Note: Descriptions are shown in the official language in which they were submitted.
~LH ABR 5~80.1
PATENT
Z01664(~
PROCESS FOR RECOVERY OF GOLD FROM REFRACTO~Y ORES
8ackground of the Inven~i~n
This invention relates to the recovery of gold from
ores and, more particularly to an improved pressure oxidation
process for the recovery of gold from refractory ores.
In order to remove sulfide sulfur, refractory ores
are conventionally treated by pressure oxidation before
cyanide leaching. If the sulfide sulfur is not substantially
oxidized, leaching is inhibited and leached gold remains
locked in the sulfides. By treating the ore in an aqueous
slurry at elevated temperature and oxygen pressure, the sulfur
is oxidized and removed from the ore before it is contacted
with cyanide leaching agent. Thereafter the gold is leached
by the cyanide and acceptable yields are produced.
Pressure oxidation is an exothermic process but
requires the use of a substantial amount of energy in
pre-heating the ore slurry to a temperature at which the
reaction is self-sustaining. The oxidized slurry may contain
substantial amounts of iron, arsenic and other heavy metals
which it is desirable to remove before cyanidation. These
various metals are typically oxidized during the pressure
oxidation step, but further measures are required if the salts
and oxides of these undesired metals are to be removed from
the process.
Weir et al U.S. patent 4,571,263 describes a process
for pressure o~idation of refractory ores in which the effluent
from the pressure osidation autoclave is subjected to a two
step repulping operation with solids-liquid separations after
each step. Liquid from the second separation step is recycled
to the first repulping step. Liquid from the first separation
step is in part recycled to pressure oxidation and in part
subjected to a two step precipitation first with limestone and
then with lime. Effluent slurry from the second precipitation
4LH ABR 5080.1
PATENT
~0~6640
step is subjected to solids-liquid separation and the liquid
fraction is passed through a cooling pond and recycled to the
second repulping step and the pressure oxidation step.
Weir 4,571,264 describes a pressure oxidation gold
recovery process in which the effluent from the pressure
oxidation step is repulped, thickened and then subjected to a
two stage washing process. Water for washing derives from a
liquid fraction produced in thickening the ore slurry after
aci~ pretreatment prior to pressure oxidation. This liquid
fraction is subjected to a two stage precipitation with
limestone and lime, respectively. The effluent slurry from
the second precipitation is-thickened, and the liquid overflow
from the latter thickener is used as water in the second
washing stage. A solids-liquid separation after the second
washing stage produces a liquid fraction which is recycled and
ser~es as the wash water in the first washing stage.
In both Weir et al '263 and Weir et al ~264, the
neutralized oxidized slurry is subjected to cyanidation,
followed by an eight stage carbon-in-leach adsorption process.
~oth patents disclose pressure oxidation at 160 to
200C and 700-5000 kPa (total pressure).
Weir 4,606,763 describes pressure oxidation at 165C
and 50-2000 kPa total pressure, using a compartment autoclave
in which the fir~t compartment is approximately twice the size
of each of the other compartments. Weir U.S. patent 4,605,439
di~clo~es a pressure oxidation process operated at 120 to
25~C and 350-6000 kPa. Mason et al U.S. patent 4,552,589
discloses alkaline pressure oxidation at 220-250C and 10-25
psia o~ygen partial pressure for 30 to 90 minutes. Matson et
al U.S. patent 4,289,532 describes alkaline pressure oxidation
at 140-190F using air.
4LH ABR 5080.1
PATENT
Z0166~!~
Summary of the Invention
Among the several objects of the present invention
may be noted the provision of an improved process for the
recovery of gold from refractory ores; the provision of such a
process which effectively removes sulfur, iron, arsenic, and
other heavy metals; the provision of such a process which is
effective for the removal of oxides, salts and any other
o~idation products of iron, arsenic and other heavy metals;
the provision of such a process which can be implemented at
relatively modest capital investment; the provision of such a
process which is energy efficient; and the provision of such a
process by which gold is recovered in high yield from
relatively lean refractory ores.
~riefly, therefore, the present invention is
directed to an improvement in a process for the recovery of
gold from refractory sulfidic auriferous ores. The process
comprises treatment of a slurry of the ore with sulfuric acid,
oxidizing the treated slurry with oxygen gas under pressure in
the pzesence of sulfuric acid, neutralizing the oxidized
81urry, cyanidizing the neutralized slurry to leach gold
therefrom, and recovering gold from the resultant leachate.
In accordance with the improvement, the oxidized slurry is
contacted with wash water in an oxidized pulp washing stage,
wash water as introduced into the pulp washing stage being at
a temperature lower than that of the oxidized slurry. A
partial liquid/solids separation is effected within the
washing stage, and a relatively high solids fraction
comprising washed osidized slurry and a liquid fraction
comprising spent wash liquor are separately removed from the
washing stage. The ~pent wash liquor is neutralized by mixing
it with a base which forms a substantially insoluble sulfate
salt upon reaction with sulfuric acid, after which the
neutralized wash liquor is cooled. Precipitation solids are
4LH ABR 5080.1
P~TENT
X0~664S~
separated from the neutralized spent wash liquor and the
cooled neutralized wash liquor is recycled to the pulp washing
stage to provide wash water for contacting the oxidized
slurry.
S The invention is further directed to an improvement
in the aforesaid process in which a sulfuric acid treated
auriferous ore slurry having a solids content of at least
about 30% by weight is subjected to pressure oxidation in a
horizontal autoclave at a temperature of between about 180
and about 225C, a total pressure of between about 275 and
about 490 psia, and an oxygen partial pressure of at least
about 25 psia for a period of at least 60 minutes. The
sulfuric acid concentration of the slurry is between 5 and 40
gpl after the pressure oxidation is complete. The resultant
oxidized slurry is washed with relatively cool water to reduce
the iron and arsenic content of the slurry and cool the washed
slurry to a temperature not greater than about 45F.
Other objects and features will be in part apparent
and in part pointed out hereinafter.
~Lief Description of the Drawinqs
Each of Figs. 1-4 is a flowsheet of a particular
embodiment of the process of the invention;
Fig. 5 is a more detailed flowsheet illustrating the
acidulation and pressure oxidation steps in a preferred
processing scheme of the invention;
Fig. 6 is a more detailed flowsheet illustrating the
o~idized slurry washing, cooling and neutralization steps in a
preferred embodiment of the invention; and
4LH ABR 50~0.1
PATENT
Z0~6640
Fig. 7 is a more detailed flowsheet for the
cyanidation, carbon-in-leach, and gold recovery steps in the
process of the invention.
Corresponding reference characters indicate
corresponding process and equipment features in the several
views of the drawings.
~escription of the Preferred Embodiments
The present invention provides an improved process
for recovery of gold from refractory auriferous ores,
including relatively lean ores containing as low as 0.10 oz Au
per ton. The process is effective for recovery of gold from
ores such as those found at the American Barrick Goldstrike
property in Nevada, which are sulfidic, and contain iron,
arsenic and other heavy metals. In accordance with the
process, the various contaminants are oxidized under acidic
conditions in a pressure oxidation operation, the sulfuric
acid, oxides and salts produced in the pressure oxidation are
removed in a combined washing and neutralization operation,
and the washed neutralized slurry is subjected to
carbon-in-leach cyanidation, preferably in a continuous
countercurrent manner, for recovery of gold.
In order that the pressure oxidation step of the
process of the invention operate autogenously, the ore used as
feed to the process preferably contains at least about 3~ by
weight sulfur in the form of sulfides. Exothermic oxidation
of the sulfide sulfur generates the heat which brings the
slurry to the temperature at which not only the sulfur but
also the iron and other heavy metals are oxidized. However,
by addition of steam to the pressure oxidation step, the
process of the invention is also effective for the treatment
of refractory sulfide ores containing as low as 1.5~ by weight
s
4LH A~R 5080.1
PATENT
2016~
sulfide sulfur. As a further alternative, pyrite concentrates
may be blended with the ore feed to provide an additional
source of sulfide sulfur and assure that autogenous heat is
sufficient to bring the autoclave to the desired reaction
temperature and pressure. The latter alternative may provide
a further advantage in allowing recovery of gold from the
pyrite concentrate, where it is sometimes present in
concentrations otherwise too low for economical recovery.
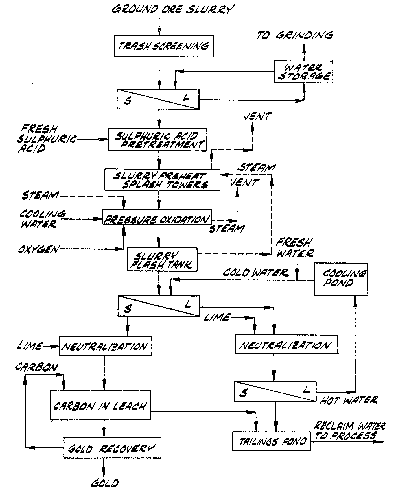
Illustrated in Fig. 1 is a preferred process of the
invention. According to the process of this flowsheet, the
ore is crushed and wet milled, and the ground ore slurry
screened for trash or tramp material. Next the ground ore is
thickened by removal of escess water in a solid-liquid
separation operation. The carbonate content of the ore slurry
is then substantially reduced by an acidulation treatment
using fresh sulfuric acid. Removal of carbonate as CO2 in the
acidulation step substantially reduces the volume of CO2 that
must be vented from the autoclave, where it is generated from
residual carbonate in the pressure oxidation step. Acidulation
may be carried out in either a batch or continuous manner.
Next the acid treated ore slurry is subjected to pressure
oxidation in the presence of sulfuric acid using oxygen gas at
elevated pressure. The pressure oxidation step is typically
conducted in a horizontal multi-compartmented autoclave, the
compartments of which are preferably of substantially equal
volume. Energy from the exothermic pressure oxidation is
recovered by heat eschange between the oxidized slurry and
acidulated feed to the autoclave. As indicated in Fig. 1,
this heat e~change is preferably effected by letting down the
pressure of the oxidized slurry, and using the steam which is
flashed from the oxidized slurry to heat the acidulated
autoclave feed, preferably by direct contact in splash
condensers positioned ahead of the autoclave.
4LH ABR 5080.1
PATENT
201~
After it is partially cooled by flashing of steam,
the oxidized slurry is directed to a washing operation where
it is diluted with relatively cool water, and then subjected
to a solids/liquid separation in which sulfuric acid and
soluble metal salts produced in the pressure oxidation are
separated in a spent wash solution liquid fraction. The spent
wash solution is neutralized with lime or other base, thereby
precipitating sulfate and heavy metals, clarified by
solids/liquid separation, cooled, and recycled to the washing
step where it serves as the source of fresh wash water. The
solids stream from the clarifier is disposed of in a tailing
facility.
Following the washing step and separation from the
spent wash water, the cooled oxidized slurry is directed to a
neutralization operation. Here lime or other base is added to
increase the pH to allow for subsequent cyanide leaching.
Autoclave vent gas is added to oxidize the heavy metals
remaining in the ore slurry. Gold is recovered from the
washed and neutralized o~idized slurry by carbon-in-leach
cyanidation, preferably in a continuous countercurrent system.
Referring to Fig. 5, ground ore slurry, a substantial
fraction of which, for esample 65-85% by weight, passes 200
mesh, is directed to a trash screen 1 where rock, wood fiber
and plastic larger than 20 mesh are separated and removed.
The ore slurry passing through the screen is directed to a
mechanical thickening device 3, typically a vertical tank of
large diameter which provides a net vertical flow low enough
to permit sedimentation of the solid particles, In the
thickener, the concentration of the ore slurry is increased
from a range of about 10-25% by weight to a range of about
40-55%, preferably 45-50%, by weight. To promote separation
of solids, a flocculant is preferably added to the thickener,
for example, the polymeric thickener sold under the trade
designation Percol 351, at a dosage of about 0.05 to about 0.2
4LH ABR 5080.1
PATENT
20166~0
pounds per ton of ore and a concentration in the thickener
feed of between about 0.05% and about 2% by weight. Overflow
from the thickener is recycled to the grinding circuit.
Thickened ore slurry underflow from the thickener is directed
by a transfer pump 5 to a series of stirred acidulation tanks
7, 9 and 11, through which the slurry passes continuously. A
fresh sulfuric acid stream 13 is added to the acidulation
tanks in order to release carbon dioxide from the carbonate
contained in the slurry, and thereby reduce the equivalent
carbon dio~ide levels in the ore to between about 0.05 and
about 0.7% by weight, preferably not more than about 4 lbs/ton
of ore. To promote removal of CO2, compressed air may be
sparged into the acidulation tanks.
Residue slurry leaving the acidulation tanks, having
lS an adjusted solids content of at least about 30%, preferably
40-SS~, optimum of 45-50~ by weight, i~ fed by a transfer pump
lS to the first of a series of brick lined splash condensers
17, 19 and 21, in which the treated feed slurry for the
pressure oxidation step is preheated by contact with steam
flashed from the o~idized ~lurry leaving the pressure
o~idation. The successive ~plash condensers are each,
preferably, internally baffled to promote contact between
steam and liquid, and are respectively operated at
progressively higher pressure and temperature. Pumps are
interposed to increa8e the pressure of the slurry between
condensers, pump 23 transferring the slurry from condenser 17
to condenser 19, and pump 25 transferring the slurry from
conden~er 19 to condenser 21. Preferably, condenser 17 is
operated under a slight vacuum, condenser 19 is operated at
substantially atmospheric pressure, and condenser 21 is
operated under steam pressure.
Pressure o~idation is carried out in an autoclave
29, preferably multilined, the last lining being brick, to
which the slurry i8 transferred, preferably by a diaphragm
B
4LH A~R 5080.1
PATENT
X0166~
pump 27, from the last splash condenser 21. Addition of live
steam to the slurry leaving the last splash condenser may be
indicated for bringing the slurry to a temperature of at least
about 175-180C, at which the exothermic pressure oxidation
reactions become self-sustaining. In the autoclave, the slurry
is passed through a plurality of compartments, where it is
contacted in the presence of sulfuric acid with oxygen gas at
a temperature of between about 180 and about 225C, an oxygen
partial pressure of at least about 25 psia and a total pressure
of between abaut 275 and about 490 psia. The final acidity of
the slurry leaving the last compartment of the autoclave is
between 5 and 40 grams sulfuric acid per liter of solution,
and the final emf of the slurry is between about 480 and about
530 mv.
Noncondensables and steam generated during the
pressure oxidation operation are vented preferably through a
cyclone 31 which separates entrained solids for return to the
autoclave. In the course of the autoclave operation, iron
sulfides are oxidized to ferrous sulfate and sulfuric acid,
further oxidation producing ferric sulfate; FeAsS is oxidized
to arsenous acid and ferrous sulfate; and arsenous acid is
oxidized to arsenic acid:
FeS + 72 + 2H20 - 2FeS04 ~ 2H2S04
4FeS04 + 2H2S04 + 2 ~ 2Fe2(S4)3 + 2H2
4FeAsS + 1102 + 2H20 ~ 4HAsO2 + 4FeS04
2HAs02 + 2 + 2H20 - 2H3As04
Oxidized slurry leaving the autoclave is passed through a
choke 33 to reduce its pressure, then through a series of
flash tanks 35, 37, and 39 where steam is flashed off to cool
4LH ABR 5080.1
PATENT
201664~
the slurry. Pressure of the slurry is progressively reduced
by passage through chokes 41 and 43 between each flash tank.
Steam from each flash tank is recycled and contacted with
autoclave feed slurry in a complementary splash condenser,
operated at substantially the same pressure as the flash tank,
for preheating the feed slurry. Thus, in the series as
illustrated in the drawing, the first flash tank 35 is coupled
to the last splash condenser 21, the second flash tank 37 is
coupled with the second condenser 19, and the last flash tank
39 is coupled with the first splash condenser 17.
Steam leaving each of flash tanks 35, 37 and 39 is
preferably passed through a cyclone 45, 47 or 49, respectively,
for recovery of entrained solids. The recovered solids are
blended back into the oxidized slurry.
Preferably, the temperature of the pressure
oxidation is controlled at a level no higher than about
225C. Significantly higher temperatures can result in a
runaway reaction and resultant overpressure of the autoclave.
Temperature can be controlled by a variety of means, including
venting tailgas from the autoclave, venting steam from the
first splash tank 35, and/or injecting cold water directly
into the autoclave compartments.
Oxidized slurry having a solids content of at least
about 30~ by weight and containing soluble sulfates, iron
salts, arsenates, etc., leaves the last of the flash tanks at
temperature in the neighborhood of 85 to 100C. Neither the
soluble contaminants in this slurry nor the temperature
thereof is conducive to efficient cyanidation for leaching of
gold. In order to condition the slurry for gold recovery
operations, the improved process of the invention subjects the
hot contaminated oxidized slurry/to the novel washing, cooling
and neutralization operation illustrated in Fig. 6. In
accordance with this flow scheme, the slurry containing at
least about 30% by weight solids is introduced continuously
4LH ABR 5080.1
PATENT
2016640
into an o~idized pulp washing stage comprising a
washer/thickener tank 51 where it is diluted with relatively
cool water in such relative proportions as to reduce the
slurry temperature to between about 40-70C and dilute the
solids content to 10-25%. Preferably, the slurry is cooled to
a temperature no higher than about 45C and is diluted to a
solids concentration in the neighborhood of 15% by weight.
Partial solids/liquid separation occurring in thickener 51
produces a continuous spent wash liquor overflow and a
continuous washed oxidized slurry underflow having a strength
of between about 35% and about 50%, preferably about 40% to
about 45%, by weight solids. To assist in the separation, a
flocculant is advantageously added to Lhickener 51.
Preferably, a nonionic polymeric flocculant, such as that sold
under the trade designation Percol 351, is added at a
concentration of about 0.05 to 0.2 lbs/ton of ore and about
0.05% to about 0.2% by weight solids.
The spent wash liquor is not recycled to the
pressure oxidation or other early steps of the process, as in
the prior art, but instead i8 reconditioned, cooled and
recycled to thé step of washing the oxidized slurry. Thus, as
shown in Fig. 6, the spent wash liquor is passed continuously
through a series of stainless steel and carbon steel
neutralization tanks 53, 55 and 57 where it is neutralized
with lime to raise its pH to the neighborhood of 9.5 to 11,
preferably about 10.0, and precipitate sulfates and compounds
of iron, nickel, zinc and arsenic. Lime is highly preferred
but the neutralization may be carried out with other bases
which form substantially insoluble sulfate salts on reaction
with sulfuric acid and are capable of raising the pH to a
level at which iron and arsenate salts are precipitated.
Effluent from the spent wash neutralization is passed to a
clarifier 59 where precipitated solids are removed in an
underflow stream, typically containing 5-30% solids, that is
11
4LH ABR 5080.1
PATENT
Z0~664~
directed via a pump 61 to a tailings pond. Preferably, a
flocculant is used in the neutralized spent wash clarifier
also. In this instance, it is preferred to use a cationic
polymeric flocculant such as that sold under the trade
designation Percol E24 at a dosage of 1-4 ppm and a feed
concentration of 0.02 to 0. 2 weight % solids.
Overflow from the neutralized spent wash clarifier
is transferred by a pump 63 through open spray nozzles 65 to a
cooling pond 67. Spraying of the neutralized and clarified
spent wash liquor cools it by evaporation to a temperature
near ambient, preferably not greater than about 32-40C,
typically 20-38C. After it is cooled, the neutralized and
clarified spent wash liquor flows to a wash maker making tank
69, where it is mixed with fresh water; and the combined
stream is recycled via a pump 71 to washer/thickener 51 where
it serves as the source of cool water for washing and cooling
the oxidized slurry.
Underflow from washer/thickener 51 is transferred by
a pump 73 through a series of rubber lined neutralization
tanks 75, 77 and 79 where the pH of the slurry is raised to
about 10.5 to 11.5, preferably about 1].0, by treatment with
lime to condition it for cyanidation. Recovery of gold from
the o~idized slurry by carbon-in-leach ~C-I-L) cyanidation is
illustrated in Fig. 7. The washed and neutralized slurry is
pumped, 81, to the first of a series of agitated carbon-in-
leach tanks 87, 89, 91, 93, 95, and 97, and possibly more
depending on retention time, countercurrently to a flow of
granular activated carbon. Loaded carbon recovered from the
carbon-in-leach operation is stripped with hot alkaline
cyanide solution in a stripping vessel 99, and gold is
recovered from the stripping solution by conventional means
such as electrowinning and refining (not shown).
The process of the invention provides for high
recovery of gold, for example, in a yield exceeding 80%, from
12
4LH ABR 5080.1
PATENT
~0~66~
relatively lean refractory auriferous ores containing 0.10 to
0.50 oz gold per ton. It is effective for removing
contaminating elements such as iron, arsenic, nickel, and zinc
from the oxidized slurry, and can be implemented with
relatively modest capital investment. The autoclave
conditions and means for recovery of exothermic reaction heat
provide not only efficient gold recovery but efficient use of
energy.
Illustrated in Figs. 2-4 are various alternative
embodiments of the process of the invention. Fig. 2 varies
from Fig. 1 in that an indirect heat exchanger 101 rather than
coupled splash condensers and flash tanks are used for
exchange of heat between the oxidized slurry and the feed to
the pressure osidation autoclave. After leaving heat exchanger
lOl, the oxidized slurry is further cooled in a slurry flash
tank 103. Figs. 3 and 4 both use an indirect heat exchanger
105 rather than a cooling pond for cooling of the recycled
neutralized and clarified spent wash liquor. The cooling
fluid for the indirect heat exchanger comprises water
circulated in a closed circuit through a cooling tower 107.
In the process of Fig. 3, heat exchange between oxidized
slurry and autoclave feed is by means of coupled flash tanks
and splash condensers as in Fig. 1, while in Fig. 4, heat
exchange between oxidized slurry and autoclave feed is
accompli~hed via an indirect heat exchanger 101 as in Fig. 2.
In those embodiments in which transfer of heat from
the oxidized slurry to the treated slurry autoclave feed is
accomplished by indirect heat exchange rather than by coupled
flash tanks and splash condensers, the indirect heat exchanger
is preferably a double pipe exchanger in which the inner pipe
is constructed of titanium and the outer pipe of steel. The
oxidized slurry is passed through the interior of the titanium
pipe and the relatively cold pressure oxidation feed slurry is
passed through the ar.nular space between the pipes.
13
4LH ABR 5080. 1
PATENT
2016640
Further illustrations of the process of this
invention are given below.
Three samples of each Betze and Deep Post ore were
pulverized to minus 200 mesh and identified as Head Samples A,
B, and C. Each of the three head samples was fire assayed for
gold and silver, and the A samples for each ore type were
assayed for other elements of interest.
The analyses are listed in Table 1 and show average
gold analyses of 0.205 and 0.336 oz Au/ton for the Betze and
Deep Post composites, respectively. The average of 75 assay
determinations for the Betze ore during the course of testing
was 0.201 oz Au/ton. Likewise, the average of 11
determinations for the Deep Post ore was 0.328 oz Au/ton.
14
4dfb `_ Patent
~01664
a~ ~. r-~
~c l oo
C4 r-l
C n~ a j o o
~ ~ o r~ o ~ r~ co o r~l
o o o o o~ ~r~ t~ o
O o o o o ~ ~r o ~ o~ u~ o
a l
~ l ~
c l lu ~r ~ ¦ o O
N o ~P
m a o o
~ ~ ~0 0 ~ O
o o o o O~ ~ O 1` r~
o o o o o ~ o ~I
O ~ O C4 C4 ~
~o 0~ 0~ d o~ o~
N " O~o ~ .r ` C C C ~ d 3
o ,~ o O ~ I n~ n~ n~
, ~ u~ o ~ ~ ~ ~,q ~.q t~ ~ o ~ ~;
4LH AB~ 5080.1
PATENT
E~AMPLE 1 20~66~
A pilot plant arranged in a manner generally
corresponding to the flow sheet of Fig. 1, initially was
operated continuously for about 2 1/2 days (62 hours) using
the Betze ore. The total retention time in the circuit was
estimated to be approximately 36 hours, so that a large
portion of the time during this first period of operation was
required to fill the autoclave thickener and the various
neutralization and CIL tanks.
The autoclave conditions during this period are
listed in Table 2. Sulfuric acid in a proportion of 100 lbs.
per ton of ore feed was added to the autoclave feed. This was
the stoichiometric amount required to neutralize the natural
carbonate in the ore.
16
~`
4LH AaR 5080. 1
PATENT
TAI~LE 2 Z01664~
Start-Up Conditions
for ~ilot Plant ~utoclave
Retention time 90 minutes
Feed 40% solids by weight
~85% passing 200 mesh
Temperature 435F (225C~
Pressure 420 psig total
O~ygen 2 standard liters f 2 per
stage (total of 8 SLPM)
Acid addition 100 lb H2SO4/ton ore
Mixers 500 rpm
The circuit finished filling during the morning of
the secona 24 hour period and was shut down a day later. The
CIL and neutralization circuits were both profiled and the
gold fire assays of the solids determine~. The data indicated
rapid gold leaching; i.e., the CIL tailings after 2 hours of
leaching in the first stage assayed 0.024 oz Au/ton compared
to 0.019 oz Au/ton for the last stage after 16 hours of
leaching. The calculated gold dissolution was 90% based on
final tailings of 0.019 oz Au/ton and the average
neutralization product ~CIL feed) which assayed 0.190 oz
Au/ton.
17
4LH A~R 5080.1
PATENT
The solution in the autoclave discharge ~ rlng this
period contained approximately 22 to 25 grams of free acid per
liter and the off-gas from the autoclave measured approximately
96% oxygen and 3% carbon dioxide. Typical solution analyses
show arsenic concentrations of approximately 70 to 100 mg
As/liter in the autoclave discharge liquor and 0.2 to 0.3 mg
As/liter in both the CIL tailings and clarifier overflow
liquors. The mercury in all the liquors was below the
detection limit of 0.005 mg per liter. The iron in the
autoclave discharge liquor was as high as 996 mg per liter but
assayed 1.1 to 1.7 mg Fe/liter in the CIL tailings and
clarifier overflow liquors.
Cyanide addition of 1 lb NaCN/ton of ore was
initially added to the first leach stage. The cyanide
concentration in the final CIL tailings solution measured 0.06
g NaCN/liter (<0.12 lb NaCN/ton solution). The cyanide
addition was increased to as high as 2 lb NaCN/ton of ore in
sùbsequent operations, but good leach results were obtained at
levels averaging 1.5 lb NaCN/ton of ore feed.
Analysis of the laboratory CIL tests indicated that
the sulfide sulfur in the autoclave solids averaged 0.05~ S~,
the gold assay of the CIL tailings averaged 0.013 oz Au/ton,
and the calculated gold dissolution averaged 93.4%.
EXAMPLE 2
In this sxample the pilot plant of Example 1 was
operated continuously for 60 hours. The acid addition to the
autoclave feed was reduced from 100 to 80 pounds of sulfuric
acid per ton of sre feed, but otherwise the autoclave
conditions were the same as for Example 1. Plant recycle
water was used to dilute the autoclave discharge slurry prior
to thickening.
18
9LH A~R 5080.1
PATENT
2016640
A summary of analyses for this example is given in
Table 3. The average ore feed assayed 0.207 oz Au/ton and,
with the exception of one 12-hour composite, the CIL tailings
averaged 0.014 oz Au/ton. This corresponds to a calculated
gold dissolution of 93.2% (assuming no weight changes).
19
4dfb _ A~R 5080
PATENT
~016640
,~ ~
C~ o ~o
~ ~ o
~ o v
o, CO o ~o " o o ~ o
~ e c O O ~ O ,~ O O ~ O ~
P- t~ V ~ V V ~ V O
' ~ I~ U r~ ~ ~ Ul,
u~ ~¢ & E ~1 0 r I cO ~1 u7 o _I O ~1 0 _~
P~ . o~
I ~o ~
.- ~ .- ~r ~ ~ u, ~ u~ ~ I
t~l t O ~ 1` ~ o ~r ~D O ~ a~
~ _ ~ ~ v ~ vl ~ v o~o~
r l ~ U ~ o ' o
E ~ O O O CO. _~ 'JJ
C~
tl ~ rO` o o ~o o, ~r o ,~ ~" , o ,~
~ ~ ~1 0~1 0 r l O 0 ~1 ~O O O o ~o
N vv v r-l ~
O ~0
l ~ O ~ 1 o .
, .,~ ~ '~ ~
o ~ O t~ O ~ ~
~a o ,,~ ~a o u, ,~ :~ O
3 ~ U~
O ,~ ~ V~ ,0~ 30 3 0 ~ tn tn
rc v1 ~1 la ,a ~ ,1 _I u~ . .
~ ~ ~ U ~ Q
O u~ :~
~ b P ~ ~ ~ ~' ,~
14 14 ~ C~ t~ O O ~ Is~ E~ ~ n1
~ ~ ~ 3
ul n~ C '~u nl
~ C I O X ~
P~ V .~ _) n1
4LH ABR 5080.l
PATENT
Z0~6640
There was one 12-hour period when the CIL tailings
composite assayed 0.027 oz Au/ton. The exclusion of this
value during averaging produced the numbers in parentheses.
It is of particular interest to note the presence of
gold in the CIL feed liquor. There was no cyanide in the
process at this point and it is not known why there should be
soluble gold. However, the presence of soluble gold was
substantiated by solution assays for other periods of pilot
plant operation.
Also, the low gold assays for many of the CIL feed
solids are indicative of soluble gold. These solids assayed
only 0.152 oz Au/ton compared to the autoclave discharge
solids which assayed 0.204 oz Au/ton. This is a significant
difference and could occur only if (1) there was a substantial
weight gain ~appro~imately 30%) during neutralization, or (2)
if there was soluble gold in the CIL feed liquors. It is
considered e~tremely improbable that there was such a large
weight gain and, in fact, later laboratory tests demonstrated
a minimal weight gain of les~ than 2%. Calculations indicate
a gold balance is achieved when the solubilized gold is
considered in the balance.
The mercury analyses in Table 3 show less than 0.005
mg/l in ~olution throughout the c1rcuit except for the CIL
tailings ~olution which as~ayed 0.212 mg Hg/liter. The
arSeniC values show a concentration of less than 100 ppm in
the autoclave discharge solution, 0.08 mg As/liter in the
clarifier overflow, and appro~imately 0.3 mg As/liter in both
the CIL feed and tailings liquors.
A Jummary of the laboratory CIL tests showed that
the average CIL tailings assayed 0.015 oz Au/ton with a
corresponding average gold dissolution of 93.0%. These values
agree extremely well with the results obtained in the plant
and summarized in Table 3. The average sulfide sulfur analyses
in the autoclave discharge products used for bath CIL leaching
was 0.05%S'.
21
4LH A~R 5080.1
PATENT
X016640
During the last two days (48 hours) of the 60 hour
operation of Example 2 the CIL circuit was profiled. The
solids and li~uor gold analyses are listed in Table 4. Both
sets of data show very rapid rates of gold dissolution. The
assays from the first day show that the gold in the solids
from the first stage are not significantly different from those
in the last stage. On the second day, the solids assays in
the first and last stages differed by only 0.004 oz Au/ton.
Both sets of data show high tailings at mid-points in the CIL
circuit. On day one, for example, the solids in Stages 3 and
4 assayed 0.027 and 0.024 oz Au/ton, respectively. It is not
known if these are anomalous data or if they are indicative of
aberrations in the operation of the autoclave of CIL circuit.
TABLe 4
Profiles of the Pilot
Plant CIL Circuit
Day 1 Day 2
CIL SolidsSolution SolidsSolution
~ta~e oz Au/ton ma Au/l oz Au/ton ma Au/l
1 0.017 1.33 0.020 0.65
2 0.019 0.24 0.021 0.07
3 0.027 0.04 0.017 0.013
4 0.024 0.01 0.016 <0.004
0.017 0.01 0.027 <0.004
6 0.015 0.01 0.027 >0.004
7 0.017 0.01 0.016 <0.004
8 0.016 <0.01 0.014 <0.004
4LH ABR 5080.1
PATENT
20166~
E~AMPLE 3
For this example the pilot plant of Example l was
operated so as to study autoclave retention times of 90, 80,
70 and 60 minutes using the Betze ore and a sulfuric acid
addition of ~0 pounds per ton of ore. Other autoclave
conditions were the same as in Example l, i.e., a teMperature
of 435F and a total pressure of 420 psig ~50 psig
overpressure).
The retention times were varied by changing the feed
rate to the autoclave and adjusting the acid and 02ygen
additions accordingly. After each change the autoclave was
allowed to equilibrate for a time period equal to three times
the tested retention time. For example, 240 minutes (4 hours)
were allowed for equilibration after switching to 80 minutes
retention time.
The data for each test include sulfide sulfur
analyses for the solids in each autoclave compartment, the
corresponding redox potentials (emf), free acids, and pH
values for the liquors, and gold dissolutions from the
solids. The gold dissolutions for the compartment solids were
determined by shaking 10 grams of sollds with an excess of
cyanide and carbon in a test tube for 16 hours. The gold
dissolutions of the final autoclave products were determined
by dupllcate standard CIL tests of splits from a 2-liter
sample of the autoclave discharge.
The data in Table 5 show that each of the tested
retention times achieved e~cellent sulfide sulfur oxidations.
In every case the autoclave discharges contained 0.07~ or less
sulfide sulfur corresponding to oxidations in excess of 95%.
The other data indicate that the final autoclave products
leached very well and the tailings from the batch CIL tests
were in a range of O.OlS oz Au/ton with calculated gold
dissolutions of 92% or better.
23
- 4LH ABR 5080.1
PATENT
Z0~664~3
The data in Table 5 for the four tests using 80
pounds of acid addition and retention times of 60 to 90
minutes were used to determine the rate of sulfide sulfur
oxidation. The percentages of sulfide sulfur oxidation were
5 calculated based on analyses for the solids in each of the
autoclave compartments and a head value of 2.4%S~.
The data showed rapid rates of oxidation:
approximately 95% of the sulfide sulfur is oxidized within the
first 30 minutes. Longer times increased the oxidation by
only 2 to 3 percentage points.
The data in Table 5 include two periods of pilot
plant operation using retention times of 75 and 90 minutes and
no acid addition to the autoclave feed. The sulfide sulfur
analyses of 0.06 to 0.07%S~ for the autoclave discharge solids
were comparable to similar tests using acid. The batch CIL
tailings appear to be slightly higher than tests with acid,
but the gold dissolutions of the final autoclave discharges
remained in excess of 90%.
29
4dfb -- -- ABR 5080
PATENT
20~66~0
~ 1~ o o o I o ~o
~ ~ ~ u~
o o ~ o ~
O O ~ , I O
c a . aa)
g' .~ 'u~
_~ C _ ~ O~ ,~ u~ o o 1~ N 1~ ~ ~ 0 t` o
~J ~- ul a~ r~N ~ ~ ~ i ~
e
v a r ~ a , o
.~ ~ ~ ~ I~ , o
,~ oo oooo oo oo oo v~
i~ i~ ~- ¦~NO O O O O O O O O O O O ~N
'C O ~uOl
o ~ ~ ~ u~ r~ a o
C~ C , ~ D~ ~ ~ U WO
rc +
:~ ~ ~
r~ 1~ ~ ~ o o o o o o o o o o O O O c
~i c r~ o o o o o o o o o o o o ~
~ t Ct N ~
~ JJ a ~ a o c o o o o o o o o v~ u~
.~ ~3 ~$ ;~ o~ o~ a~ a~ ~ o
c u
o W V
~-~1 v o o o o o o o o o o o o
~ 3 ~ ~q oo ~ oo a~ o a~
a 3
u~ o ~ I ,~ ~~ o E~ u~
D
4LH ABR 5080.1
- PATENT
ZO:16fi4~
EXAMPLE 4
Autoclave oxidation of the Betze ore using reduced
oxygen additions with 80 pounds of acid addition was
investigated during the runs of Example 3. The amount of
oxygen used was measured using a high-pressure mass flowmeter
calibrated to indicate standard liters of oxygen flow per
minute. Oxygen flow rate was controlled by precision needle
values. The efficiency of the autoclave treatment was
evaluated by sampling the four autoclave compartments and
assaying the solids for sulfide sulfur and the liquors for
free acid, pH, and emf. Samples of the final autoclave
products were batch leached in the laboratory; however, none
of the solids from the individual autoclave compartments were
leached.
Results of these tests and others, summarized in
Table 6, show good results even with the oxygen reduced to as
low as 92 pounds added per ton of ore feed. The use of the
lowest oxygen producéd sufficient oxidation to make the ore
amenable to cyanidation. Tests 67 and 68 are replicate tests
of the autoclave product using only 92 pounds of oxygen; CIL
tailings were 0.017 and 0.018 oz Au/ton with calculated gold
dissolutions in excess of 90%.
There were, however, differences in the rates and
completion of the sulfide sulfur oxidation. For example,
using the 92 pounds of osygen achieved a sulfide sulfur
analysis of 0.11% for the solids in the second autoclave
compartment. By comparison, the use of 154 pounds of oxygen
achieved solids assaying 0.07~ sulfide sulfur from the same
compartment.
4dfb -- -- ABR 5080
PATENT
Z01664~
~ ~o
D J ~ N
J,~3J. ~'q ~
a
o
VVo .~
~~ O N O ~ r 0
P~ ~ ~ ~ ~ ~ ~ I 1~ ~ ~ N O~
, t. 1 O~
~g ~8, ~
~ u.,a ~
~9~ % a ~ a ,, ~ .,7 ,, ~ ~ '',, _, '` ~
~ ~ ~ o o o O O O O O O O O
o.~ ~ ."~3 o o o o o o o o o o o
~ ~ ~ . ~ O
C r. ~'D r~
o c ~1 o oo o o oo o o o o ,~
.,~ ~q .. .. .. .... ..
.,1., ~ ~ oo oo oo oo oo oo
u~c,
. , J ~ V~
3 P le ~ u~ o; o
.~ ~ 1 o~ a~
c
` ~
~J~ OO OO OO OO OO OO
~ ~ o 0 oD ~ c~
~ ~ h 2 7
:,
4LH ABR 5080.1
PATENT
PLE 5 201664~
The pilot plant of Example 1 was also operated
continuously for approximately 52 hours using Deep Post ore
feed. The autoclave conditions included the following: a feed
of 40% solids by weight, a retention of 90 minutes, 435F
(225OC), and 420 psig total pressure (50 psig overpressure).
An acid addition of only 12 pounds per ton of ore feed was
required to achieve 10 to 15 g H2SO4/I in the final autoclave
discharge solution. During one six hour period, the temp-
10 rature was reduced to 410F and the pressure to 325 psig total
(50 psig overpressure).
The autoclave oxidation was evaluated by leaching
samples of the autoclave slurry in the laboratory using the
standard CIL procedure described earlier.
The entire pilot plant was operated during this
period but only the autoclave portion of the circuit was in
equilibrium. This is shown by the low gold assays for the
solids in the thickener underflow and CIL feed samples for the
last day of preparation. Both samples assayed only 0.2 oz
Au/ton compared to the average aùtoclave discharge solids
assay of 0.35 oz Au/ton and the Deep Post ore assay of 0.336
oz Au/ton.
Although there was approximately 1.4 mg Au/liter in
the CIL feed solution, the inclusion of this gold would
increase the eguivalent CIL feed solids assay to only 0.27 oz
Au/ton. This is significantly lower than the Deep Post but
higher than the Betze material and it would seem, therefore,
that the slurries in the neutralization and CIL circuits were
mixtures of the Betze and Deep Post materials.
Results of the laboratory batch CIL tests are
summarized in Table 7. The CIL tailings of the material
produced using the standard autoclave conditions (420 psig
total pressure, 50 psig overpressure, and 435F) averaged
28
9LH ABR 5080.1
PATENT
20166~0
0.019 oz Au/ton with a corresponding calculated gold
dissolution of 94.6%. The reduced temperature and pressure
conditions resulted in a CIL response which was only slightly
less; the tailings assayed 0.022 oz Au/ton and the calculated
gold dissolution was 93.8%.
Table 7
Summary of Laboratory Batch CIL
Tests of DeeD Post Autoclave Product
Laboratory Batch
Autoclave Conditionsl CLL_Tests
Temp, Pressure, Acid Product,Tailings %Au
F psia Total lk/ton %Soz Au/ton Dissolution
435 420 lZ 0.~8 0.019 94.6
410 325 12 0.07 0.022 93.8
1/ Other conditions include 40~ solids, 90 minutes retention,
and 154 pound 02/ton of ore feed.
The results indicate that the Deep Post ore was very
amenable to cyanidation after autoclave o~idation using
conditions which were at or near the standard conditions used
for the Betze ore.
,~
Three batch laboratory autoclave tests were made
using the ~etze ore auger composite. The products were
cyanide leached at temperatures of 25, 37 and 49C. The
29
4LH ABR 5080.1
PATENT
20166a~3
autoclave products from two different pilot plant tests using
the Betze ore feed were also leached in the laboratory at
temperatures of 25, 30, 40, 50, and 65C.
Results for the laboratory batch autoclave tests in
S Table 8 show a trend of increasing gold dissolutions
corresponding to increased leaching temperatures. The leach
tailings, for example, decreased from 0.020 to 0.016 oz Au/ton
when the temperature was increased from 25 to 49C.
4dfb . _ ABR 5080
PATENT
20~6
o U~
o~ll o o o
a~
C n~ ~ ,~ o~ r o ,~ n o r~ ,~
1 ::~ ~ r~ N ~ ~ ~ ~ ,~ o ,~
~ U~¢ ~ ,
n N O o o O O o o O O O O o O ~
~1~ r1
~) ~
.C~ ~ ~ ~ ~ O O~
n t_) Z ~ ~ ~ ~ ~ ~ ~ n
. ,-1 N ~.
~ql , .~
.~: c ~ 0 ~ D O CO ~O Y
,~
O O O O O O O O O O O O O
~ ~ ~ oOOOO oOOOO OOo a
~3 ~ .
c ~a a)
E~ ~ ~ t~ u~ ~ N 0~ r al
~ ,~1 p ~,) ~ ~ N ~ ~ N N N O ,~1 N P
~ ~ ~ cn ~ 'U
~, n~
~ .d
n ~ ~ O O O ~ o O O u u~
l~ l~ N ~ ~ N ~ 0 N
. J~
. ~0
P ~ ~I N . _I
~ ~ ~ ~ 3
J~ ~ ~
U E~ ~ D t~ 0 ~N N N N N ~ ~ a~ U O
31
9LH ABR 5080.1
`~ PATENT
Z0166~3
Results obtained by leaching the pilot products
indicate no trend associated with the leaching temperature.
There were only minimal differences in the tailings assays
which were within normal expected experimental and analytical
precision. However, the data, and particularly those from
Example 2, indicate increased cyanide consumptions with
increasing temperature. This is not unexpected and is
indicative of either increased reactivity with the cyanicides
or higher rates of cyanide volatilization.
Autoclave discharge slurry from Example 2 (using the
Betze ore) was tested to determine what effects, if any,
different neutralization techniques might have on the
subsequent cyanide gold dissolutions.
In test CIL-17, -18, and -19 the autoclave slurry
was neutralized at temperatures of 25, 40, and 70C,
respectively. The neutralization in each case was made by
adding milk of lime directly to the autoclave slurry to
achieve pH lO.S, holding the slurry at the temperature and pH
for 2 hours, and batch CIL testing for 16 hours.
For test CIL-21, the autoclave slurry was heated to
90C, diluted to 10~ solids ~by weight) using fresh water,
thickened to 40~ solids, and then decanted. The thickened
slurry, which wa~ now at ambient temperature, was neutralized
to pH 10.5 using milk of lime. This procedure simulated the
pilot plant operation.
The results are summarized in Table 9 and with the
exception of Test 18 at 40C, the leach tailings all assayed
0.014 oz Au/ton. The higher tailings assay for Test 18 make
interpretation of the results difficult. However, these
results appear to be anomalous and, if so, the gold
dissolutions were independent of the tested neutralization
techniques.
32
4dfb `--- PATENT
Z0166~1
.~
C C U~
o-,l 3 ~
.0 ~ rl ~ ~ E
nl 5~ ~ ~ ~ ~ ~ a~ E
rl U ~ 1 Orl O rl n~ O
~ ¢ ~ S ~ vl
~, ~ N O O O O ~ ~ O
n O ,~~ r1
a) ~ e
',~
~ ~ ~ Z ~a
~ e ~ ~ ~ 3 ~1
", z :~ ~ ~ a) ~ u~ u~ ~ JJ ~ ~ 3
o~ o~
C C E4 . . . . a~
~ Z ~ ~ O ~1 ~1
E~ .C n~ C ~ '~
O C: 3 p E~ ~ n
,~ o 3
N ~ ~ C ~ ~ ~ ~r O a
.,~ ~ n .-1 ~'C O O O o ~ e;~ N
~ NO O O O I~
:~ ~ ~ 01 ~
Z '~ ~1 ~ O O E-~ :1 nl ,C O~O a~
O ~! ~ ~ ~ .a ,~r~ o ~D V ~ ~ O
~ . O O n~ ~u~ ~ o 1~ 0 3
.~0 ~ 1,) O~ ooo~ cn ~ ~ ~ o nl
~1 ~I P ~ P~
:~ ~ ~ 'V ~ C
i N o ~ m o O a~
C n~ ~ > '1
_ JJ ~u~ o O u~ U d ~ ~ ~ V
~ :1~ ~ a c~
:z; ~ s ~ ~ ~ a
~ ~ o ~
~ s~
U 11 E~
~n In ..
oO~o o c
~ a
Q) I~ a~ a~ ,1 O
,
33
4LH ABR 5080.1
PATENT
Z0~66~0
The cyanide consumptions for Tests 19 and 21 were
significantly higher than the other two tests. In the case of
Test 19, this was probably due to starting the cyanide
leaching immediately after neutralizing while the slurry was
still at 70C. It is not known why the consumption for Test
21 was higher since the dilution and thickening process
allowed the slurry to cool to ambient conditions prio~ to
cyanidation. However, since this process simulated plant
conditions, it correlates with experience in the plant.
E~N~PLE 8
A sample of autoclave slurry (Betze ore) was split
into six equal portions which were each aged (held in storage
at ambient temperature) for times of 0 to 96 hours followed by
CIL testing. Prior to cyanidation, the aged slurries were
filtered, the solids washed using three water displacements
and repulped with fresh water. The objective was to determine
whether aging the autoclave slurry had any deleterious effects
on the gold dissolutions.
The results summarized in Table 10 show no
significant differences in the gold dissolutions, but there
were measurable differences in the cyanide consumptions. The
latter two tests using 72 and 96 hours of aging demonstrated
lower consumptions than the other tests.
34
ABR 5080
4df b ~ PATENT
~0166~
V~q O O~ ~ ,, ~ ~r o
,~ ~ ,~
U ~ ~ ~ O O O O O O ,
~ o
~J
o `
a CD O r~
~3 3 ~ 11 u~ o o o r- OD
~; d Z C ~ ~ ~ ~r~
~.)r~
Q~
PIn d
r ~ ~ ~ O
O 1~ ~
J~ 'C ~ _~ ~ O O O O O O
~ I P N O o o o o o
q J~_
.~~ ~ ~
~1~ O ~ ~ . . ~ . .
u o ~ ~ ~ rJ~r
.C~ p O
O P
~,~
~C~
~." ~ ~ O U~ ~CO ~ ~D
~;v ~
~ r ~ ~r ~
u~ oooooo
~ o o o o o o
~I
J~
co ~ o
r~t
4LH ABR 5080.1
PATENT
A sample of the pilot plant thickener un~erflow
(~etze ore) taken during the same time period during which the
autoclave slurry samples were taken was neutralized in the
laboratory from pH 1.3 to 10.5 using milk of lime to determine
weight changes during neutralization. A worst case weight
change would occur if all the lime was converted to CaSO42H2O
(gypsum), and this would result in a weight gain of 1.6% with
the lime addition of 11.5 pounds of CaO (90% basis) per short
ton of dry autoclave solids.
During the run of Example 2 samples of the Betze ore
autoclave feed and discharge were screened at sizes from 48 to
400 mesh and each fraction was assayed for sulfide sulfur and
gold. In each case there was only a trace of plus 48-mesh
material and it was not assayed. There was insufficient
material to assay the minus 65- and plus 100-mesh fractions of
gold.
The results, given in Table 11, show sulfide sulfur
values in the feed which were more or less evenly distributed
throughout the various size fractions. There was a slight
concentration of gold in the minu8 400-mesh fraction and it
contained 67.6% of the gold compared to 60.7~ of the total
solids weight.
By comparison, results for the autoclave discharge
show major differences in the weight and gold or sulfide
sulfur distributions. Approximately 42% of the sulfide sulfur
values were contained in the plus 270 size fractions compared
to a of only 22% of the total solids, and the minus 400-mesh
fraction contained 72~ of the total solids and only 50% of the
sulfide sulfur.
The results also show a concentration of almost 89%
of the gold values in the minus 400-mesh fraction of the
36
4LH ABR 5080.1
PATENT
;~0166~
autoclave discharge slurry compared to 72% of the total
solids. This high concentration of gold in the minus 400-mesh
size fraction is probably a major reason for the rapid gold
dissolutions which were demonstrated in the pilot plant CIL
circuit.
In view of the above, it will be seen that the
several objects of the invention are achieved and other
advantageous results attained.
As various changes could be made in the above
process without departing from the scope of the invention, it
is intended that all matter contained in the above description
or shown in the accompanying drawings shall be interpreted as
illustrative and not in a limiting sense.