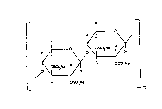Note: Descriptions are shown in the official language in which they were submitted.
z;~
B~ERINGhERR~ ARTI~NOE S~L~SC~a~T 89/B 024 - Na 730
Dr. ~a/Sd
The ~e of ~ylan polyhydrogensulfates for ~he therapy of
di~orders ba~ed on ~ell proliferation
_
The in~ention relates to the use of xylan p~lyhydrogen-
sulfates for the preparation of a pharmaceutical for the
therapy of disorders basQd on uncontrolled and undif-
ferentiated cell growth.
Xylan polyhydrogensulfates are disclosed in DE-
~36 01 136. The following formula I is given therein for
these compounds;
H~
: /O~H/
H OSO~N- _ n
An anti-retroviral action i~ al~o de~cribed for these
compounds therein. It has furthermore been dis~losed that
xylan polyhydrogensulfate inhibits ~he binding of bFGF
(basic fibroblast growth fac~or) to an adrenocortical
carcinoma cell line (SW 13) (A. Wellstein et al., Proc.
of the American Association for Cancer Research (1989)
30, 583).
It has now been found/ surprisingly, that compounds of
the formula I inhibit oncogene-encoded Xinases and growth
factor receptor tyrosine kinases. Hence they are suitable
for controlling diseases in which growth factor receptor~
play a part, especially p60riasis and onco~es.
~he expression of oncogenes in a mammalian cell i8
associated with the transition from the normal to the
transformed type of cell, which then becomes a cancer
-- 2 --
cell. The transformation can be induced by infection of
a cell with a retro~irus. A well-known e~ample i8 RoU8
sarcoma virus infection of chickens, which subsequently
develop cancer. The relevant oncogene which is
responsible for the malignant transformation has been
called the "SRC" (sarcoma) gene. Many of the oncogenes
now known are characterized by the expression of a
protein with kinase activi~y. The anzymes catalyze the
transfer of the terminal phosphate group of ATP to an
amino acid. In contrast to many other protein kina~es
which transfer the phosphate group ~o a seryl or threonyl
radical, most of the oncogene-encoded kinases phosphory-
late a tyrosyl radical of the protein chain. Apart from
this, it is known that products of oncogenes, namely
those of the v-mos, v-mil and v-raf oncogenes, have
serine/threonine-specific protein kinase activity.
Tyrosine kinase activity is also expressed in growth
factor receptors; recent results have now shown that
various diseases in ~hich the proliferation of cell~
plays a part, for example psoriasis~ and the growth of
many tumors depend on the presence of growth factors,
such as epidermal growth factor (EGF), transforming
growth factor alpha (TGF alpha), platelet derived growth
factor (PDGF) or fibroblas~ growth factor ~basic FGF,
acidic FGF). Binding of the growth factor to its receptor
is followed by stimulation of tyrosine kinase which is an
intrinsic component of the growth factor receptor.
~his is why an inhibitor of the tyrosine kinase of a
growth factor receptor inhibits cell growth and thus, for
example, also tumor growth and the ~preading of tumors.
It can therefore be employed in tumor therapy and in the
therapy of all diseases in which cell growth plays a part
(for example psoriasis~.
~he invention therefore relates to the use of a compound
of the formula I
2~
- 3 ~
~H ~1 I
/o~/ CSO,N~
_ ' _ n
in which n is an integer from 1 to 20, preferably 8 to
10, especially 9, or of mixture~ with a mean molecular
weight o~ about 1,000 to 20,000 Dalton~ preferably of
about 5,000 to 12tO00 Dalton, especially about 6,000
Dalton (n = 9), for the preparation of a pharmaceutical
for inhibiting oncogene-encoded tyro~ine kinases, growth
factor receptor ~yrosine kinases and growth factor/-
receptor interaction and thus for controlling disorders
based on cell proliferation, preferably psoriasis, and
oncoses.
Tumors of this type are, in particular, carcinomas and
sarcomas such as renal carcinoma, mammary carcinoma,
prostate carcinoma, pulmonary carcinoma, ovarian car-
cinoma or rhabdomyosarcoma or melanoma.
The compounds according to the invention have advan-
tageous pharmacological properties in that they inhibit
the growth and the spread of tumors and can therefore be
used in tumor therapy and in the therapy of all disorders
based on cell proliferation, that is to say disorders
which are characterized by uncontrolled and undifferen-
tiated cell growth.
For this purpose it is possible to use the compounds of
the formula I themselves also in combination or their
pharmacologically acceptable acid addition sal~s.
A suitable pharmaceutical is prepared by converting the
33
-- 4 --
active substances, where appropriate wi~h auxiliaries
and/or excipients, into a sui~able dosage form.
The examples which follow explain the in~ention.
Examples
Assay for inhibi~ion of EG~ receptor tyrosine ki~ase
The star~ing material for the tyrosine kinase activity
was the human tumor cell line A 431 (ATCC CRL 1555),
which was cultivated in ~PMI 1640 medium ~ 10% FCS. This
cell line expresses on the cell surface a large number of
EGF receptors which have tyrosine kinase acti~ity.
The cells were cultivated almo~t to confluence, washed
with PBS (phosphate-buffered saline, p~ 7.2), scraped off
the culture flask and incubated at 4C for 1 h, treated
20x in a Potter and centrifuged at lOOOxg for 30'. The
supernatant was centrifuged at 20,000xg for a further 20
min, and the pellet was taken up in 100 ~1 per 1 x 106
cells as membrane preparation.
The tyrosine kinase activity of the EGF receptor was
measured with poly(Glu, Ala, Tyr 6:391) as substrate. The
cell membranes were treated with 1000 nM EGF at RT for
15' and then added to the mixture which contains the
inhibitor, substrate (3 mg/ml), Mg2'/Mh~ (8 mM/ 1.6 mM)~
0.16% Triton X-100 and sodium ortho~anadate ~20 ~M) in
~ 100 mM HEPES (N-2-hydroxyethyl-piperazine-N~-2-ethanesul-
fonic acid), pH 7.5, and preincubated, and the reaction
was started by adding gamma~32P-ATP (32 ~M). After 15~ at
30C, the substrate was precipitated with 10% TCA (tri-
chloroacetic acid), filtered on a hillititer fil~ration
plate (Millipore Corporation, Mass., US~), washéd and
dried. The incorporation of 32p was determined using a
1 iquid scintillation counter.
Results:
23~ 3
~ 5 -
Xylan polyhydrogensulfate (n = 9) ~as tested at a maximum
concentration of 1460 ~g/ml and subjected to stepwise
1:10 dilution. The IC50 was defined as the concentration
at which 50% of ~he initial enzyme ac~ivity was
inhibited. A fiyure of 6 ~g/ml was determined for EGF
receptor tyrosine kinase (Table 1).
Table 1
Inhibition of the enzymatic activity of protein kinases-
by xylan polyhydrogensulfate (formula I in which n = 9)
IC50 in ~g/ml ICsOin~g/ml
EGF receptor cAMP-depen-
kyrosine dent protein
kinase kinase
_
Xylan polyhydrogensulfa$e
(n=9) 6 884
.
Assay for inhibition of 3', 5'-cAMP~dependent protein
kinase
The catalytic subunit of cAMP-dependent protein kinase
(Sigma) was reconstituted as described by Sigma (Sigma
Chemical Co., St. Louis, MO, USA). The enzyme activity
was measured using kemptide (Sigma) (Leu-Arg-Arg-Ala-Ser-
Leu-Gly) as substrate. The inhibitor was preincubated
with enzyme, substrate (190~ M), Mg2~ (5 ~M), 0.25 mg/ml
BSA and 3.75 mM mercaptoethanol in 50 mM MOPS (4-morpho-
linopropanesulfonic acid), pH 6.9. The reaction was
started by adding gamma-32P-ATP (40 ~ N). After 15' at
30C, an aliquot was applied to p81 ion exchanger (2 x 9
2 cm; Whatman Paper Ltd, Great Britain), dipped i~ 75 mM
H3PO4, washed and dried, and $he incorporation of 32p was
determined using a liquid scintillation counter.
21J~131 33
-- 6 --
Results:
Xylan polyhydrogensulfate (n=9) was tested at a ma~imum
concentration of 1142 ~g/ml and sub~ected to ~tepwise
1:10 dilution. The IC50 was de~ined a~ the concentration
at which 50~ of the ini~ial enzyme activity was
inhibited. The IC50 for cAMP-dependent protein kinas~ was
884 ~g/ml (Table 1~, i.e. xylan polyhydrogensulfate (n=9)
is a specific inhibitor of tyrosine kinase.
Inhibition of non-adherent growth of A 549 (human pul-
monary carcinoma cell line~, A 204 (human rhabdomyosar-
coma cell line), Ovar Cal (human ovarian carcinoma cell
line), MDA-MB231, MDA-MB468 (human mammary carcinoma cell
lines), ~nCaP (human prostate carcinoma cell line) and
NRK (normal rat kidney fibroblasts).
The assay was carried out by the method de~cribed by
Hamburger and Salmon (J. Natl. Cancer Inst. 6S, 981-988,
1981) with the modifications described below.
Conditioned medium was replaced by RPMI 1640 Medium (A
549, A 204, Ovar Cal) or IMEM (all other cell lines) +
10% FCS (fetal calf serum). As a consequence of the high
cloning rate of the tumor cell lines in soft agar, the
number of tumor cells per plate was reduced to 3 x 103 for
A 549 and to 10 x 103 for all the others.
The cells were plated out as the upper layer in a two-
layer agar sy~tem in accordance with the Hamburger and
Salmon method (J. ~atl. Cancer Inst. 66, 981-988, 1981),
with various concentrations of the test substance +
additional EGF (epidermal growth factor) (1 nM3 or
additional bFGF (basic fibroblast growth factor) (100
ng/ml) being mixed with the upper agar layer before the
cells were plated out.
The plates were incubated in an incubator with 5% CO2,
20~ 2 and 95~ relative humidity for 10 days ~A 549 and A
;2~?1~
-- 7 --
204), 18 ~ays (Ovar Cal) or 1~ days (all other cells).
After this tLme, colonies with a diameter greater than
60 ~m were counted using an Lmage analyzer. The IC50 has
been reported as the concentration of substance at which
the number of colonies was reduced to one half.
The coefficient of variation on repetition of the experi-
ments was less than 15~.
Result:
Xylan polyhydrogensulfate (n=9) inhibits the prolifera-
tion of cell lines which have receptors, containingtyrosine phosphokinase, for various growth factors. This
is why cell growth is inhibi~ed irrespective of whether
the particular growth factor was also added or not (see
1, 2 and 4 in Table 2).
There is likewise inhibition in the case of cells which
grow only in the presence of a growth factor (in the cell
culture) (see 3). Cells not dependent on growth factors
(see 5) are unaffected.
The IC50 of xylan polyhydrogensulfate (n=9) for continuous
incubation was determined from the dose-effect plot. The
results were:
-- 8 --
Table 2
Tumor cell line IC50 (~g/~l)
1. A 549 29 (- E~F)
S 59 (~ EGF~
2. Ovar Cal 4 (- bFGF)
6 (~ bFGF)
3. A 204 34 (+ b~F) `
(without bFGF no
colonies)
4. LNcap 200 (~ bFGF)
MDA 468 40
NRK ~GF-dependent 60
5. MDA 231
Inhibition of human tumor growth in the renal capsule
assay (nude mouse)
1. Preparation of the tumor
The human tumors to be tested are routinely maintained
and passaged in nu/nu CDl mice~ The tumor is removed
under sterile conditions, connective tissue and necro~ic
portions are removed, and the tumor is comminuted and
disintegrated with 20 ml of the following solution
(100 ml of PBS without Ca2+ and Mg2~ with 800 mg of cul-
lagenase (Serva 0.6-0.8 U/ml) + 2 mg of DNA~e). Incuba-
~ion: 2 h, 37C with shaking. The cell ~uspension is then
filtered through a nylon net (pore diameter 51 ~m) and
washed three times with Ca2+- and Mg~-free PBS. The cells
are then counted and pelleted.
20~ ~ 3~
g
2. Embedding the cells in fibrin pieces
The interior wall of a 50 ~1 glass capillary ~diameter
1.5 mm) is wetted with a thrombin/CaCl2 ~olu~ion (50 unit
of thrombin in 1 ml of 40 mmol/l CaClz).
~he tumor cell pellet is suspended in a coagulable
fibrinogen solution~ such as RBeriplast (Behringwerke AG),
and diluted 1:2 with RPNI ~ 15% FCS to a final c~ll
concentration of 107 cell~/50 ~1. This ~uspension is
rapidly a~pirated into the qlass capillarie~.
After the cell-fibrin mixture has Rolidified (about 5 min
at room temperature) the fibrin adhesive is forced with
compressed air out of the glass capillaries into Petri
dishes. The fibrin adhesive is cut up into 2 mm pieces
(corresponding to 5 ~ 105 cells/piece), and the pieces are
stored in RPMI + 15~ FCS until implanted.
3. Implantation
The animals are anesthetized with Nembutal (diluted 1:7
with physiological saline; 0.1 ml/10 g of mouse). The
kidney is exposed by a 1.5 cm-long incision into the
flank. The renal capsule is cut open and a single fibrin
piece is transferred under the renal capsule. Then the
two diameters of the implanted piece are measured using
a microscope with an ocular micrometer. Th~ t~mor size is
determined from V = a x b, where V = tumor size, a =
largest diameter of tumor and b = tumor diameter at right
angles to a.
The peritoneum is then closed with tissue adhesive
(Histoacryl~, and the skin is ~lipped.
Treatment
The substance is administered i.v. each day on days 2-10
with the maximum tolerable dose and with 2/3 of the
L3~
-- 10 --
maxLmum tolerable dose found in preliminary tests.
Measurement
On day 20 after the implantation, the animals are sacri-
ficed, the kidney is exposed and the tumor size iS again
measured. The efficacy of the ~est substance is deter-
mined by the inhibition of tumor growth.
The relative tumor size VR is calculated using YR = Vt/VO~
where ~t is the tumor size at the end of the experiment
(day 20) and V0 is the size of the tumor fibrin piece on
the day of implantation.
Then the median relative tumor size in the treated group
(VT) is related to the corresponding median relative tumor
si~e of the controls (VC)~ snd T/C % = VT~VC X 100 i8
calculated.
~he statistical significance of the antitumor effect i8
determined usin~ the Wilcoxon U test. ~he relati~e tumor
si~es in the treated group are compared with the cor-
responding relative si~es in ~he control group, and the
change in the tumor size is regarded as substance-
specific only if it is statistically significant with pless than 0.05.
~J~ 3~
Result:
Table 3
Tumor Dose Activity
(T/C %)
.
LXF 529 20 mg/kg 70
(human bronchial
carcinoma) 30 mg/kg 71
MCF7 20 mg/kg 66
10~human mamMary
carcinoma) 30 mg/kg 65 n.s.
Ovar-6 20 mg/kg 78 n.s.
(human ovarian
carcinoma) 30 mg/kg 66
PaTu 8902 20 mg/kg 56
(human pancreatic
carcinoma) 30 mg/kg 27
~T 29 20 mg/kg 71
(human colon carcinoma) 30 mg/kg 76 n.s.
n.s. = not significan~
- 12 -
Inhibition of human tumor growth in subcut~neously
growing human t~mors (nude mou~e)
The tested human tumors are routinely maintained and
passaged in nu/nu CD~ mice. ~he $umors are tested at each
S third passage for their human charactPr by immunohist-
ology using monoclonal an~ibodies. ~he ~umor is remo~ed
under s~erile conditions and cut into small pieces of
volume about 1~10 mm3. One tumor piece is implanted
subcutaneously in the side of each nude mouse. Af~er 7-
14 days the tumor piece has grown into the surroundingtissue, and the ~umor size is determined from the 2
diameters using the following fonmula: V = a x b (see
above).
This procedure is repeated twice a week, and only animals
1~ with progressive tumor growth are randomi~ed to the
control group and the group to be ~rea~ed. Five mice are
used per group. Starting on the day of randomization the
animals are ~reated intravenously in accordance with ~he
treatment regimen which is indicated in the results
section. Two different dosages are used for each sub-
stance. The selected dosages are identical to the maximum
tolerable dose (MTD) which was determined beforehand for
each test substance in an extra experiment with nude
mice.
The two tumor diameters are measured for each mouse twice
a week, and the individual tumor area is calculated using
the abovementioned formula.
Evaluation:
The relative tumor size VR and T/C ~ are calculated as
above.
The statistical significance of the antitumor effect is
determined using the Wilcoxon U test. The relative tumor
sizes in the treated group are compared with the cor-
3~:~
13 -
responding relative sizes in the control group on the
same day of the experiment. The change in the tumor area
is regarded as substance-speci~ic only if it is statisti-
cally significant with p less than O . 05 .
Result:
Table 4
5~e Tm~r Dose Reyime Acti~ity
(day 9);
(T/C %)
.
Xylan poly- B 17 15 mg~bg QD9 x i.v. 61.4
~- (~aan
sulfate bron~
(n=9) camL%~)
20 mg/hg QD9 x i.v. 68.