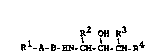Note: Descriptions are shown in the official language in which they were submitted.
2V~07~L
I
HOECHST ARTIENGESELLSCHAFT HOE 89/F 129 Dr.MY/gm
Description
Renin-inhibiting dipeptides, a proce~ for their prepara-
tion, agents containing them and their uffe
Di- and tripeptide derivatives and their use as renin
inhibitors are known from EP-A-152,255, EP-A-155,809,
EP-A-163,237, EP-A-172,346, ~P-A-172,347, EP-A-179,352
and EP-A-255,082.
Novel peptide derivatives have now been found which
highly effectively inhibit the enzyme renin in vitro and
in vivo.
The invention relates to compounds of the formula I
l A B HN CH CH CH R4 (I)
in which
Rl denotes a radical of the formula II
R~-W- (II)
: where W stands for -CO-, -O-CO-, -SO2- or -NH-CO-
and RA denotQs hydrogen, (C1-CI0)-alkyl which is
optionally monounsaturated or diunsaturatQd and
which i8 optionally substituted by up to 3 identical
or different radicals from the series comprising
hydroxyl, (C~-C,)-alkoxy, (Cl-C7)-alkanoyloxy, car-
boxyl, (Cl-C7)-alkoxycarbonyl, halogen, amino, (Cl-
C7 )-alkylamino,di-(Cl-C7)-alkylamino,(Cl-C5)-alkoxy-
csrbonylamino, (C7-Cls)-aralkoxycarbonylamino and
9-fluorenylmethyloxycarbonylamino, (C3-ca)-cyclo-
alkyl, (C3-Ca)-cycloalkyl-(C1-C6)-alkyl, (C6-C~4)-aryl
which is optionally substituted by one or two
identical or different radicals from the series
: 30 comprising F, Cl, ~r, I, hydroxyl, (Cl-C~)-alkoxy,
( Cl-C7 ) -alkyl, (Cl-C,)-alkoxycarbonyl, amino, anilino
optionally substituted by up to 2 halogens and
trifluoromethyl, (C6-Cl4)-aryl-(Cl-C6)-alkyl, where
the aryl moiety is op~ionally sub6tituted by ons or
two identical or different radicals from the seriPs
comprising F, Cl/ Br, I, hydxoxyl, ~C1-C7)-alkoxy,
(C1-C7)-alkyl~ (Cl-C7)-alkoxycarbonyl, aminoS (C1-C7)-
alkylamino, di-(C1~C7)-alkylamino,carboxyl,carboxy-
methoxy, amino-(Cl-C7~-alkyl~ (C1-C7)~alkylamino-(C1-
C7 )-alkyl, di-(C1-C7)-alkylamino~(C1-C7)-alkyl t ( Cl-
C7)-alkoxycarbonylmethoxy,carbamoyl, sulfamoyl,(C1
C7~-alkoxysulfonyl, sulfo and guanidinome hyl, or
6tands for the radical of a 5- or 6-membered mono-
cyclic or 9- or l0-membered bicyclic heterocycle
having at least l carbon a~om, 1-4 nitrogen a~oms
and/or 1 sulfur or oxygen atom in addition a~ ring
membersl which is optionally disubstituted or
lS trisubstituted by one or more radicals from the
series comprising F, Cl, Br, hydroxyl, (Cl-C7)-
alkoxy, (C1-C7)-al~yl, (C2-C~)-alkoxycarbonyl, amino
or trifluoromethyl,
R4 denotes a radical of th0 formula III
-X-(CH~)n-R7 5III)
A denotes a radical linked to R1 at the N te~minus and
to B at the C terminus of an amino acid from the
series comprising phenylalanine, histidine,
tyrosine, tr~ptophan, methionine, leucine, isoleu-
cine, asparagine, aspartic acid, ~2-thienylalanine,
~-3-thienylalanine,~-2-furanylalanine,~-3-furanyl-
alanine, lysine, ornithine, valine, alaIIine~ 2,4~
diaminobutyric acid~ arginine, 4-chlorophenylala-
nine, methionine sulfone, meth.ionine sulfoxide, 2-
3D pyridylalanine, 3-pyridylalaninel 4-pyridylalanine,
cyclohexylalanine, cyclohexylglycine,im-methylhis-
tidine, O-methyltyrosin~, O-benzyltyrosine, O-text.-
butyltyrosinel phenylglycine, l-naphthylalanine, 2-
naphthylalanine, 4-nitrophenylalanine, norvaline, ~-
2-benzo(b)thienylalanine, ~-3-benzo(b)thienylala-
nine, 2-fluoroph~nylalanine, 3-fluorophenylalanine~
4-fluorophenylalaninet norleucine, cysteineJ S-
methylcysteine, l,2,3,4-tetrahydroisoquinolin~-3-
carboxylic acid, homophenylalan.ine, DOPA,
f~ Q 7 1
0-dimethyldopa, 2-amino-4-(2-thienyl)butyric acid,
2-amino-4-(3-thienyl)butyric acid, 3-(2-thienyl)-
serine, (Z)-dehydrophenylalanine and (E)-dehydro-
phenylalanine, B denotes a radical of an amino acid, as defined under
A,
R2 denotes hydrogen, (Cl-C10)-alkyl, (C~-C7)-cycloalkyl,
(C4-C7)-cycloalkyl-(Cl-C~)-alkyl, (C6-Cl4)-aryl or (C6-
Cl4)-aryl-(Cl-C~)-alkyl,
R3 denotes hydrogen, (Cl-C10)-alkyl, (C6-Cl~)-aryl or (C6-
Cl~)-aryl-(Cl-C4)-alkyl,
X can either be absent or denotes 0 or S,
n can be 2, 3 or 4 and
R7 denotes hydroxyl or amino or stands for the radical
of a 5-membered heterocycle having at least 1 carbon
atom, 1-4 nitrogen atoms and/or 1 sulfur or oxygen
atom, which is optionally substituted by one or ~ore
radicals from the series comprising F, Cl, Br,
hydroxyl, (Cl-C7)-alkoxy, (Cl-C7)-alkyl, (Cz-C~)-
alkoxycarbonyl, amino or trifluoromethyl, where
oxazoline is excluded, and their physiologically
tolerable salts.
The carbon atoms sub~tituted by R2, hydroxyl and R3 can in
each case have the R, S or R,S configurstion.
Alkyl can be straight-chain or branched. The same applies
to radicals derived therefrom, such a~, for example,
alkoxy, alkylthio, alkylamino, dialkylamino, alkanoyl and
aralkyl.
Cycloalkyl is also understood as meaning alkyl-
substituted radicals, such as, for example, 4-methyl-
cyclohexyl or 2,3-dimethylcyclopentyl.
(C6-Cl4)-Aryl is, for example, phenyl, naphthyl, biphenyl-
yl or fluorenyl; phenyl is preferred. The same applies to
radicals derived therefrom, such as, for example, aryl-
oxy, aroyl, aralkyl and aralkyloxy. Aralkyl is understood
20~507~
-- 4 --
as meaning an unsubstituted or substituted (C6-C~ aryl
radical linked to (Cl-C6)-alkyl, such as, for example,
benzyl, ~- and ~-naphthylmethyl, halobenzyl and alkoxy-
benzyl, but where aralkyl need not be limited to the
compounds mentioned.
A radical of a 5- or 6-membered monocyclic or 9- or 10-
membered bicyclic heterocycle having at least 1 carbon
atom, 1-4 nitrogen atoms and/or 1 sulfur or oxygen atom
as ring members and standing for R^ is understood as
meaning heterocycles such as are defined, for example,
in Katritzky and Laqowski, Chemie der Heterocyclen
[Heterocyclic Chemistry], Berlin, Heidelberg 1968, pages
3-5. Monocyclic heterocycles are, for example, thiophene,
furan, pyrrole, imidazole, pyrazole, pyridine, pyrazine,
pyrimidine, pyridazine, 1,2,4-triazole, thiazole, tetra-
zole, isothiazole, oxazole and isoxazole and the com-
pletely or partially hydrogenated heterocycles derived
therefrom. Bicyclic heterocycles are, for example,
benzothiophene, benzofuran, indole, isoindole, indazole,
benzimidazole, quinoline, isoquinoline, phthalazine,
quinoxaline, quinazoline and cinnoline and the completely
or partially hydrogenated heterocycles derived therefrom.
A radical of a 5-membered heterocycle having at least 1
carbon atom, 1-4 nitrogen atoms and/or 1 sulfur or oxygen
atom standing for R7 is understood as meaning, in par-
ticular, thiophene, furan, pyrrole, imidazole, pyrazole,
1,2,4-triazole, thiazole, tetrazole, isothiazole, oxa-
zole, isoxazole and completely or partially hydrogenated
cycles derived therefrom, where oxazoline is excluded.
The amino acids A and B in formula I are linked to one
another by an amide bond, and they are natural or un-
natural ~-amino acids of the L, D or D,L configuration,
preferably the L configuration.
Salts of compounds of the formula I are understood as
meaning, in particular, pharmaceutically utilizable or
non-toxic 6alts.
Such salts are formed, for e~ample, by compounds of the
formula I which contain acidic groups, for example
carboxyl, with alkali metals or alkaline oarth metal~,
such as Na, R, Mg and Ca, and with physiologically
tolerable organic amine~, 6uch as, for example, triethyl-
amine and tri-(2-hydroxyethyl)amine.
Compounds of the formula I which contain basic groups,
for example an amino group or a guanidino group, form
salts with inorganic acid~, ~uch a~J for example, hydro-
chloric acid, sulfuric acid or phosphoric acid and with
organic carboxylic or sulfonic acids, su~h as, for
example, acetic acid, citric acidl benzoic acid, maleic
acid, fumaric acid, tartaric acid and p-toluenesulfonio
acid.
R1 preferably denotes (C2-Cl1)-alkanoyl, ~uch as
n-decanoyl, formyl, acetyl, pi~aloyl, i~ovaleryl or
isobuty]~yl; substituted (C2-Cll)-alkanoyl, such a~ 2-
hydro~n?ropionyl, 2-hydroxy-3-methylbutyryl or
optiona:Lly protected amino-(C2-Cll)-alkanoyl, such as
4 aminobutyryl, 5-aminopentanoyl, 6-aminohexanoyl,
4-N-tert.-butoxycarbonylaminobutyryl, 5-N-tert.-
butoxycarbonylaminopentanoylor6-N-tert.-butoxycar-
bonylaminohexanoyl or di-(C1-C7)-alkylamino-~C2-C~
alkanoyl, such as dim~thylaminoacetyl; [C~-Cg)-
cycloalkylcarbonyl, such as cyclopropylcaxbonyl,
cyclobutylcarbonyl, cyclopentylcarbonyl or cyclo-
hexylcarbonyl;( C6-Clo ) -aryl-( C2-Cll )-alkanoyl,~uchas
phenylacetyl, phenylpropanoyl or phenylbutanoyl, 2
(2~6-dichloroanilino)-phenylacetyl, 2-(N-benzyl-2,6-
dichloroanilino)-phenylacetyl; benzoyl optionally
substituted by halogen, (~l-C7)-alkyl, (Ct-C~)-alkoxy
or (C1~C7)-alkoxycarbQnyl, such as 4-chlorobenzoyl,
4-methylbenzoyl, 2-metho~ycarbonylbenzoyl or 4-
metho~ybenzoyl, pyrrolyl-2-carbonyl, pyridyl-3
carbonyl, b~nzylsulfonyl; (C2-C~ alkoxycarbonyl,
2 0 ~
such as methoxycarbonyl, ethoxycarbonyl or tert.-
butoxycarbonyl; ( C2-Cll )-alkoxycarbonyl substituted
by halogen, such as 2,2,2-trichloroethoxycarbonyl or
1,1-dimethyl-2,2,2-trichloroethoxycarbonyl; or ( C6-
S C14)-aryl-(C2-C,)-alkoxycarbonyl, such as benzyloxy-
carbonyl or 9-fluorenylmethylcarbonyl.
R2 is preferably isobutyl, benzyl or cyclohexylmethyl.
R3 is preferably hydrogen, isopropyl or isobutyl.
Preferred amino acids A and B independently of one
another are phenylalanine, histidine, tyrosine, trypto-
phan, methionine, leucine, isoleucine, asparagine,
aspartic acid, ~-2-thienylalanine, ~-3-thienylalanine, ~-
2-furylalanine, lysine, ornithine, 2,4-diaminobutyric
acid, arginine, norvaline, 4-chlorophenylalanine, methio-
nine sulfone, methionine sulfoxide, 2-pyridylalanine, 3-
pyridylalanine, 4-pyridylalanine, cyclohexylalanine,
cyclohexylglycine, im-methylhistidine, 0-methyltyrosine,
0-benzyltyrosine, 0-tert.-butyltyrosine, phenylglycine,
l-naphthylalanine, 2-naphthylalanine, 4-nitrophenylala-
nine, norleucine, valine, alanine, 1,2,3,4-tetrahydroiso-
quinoline-3-carboxylic acid, homophenylalanine, 2-~mino-
4-(2-thienyl)butyric acid, (Z)-dehydrophenylalanine or
(E)-dehydrophenylalanine.
X is preferably absent.
n preferably denotes 2 or 3, preferably 2.
R7 preferably stands for the radical of a 5-membered
heterocycle having one or two heteroatoms, which is
optionally substituted by (C1-C,)-alkyl.
R7 particularly preferably stands for 2-imidazolyl, 2-N-
methylimidazolyl, 2-N-ethylimidazolyl, 2-N-n-propylimi-
dazolyl and 2-N-isopropylimidazolyl.
The invention furthermore relates to a process for the
preparation of compounds of the formula I which comprises
coupling a fragment having a terminal carboxyl group or
its reactive derivative to a corresponding fragment
201~7~
- 7 -
having a free amino group, if appropriate removing (a)
protective group(s) temporarily introduced for the
protection of further functional groups and optionally
converting the compound thus obtained into it~ physio-
logically tolerable salt.
Fragments of a compound of the formula I having a ter-
minal carboxyl group have the formulae IVa-IVc below:
Rl-OH (IVa)
Rl-A-OH ~IVb)
Rl-A-B-OH (IVc)
Fragments of a compound of the formula I having a ter-
minal amino group have the formulae Va-Vc below:
~2 OH ~3
H2N-A-B-NH-CH-CH-CH-R4 (Va)
R2 OH ~3
I ~ 4
H2N-B-NH-CH-CH-CH-~ (Vb)
~2 pH ~3
H2N- CH- CH- CH- R4 . ( Vc )
Methods which are suitable for the preparation of an
amide bond are described, for example, in Houben-Weyl,
Methoden der organischen Chemie [Nethods of Organic
Chemistry], Volume 15/2; Bodanszky et al., Peptide
synthesis, 2nd ed. (Wiley & Sons, New York 1976) or
Gross, Meienhofer, The Peptides. Analysis, synthesis,
biology (Academic Press, New York 1979). The following
methods are preferably used: the active ester method
using N-hydroxylsuccinimide as the e~ter component,
coupling with a carbodiimide such as dicyclohexyl-
carbodiimide or with propanephosphonic anhydride and the
mixed anhydride method using pivaloyl chloride.
2 ~ 7 ~
The preparation of the optically active amines used as a
starting compound, of the formula Vc
H2N- CH- CH- CH- R4 ( Vc )
OH
wherein R2, R3 and R~ are as def$ned above, is carried out
starting from optically active ~-amino acids, their
asymmetric center being retained. For this purpose, an N-
protected amino acid aldehyde i6 prepared in a known
manner and converted into a corresponding heteroarylalkyl
building block after an aldol addition. After removal of
the N-protective group, aminoalcohols of the formula Vc
are obtained. Nixtures of diastereomers relative to the
OH-carrying center are obtained, which are separated in
a manner known per se, for example by fractional crys-
tallization or by chromatography. Checking of the dia-
stereomeric purity is carried out by means of HPLC, andthe checking of the enantiomeric purity can be carried
out in a known manner by converting into Mosher
derivatives (H.S. Nosher et al., J. Org. Chem. 34, 2543
(1969)).
The preparation of N-protected amino acid aldehydes is
carried out according to B. Castro et al. (Synthesis
1983, 676).
The aldol-analogous addition to N-protected amino acid
aldehyde~ (preferably N-tert.-butoxycarbonyl and benzyl-
oxycarbonyl protective groups) is carried out in asolvent inert to bases, such as ether, THF, toluene, DMF,
DNSO or dimethoxyethane.
Bases which can be used for the deprotonation of the
heteroarylalkyl component are alkali metal alcoholates,
such as potassium o-tert.-butylate or sodium methylate,
alkali metal hydrides, such as sodium hydride or potas-
sium hydride, organometallic bases, such as n-butyl-
201~07~
lithium, s-butyllithium, methyllithium or phenyllithium,
sodium amide and alkali metal ~alts of organic nitrogen
base6, such as lithium dii~opropylamide.
The 6ubstituted 4-amino-3-hydroxybutyric acids are known
from the literature and are prepared according to
D.H. Rich et al., J. Org. Chem. 43, 3624 (1978).
The preliminary and subsequent operations necessary for
the preparation of compounds of the formula I such as
introduction and removal of protective groups are known
from the literature and are described, for example, in
T.W. Greene, "Protective Groups in Organic Synthesisn.
Salts of compounds of the formula I containing salt-
forming groups are prepared in a manner known per se by
reacting, for example, a compound of the formula I
containing a basic group with a stoichiometric amount of
a suitable acid. Mixtures of stereoi~omers, in particular
mixtures of diastereomers which are obtained using
racemic amino acids A or B can be separated by fractional
crystallization or by chromatography in a manner known
per se.
The compounds of the formula I according to the invention
exhibit enzyme-inhibiting properties; in particular, they
inhibit the action of the natural enzyme renin. Renin is
a proteolytic enzyme of the aspartyl protease class
which, as a result of various stimuli (volume depletion,
sodium deficiency, ~-receptor stimulation), is ~ecreted
into the blood circulation by the ~uxtaglom~rular cells
of the kidney. There, it cleaves the decapeptide
angiotensin 1 from the angiotensinogen 6ecreted from the
liver. This angiotensin 1 is converted by the ~angio-
tensin converting enzyme~ (ACE) into angiotensin 2.
Angiotensin 2 play6 an essential role in blood pressure
regulation, since it directly increase6 the blood pres-
sure by vascular contraction. It additionally stimulates
the secretion of aldosterone from the adrenal gland and
increase6 the extracellular fluid volume in this manner
2~07~
-- 10 --
via the inhibition of sodium excretion, which in turn
contributes to an increase in blood pressure. Inhibitors
of the enzymatic activity of renin cause a reduced
formation of angiotensin 1 which results in a reduced
formation of angiotensin 2. The lowering of the con-
centration of this active peptide hormone is the direct
cause of the hypotensive action of renin inhibitors.
The activity of renin inhibitors can be checked by in
vitro tests. The reduction in the formation of angioten-
sin 1 in various sytems (human plasma, porcine renin) i~measured in this connection. Por this purpose, for
example, human plasma which contains both renin and
angiotensinogen is incubated at 37C with the compound to
be tested. The concentration of the angioten~in 1 formed
during the incubation is then measured using a radio-
im~unoaggay. The co~pounds of the general formula I
described in the present invention show inhibitory
effects at concentrations of about 10-5 to 10-1~ mol/l in
the in vitro tests used.
Renin inhibitors cause a lowering of blood pressure in
salt-depleted animals. Since human renin differs from the
renin of other species, primates (marmosets, rhesus
monkeys) are used to test renin inhibitors in vivo.
Primate renin and human renin are substantially homolo-
gous in their sequence. An endogenous effusion of reninis initiated by i.v. in~ection of furosemide. The test
compounds are then administered by continuous infusion
and their effect on blood pressure and heart rate is
measured. The compounds of the present invention are
effective here in a dose range from about 0.1-5 mg/kg
i.v. The compounds of the general formula I described in
the present invention can be used as antihyperten~ives
and also for the treatment of cardiac insufficiency.
The invention therefore also relates to the use of
compounds of the formula I as pharmaceuticals and to
pharmaceutical preparations which contain this compound.
2015~ ~
Use in primates is preferred, in particular in humans.
Pharmaceutical preparations contain an effective amount
of the active compound of the formula I together with an
inorganic or organic pharmaceutically utilizable exci-
pient. Administration can take place intranasally,intravenously, subcutaneously or perorally. The dose of
the active compound depends on the warm-blooded species,
body weight, age and the manner of administration.
The pharmaceutical preparations of the present invention
are prepared in a dissolving, mixing, granulating or
tablet coating proces~ known per se.
For a fo~m for oral administration, the active compounds
are mixed with the additives customary therefor such as
excipients, stabilizers or inert diluents and brought
into a suitable form for administration, such as tablets,
coated tablets, hard gelatin capsules, aqueous, alcoholic
or oily suspensions or aqueous, alcoholic or oily solu-
tions by cu6tomary methods. Inert excipients which can be
used are, for example, gum arabic, magnesia, magnesium
carbonate, potassium phosphate, lactose, glucose,
magnesium stearyl fumarate or starch, in particular corn
starch. Preparation can be carried out both as dry or
mcist granules. Suitable oily excipients or solvents are,
for example, vegetable or animal oils, 6uch as sunflower
oil and cod liver oil.
For subcutaneous or intravenous administration, the
active compounds or their physiologically tolerable salts
are brought into solution, suspensions or emulsions, if
desired with the substances customary therefor such as
solubilizers, emulsifiers or other auxiliaries. Possible
solvents, for example, are: water, physiological saline
solutions or alcohols, for example ethanol, propanediol
or glycerol, and in addition also sugar solutions such as
glucose or mannitol solutions, or even a mixture of the
various solvents mentioned.
2015~7~
- 12 -
List of _he abbreviations used:
Ac Acetyl
ACHPA t3S,4S]-4-Amino-3-hydroxy-5-cyclohexylpentanoic
acid
S BOC tert.-Butoxycarbonyl
TLC Thin layer chromatography
DCC Dicyclohexylcarbod~imide
DNP 2,4-Dinitrophenyl
DNF Dimethylformamide
DNSO Dimethyl sulfoxide
EA Ethyl acetate
Etoc Ethoxycarbonyl
FAB Fast atom bombardment
H Hexane
HOBt l-Hydroxybenzotriazole
Iva Isovaleryl
M Nolecular peak
MeOH Methanol
MS Nass spectrum
NTB Methyl tert.-butyl ether
R.T. Room temperature
M.p. Nelting point
Thi ~-2-Thienylalanine
THF Tetrahydrofuran
Z Benzyloxycarbonyl
The other abbreviations used for amino acids correspond
to the three-letter code customary in peptide chemistry
as i8 described, for example, in Europ. J. Biochem. 138,
9-37 (1984). If not expressly stated otherwise, the amino
acids are always in the L-configuration.
The examples below serve to illustrate the present
invention, without limiting it thereto.
2015~071
- 13 -
Bsanple 1
Iva-Phe-His-l(S)-cyclohexylmethyl-2(S)-hydroxy-5-(2-
imidazolyl)-pentylamide
70 my of Iva-Phe-His-l(S)-cyclohexylmethyl-2(S)-hydroxy-
5-(2-N-benzyloxymethyl-imidazolyl)-pentylamide are
dissolved in 15 ml of ethanol. After addition of 35 mg of
10~ strength palladium on active carbon and 1.5 ml of
glacial acetic acid, the mixture is hydrogenated at room
temperature and normal pressure for 20 h. After filter-
ing, it i8 concentrated. After addition of 20 ml ofsaturated NaHCO3 solution, the mixture i~ extracted 3
times using EA. After drying the EA phases with Na2SO4,
they are concentrated, the title compound being obtained
as a faintly yellow substance.
Rf (EA~MeOH 2/1) = 0.1 MS(FAB) = 634 (M+l)
!
a) Iva-Phe-His-l(S)-cyclohexylmethyl-2(S)-hydroxy-5-(2-
N-benzyloxymethyl-imidazolyl)-pentylamide.
200 mg of Iva-Phe-His(DNP)-l(S)-cyclohexylmethyl-2(S)-
hydroxy-5-(2-N-benzyloxymethyl-imidazolyl)-pentylamide
are stirred at room temperature for 3 h in 5 ml of
acetonitrile containing 0.5 ml of thiophenol. The mixture
is concentrated in vacuo and digested 3 times using
diisopropyl ether. After chromatography on silica gel
(eluent EA/methanol 10:1) and concentration of the
product-containing fractions, a colorless powder is
obtained.
Rf (EA/MeOH 10/1) = 0.2 MS(FAB) - 754 (M+l)
b) Iva-Phe-His(DNP)-l(S)-cyclohexylmethyl-2(S)-hydroxy-
5-(2-N-benzyloxymethylimidazolyl)-pentylamide.
44 ~1 of pivaloyl chloride are added at -5C to 192 mg
of Iva-Phe-His(DNP)-OH, 30 ~1 of pyridine and 50 ~1 of
N-ethylpiperidine in 10 ml of CH2Cl2. The mixture is
stirred at +5 to +10C for 30 min, cooled to -10C and
l(S)-cyclohexylmethyl-2(S)-hydroxy-5-(2-N-benzyloxy-
20~ ~7~
methyl-imidazolyl)-pentylamine in 5 ml of CH2C12 is added
dropwise. The mixture is stirred for 16 h without cool-
ing, concentrated in vacuo and taken up in 100 ml of EA.
The solution is extracted 3 times using aqueous K2C03
solution, once using H20, dried over Na2S04 and concentra-
ted in vacuo. After chromatography on silica gel (eluent
EA/methanol 10:1), the title compound is obtained as a
faintly yellowish powder.
Rs (EA/methanol 10/1) = 0.4 MS(FAB) = 920 (M+l)
c) l(S)-Cyclohexylmethyl-2(S)-hydroxy-5-[2-N-benzyloxy-
methyl-imidazolyl]-pentylamine.
500 mg of 3-Boc-4(S)-cyclohexylmethyl-2,2-dimethyl-5-[3-
(2-N-benzyloxymethyl-imidazolyl)-propyl]-oxazolidine are
dissolved in 15 ml of dioxane. 10 ml of 12% strength HCl
in dioxane are added with slight cooling (+10C). After
3 h at room temperature, the mixture is concentrated in
vacuo, and the residue is taken up in H20, ad~usted to
pH 9-10 using saturated aqueous Na2C03 ~olution and
extracted 3 times using EA. The title compound, which can
be used without further purification in the next coupling
steps, i8 obtained after drying with Na2S0~ and concen-
trating in vacuo.
d) 3-Boc-4(S)-cyclohexylmethyl-2,2-dimethyl-5-[3-(2-N-
benzyloxymethyl-imidazolyl)-propyl]-oxazolidine
550 mg of 2-methyl-N-benzyloxymethyl-imidazole are
dissolved in 5 ml of dry THF. 1.9 ml of 1.5 M n-butyl-
lithium in hexane are added at -60 under an argon
atmosphere. After 15 min, 500 mg of 3-Boc-4~S)-cyclo-
hexylmethyl-2,2-dimethyl-5-(2-bromoethyl)-oxazolidine in
5 ml of THF are added. After 1 h at -60C, 10 ml of
saturated NaHC03 solution are added. After warming to room
temperature, the mixture is extracted 3 times using EA,
and the extracts are dried using Na2S04 and concentrated.
The title compound is obtained by chromatography on
silica gel (eluent EA).
2015~7~
-- 15 --
Rf ~EA) = 0.3 MS(FAB) = 526 (M+l)
e) 2-Methyl-N-benzyloxymethyl-imidazole
5 g of 2-methylimidazole are added in portions to 16.8 g
of potas6ium hydroxide in 200 ml of dry acetone. After
5 min, 8.5 ml of benzyloxymethyl chloride are added
dropwise at room temperature. After 15 min, the mixture
is concentrated and ad~usted to pH 1 using 2 N HCl. It is
then extracted 3 times using EA, and the organic pha~es
are discarded. The aqueous phase is ad~usted to pH - 9-10
using KOH and extracted 3 times using EA. The combined EA
phases are dried using Na2SO4 and concentrated. Chromato-
graphy on silica gel (eluent EA/MeOH 10/1) yields the
title compound.
R~ (EA/MeOH 10/1) = 0.4
f) 3-Boc-4(S)-cyclohexylmethyl-2,2-dimethyl-5-(2-bromo-
ethyl)-oxazolidine
1.6 ml of diethyl azodicarboxylate are added dropwise at
20C under argon to 690 mg of 3-Boc-4(S)-cyclohexyl-
methyl-2,2-dimethyl-5-(2-hydroxyethyl)-oxazolidine, 2.6 g
of triphenylphosphine and 1.6 g of pyridinium hydro-
bromide in 15 ml of CH2Cl2. After 16 h at R.T., H2O is
added and the mixture is diluted with 100 ml of CH2C12.
The organic phase i8 washed twice with saturated NaHCO3
solution and once with saturated NaCl solution. The
organic phase dried using Na2SO4 i8 concentrated, the
residue is taken up in a little EA and filtered to
separate off PPh3. Purification on silica gel yields the
title compound (eluents H/EA 15:1).
R~ (H/EA 15/1) = 0.3; MS 404 (M)
g) 3-Boc-4(S)-cyclohexylmethyl-2,2-dimethyl-5-(2-hydroxy-
ethyl)-oxazolidine
10 g of Boc-ACHPA-OEt (preparation according to J. Med.
Chem. 28 [1985], 1779), 500 mg of p-toluenesulfonic acid
2 0 ~
- 16 -
and 7.2 ml of dimethoxypropane are heated at 80C in
160 ml of toluene under argon for 2 h. The mixture is
then concentrated. The residue is added dropwise at 0C
to a suspension of 2 g of LiAlH4 in 200 ml of THF. After
2.5 h at 0-C, 100 ml of 5~ strength NaHSO4 solution are
added and the mixture is extracted 3 times using EA. The
combined organic phases are washed once using saturated
NaHCO3 solution. After drying with Na2SO4, the solution is
concentrated and chromatographed (eluent: H~EA 2:1).
R~ (H/EA 4:1) = 0.1; MS=342 (M+1)
Examples 2-10 are prepared analogously to Example 1.
Example 2
Iva-Phe-His-l(S)-cyclohexylmethyl-2(S)-hydroxy-5-(2-N-
methyl-imidazolyl)-pentylamide
Rf (EA/MeOH 3/1) = 0.1 MS(FAB) = 648 (M+l)
E~ample 3
Iva-Phe-His-l(S)-cyclohexylmethyl-2(S)-hydroxy-5-(2-N-
ethyl-imidazolyl)-pentylamide
Rf (CH2C12/NH40H/NeOH 10/0-1/1) = 0.35 NS(FAB) = 662 (M+l)
F a ple 4
Iva-Phe-His-l(S)-cyclohexylmethyl-2(S)-hydroxy-5-(2-N-n-
propyl-imidazolyl)-pentylamide
Rf (EA/MeOH 3/1) = 0.1 MS(FAB) = 676 (M+1)
~a ple S
2S Iva-Phe-His-l(S)-cyclohexylmethyl-2(S)-hydroxy-5-(2-N-
isopropyl-imidazolyl)-pentylamide
R~ (EA/MeOH 3/1) = 0.1 MS(FAB) = 676 (M+1)
The preparation of 2-methyl-N-alkyl-imidazoles is des-
cribed in the following as exemplified by 2-methyl-N-n-
propyl imidazole:
2 ~ 7 .~
- 17 -
2-Methyl-N-n-propyl-imidazole
10 g of 2-methylimidazole are heated to boiling for 2 h
in 40 ml of n-propyl bromide. The mixture is then poured
into 150 ml of 5% strength NaHSO4 solution, extracted 3
times using FA, dried using Na2SO4 and concentrated. The
residue is taken up in ether and insoluble starting
material i8 removed by filtration. In this manner, the
title compound is obtained as a colorle~s oil.
R (EA/MeOH 10/1) = 0.2
E-a ple 6
Iva-Phe-Nva-l(S)-cyclohexylmethyl-2(S)-hydroxy-5-(2-
imidazolyl)-pentylamide
Rf (EA/MeOH 5/1) = 0.1 MS(FAB) = 596 (M+l)
E~a~ple 7
Iva-Phe-Nva-l(S)-cyclohexylmethyl-2(S)-hydroxy-5-(2-N-
methyl-imidazolyl)-pentylamide
R (CHzCl2/MeOH/NH~OH 20/1/0.1) = 0.15 MS(FAB) = 610 (M+l)
E~a ple 8
Iva-Phe-Nva-l(S)-cyclohexylmethyl-2(S)-hydroxy-5-(2-N-
ethyl-imidazolyl)-pentylamide
Rr (CH2Cl2/NeOH/NH40H 20/1/0.1) = 0.15 MS(FAB) = 624 (N+l)
E~ample 9
Iva-Phe-Nva-l(S)-cyclohexylmethyl-2(S)-hydroxy-5-(2-N-n-
propyl-imidazolyl)-pentylamide
R (EA/MeOH 10/1) = 0.3 MS(FAB) = 638 (M+l)
E~a ple 10
Ival-Phe-Nva-l(S)-cyclohexylmethyl-2(S)-hydroxy-5-(2-N-
isopropyl-imidazolyl)-pentylamide
R (EA/MeOH 10/1) = 0.3 MS(FAB) = 638 (M+l)