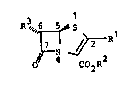Note: Descriptions are shown in the official language in which they were submitted.
5~
HO~CHST ~RTIENGES~LLSCHAFT HOE 89/F 135K Dr. v.F./PP
D~cription
A proce~s for the pr~paration of penem co~pounds
The invention relates to ~ process for the preparation of
penem compound~.
Penem derivativ~s of th~ formula I ~re valuable compound~
with antibiotic propertie~. A synthetic process di~closed
in the literature for penem derlvatives I entails intra-
molecular cyclization of a~etidinone derivatives o~ the
foxmula II with trialkyl phosphites. However, this
process often provides only poor yields. Moreover, the
reaction i~ ~low and requires elevated reactlon ~emper-
atures, for example reflux in toluene. Under these
reaction conditions there ~8 frequently partial inver~ion
of configuration a~ C-5, which re~ults in undesired
(5S,6S~-penems.
It has now been found that the de cribed di~adv~nt~ges of
the known proc~ss can be avo~ded if dialkyl alkylpho~pho-
nites are uæed in place o~ trialkyl phosphites for the
cyclization reaction. These novel roagent~ permit the
reaction ~emperature~ to be lower, for ~xample room
temperature, ~nd re~ult ~n ~horter reaction t~mes. ~i~her
yield~ and purer products nre achieved ~hereby. In
addition, the unde~ired ~so~erization to (5S,65)~pen~m6
25 i8 avoided. ~he dialkyl al~ylphosphonates and dialkyl
alkylthiophosphonate6 formed as by-products c~n bs
remo~ed in a ~trai~htforw~rd ~anner.
Hence the inventisn relates to a process for the prepar-
ation of penem derivatives o~ the formula I, in which the
preferred configuration i~ ~5~,6S)
- 2
R3~"6
C02R2
and in which
Rl denote~ hydrogen, (Cl-C~)-alkyl, (C~-C~)-alkoxy,
(Cl-C~)-alkylthio, phenoxy, phenyl (the phenyl ring~
bei~g unsub6tituted or sub~tituted oncs or twice by
carboxyl, (Cl-C~)-alkoxycarbonyl, allyloxyc~rbo~yl,
aminocarbonyl, (C1-C4)-alkylaminocarbonyl, cyano, F,
Cl or Br), (C3-C6) cycloalkyl, (C3-C6)-cycloalkyloxy,
(C5-C~)-oxacycloalkyl (saturated or ~ingly or doubly
unsaturated~, (c3-c6)-oxocycloalkyl~ (C3-C6)-[l,l-bis-
~cl-c3)-alkyloxy]-cyclo~lkyl~ (C3_CB)_1(C1-C3) alkYl-
imino]-cycloalkyl, (C3-C6)-[arylimino~cycloalkyl,
(c3-c6)-hydro~yiminocycloalkyl~ (C3-C6)-(C~-C3-alkYl-
oxyimino)-cycloalkyl, in which the cyclo~lkyl
radical i8 unsubstituted or ~ubstituted onGe or
twice by Cl-C3-alkyl, preferably methyl, by (Cl-C3)-
alkoxy, preferably methoxy, by halogen, preferably
chlorine, or by methylene and i~ ~aturated or c~n
contain one or two double bond~,
RZ denotes hydxog~n or 8 customary carbo~yl protective
group which can be elimin~ted by hydroly~i~, photol-
y8i8, oxidation, reduction or enzymatically,
R3 denote~ hydrogen, ~Cl-C4)-alkyl J (Cl-C4)-~lkenYl,
(Cl-C4)-alkoxy,(C~-r7~-cycloalkyl,phenyl,2-oxo-1,3~
dioxolyl, triazolyl, th~zolyl, ~mino, ~cyl~mino or
alkoxy.
Suitable and parti~ularly preferred ~ub~t~tuents ~re tne
~ollowings
Rl hydrogen, (Cl-C4)-alkyl (for e~ample ~ethyl, ethyl,
hydro~ymethyl and aminsmethyl)~ (Cl-C4)-alkoxy (for
example methoxy ~nd ethoxy)~ (Cl-C3~-~lkylthio ~ior
example methylthio, ethylthio ~nd propyl~hio~,
phenoxy (for example 4-carboxamldophenoxy or 4-
cy~nophenoxy), phenyl (~or sxample 4
-- 3 --
carboxamidophenyl or 4-cyanophenyl), ~a~urated or
un~aturated (C5-c~) -oxacycloalkyl (for example
tetrahydrofuryl or furyl), (C4-C6)-oxocycloalkyl (for
example l-oxo-3-cyclobutyl), 3-
hydroxyiminocyclobutyl,3~methosyiminocyclobu~yland
3,3-dimethoxycyclobutyl.
R3 l-hydroxyethyl ~in which the OH group iB free or
pro~ected by trimethylsilyl, diphenyl-tert.-butyl-
8ilyl, allyloxycarbonyl, trichloroethylogycarbonyl
or 4-nitrobenzyloxyc~sbonyl)~ (Cl~C3)-alkoxy (for
ex~mple methoxy or ethoxy), (Cl-C3)-alksnyl, ~or
example triazolylethylene or ~hiazolyle~hylene.
The (Cl-C4)-alkyl and (Cl-C4)-alkoxy group~ in the 8ub-
6titutent Rl are either unsubatituted or sub~tituted once
or twice by hydro~yl, (C1-C4)-alkoxy, (C~-C~)-acyloxy,
amino, (Cl-C4)-al~ylamino, (Cl~C~)-acylamino, ~er~apto,
(C~-C4)-alkylthio or heterocyclylthio, for example thiaz-
olyl-, thiadiazolyl-, pyridylth~o.
Phenyl nuclei are likewise un~ubstituted or substituted
once or twice by carboxyl, (Cl~C~ lkoxycarbonyl, ~llyl-
oxycarbonyl, ~minoc~rbonyl, (C1 C~)-al~ylaminocarhonyl~
cyano or haloqenl preferably F9 Cl, Br.
The (C,-C4)-alkyl and (C1~C~)-alkoxy group~ in R3 are
~ither unsubstituted or substituted by hydroxyl, (Cl-C~)-
alkoxy, (C1 ~4~-~cyloxy~ amino, ~ C,-C4 )-alkyl~mino,
(Cl-C4)acylamino, merc~pto, (C1-C~ ylthio or hetero-
cyclylthio, it being pos~ible for ~ OH group to b~ ~ree
or protected by trimethylsilyl, diphenyl-tert.-butyl_
eilyl, allyloxycarbonyl, trichloro0tho~ycarbonyl or 4-
nitrobenzyloxycarbonyl.
In the process ~ccording to the invention, the c~mpounds
of the formula I are prepared by reacting a compound of
the formula II
R3 S ~
`r~
~a~o
~ ~2R2
in which
X denote~ oxygen or sulfur, ~nd
Rl, R2 and ~3 have the above ~neaning, wi~h ~ ~rivalent
S organic pho phorus compound of the ~ormula III
oR4
R6 _ p ~'
~ oR5
in which
R6 denotes (C1-C~ alkyl, for example methyl, e~hyl or
trifluoromethyl, phenyl ~hich can be ~ubstitu~ed by:
(cl-c3)-alkyl or (C~-C3)-alkoxy, and
R4 and R5 are identical or dif ferent and denote ( C1-C4 ) -
alkyl/ allyl, b~nzyl, or phe~yl which can 1~ ~ub~ti-
tuted by ( Cl-C3 ) -allcyl or ~ Cl-C3 ) -alkoxy .
The reaction between a compound II and a ~o~pound III can
be carried out in a ~uitable ors~anic 801VeIlt~ for example
in tetrahydrofuran, ethyl ~cetate, an ~rQ~n~tic hydro~
carbon such as benzene, toluene or xyl~3ne, or ~ halo~eJl-
ated hydroc~rbon ~uch a~ dichlor~methane, trichloro-
methan or 1,1, 2 -trichloroeth~ne .
The reaction teinperature can v~ry between ~10C and 160C,
preferably between +20C and ~70~C.
~he concentration of the compound II to be cycli~ed i6
between 1 mmol/l and 100 ~mol/l, preferably between
2 mmol/l and 20 ~mol/l .
% ~
~he nmount of the compound III can be bet~een 2 and
8 mole-eguivalents, preferably between 2 and 6 mole-
equivalents, relative to II.
The compounds of the foxmulae II and III ~re known or can
be prepared by processe~ di~closed in the literature.
The examples which follow ~erve to Illustrate ~he inven-
tion further.
~sample 1
4-Nitrobenzyl (5R,6S)-6-~(lR~-tert.-butyldimethyl~ilyl-
oxyethyl]-2-(4-aminocarbonylphenoxy)pen~m-3-carboxylate
130 mg (0.2 mmol) of (3S,4R)~4-[(sminocarbonyl)-pheno~y-
thiocarbonylthio]-3-[(lR)-tert.-butyldimethyls~lyloxy-
ethyl)3-1-(4-nitrobenzyloxycarbonyl)-azetidin-2-one were
dissolved in 90 ml of CHCl3 under an argon atmo~phere at
55C. To this was added within 30 minutes a ~olution of
90 mg (0.8 mmol3 of dimethyl methylpho~phonite CH3P(OCH3) 2
in 10 ml of CHC13, and, after addition wa~ complete, the
mixture was then ~tirred for 2 hours and, after cooling,
worked up. Extraction with 1 N NhHCO3 ~olution, 1 ~ XHSO~
~olution and water wa~ carried out, and the or~anic pha~e
was dried with magnesi~m Rulfa~e and ~oncentrated in
vacuo. The crude product proYided, a~ter ~lash c~romato-
graphy (SiO2 70-20~ ~m + 10 % H2O; ~ir~t toluene + ~thyl
acetate 20 ~ 1, then 1 ~ 1 for elution)~ the ti~le
~ompound in 83 % yield.
The compounds I listed in Table 1 w~re obtained analo-
gously from the stArting ~ub~tances II li~ted in ~able 2
under the reaction condition~ ted ln Table 3.
- 6~ is~30
^ ~ ~ ~ s
X~ I ~ I " oô ~ ~ S ~
~ O ~ E ~ o N ~
S ~ I ~ E ~ ~ C
_ 1~ ~ _5 ~ 0 a~ S ~ L
G ff~ I E I ~ ~ o ~ ^ E
oe .o co ~o x 0 ~ ~ ~ ~ 5 N o a O ~1) o _~
~'d .. ~ ~ C ~ ~ S '~
_ ~ t ^x - o~--S
~ ~o~ z ~ ~ ~ 11 0 u~ X O ~ u E ~ o æ 1-
u~ _ ~ ~ ~ ~ ~ _3
~ ~ 5 X t,~ S t ~ 1 ~ C
1~ ~ I` e 0 s~J ~ 1. s3 ~ ~~ a~ s o
~ o 0~ n ~ D--~
o~ 7 ~ ~ 7 C~ o ~ ~--~ ~ V) Z
. r~
o ~ Ei
c
~a ~
S ~ ~ S~
a
c.
.
~ K
s~
-- 7 --
~ ~ E ", ,' ~ ^ E S
.~ ~ ~ O ~ O ~ ~
^ ~ Il o _ o In ~ ~o ^
- ~ o x~ I~ a ~
^ E E ~^ ^ ^ L-- ^ ^-- o ^~ -- --~-.D ^
T _~ ~ --'~ ~ i O 11 QO
_S S ~ ^S ~ S ^Vl
E Ll~---- ~ > ~ ^ O ~ ~N ~ C u~ ~
G U~ ~ _ ~ ~t ~ --~ O ~ ~-- ~ ~ C~ ^
c ~ t 0 S,,~
~o . ,~v ~ 1 1I y N ~ N ^~ co E O ~~ ~ c S
. . _ _ _1 L `~ ~ 0 S
_ ~ O N ~ 6D ~ ^N
_ ~ ~ O ~ T 'r O
E ~ o ~ s ~ ~ l s ll ~ L
----E--` O t~ ~ N _
~ o
N ~ _ O ~ O ~ ~ ~ X ~n X L
~ -~ ^ - Z ~ L lv~ z _--11 CL_
u~ --~, ~ ô & ~ D ~ O "~ o
X ~ ~9 T ^ P~ X
T
~ .
.
11 S
t~ ~
oS~ ,~
.
c
0 _~
~ ~ I~
O C:
~V
-
~I ~r ~ ~ ~
X
w
- 8~ 5~
N ~ ~ ~ ~ E ~ ,o ,~ s
N ~ 0 ~ O O ~ 11 5 5 19 ~ ~ CO ^O
^ O ^ ~ ~ O
L ~ J ^ 11 ^ ~ ~
u~ X . ~ ~ 5 ~ ~ o E _l
_ ~ , S 4~ Y ~ _.~ S O
E ~ ~ , O N N ~ ~D N 8 ~ ~ . ~ I ~s , . o _ . ~
0 ~ C C~~ E
~----5 ~ o Lr~
o ~ __~, x s ~ E ~ ~,~ C ~ Il G
e~ J O ~_ x :~: ^0 ~ X
:1~ ~ eC E 11 î~ N N~__ N C --~ ~ <--~ N ~ ~ _ ^~
z~ ^ ^ ^ 3 . ~ 5 0 ~~
S~N-- ~ ~ O Y 11 ~D O 1~ a~ X ~ ~ O ~ X I ~ a V~
z _--~o E ~-- ~ ^ ^ E s ~ Z ~ E ~ vl
S OD K
G ~
C~ O
S
~ a~
O S S:
a~ 1 m s:
CL ~ Q. L~
._
I
~ s~
6,,
o~ o
- 9 ~
~ T ~ _ . ~
_ T ~J ~ 1 2 ~ ~ e
",, 2 ^ ^ ~ --
u~1 _ N ~^-- L ~
E ~' ~ ~ , X:S:-- ^ N
t~ ' ~
~ ~ N ~ NE ~ ~--~ E i~) E NN E ~`
_ ~ Q K ~ ~ S
~ ~ ~ o .-- ~
~ ~ O ~ ~ ~ O
_ ~ ~ , ~ E _, e _~ ~
~ _, ^~t S ~ t ~
.. -- ~ X ~ Q O t~ O O
_ ~ , 5 e V~ O, ~ o 5 _cr t~D t~
t_~ o ~ 0 ^ ~ E ~ ~ ^ U O -- ~ er O
1~ ~ E ~ N E t`J ~ ~ `~--' N fll S ~
O ~_ I I.D T ^ ~0 11 ~D S ^ ~ ^ O ^
_ ~ T ~ ~ _ ~ ~ ~D ~ ~ ' ~t -- ^ S
Y ~ ~ ~
~ ~_o ~T _ E ~ ~ E ~ o~ ,
2 ~ ~ O T-- o ~ 0 ~ ~ ~ ~ O ~ ~ L
~1 0 ~ 2 1 ^__0 ~ S ~o" ~ ', ~ ~ o N ~ X V
. . ~ S S I ~ ~., L
E E E _1 0 0 ~ n E ~
O
. _ .
t~
a: 4
C~: l~
O e~
L--
O = ~ ~ ~ .
e ~
o a 3
o ~ I Q ~` ~ '
~ ~ ~ ~ ~n
K
2~5~
10 --
S "~ 5
-- E-- , u~ ^ ~ S ~ E ~~
. ,.~ T ~ L
EL ~ o I ~, O _ C--~ ~ T II~ D ~ N O E _
C ~T~tS~ 1 E ~ ~7o ~3~ .s
_ ~ ~ E r ~ ~ t'~ ~ ~ '--.
~ m ~~ E _~ 0 ~'~ o-- ~9 ~ ~ W--~ ~ E N
æ ~ s ~ ~ . ~ ~ . . ~ ~ ~ ~ . s
~ ~ x ~ ~ ~. ~ 3 ~ ~ " " .N 7--
T ~ ~ _ E E ^ 11 ~ _ 0 o
--~ -- Ew mo~ ~ ~ S ~ __s~ ~cn ~,_~~ x-- O
r` ~ ~ ~ ~ ^~ ^ô 3 -o a E ~ ~ IE ~ 8 ~ N ~T ~
o ~ x 0 r~ a~~ N ~
~3 ~-~o~t ~v~o~u ~t~cO ~ ,~,3_~
~ O
O
o 1 ~
:~:
nl N
; ~
~ ~ ~- ~ _
~able 2
Starting compounds
R3 S - C - R
C 2
Ex~ple Rl _ R2 -- R3 - X
-O~H2 -PNB -CH(OT3DMS) S
2 -O~CONH2 -CH2CH~H2 " S
3 -O~CON112 -cH2CH?S~e3 " . S
4 OCH3 - CH2C14=CH2 S
OCH3 - PNB ,1 5
OCH3 -CH~CH251Me~ " S
7 a -PNB " S
8 O PNB 1l S
9 SCH3 CH2CH-~H2 S
- 12 ~ 5~
Ex~pl ~ Rl R2 R3 X
SCH3 -PN~ " S
11 SCH3 -cH2cH2s~Me3
12 ~ -CH~CH2SiMe3 " O
13 ,~ -cHzcH2s~Me3 " O
14 ~ -CH2CH2S~Me3 "
~ -CH2cH2siMe3 " s ..
16 ~C02CH,~CH-CH2 -cH2cH2s~Me3 " O
O -CH2CH2SiMe3 'l o
O - CH2CH-CH2 ~ o
19 O~H3 -CH2cH2siMe3 "
-
- 13 - ~$~55~
Table 3
Reaction condi~ions for the cycli~ation reaction
~ o~ ~~ ~n oo o o ~ o u ~ C~ ~
_ .
_
r~ ~O
o ~ u~ ~7 .n u~ u~ Y'>
5~ O~O~
E ~ u o n 0 1~ 0 0
~- ~ L~
C
~: >
~ o oo~ooo~c~ooooooooooo
_.
._
o
~1 N
~ N
_ ~ ~ ~-~
_.
S ~ S ~ S
~5' ~ x:r
C~ ~ ~ ~5~
- ~ ~
'