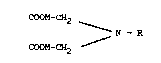Note: Descriptions are shown in the official language in which they were submitted.
~016i~2~
CM-300
NOVEL CEELATING AGEN5S AND DETERGENT AND
CLEANING CONPOSITIONS CONTAINING THEM
Eddy VOS
Axel KONIG
Ian CORN
Technical Field
ffl e present invention relates to novel chelating agents
derlved from iminodiacetic acid, and detergent and cleaning
compositions in particular hard-surface cleaning
compositions, containing them.
,
ackoround of the Invention
Several compounds of the iminodiacetic acid type have
been synthesised and investigated for the purpose of metal
sequestering propertles.
~; .
.
: ~ ,
~ ,
i .~ . - . -.. :
,
. ,.~, . ~
- - 2~64~
For example, methyl iminodiacetic acid, whose synthesis
is described in Chemical Abstracts 104(6)45062d, Acetamido
Imino Diacetic acid, described in Biochemistry 5, p 467
(1966), Glyceryl Imino Diacetic acid, described in Chem.
ZUESTI 34(1) p. 93-103 (1980) MAYER, RIECANSK~, et al
publication of 26 March 1979.
m e compounds 2-hydroxyethyl diacetic acid,
diethyleneglycol diacetic acid and l-hydroxypropyl imino
diacetic acid have been disclosed in Japanese Laid-Open
Application 59/70652;
Other iminodiacetic derivatives such as
2-hydroxypropylimino diacetic acid, and di-hydroxypropyl
imino diacetic acid have been disclosed in DE-OS 23 14 449,
and DE-OS 25 42 708;
Several of the above compounds have been described as
chelating agents in detergent compositions. ~-~
EPA 262 112 describes the use of diethyleneglycol diacetic -
acid as builder in detergent compositions, including
hard-surface cleaning compositions. ~ ~ ~
-, ~ .- .
Copending EP-A-317 542 discloses the combination of
specific imino acetic derivatives such as Glyceryl Imino
Diacetic Acid and Acetamido Imino Diacetic Acid with
certain solvents, in hard-surface cleaning compositions. ~ ~
,:
Novel iminodiacetic acid derivatives have now been
synthesized, and have been found particularly performing as
chelating agents in detergent/cleaning compositions
' ' '. , :
' . . ~ ' ~ , `
- 2~16~2~
- 3 -
. Summary of the Invention
The present invention relates to novel chelating agents
derived from iminodiacetic acid, having the formula
COOM-CH2 ,
N - R
COOM-CH2
wherein R is selected from the group of
- (CH2)20CH2CH3; -(CH2)30CH2CH3;
- CH(CH3)CH20H; -(CH2)30(CH2)2CH3;
and M is either hydrogen or a salt-forming cation.
The present invention also encompasses detergent
compositions and in particular har~-surface cleaning
compositions, containing the novel compounds herein.
Detailed Description of the I~vention
The chelat~ngLagents
m e novel chelating agents of the present invention are
of the formula :
COOM-CH2 ~
N - R
COOM-CH2 ~
wherein R is selected from the group of
-(CH2)20CH2CH3 ; -(CH2)3(CH2)2CH3
-CH(CH3)CH20H ; -(CH2)30(cH2)2cH3
and M is either hydrogen or a salt-forming cation,
preferably Na, K, NH4+ or substituted ammonium cations
~ r!._ .. .. .
,: ~" ' '' ~ ' .
-- ` 20~6~2~
containing from 1 to 4 short-chain alkyl or hydroxy alkyl
groups each of which containing from 1 to 3 carbon atoms.
m e following chelating agents are thus encompassed by
the present invention (M being hydrogen) :
Ethoxy Ethyl Imino Diacetic Acid (EOEInA), having the
following structure :
:
COOH CH2 `
N(CH2)20CH2CH3
COOH - CH2 ~ ~
miS compound is prepared by the following reaction : ~ ~:
2 clcH2cooNa + H2N(CH2)20CH2CH3 + NaCO3
--> 2 NaCl + CO2 + H20 + COONa
(CH2)2
COONa
.: ~
EthQxy Propyl Imino Diacetic Acid (EOPIDA), having the -~
follow mg structure ~
~ ::
COOH-CH2
~N(CH2) 3CH2CH3
COOH-CH2
mis compound is prepared by the following reaction :
2 clcH2cooNa + H2N(CH2)30CH2CR3 + NaC03
--> 2NaCl + CO2 + H20 + COONa-CH2
N(cH2)3ocH2cH3
COONa-CH2
- 201642~
Iso Hydrox,y Propyl Imino Diacetic Acid (IHYPIDA),
having the following structure :
COOH-CH2
~ N - CH - CH2 - OH
COOH-CH2 CH3
mis compound is prepared by the following reaction :
2ClCH2COONa + H2N CH(CH3)CH20H
--> 2NaCl + CO2 + H20 + COONa-CN2 ~
NCH(CH3)CH2H
COONa-CH2
Propox,y Propyl Imino Diacetic Acid (POPIDA), having the
following structure :
COOH-CH2
N - (c~2)3o(cH2)2clH3
COOH-CH2
mis compound is prepared by the following reaction :
2 clcH2COONa + H2N(CH2)30(CH2)2CH3 + NaCO3
--> 2 NaCl+C02+H20+COONa-CH2
N(CH2)30(CH2)2cH3
COONa~H2
.. .. .. . . , ~
,, .... ~ . ~ ~
: .
,
..
2016~
Detailed method of preDaration of the chelating aaents
(sod um salt)
About 0.86mole of - aminoethoxyethane (for EOEIDA)
- aminopropoxyethane (for EOPIDA)
- aminoisopropanol ~for rHypIDA)
- aminopropoxypropane (for POPIDA)
and about 1.7mole sodium chloroacetate are slurried in
500mls water in a 1 litre conical fitted with a reflux
condenser. 0.86mole sodium carbonate is added carefully
and the mixture heated to at least 50-C for over 2 hours
then at least 95-C for over 3 hours. On cooling, the
solution is acidified to pH 2 with conc. HCL and evaporated
under reduced pressure. The solid is hot ethanol extracted
and evaporated to dryness again. A slurry of the solid in
water is resaponified at pH 11 (conc. sodiu~ hydroxide) for
over 1 hour at least 60-C then evaporated to dryness.
::
_teraent/cleaning compositions
m e new iminodiacetic acid derivatives herein are
useful as chelating agents in detergent/cleaning
compositions which encompass laundry detergent compositions
as well as hard-surface cleaning compositions. The
application as chelating agents in hard-surface cleaning
compcsitions is especially preferred.
Detergent compositions according to the present
invention contain from 0.01% to 95% of an organic synthetic
surfactant and from 0.05% to 95% of a novel chelating agent
herein.
organic synthetic surfactants useful herein include
well-known synthetic anionic, nonionic, cationic,
.,: .
. .
-`:` 201642~
amphoteric and zwitterionic surfactants and mixtures
thereof. Typical of these are the alkyl sulfates, alkyl
benzene sulfates and sulfonates, paraffin sulfonates,
olefin sulfonates, alkoxylated (especially ethoxylated)
alcohols, alkyl phenols, and sulfates, amine oxides,
sulfonates of fatty acids and of fatty acid esters, and the
like, which are well-known in the detergency art. In
general, such detersive surfactants contain an alkyl group
in the C10-Cl8 range; the anionic detersive surfactants
can be used in the form of their sodium, potassium or
ammoniu~ salts. The nonionic generally contain from 3 to
17 ethylene oxide groups per mole of hydrophobic moiety.
Cationic surfactants will generally be represented by
quaternary ammonium compounds such as ditallow dimethyl
ammonium chloride, and will be preferably used in
combination with nonionic surfactants.
Especially preferred in the compositions of the present
invention are : C12-C16 alkyl benzene sulfonates,
C12-C18 paraffin-sulfonates and the ethoxylated
alcohols of the formula RO(CH2CH20)n, with R being a
Cl2-C15 alkyl chain and n being a nu~ber from 6 to lO,
and the ethoxylated alcohol sulfates of formula
RO-(CH2CH20)n-S03M, with R being a C12-C18
alkyl chain on a number from 2 to 8, and M is H or an
alkalimetal ion.
Laundry detergent compositions according to the present
invention typically contain from 1% to 40% preferably 5% to
30% of organic synthetic surfactant, and from 1% to 40%
preferably 5% to 30% of a novel chelating agent herein.
, ~
. .
. ~ . .
` 2~16~2~
Said laundry detergent compositions may be formulated
into granules, liquids, solid tablets or bar forms; said
compositions may contain the various adjuncts which are
known to the art for laundry detergent compositions.
Non-lLmiting examples of such adjuncts are :
Additional detergency builders such as polyphosphates
(e.g., potassium pyrophosphate), nitrilotriacetates (e.g.,
Na~NTA), sodium ethylenediaminetetraacetate, sodium
ethylenetriaminepentaacetate, sodium citrate, sodium
carbonate, sodium metasilicate and zeolites, e.g., zeolites
having a cation exchange capacity (measured as CaCO3) of
200 mg or greater per gram of zeolite;
Enzymes such as proteases and amylases;
Bleaches such as sodium perborate, diperoxydodecanedioic
acid, sodium dicholorisocyanurate and m-cholorperoxybenzoic
acid;
Soil suspending agents such as sodium carboxymethyl
cellulose; ~_
Bleach activators for use with sodium perborate, such as
tetraacetyl ethylene diamine and sodium nonanoyloxybenzene
sulfonate:
Bleach stabilizers such as sodium
diethylenetriamine-pentamethylenephosphonate and sodium
diethylenetriaminipentaacetate;
Hydrotropes such as scdium toluene sulfonate, sodium cumene
sulfonate and potassium xylene sulfonate;
Fabric softening ingredients such as smectite clay,
ditallowdimethylamine, ditallowacetamide,
ditallowbenzamide, ditallowdimethylammonium chloride.
Hard-sur~ace cleaning comDositions according to the
present invention typically contain from 0.01~ to 45% of
organic synthetic surfactant, and from 0.2% to 20%,
preferably 0.2% to 10% of a novel chelating agent herein.
2~642~
It is preferred that the hard-surface cleaning
compositions herein also contain from 1% to 20% of an
organic solvent having a boiling point above 90-C;
It has been found indeed that such solvents are
particularly suitable for use in combination with the
present chelating agents, the combination giving unexpected
soil-release benefits.
Such benefits are particularly noticeable in ter~s of
calcium soap-soil removal from surfaces such as bathtub
surfaces.
It has been found that, in order to obtain such an
effect in hard-surface cleaning compositions not of the
spray type, the weight ratio of said organic solvent to
chelating agent should be in the range from 2/3 to 2/1,
preferably 1/1 to 2/1.
In the non-spray type compositions, it is preferable
not to include Cl-C3 aliphatic alcohols like
isopropanol (~.P. 82-C) which do not constitute such an
effective combination with the present chelating agents.
In spray cleaners however, Cl_3 aliphatic alcohols
such as ethanol and isopropanol are preferably used, in
combination with a solvent having a boiling point above
90-C.
Representatives of organic solvents with boiling point
above 90-C which are effective in the present context are :
C6-Cg alkyl aromatic solvents, especially the C6-Cg
alkyl benzenes, alpha-olefins, like l-decene or l-dodecene,
benzyl alcohol, n-hexanol, phthalic acid esters.
.. .. . . .
~.
. .
... ., .. .. ~.,
2 ~ 2 ~
- 10 -
A type of these solvent especially suitable for the
compositions herein comprises diols having from 6 to 16,
preferably 8 to 12, carbon atoms in theix molecular
structure. Preferred diol solvents have a solubility in
water of from about 0.1 to about 20g/lOOg of water at
20-C. The most preferred diol solvents are
2,2,4-trimethyl-1,3-pentaediol, and 2-ethyl-1,3-hexanediol.
Glycol ethers are another class of particularly
preferred solvents.
In this category, are : water-soluble CARBITOL R
solvents or water-soluble OE nT~OLVE R solvents.
Water-soluble CARBITOL R solvents are compounds of the
2-(2-alkoxyethoxy)ethanol class wherein the alkoxy group is
derived frcm ethyl, propyl, butyl pentyl hexyl: a preferred
water-soluble carbitol is 2-(2-butoxyethoxy)ethanol also
known as butyl carbitol. Preferred are also hexyl carbitol
and 2-methyl pentyl carbitol. Water-soluble ~FTI~SOLVE R
solvents are compounds of the 2-alkoxyethoxy ethanol class,
wherein the alkoxy group is preferably butyl or hexyl.
Still in the glycol ether catergory, certain
propylene-glycol derivatives have been found to be
particularly efficient in the present context; these
species include l-n-butoxypropane-2-ol, and
1(2-n-butoxy-1-methylethoxy)propane-2-ol
(butoxypropoxypropanol), with the latter being especially
preferred.
Mixtures of the above solvents can also be used, like
Butyl carbitol and/or Benzyl alcohol together with diols
and/or glycol ethers.
, ; ~ . . . .. .
.~: . , . :
20~6~2~
The organic solvent having a boiling point above 90C
is preferably present at levels of from 1% to 10% of the
total composition.
In spray-cleaners, preferred are mixtures of
l-n-butoxypropane-2-ol with ethanol or isopropanol, in a
ratio of from 1 to 20 to 1 to 2.
Hard-surface cleaning compositions according to the
present invention may contain additional (optional)
ingredients.
Such ingredients can be represented by conventional
detergency builders, which may be used in addition to the
chelating agent herein; compounds classifiable and
well-known in the art as detergent builders include the
nitrilotriacetates (NTA), polycarboxylates, citrates,
water-soluble phosphates such as tri-polyphosphate and
sodium ortho- and pyro-phosphates, silicates, ethylene
diamine tetraacetate (EDTA), amino-polyphosphonates
(DEQUEST), phosphates and mixtures thereof.
Highly desirable ingredients for use herein are
represented by conventional detergent hydrotropes.
Examples of suitable hydrotropes are urea,
monoethanolamine, diethanol~lne, triethanolamine and the
sodium potassium, ammonium and alkanol ammonium salts of
xylene-, toluene-, ethylebenzene- and isopropyl-benzene
(cumene) sulfonates.
m e hard-surface cleaning compositions of the invention
may also contain an abrasive material.
..... . . .
. ' ' '.
2 0 1 6 ~
- 12 -
~ he abrasives suitable herein are selected from
water-insoluble, non-gritty materials well-known in the
literature for their relatively mild abra-~ive properties.
It is highly preferred that the abrasives used herein not ~ -
be undesirable "scratchy". Abrasive materials having a
Mohs hardness in the range of about 7, or below, are
typically used; abrasives having a Mohs hardness of 3, or
below, can be used to avoid scratches on aluminium or
stainless steel finishes. Suitable abrasives herein
include inorganic materials, especially such materials as
calcium carbonate and diatomaceous earth, as well as
materials such as Fuller's earth, magnesium carbonate,
China clay, actapulgite, calcium hydroxyapatite, calcium
orthophosphate, dolomite and the like. m e aforesaid
inorganic materials can be qualified as "strong abrasives".
Organic abrasives such as urea-formaldehyde, methyl
methacrylate melamine-formaldehyde resins, polyethylene
spheres and polyvinylchloride can be advantageously used in
order to avoid scratching on certain surfaces, especially
plastic surfaces.
Typically, abrasives have a particle size range of
10-1000 microns and are used at concentrations of 5% to 30%
in the compositions. m ickeners are frequently added to
suspend the abrasives.
Thickeners will preferably be included in the
compositions of the inventions, mainly in order to suspend
the abrasive; high levels of thickener are detrimental to
the performance because they are difficult to rinse from
the cleaned surfaces. Accordingly, the level will be kept
under 2%, preferably from 0.2% to 1.5%. Common thickeners
such as the polyacrylates, xanthan gums, carboxymethyl ;
celluloses, swellable smectite clays, and the like, can be
used herein.
:'. , :
. ~ . .
~ .
~ ~ 1 6 4 2 . b
Soaps can be included in the compositions herein, the
soaps prepared from c~conut oil fatty acids being
preferred.
Optional components are also represented by ingredients
typically used in commercial products to provide aesthetic
or additional product performance benefits. Typical
ingredients include perfumes, dyes, optical brighteners,
soil suspending agents, detersive enzymes, gel-control
agents, thickeners, freeze-thaw stabilizers, bactericides,
preservatives, and the like.
The hard-surface cleaning compositions herein will
advantageously be executed in the form of an aqueous liquid
compositions, including concentrates, containing as
essential ingredients a surface-active agent, and a
solvent/chelating agent binary mixture such as described
above. ~-
Liguid executions at nor?~al dilution usually contain1-6% surfactant and 8-20% solvent/chelating agent binary
mixture.
. .
Concentrated liquid executions usually contain 6-10%
surfactant and 16-24% solvent/cheldting agent binary
mixture.
The present invention also encompasses window-cleaners
or all-purpose cleaners designed to be applied via sprays,
wherein the surfactants are typically used at levels of
from 0.01 to 2% and chelants are used at levels of from
0.2% to 3%.
;~.. ..... .. ,; ,.. . .
?
'' " ' ' ' '
` ` 20164~
- 14 -
Alternatively, the compositions herein will be in the
form of a creamy scouring cleanser, containing an abrasive
material, surface-active agent, and the solvent/chelating
agent binary mixture of the invention.
In above executions, the pH of the compositions will be
neutral or in the alkaline range, generally in the range of
pH 5-11.
m e following exa~ples are given by way of illustrating
the compositions herein, but are not intended to be
limiting of the scope of the invention.
The following abreviations will be used :
EOEIDA Ethoxy Ethyl Imino Diacetic Acid
EOPIDA Ethoxy Propyl Imino Diacetic Acid ::
IHYPIDA Iso Hydroxy Propyl Diacetic Acid
POPIDA Propoxy Propyl Imino Diacetic Acid
HCnFA Narrow cut, hardened, coconut fatty acid
ETWD 2-Ethyl-1,3-hexanediol
BPP Butoxy Propoxy Propanol=1(2-n-butoxy-1-
methylethoxy)propane-2-ol
BP Butoxy Propanol = l-n-butoxy propane-2-ol
NaCS Sodium cumene sulfonate
Sokola ~ PffC25 Crosslinked polyacrylate thickener
:,
.. ~ .. , .... : :
. . . .
.
. . . ,.
- ~016~
- 15 -
EXAMPLE I
A spray-dried laundry detergent granule according to
the present invention is prepared according to the
following formula :
Inoredient Wt. %
Alpha-Sulfonated Coconut Fatty Acid 8
(methyl ester)
Cll-C13 n-Alkyl Benzene Sulfonate (Na) 6
C13-C15 Alcohol Ethoxylate (EO 5-8) 12
Hydrated Zeolite A (1-10 microns) 20
EOEIDA 5
Silicate Solids 2.5
Sodium Sulphate 20
Sodium Perborate . 4H2O* 13
Tetraacetyl Ethylene Di~;ne* 1.0
Diethylene Triamine Penta-Methylenephosphonate 0.15
Sodium Toluene Sulfonate 0.6
Protease Enzyme* 0-5
Na Carboxymethylcellulose 2
Brightener/Perfume*/Minors 3
Moisture/~iscellaneous Balance
* me co~pcsition of Example I is prepared by conventional
spray-drying procedures. Ingredients indicated by an
asterisk (*) are dry mixed into the spray-dried product to
avoid decomposition.
.. , . : .. ... :, . , :
. .
. ,: , , . `
. . :
: : .
~` 20~6~
- 16 -
EXAMPLE II
A liquid heavy du~y laundry detergent according to the
present invention is prepared according to the following
formula :
Inaredient . Wt. %
C12 alkyl Ethoxy sulfate (E03)(Na)11.6
C12_14 alcohol ethoxylate (EO)7 21.5
EOEIDA 10.0
Ethanol 10.0
Brightener/perfume/enzyme/minors 3.0
Water Balance to 100%
The following liquid hard-surface cleaning compositions
accordLng to the present invention are prepared
~'- '. . :' ' ' '
~ . . ~ . .
- 17 -
~1642`~
X I o o ~ o o m In m
H j OU~ O
X I ~ I I I I ~ ~ I I I ~ I ~ 0
'' 1
H
~H l O ~ O ~ O ~ O ~ ~ O
~ !
H
X ! ~ !
H!
~ ! ~ o o o ~ o o
~ I
X I , , o, o o o
.
.
~o~ol ~olool ~ ~ou~ j
~ I 11- 0 N ~ ~ ~ O O O O
_ _
Z ~3 ~
__
~ X~
I ~1 ~
O O O O
C
a ~ a a o
æ ~
~ M t.~ U C) a~ ~3 ~; & ~ H ~ ~ Z; Z
: ' , :
. . : , :
. . ~
::
. . . .
2~6~2~
- 18 -
The following creamy scouring compositions according to
the invention are also prepared :
Ex XI Ex XII
________~.______________________ ______________________ .
Cll_l3n Alkyl Benzene Sulfonate (Na) - 4.0
C13_16 paraffin sulfonate (Na) 4.0
C12_14 alcohol ethoxylate (EO7)
HCnFA 2.0 1.5
Benzyl alcohol 1.0
BPP 3.0 4.0
EOEID~ 3.0
IHYPIDA - - 3.0
Na2C3 3.0 3.0
caco3 30.0
Polyvinylchloride - 10.0
SokolanRPHC25 0.4 0.4 :;
water & mino~s -- up to 100 --
m e following spray-cleaners according to the present
invention are also prepared :
Ex XIII Ex XIV
___________________________________________________________
C13_16 Alkyl Sulfate (Na) 0.3 0.3
C13_16 Alkyl Sulfate,
Ethoxylated(EO3)(Na) 0.6 0.6
Ethanol 4.0 4.0
BP 4.0 4.0
EOEIDA 1.2
IHYPIDA - 1.2
Perfume, water, and minors -- up to 100 --
pH 10.3 10.4
.. . .