Note: Descriptions are shown in the official language in which they were submitted.
i~:'~~L~~''~~ -.
P1~DUCI' FoR LUBRICATII~ CARHON FIBRES FUR A CQ~POSITE MATERIAL
AI~ PROS FOR PF~DiICING SAID MATERIAL
T1G~~l'1?TDT' TllA7
The present invention relates to a product for lubricating carbon
fibres for embedding in a resin hardenable by radiation in accordance
with a radical mechanism, which serves as an interface between the
fibres and the resin of a site material. It also relates to a
process for producing a ccr~osite material having lubricated fibres,
as well as to the material obtained.
The carQosite mater_als can be of a simple or canplPx nature and are
in particular used in the motor vehicle, aeronautical, space, navi-
gation and similar fields. In the aezronautical and space field, they
are more particularly used for producing engines, floor plates and
leading edges of aircraft.
Fig. 1 diagrammatically shags a simple structure composite material.
Sari canposite material has carbon fibres 2 embedde3 in a matrix 4
constituted by a polymerized and/or crosslinked resin. It is also
possible to see the interfaces 6 between the fibres 2 and the matrix 4.
In such a car~osite material there are two mechanical stressing types,
one being longitudinal and corresponding to the direction of the
fibres, as represented by arrows fl, whilst the other is transverse
and perpendicular to the fibres and is represented by arxro~ws f2.
Transverse stresses are those of the shear and/or cxmpressive type.
These stresses have repercussions at the fibre-matrix interfaces 6
and in the case where the latter constitute the weak point of the
ca~osite material, the mechanical perforn~ance characteristics obtained
are lager than those of the matrix. In addition, when said canposite
materials are subject to shear stresses, there is generally a delamin-
ation between the fibres and the matrix, which can lead to the frac-
ture of the material. The same applies with regards to carq~lex
structure ca~posite materials, which essentially differ by the
SP 5552.69 LC
~fl~~~~~
_ 2 _
presence of metal inserts or objects.
In order to obviate these disadvantages, a certain number of inter-
face products deposited on the carbon fibre have been envisaged ~d
which are called lubricating or sizing products for the purpose of
facilitating the impregnation conditions of the fibres by the resin
during the production of the carposite material and with a view to
obtaining a "fastening" between the matrix and the fibres.
The known lubricating materials are generally complex copolymers or
polymers leading to often long and tedious lubricating processes.
Polymeric lubricating materials are in particular described in FR-A-2
129 905, FR-A-2 129 906, FR-A-2 558 842 and FR-A-2 483 395.
More specifically, the prc-sent invention relates to a pmduct for
lubricating carbai fibres for ding in a resin which, according
to a radical mechanism, can be hardened by radiation not causing any
1,5 temperature rise in the material, such as X or beta radiation. Thus,
the composite materials produced from resins which are polymerized
and/or csrossllnked by cold ionizing rays, according to a radical
mechanian, have under certain stresses, mechanical perforniance charac-
teristics equal or superior to those polymerized by the thermal
procedure. The production of high performance canposite materials
with a single or carplex stmcture and using X or beta radiation has
in particular been described in FR-A-2 564 029. However, the presently
known lubricating materials are nGt car~patible with a process for
hazdening resin by ionizing rays, according to a radical mechanism,
a~ theY t~ l~ to inadequate interface bonds in the case of
severe transverse stresses.
'Thus, the most widely used high performance or stnictural carq~osite
materials (mechanical properties) are obtained by theanal polymeriz-
ation and/or czrosalinking in the presence or absence of a hardener,
3p cf. the aforementioned documents.
The resins used are then epoxy resins or polyesters and the fibres
SP 5552.69 LC
~0~..,~~,4'~s
-3-
are carbon or ararnide glass fibres, whereof the surface preparation
or lubricating material is car~patible with the thermal process used
for hardening these resins.
Reference can be made in this ccnnection to US-A-3 398 210, which
refers to the lubricating of glass fibres for embedding in a polyester
resin with ethylena unsaturations and which can be hard~ed thernially
or by irradiation. The fastening or attacYment of the lubricating
product to the fibres is ensured by physical bonds of the hydrogen
type between silanol groups Si-0H of the fibres and those of the
~bricating products, which are products obtained by the hydrolysis
of silane derivatives.
Reference can also be made to Wt0-A-85/04200 concerning the prepar-
ation of cellulose fibres for anbedding in a thermally hardenable,
unsaturated or non-unsaturated resin. The coating of the fibres by
this preparation is followed by a hot alkaline treatment. This takes
a long time, is tedious and relatively complex.
Reference can also be made to EP-A-256 852 concerning the lubricating
of carbon fibres by a dimethacrylate-urethane product and which are
to be embedded in a thermally hardenable, unsaturated resin.
The attachment of the lubricating pmduct to the fibres is brought
about solely by Physical bonds, the polar urethane functions having
affinities with the reaction sites of the fibres.
The invention also aims at improving the transverse stress character-
istics of a carbon fibre canposite material, whereof the matrix is
°btained by Polymerizing a resin under radiation, according to a
radical mechanimn, so as to have identical perforrnance characteristics
to the thermally polymerized canposite material, with regards to the
compression and shear strength. The imprwemen t to these character-
istics involves the use of a specific, radiation-sensitive lubricating
product. In addition, lubrication must be relatively simple and
industrially usable.
SP 5552.69 L~
~o~.s~~6
- 4 -
The invention more specifically relates to a reactive carbon fibre
lubricating product having oFi reaction sites and for embedding in a .
resin cold haxdenable by radiation in accordance with a radical
mechanism and constituted by a monomer having at least one functional
gxnup able to thermally four covalent chenical bonds with these
reaction sites and at least one second functional group differing fran
the first and able to form covalent chemical bonds with said resin
during its hardening under said radiation, the first group being
chosen fran among isocyanate, carboxylic acid anhydride, methylol and
c~xYlic acid chloride groups.
The term lubricating is understood to mean the operation consisting
of coating the non-surface treated fibres with an accurately metered
material quantity. Generally, this quantity represents 0.3 to 1.5%
by weight and preferably 0.3 to 0.9% by weight of the fibres.
The bifunctional lubricating product according to the invention makes
it possible to assure a "windability" of the fibres at the time of
impregnation by the resin, as well as a good interface quality between
the fibre and the matrix (in order to have a good resistance to trans-
verse stresses). The term "windability" is understood to mean that
the fibre retains its shape and does not undergo fibre separation.
The first functional group F1 of the lubricating product acco~iing
to the invention ensures a cavalen t chanical bond between the chanical
reaction sites of the fibres and the lubricating product.
The reaction sites of the fibres are dependent rn the nature of the
latter, as well as the heat treatments perforn~ed an then during their
production. Thus, the reaction sites of the presently known carbon
fibres are CH, N2t-!, R~=0 with R and R'=H or an alkyl, aryl or phenyl
group, R'
The invention solely relates to carbon fibres having mainly CH sites
~ ~ reaction sites.
SP 5552.69 LC
2015~'~~
- 5 -
The first functional groups of the lubricating material according to
the invention have the advantage of thermally forming chemical bonds
with the CH sites, at tarperatures which do not trigger a txxnopolymer-
ization chemical reaction of the second grr~up of the habricating
product. The choice of the reaction sites present in.a larger quantity
on the fibres is aimed at improving the attachment of the resin matrix
to the fibres.
The functional grips of the carboxylic acid anhydride type can result
fran a mono-di or tri-carboxylic acid. Preferably, the isocyanate
~Ction is used for the first group. Under these conditions, the
covalent chemical bond established with the CH sites of the fibres is
a urethane bond.
The second functional group F2 of the lubricating product according to
the invention favours the connection between said interface product
and the resin of the ornposite material matrix. This connection or
bond is a covalent chgnical bond and is ensured during the cold
polymerization and/or crosslinking of the ream of the oanposite
material under radiation according to a radical mechanism. In
particular, the second functional group must be able to copolymerize,
according to a radical mechanism, with the resin of the matrix during
its tsazdening and wider beta or X radiation. The second functional
gsroup must also be of the same nature as that constituting the resin
of the carposite material.
In order to obtain high performance carposite materials, preference
~ 91v~ to the use of resins with ethylene unsaturations such as
epoxy, polyester or polyurethane resins with (meth)acrylic teirninations
or mixtures of these resins. It should be noted that epoxy resins
with ethylene unsaturations are resins having an epoxy origin having
no longer any epoxy function. Moreover, the F2 gxnup of the lubri-
cating product according to the invention is advantageously chosen
without ethylene unsaturations. However, it is possible to use F2
groups of the maleimide type for resins of the bisrnaleimide type.
SP 5552.69 LC
~C1~~'~~6
- 6 -
In particular, sail second group F2 is of formula (I)
~\
C=C
R2 / \ R4 _
in which R5 represents a hydrogen star, a benzene nucleus, -C~I~ and
O
R2 represents a hydrogen star or -~Oi~, with F~ representing a straight
or branched aryl or alkyl radical having 1 to 12 carbon stars, R3
represents a hydrogen star or the methyl radical and R4 an aryl radical,
(-0-, r-l~i_, ~_.
0 O O
The aryl groups are in particular of the phenyl, naphthyl, etc. types.
The lubricating material according to the invention serves to impr~ave
the adhesion of the carbon fibres to the resin matrix of a composite
material. The invention also relates to a opposite material having
caxbon fibres enbedded in a resin hardened by beta or X radiation
accoxriing to a radical mechanism and having a reactive fibre lubri-
cating product of the type defined hereinbefore. ThQ invention also
relates to a process for producing sair3 canposite material.
According to an essential feature of the sail process, the latter
comprises the following stages:
a) dissolving the aforsnentiored lubricating product in an organic
solvent:
b) depositing the solution obtained in a) on the fibres;
c) heating the fibres obtained in b) in order to evaporate the
solvent and solely trigger the chemical reaction between the first
group and the reaction sites of the fibres;
d) irrQregnating the fibres obtained in c) with a resin hardenable by
SP 5552.59 LC
~s~.~~°~s
_ 7 _
radiation according to a radical mechanism and
e) subjecting the impregnated resin fibres to said radiation in order
to copolymerize the lubricating product with the resin via the second
group and ha~i~ the said resin.
The functions F2 canpatible with matrixes hardenable by radiation are
generally terrgerature-sensitive. Thus, stage c) of the px~xess must
be carried out at a ter~erature not permitting the homopolymerizatiora
of the lubricating pnxluct via the second functional group. It is
also preferable to use as the organic solvent, solvents having a low
v~o~ Pressure or tension Pv and a low boiling point Te. In partic-
ular, the organic solvent must have a boiling point below 100°C and a
vapour t~sion above 95 rob (9.5 kPa) at 20°C.
For example, the solvent is constituted by dichloranethane Pv=45.3 kPa,
Te=40°C), chlox~ofonn (Pv=21 kPa, Te=60°C), tetrahydrofuran
(Pv=20 kPa,
Te=66°C), acetone (Pv=23.3 kPa, Te=56.5°C), Dichloroethane
(Pv=24 kPa,
T~83°C), methyl ethyl ketone (Pv=10.5 kPa, Te=79.6°C) or
ethyl acetate
(Pv=9.5 kPa, Te=77°C).
Moreover, the temperature designated T1 during stage c) is depend~t
rn the reaction, designated R1, between the OH reaction sites of the
fibres and the functional group F1 (and therefore the function F1),
as well as the reaction between the functional groups F2 of the
lubricating product.
For example, for a function F1 of the isocyanate type and a function
F2 of the (meth)acrylate type (CH2=CY-COZ- with Y=H or CH3), a reaction
temperature T1 of approximately 60°C is used, bearing in mind that the
(meth)acrylate function is very temperature-sensitive. Under these
terr~erature conditions, the duration of stage c) will obviously be
sufficiently long to sure a sufficiently high reaction level R1.
By only replacing the funct:lon F2 (meth)acrylate by a cinnamate
3p function (Ph-CH=CH-002-) maleate function or fumarate function
( HO O-Cti=C'~~1~-0 ) , or by a styrene function ( CH2=CH-Ph- ) , with pH
SP 5552.69 LC
_8_
representing the phenyl radical, it is possible to increase the tgrQer-
ature T1 to approximately 110°C. Under these conditions, at the end
of stage c), the reaction level R1 is higher in the case of the
(meth)acrylate function on leaving the resin impregnation chain.
The other types of functions F1 which can be used, whilst retaining
the function F2 (meth)acrylate are carboxylic acid anhydride, acid
chloride and N-methylol.
In the same way, it is possible to carbine these new functions F1 by
replacing the function F2 (meth)acrylate by a cinnanate, maleate,
fumarate or styrene function. In this case, the reaction terr~erature
T1 can be chosen between 60 and 110°C.
As the lubricating product having as the function F1 an acid anhydride
and as the function F2 an ethylene unsaturation, reference can be made
to maleic anhydride of formula 0=C~1=CH-C=0.
As the lubricating product having as the function F1 an acid chloride
and as the function F2 an ethylene unsaturation, reference can be
made to the cynnanoyl chloride of forn~ula:
0
Ph-CHmCti-C \
C1
As the lubricating product having as the function F1 a N-methylol and
as the function F2 an ethylene unsaturation, reference can be made to
the N~nethylol acrylamide of fornnala OHCH -NH-C-Ct1=CH .
2 II 2
0
In order to favour and/or accelerate the chemical reaction Rl betwe~
the reaction sites of the fibres and the first functional group to an
even greater extent, it is possible to add a catalyst or a catalyst
mixture to the solution centaining the lubricating product. It is
also possible to add to the lubricating product solution an inhibitor
of the hanopolymerization reaction between the functions F2 of the
SP 5552.69 LC
201 a4'7fi
- 9 -
lubricating product.
When the first functional group is the isocyanate group, the catalyst
is constituted by DBTDL (dibutyl tin dilaurate) optionally associated
with DAHO~ (1,4-diazobicyclo(2,2,2)-octane).
The lubricating product according to the invention e.g. has the
following forn~ula ( II )
R5 ~ R3
C=C
R2 R4 - Ax- (~)y - Bz - F1
li
0
with x, y and z representing 0 or 1, F1 representing -N=C=0, -C1,
-CH OH, -C-C1, R5, R2, R3 and R4 having the sane meanings as herein-
2 t.
0
before, A representing a straight or branched alkyl radical with 1 to
12 carbon atone and B representing a straight or branched alkyl
radical with 1 to 6 carbon atoms or an aryl radical of type:
v
O ~ 0 - ~3 ~ O -~ 4'13r O --1712-
Other features and advantages of the invention can be gathered fnxn
the follcxNing description given in an illustrative and non-limitative
manner with reference to the attached figs. 2 to 4, fig. 1 having
already been described.
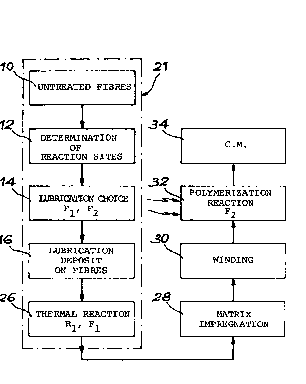
Figs. 2 to 4 are block diagrams illustrating the process for producing
a canposite material iron carbon fibres lubricated with the lubricating
product according to the invention. Fig. 3 diagrammatically represents
the lubricating of a carbon fibre with the lubricating product accor-
ding to the invention.
SP 5552.69 LC
20154'6
-lo-
With reference to figs. 2 and 3, a description is given hereinafter
of the pxnduction of a carposite material part having fibres lubri-
cated in accordance with the invention and anbedded in a radiation-
hardened matrix. Lubrication is carried out on untreated,carbon
fibres, which have undergone no surface treatment. The use of
untreated fibres is symbolized by block 10 in fig. 2.
Firstly the surface state of the untreated fibres is investigated by
the knaan electron spectroscopy ESCA. This stage is represented by
block 12 in fig. 2. It makes it possible to deternzine the reaction
sites of the fibres. With a view to an optimum fastening'of the
lubricating product, the statistically most numerous reaction sites
are chosen. In the case of carbon fibres of the intermediate modulus
type, it is found that the preponderant reaction sites are hydroxyl
groups.
This is followed by the determination of the ctnice of lubricating
product, as symbolized by block 14 in fig. 2. The chosen lubricating
psbduct is obviously a function of the reaction sites determined by
the ESCA method, but also the resin type used for forming the matrix
of the canposite material. The lubricating products are as defined
hereinbefore.
The chosen lubricating product is then dissolved in an organic solvent
with a low vapour tension in proportions permitting a lubricating
rate of 0.3 to 2%. The solvent is necessary for aiding the distri-
bution and impregnation of the fibres by the lubricating product,
taking account of the sought low final lubricating level percentage.
In order to optimize the reaction R1 between the reaction sites of
the fibres and the function F1 of the lubricating product, it is
possible to add to the lubricating solution one or more catalysts of
the reaction R1, as well as an inhibitor of the har~opolymerization
action between the functions F2 of the lubricating product.
The fol7.owing stage of the product represented by block 16 in fig. 2
SP 5552.69 LC
2~l.,.r"~~"'J6
- 11 -
consists of depositing the lubricating solution obtained on the
untreated fibres. As represented in fig. 3, this deposition takes
place by passing at constant speed each fibre 18 to be lubricata3 into
a tank 20 ccxitaining the lubricating solution. After passing into a
spinning nozzle 22, the lubricated fibre 18 enters an oven 24 raised
to the maxirn~n temperature T1 allowed for the function F2.
The passage of each fabre into oven 24 makes it possible to evaporate
the solvent fran the lubricating solution, as well as to trigger the
chemical reaction R1 between the function F1 of the lubricating pro-
duct and the reaction sites of the fibre. This stage is represented
by block 26 in fig. 2.
The tE<rperature T1 is chosen so as to aid the reaction R1 without
bringing about any reaction by t~arx~polymerization of the function F2
of the lubricating pxnduct to be fasteners to the resin of the matrix.
The spinning nozzle 22 makes it possible to improve the lubricating
product percentage on the fibres 18, as well as the core irnpregnation
of the fibres by the lubricating product.
The storing time of each impregnated fibre is dependent on the maxim~,nn
temperature T1 authorized by the function F2, the travel speed of the
fibre and the length of oven 24. For example, the txavel speed is
24 m/min and the passage length in an oven 6 rn.
The fibre lubricating process 21 is then finished. The thus lubri-
cated fibres 18 can be stored for several days until a given ca~site
material part is manufactured.
The lubricated fibres are then impregnated in an impregnation bath by
a liquid resin of the urethane-acrylic, epoxy-acrylic or polyester-
acrylic type, or a fozmulation obtained by mixing these different
resins. This impregnation stage is perforn~ed in known manner and is
symbolized by block 28 in fig. 2.
SP 5552.69 LC
20.54'76
- 12 -
The resin-impregnated, lubricated fibres are the deposited on a
support of a mandrel type representing the shape of the car~osite
material part to be produced, in accordance with previously calculated
winding trajectories. Winding is carried out on several layers, so
as to obtain optimum mechanical performance characteristics. This
winding stage constitutes the production stage of the fibrous substrate
of canposite material and is symbolized by block 30 in fig. 2. In
place of winding the fibres, it is also possible to carry out weaving
in two or three directions.
The final stage of the process symbolized by block 32 in fig. 2
consists of polymerizing and/or cirosslinking floe resin of the matrix
and reacting the function F2 with the resin of the matrix by subjecting
the substrate to ionizing X or beta radiation.
The irradiation conditions for a ccr~osite material part are dependent
on its shape, as well as the nature of the resin constituting the
matrix. These crnditions are in particular as given in FR-A-2 564 029.
In the case of a cylindrical part, is exposed to the action under an
electron or X-ray accelerator, which is moving and rotating in such a
way that all the portions liable to be modified by the radiation
2p receive the minimum dose necessary for the same. These atn.~ctural
modifications are obviously the copolymerization of the lubricating
ps~oduct with the resin of the matrix, as well as the hard~ing of said
resin. Moreover, the ccmposite material parts, pax-ticularly of the
aircraft engine type, have adhesive portions for ensuring the various
~s ~ ~~tions which must be hardened by radiation. The
adhesive is generally based rn an acrylic-epoxy resin.
For a canposite material part produced with carbon fibres lubricated
with a lubricating product, whose function F2 is an ethyl~e unsatur-
ation and impregnated with epoxy resin with an acrylic termination,
use is made of an irradiation dose of SO kGy for the polymerization
of the resin of the matrix and the copolymerization of the function
SP 5552,69 >~
2ozs4~s
- 13 -
F2 with the resin of the matrix.
Irradiation is ensured by X-rays or electrons, as a function of the
thickness of the part in question.
In the case of a very reactive matrix resin requiring low radiation
doses, e.g. approximately ZO kGy, it is necessary to choose as the
minimum dose that necessary for ensuring the reaction F2 if said dose
exceeds that necessary for the han3ening of the matrix.
The composite material (C.M.) part symbolized by the block 34 in fig. 2
is then finfished.
Fbr the production of a ca~osite material part, it is possible to
reverse the stages of lubrication, producing the substrate and impreg-
nating the fibrous substrate by the resin and this is represented in
fig. 4. In particular, it is possible to carry out a weaving 30a of
the untreated ffibres 10 (left-hand part of ffig. 4) prior to the
~~~t~ ~~f. The lubrication represented by block 21a is then
carried out as previously.
The lubricated fibres are then impregnated by the liquid resin of the
matrix, as symbolized by block 28a. The production of the ca~osite
material 34a continues by irradiating the lubricated, impregnated
substrate.
It is also possible to carry out the lubrication of the fibres,
represented by block 21b in the right-hand part of fig. 4, just prior
to the production of the fibrous substrate by weaving or winding, as
represented by block 30b. The lubricated substrate can then be
~~ated by the liquid resin of the matrix, as indicated by block
28b, followed by irradiation, in order to obtain the ffinal camposite
material product 34b.
Various examples of lubricating products according to the invention
will now be given.
SP 5552.69 LC
- 14 - 20154'76
f~ca~le 1
This example relates to a product for lubricating carbon fibres having
hydroxyl sites for embedding in an epoxy resin, polyester or poly-
urethane having (meth)acxylic teiminations. The first functional
group is an isocyanate group and the second function gnxip a
methacrylate.
The fornulas of the compounds used for the production of said lubri-
cating pxnduct, as well as the reaction diagram are given in appendix I.
Operating Conditions
A wise addition takes place of 144 g (1 mole) of 2-hydroxypropyl
methaczylate (III) to a solution containing 174 g of a mixture of 2,4
and 2,6-toluylene diisocyanate (80/20) (IV) and 0.3 g of dibutyl tin
dilaurate (DBTDL) (catalyst of the alcohol-isocyanate reaction) in
300 g of anhydrous toluene, at a temperature of 60°C and under dzy
nitrogen bubbling.
In order to prevent the polymerization of the double bond, 100 ppm
of hydroquinone are added and stirring is continued and the temper-
ature maintained at 60°C far 5 hours. This is followed by the evapor-
atian of the solvent with the Rotavapor under 2.7 kPa (20 mmHg) at
ambient ter~gerature (20°C), followed by a vane pump at 67.5 Pa
(0.5 mrHg) for a few minutes in order to eliminate all traces of
solvent. This leads to compound (V) ta~der majority conditions (90%),
with 10% of dimethacrylate obtained by reacting 2 moles of (III) with
(N).
Access is obtained to the percentage of the isocyanate functions
according to the traditional method of dosing NOO functions by dibutyl
amine. This gives 12.8% in place of the theoretically calculated
13.4%.
SP 5552.69 LC
- is - ~~~.5~'7~
Carrying wt lubricatirn
In order to obtain a lubricating level close to 0.5% on the carbon
f fibre, a lubricating solution is prepared with 0.75 g of the mixture
previ~sly obtained and containing unsaturated isocyanate(V) in 100 g
of dichloromethane, to which is added a mixture of catalysts in the
following proportions: 0.15 g (DBTDL) of dibutyl tin dilaurate + 0.22 g
(DABCO) 1,4 ~iiazobicyclo-(2,2,2)-octane for 1 mole of isocyanate ( N).
Example 2
This example differs fran example 1 by the choice of the starting
P~ucts respectively having the isocyanate function and the meth-
acrylate function. The forniulas of the different ends used for
producing this product are given in app~dix II.
Operating Conditions
Dxvpwise addition takes place of 144 g (1 mole) of 2-hydrroxypropyl
15, methacrylate (VI) to a solution containing 168.2 g (1 mole) of hexa-
methylene diisocyanate (VII) and 0.3 g of DBTDL, as well as 100 ppm
of hyd~~quincne (polymerization inhibitor) in 300 g of ethyl acetate
at a temperature of 60°C. Stirring and the tarQerature are maintained
at 40°C for 5 hours. The solvent is evaporated with Rotavapor under
2.7 kPa (20 m~-ig) at anbient temperature (20°C). This gives compourxi
(VIII) mixed with carr~pound (IX). The dosing of the isocyanate
functions gives 12.97%, the theoretical percentage being 13.46%.
The lubricating product is obtained in the sane way as in example 1.
bcanple 3
~ the ~~le the first gmup is once again an isocyanate group and
the second group a maleate group. The products used for producing
this lubricating product, as well as the reaction diagram are given
in appendix III.
SP 5552.69 1~
~:Z.Jr~~~
- 16 -
In the first stage 60 g (1 mole) of isoprvpa»ol (XI) are reacted with
98 g (1 rmle) of malefic anhydride (X) at 100°C for 2 hours and
card (XII) is obtained quantitatively.
In the second stage 100 g (1 mole) of foxy hexane (XIII) are reacted
with 0.1% by weight (based rn all the reagents) of chrcmium diisopmpyl
salicylate (Cx~IPS) (unsaturated epoxide - acid reaction catalyst),
added to 158 g (1 mole) of (XII). The reaction takes place at 100°C
for 3 hours, which gives cad (XIV) with an 86% yield.
The third stage involves the drapwise addition of 258 g (1 mole) of
(XIV) to a solution containing 174 g (1 mole) of 2,4-toluylene
diisocyanate (TDI) (XV) in 2 litres of anhydrous hexane under a dxy
nitrogen stream at ambient ta~erature and accar~panied by stirring.
Bubbling is stopped after 7 hours, but stirring is allaaed to continue
for a further 17 hags, haW ng ensured that no moisture can enter the
reaction mixture.
The isocyanate-maleate ca~nd (XVI) obtained fornis a relatively
viscous green oil, which is insoluble in hexane. It is thus extracted
by the separation of two phases and is washed by hexane in order to
eliminate the 2,4-TDI (XV), which has not reacted. The residual
~~e ~ filtered on a frit. Carpomd (XVI) is recovered by solubil-
ization in dichlor<imethane. Finally, c~rrpomd (XVI) is obtained with
a yiel3 of 93.5%. The perc~tage of NCO functions is 9.55% in place
of the theoretically calculated 9.72%.
Lubrication is the same as in example 1.
bcanple 4
Preparation takes place of a lubricating solution containing 0.75 g
of ~nnsaturated isocyanate (V) obtained in example 1 in 100 g of
dichloranethane. Using this solution 1-IERCiULES IM6 carbon fibres,
whose majority reaction sites are hydroxyl gxnups, were lubricated.
Lubrication took place 3n accordance with the diagram of fig. 3. The
SP 5552.69 LC
~~~.J~-~~~
- 17 -
evaporation of the solvent and the reaction R1 between the isocyanate
function and the d-1 sites were carried out at 50°C. This heat treat-
ment lasted 15 seconds (passage speed 24 m/min and passage length 6 m).
The lubricated carbon fibres were the impregnated in a liquid resin
mixture containing acxylate-epoxy resin and an acrylate-polyurethane
resin.
This was follaaed by the winding of a N.O.L. ring (characterization
modulus) making it possible to define the interlaninar shear between
the fibres and the matrix. This N.O.L. ring was then irradiated with
electrons at a dose of 50 kGy. During irradiation, the characteriz-
ation ma3ulus underwent a irotation and a passage under the electron
accelerator. The travel speed was 0.12 m/min for an electron
accelerator of 6.2 MeV and T.5 kW.
The interlaminar shear characterization was performed in flexion
according to three points. This gave an interlaminar shear stress of
approximately 40 MPa.
Example 5
This example only differs from example 4 through the uae of a lubri-
cating product (VIII) obtained in example 2. The car~ogition of the
lubricating solution is the same as in exarrg>le 4.
The interlaninar shear stress measured for untreated X16 fibres
lubricated by this lubricating product and anbedded in a matrix i~den-
tical to that of example 4 is between 45 and 50 MPa.
Ccrr~arisa~ Example 1
A HERCULES IM6 carbon fibre with a lubricant G (which corresponds to
a fibre in its carmercial form) was impregnated by a resin mixture
constituted by an acrylate-epoxy resin and an acrylate-polyurethane
resin identical to that of example 4. After producing a N.O.L. ring
SP 5552.69 IBC
~~D~a~'~G
- 18 -
and polymerizing the resin under the sane conditions as in example 4,
the interlaninar shear stress of the N.O.L. ring was measured. The
measured stress was between 20 and 30 MPa and is therefore belay that
obtained in examples 4 and 5 according to the invention.
Comparative example 2
An unlubricated HERCULES DH6 carbon fibre was impregnated with a resin
mixture of aciylate-epoxy resin and aczylate-polyurethane as in
example 4. After producing a N,O.L. ring and polymerizing said ring
in accordance with example 4, the interlaminar shear stress of the
ring was measured and found to be close to 30 I~a.
It is clear fran examples 4 and 5 and comparative examples 1 and 2,
that the use of a lubricating product according to the invention
irnprnves the transverse stress characteristics of car~site materials.
Other lubricating products usable in the invention with CH group-rich
1,5 carbon fibres and a resin with acrylic texrninations, reference can be
made to those given in appendix IV.
SP 5552.69 LC
2Q~5~"~6
- 19 -
APPRNT)TX T
Isocyanate-m~thacrylate
Ruction
CH3
CH3
NCO CH2=~-i0CH2CIH-OH
0 + 0 CH3
NCO
(IV) (II1)
CH3
NCO
0
iH3
NH~O~HCH20~C=CH2 (V)
H3
SP 5552.69 LC
- 20 -
APPENDIX II
Isocyanate-m~thacrytate
Reaction
CH3
OCN-CHZ-(CH2)pCH2NC0 + CH2=C-~OCH2;H-OH
0 CH3
(V1I) (VI)
CH3
CH2=C-COCH2CH-OGNH-CH2-(CHZ)4CH2NC0
0 CH3 100
(VIII)
CH3
CHCH3-~OCH2~~O~NH(CH2)6NHb0~H~H20~C=CHZ
8C
( I if )
SP SS52.b9 LC
~0154'~~
- 21 -
APPENDIX III
Isocyanate-maleate
1st Staae
0 0
HC C~ ~ CHI
HC ~ /0 + CH3 HC C-OCH\
OPC C H
~CH-OH
H
0 CH3 ~-OH
0
(X) (XI) (XII)
2nd Stage
0
~CH3
(XII) ; CH2-CH-(CH2)3CH3 1-~ HC ~OCH \ CH3
CrDIPS HC~C=0
(XIII) d (XIV)
CH2
1
~H-(CH2)3-CH3
OH
3rd Stage s CH3
NCO CH3
(XIV) t 0 hexane NCO
NCO 0
(XV) H
C=0
I
0
~H-(CH2)3CH3
tH2
He C=o (xVI)
HC ~ =p
QH~CH3
~CH3
SP 5552.69 i_C
2054'76
- 22 -
APPENDTX TV
(XVI) CHZ= I-~0-CHZ-CH2-NCO
~H3
(XVI1) ~-CH=CH-GNH-CH2-CH2-Ut;NH- p -CH3,
UN
NCU
(XVIII) CH3
~CHO ~C/0
CH3 ~ H
jC - C/
H ~OCH2-CH-O~iNH~ CH3
I0 C4H9 0 ~/~NCO
(XIX) CHZ=CH-~CHZ-OIiNH-(CH2)b-NCU
0
SP 5552.69 LC