S)-2-amino-5-ethyl-1-cyclohexyl-6-hepten-3-ol as a
yellowish solid, MS: 240 (M~H) .
2~3~
- 60 -
2.2 g (9.19 mmol) o~ (2S,3S,5R)-2-amino-5-ethyl-1-
-cyclohexyl-6-hepten-3-ol in 20 ml of methylene chloride
a~e added dropwise at -10 to a solution of 5.7 g
(9.5 mmol) of (Fmoc)2His-OH, 2.2 g of EDC and 1.75 g of
HOBT in a mixture of 40 ml of dimethylformamide and 80 ml
of methylene chloride. ~fter completion of the addition
the reaction mixture is stirred at -10 for 2 houcs and at
room temperature for 16 hours, ~hereafter poured into 2N
sodium bicarbonate solution and extracted with methylene
chloride. The methylene chloride extracts are dried over
potassium carbonate and evaporated. The residue obtained
(7.1 g) is suspended in 100 ml of acetonitrile, treated
with 100 ml of diethylamine and stirred at room
temperature for 17 hours. Thereafter, the reaction mixture
is filtered, the ~iltrate is evaporated and the residue is
chromatographe~ on 70 g oi silica gel with methylene
chloride and increasing amounts of isopropanol and
saturated aqueous ammonia as the eluting agent, whereby
there are obtained 1.67 g (48~) of (S)-a-amino-N-
-[(lS,2S,4R)-l-(cyclohexylmethyl)-4-ethyl-2-hydraxy-5--
-hexenyl~imidazole-4-propionamide as an oil, MS: Z63
(M- C7H130) .
In an analogous manner to that described above, by
reacting ~2S,3S,5S)-2--amino-5-ethyl-1-cyclohexyl-6-
-hepten-3-ol ~ith (Fmoc)2His-OH and cleaving off the
Fmoc protecting groups with diethylamine there was
obtained (S)-a-amino-N-[(lS,2S,4S)-l-cyclohexylmethyl)-
-2-hydroxy-4-Pthyl-5-hexenyl]imidazole-4-propionamide as a
yellowish solid, MS: 377 (M~H) .
Example 15
0.30 g (0.48 mmol) of t-butyl r(S)-a-[r(S)-l-[[(lS,
2S,4R)-l-(cyclohexylmethyl)-2-hydroxy-4-ethyl-5-hexenyl]-
carbamoyl]-2-imidazol-4-ylethylcaEbamoyl]phenethyl]-
~ Gi -i , ~, ,,
- 61 -
carbamate in 3 ml o methanol is hydrogenated at room
temperature and atmospheric pressure for 7.5 hours in the
presence of 60 mg of palladium on charcoal (5%). After
completion of the hydrogen uptake the catalyst is filtered
off and the ~iltrate is evaporated, whereby there is
obtained 0.20 g (66.5%) of t-butyl [(S)-a-[[(S)-l-
-[[(lS,2S)-l-(cyclohexylmethyl)-2-hydroxy-4-ethylhexyl]-
carbamoyl]-2-imidazol-4-ylethyl]carbamoyl]phenethyl]-
carbamate as a white amorphous solid, MS: 626 (M+H) .
Example 16
0.55 g (1.05 mmol) of (S)-N-[(lS,2S,4R)-l-(cyclohexyl-
methyl)-2-hydroxy-4-ethyl-5-hexenyl]-a-(3-phenyl-L-
-alanyl)imidazole-4-propionamide in 5 ml of methylene
chloride is added dropwise at -10 to a solution of 0.23 g
(1.05 mmol) of Boc-D-Pro-OH, 0.22 g of EDC and 0.17 g of
HOBT in 5 ml of methylene chloride. After completion of
the addition the reaction mixtuLe is stirred a~ -10 ~or
2 hours and at room temperature for 18 hours, thereafter
poured into 2N sodium bicarbonate solution and extracted
with msthylene chloride. The methylene chloride ex~racts
are dried over potassium carbonate and evaporated, and the
residue is chromatographed on 10 g of silica gel with
methylene chloride and increasing amounts o isopropanol
as the eluting agent, whereby thers is obtained 0.59 g
(78%) of t-butyl (R)-2-~r(S)-a-[t(S)-l-[r(lS,2S,4R)-l-
-(cyclohexylmethyl)-2-hydroxy-4-ethyl-5-hexenyl]carbamoyl]-
-2-imidazol-4-ylethyl]carbamoyl]phenethyl]carbamoyl]-1-
-pyrrolidinecarboxylate as an amorphous solid, MS: 721
(M+H) .
The (S)-N- r ( lS,2S,4R)-l-(cyclohexylmethyl)-2-hydroxy-
-4-ethyl-5-hexenyl]-a-(3-phenyl-L-alanyl)imidazole-4-
-propionamide used as the starting material was prepared
as follows:
2~ 7~
- 62 -
0.60 g (1.5~ mmol) of (S)-a-amino-N-r(lS,2S,4R)-l-
-(cyclohexylmethyl)-4-ethyl-Z-hydroxy-5-hexenyl~imidazole-
-4-propionamide in 7.5 ml of methylene chloride i~ added
dropwise at -10 to a solution of 0.62 g (1.59 mmol) of
Fmoc-Phe-OH, 0.33 g o~ EDC and 0.24 g of HOBT in 7.5 ml of
methylene chloride. A~ter completion of the addition the
reaction mixture is stirred at -10 for 2 hours and
subsequently at room temperature for 17 hours, therea~ter
poured into 2N sodium bicarbonate solution and extracted
with methylene chloride. The methylene chloride extrac~s
are dried over potassium carbonate and eva~orated, and the
residue is chromatographed on 15 g of silica gel with
methylene chloride and increasing amounts o~ isopropanol
as the eluting agent. The thus-obtained crystalline
product (0.90 g) is suspended in 12.5 ml of acetonitrile
and subsequently treated with 12.5 ml o~ diethylamine.
Thereafter, the reaction mixture is stirred at room
temperature for 75 minutes and then evaporated, a~d the
residue is chromatographed on 10 g of silica gel with
methylene chloride and increasing amounts of isopropanol
and saturated ammonia solution as the eluting agent,
whereby there is obtained 0.62 g (74.5%) of (S)-N- r (lS,
2S,4R)-l-(cyclohexylmethyl~~2-hydroxy-4-ethyl-5-hexenyl]-
-a-(3-phenyl--L-alanyl)imidazole-4-propionamide as an
amorphous, white solid, MS: 524 (M+H) .
ExamPle 17
ln an analogous manner to that described in
Example 15, by catalytically hydrogenating t-butyl (R)-2-
-[[(S)-a-[[(S)-l-[[(lS,2S,4R)-l-(cyclohexylmethyl)-2-
-hydroxy-4-ethyl-5-hexenyl]carbamoyl]-2-imidazol-~-yl-
ethyl]carbamoyl]phenethyl]carbamoyl]-l-pyrrolidine--
carboxylate there wa obtained t-butyl (R)-2-[r(S)-a-
-r[(s)-l-[r(ls~2s)-l-(cyclohexylmethyl)-2-hydroxy-4-ethyl-
hexyl]carbamoyl]-2-imidazol-4-ylethyl]carbamoyl]phenethyl]-
2 ~
- 63 -
carbamoyl]-l-pyrrolidinecarboxylate as an amorphous, white
solid, MS: 723 (M~H) .
Example 18
The following compounds were manufactured in an
analogous manner to that described in Example 14:
- From (S)-a-amino-N-[(lS,2S,4R)-l-(cyclohexyl-
methyl)-4-ethyl-~-hydroxy-5-hexenyl]imidazole-4-prcpion-
amide and (R)-a- (pivaloylmethyl)hydrocinnamic acid the
(S)-N-[~lS,2S,4R)-l-(cyclohexylmethyl)-4-ethyl-2-hydroxy-
-5-hexenyl]-a-r(R)---(3,3-dimethyl-2-oxobutyl)hyd~o-
cinnamamido]imidazole-4-propionamide as a white foam, MS:
607 (M~H) , and
- from (S)-a-amino-N-r(lS,2S,4R)-l-(cyclohexyl-
methyl)-4-ethyl-2--hydroxy-5-hexenylJimidazole-4-propion-
amide and a-[(t-butylsulphonyl)methyl]hydrocinnamic acid
the (S) -a- r (S)-a- r ( ~-butylsulphonyl)methyl]hydro-
cinnamamido]-N-r(lS,2S,4R)-l-(cyclohexylmethyl)-4-ethyl-2-
-hydroxy-5-hexanyl]imidazole-5-propionamide as a solid,
MS: 657 (M~H) .
Example 19
In an analogous manner to that described in
Example 15, by catalytically hydrogenating (S)-a-r(S)-
-a-r(t-butylsulphonyl~methyl]hydrocinnamamido]-N-r(lS,
2S,4R)-1 (cyclohexylmethyl)-4-ethyl-2-hydroxy-5-hexenyl]-
imidazole-5-propionamide there was obtained (S)-a-~(S)-
-a-r(t-butylsulphonyl)methyl]hydrocinnamamido]-N-r(lS,2S,
4R)-l-(cyclohexylmethyl)-4-ethyl-2-hydroxyhexyl]imidazole-
-5-propionamide as an amorphous, white solid, MS: 645
~M~H) -
- 64 -
Example ~
A sterile-filtered aqueous solution of t-butyl (R)-2-
-~[tS)-a-~r(S)-l-[[(lS,2S,4~)-1-(cyclohexylmethyl)-2-
-hydroxy-4-ethyl-5-hexenyl]carbamoyl]-2-imidazol-4-yl-
ethyl]carbamoyl]phenethyl]carbamoyl]-l-py~rolidine-
carboxylate is mixed while warming with a sterile gelatine
solution, which contains phenol as a preserving agent,
under aseptic conditions so tha~ 1.0 ml of solution has
the following composition:
t-Butyl (R)-2-r[(S)-a[[(s)-l-
15 -[[(lS,2S,4R)-l-(cyclohexylmethyl)-
-2-hydroxy-4-ethyl-5-hexenyl]carbamoyl]-
-2-imidazol-4-ylethyl]carbamoyl]-
phenethyl]carbamoyl]-l-pyrrolidine-
carboxylate 3.0 mg
20 Gelatine 150.0 mg
Phenol ~.7 mg
Dist. water ad 1.0 ml
The mixture is filled into 1.0 ml vials under aseptic
conditions.
ExamPle B
5 mg of t-butyl (R)-2-[[(S)-a[[(S)-l-[~(lS,2S,~R)-l-
-(cyclohexylmethyl)~l-hydroxy-4-sthyl-5-hexenyl]carbamoyl]-
-2-imidazol-4-ylethyl3carbamoyl]phenethyl]carbamoyl3-1-
-pyrrolidinecarboxylate are dissolved in 1 ml of an
aqueous solu~ion with 20 mg of mannitol. The solution is
filtered sterile and filled under aseptic conditions into
a 2 ml ampoule, cooled to a low temperature and
lyophilized. Prior to administration the lyophilizate is
dissolved in 1 ml of distilled water or 1 ml of physio-
logical saline. The solution is used intramuscularly or
2~13~7~
int~avenously. This formulation can also be fiiled into
double chamber injection ampoules.
Example C
50~ mg of finely milled (5.0 ~m) t-butyl (R)-2-
-~(S)-a-~t(S)-l-~(lS,2S,4R)-l-(cyclohexylmethyl)-Z-
-hyd~oxy-4-ethyl-5-hexenyl]carbamoyl~-2-imidazol-4-yl-
ethyl]carbamoyl]phenethyl]carbamoyl~-l-pyrrolidine-
carboxylate are suspended in a mixture of 3.5 ml of
Myglyol 812 and 0.08 g of benzyl alcohol. This suspension
is filled into a container having a dosage valve. 5.0 g o
Freon 12 are filled into the container through the ~alve
under pressure. ~he Freon is dissolved in the Myglyol-
-benzyl alcohol mixture by shaking. This spray container
contains about 100 individual dosages which can be applied
individually.
ExamPle D
When the pro~edures described in Examples A-C are
followed, cor~esponding galenical preparations can be
manufactured from the following, likewise preferred,
compound~:
t-Butyl (R)-2-[~(S)-a-[[(S)-l-[[(lS,2S)-l-(cyclo-
hexylmethyl)-2-hydroxy-4-ethylhexyl]carbamoyl]-Z-imidazol-
-4-ylethyl]carbamoyl]phene~hyl]Jcarbamoyl]-l-pyrrolidine-
carboxylate
(S)-N-[(lS,2S,4R)-l-(cyclohexylmethyl)-4-ethyl-2-
-hydroxy-5-hexenyl]-a-[(R)-a-(3,3-dimethyl-2-oxo-
butyl)-hydrocinnamamido~imidazole-4-propionamide,
(S)-a-[(S)-a-[(t-butylsulphonyl)methyl]hydrocinnam-
amido]-N-[(lS,2S,4R)-l-(cyclohexylmethyl)-4-ethyl-2-
-hydroxy-5-hexenyl]imidazole-5-pLopionamide;
7~
- 66 -
t-butyl t(S)-a-[[(s)-l- r [ (lS, 2R or 2S)-3-cyclohexyl-
-l-(cyclohexylmethyl)-2-hydroxypropyl]carbamoyl]-2-
-imidazol-4-ylethyl]carbamoyl]phenethyl]carbamate;
(2R or S,3S)-3-(Boc-D-Pro-Phe-His-NH)-1,4-dicyclo-
hexyl-2-butanol and
(S)-N-[(lS,2S)-l-(cyclohexylmethyl)-4-ethyl-4-fluoro-
-2-hydroxyhexyl]-a-r(R)-a-(3,3-dimethyl)-2-oxobutyl)-
hydrocinnamamido]imidazole-4-propionamide.