Note: Descriptions are shown in the official language in which they were submitted.
MW/HM/89B117
2 0 ~
, ... .
HEAT TREATMENT OF METALS
-,
This invention relates to the heat treatment of metals. In particular, it
relates to the heat treatment of metals which are less readily oxidisable
than iron. Such metals include cobalt, nickel, lead, copper, palladium, -
silver and gold, alloys of such metals, and alloys of mercury.
In manufacturing articles made of metals less oxidisable than iron, it is
typically desirable to subject such articles to the step of annealing.
Although the articles are relatively difficult to oxidise, it is still ;
nonetheless necessary to maintain a reducing or non-oxidising atmosphere in
the furnace used to perform the annealing operation. It is known that in
theory nitrogen may be used to form an atmosphere that is inert for the
purposes of annealing. Typically, the nitrogen may be supplied from a
source of nitrogen which has been separated from air by distillation at ;
cryogenic temperatures and need only contain parts per million of reactive
impurities such as oxygen. Such nitrogen can be used in a heat treatment
shop as the atmosphere for a range of different heat treatments. In recent
years, it has been found that there are certain economic advantages in
producing the nitrogen on the site of its use by non-cryogenic means rather
than off-site by a cryogenic distillation process and then transporting the
nitrogen product to its site of use. There are two main ambient
temperature methods which may be used to separate nitrogen from air. The
first is by pressure swing adsorption which entails adsorbing oxygen from
the air on an adsorbent to produce a nitrogen product and then periodically
regenerating the adsorbent by subjecting it to a pressure lower than that
at which adsorption takes place. The alternative method is to separate air
by means of semi-permeable membranes. Known semi-permeable membranes
suitable for the separation of air permit oxygen to diffuse through them at
a much more rapid rate than nitrogen with the result that the non-permeate
gas becomes enriched in nitrogen.
.
Such non-cryogenic methods are able to be used to produce a nitrogen
product containing in the order of 1% by volume of oxygen more cheaply than
cryogenic methods may be used to separate nitrogen from air, provided the
cost of transporting the cryogenically produced nitrogen to its site of use
is taken into consideration. For many industrial processes the fact that
the non-cryogenically produced nitrogen contains in the order of 1% of
MW/HM/89B117
- 2 - 2~
:
oxygen as an impurity is not a drawback. However, we have found that in
the annealing of metals less readily oxidisable than iron, such an oxygen
concentration is indeed a drawback. Although at first sight it may be
thought that a suitable annealing atmosphere may be produced by mixing
hydrogen with the non-cryogenically produced nitrogen, in for example the
annealing of copper, which takes place at temperatures of 400 to 800C, the
reaction between hydrogen and oxygen proceeds sufficiently slowly at
temperatures in at least the lower part of this temperature range for there
to be a substantial risk that oxidation of the copper will still take place :
notwlthstanding the addition of a stoichiometric excess of hydrogen to the
atmosphere.
It is known to purify nitrogen containing about 1~ by volume of oxygen by
sub~ecting it to a process in which the oxygen is first catalytically
reacted with hydrogen and then the resulting water vapour is adsorbed by
means of an adsorbent or getter. The reliance on an adsorption step to
pùrify the nitrogen requires the use of a kind of apparatus in which there ;
are two parallel adsorption stages, one of which is used while the other is '
regenerated, so as to make possible continuous production of the purified
gas. The need for the adsorption stage adds considerably to the capital
and running cost of the apparatus and tends to eliminate the economic
advantage that would otherwise result from the production of nitrogen ;
on-site by non-cryogenic means. ~-
There is thus a need for a method and apparatus of annealing articles of
metal less oxidisable than iron which renders the use of non-cryogenically
produced nitrogen containing an appreciable quantity of oxygen impurity
attractive from the economic point of view.
According to the present invention there is provided a method of annealing -
an article of a metal less oxidisable than iron, comprising the steps of
subjecting the article to an annealing temperature in a heat treatment
furnace, separating nitrogen from air at the site of the annealing furnace
to produce a gas mixture containing at least 95~ by volume of nitrogen and
a minor proportion of oxygen impurity, catalytically reacting the oxygen
impurity with a stoichiometric excess of hydrogen tD form water vapour, and
passing the resulting gas mixture comprising nitrogen, water vapour and ;
unreacted hydrogen into the furnace to create an annealing atmosphere.
':,, ',. ~:'
MW/HM/89B117
_ 3
Instead of using a stoichiometric excess of hydrogen the precise
stoichiometric amount of hydrogen as required by the reactions:
2H2 + 2 ~~~ 2H20
may be employed. Accordingly the resulting gas mixture then contains no
oxygen and no hydrogen.
The invention also provides apparatus for annealing an article of a metal
less oxidisable than iron comprising an annealing furnace, means for
separating a gas mixture comprising at least 95% by volume of nitrogen and
oxygen impurity from air, and a catalytic reactor for catalytically
reacting the oxygen impurity with a stoichiometric excess of hydrogen to
form a gas mixture comprising nitrogen, water vapour and unreacted
hydrogen, wherein said reactor has an outlet in communication with the
annealing furnace so as to enable a suitable annealing atmosphere to be
created in the furnace.
The method and apparatus according to the invention are particularly suited
for the bright anneallng of the metals less oxidisable than iron.
Preferably, the nitrogen product contains 0.5-3% by volume of oxyge~
upstream of its catalytic reaction with hydrogen. In general only a small
if any stoichiometric excess of hydrogen i9 required. For example, in the
bright annealing of copper at a temperature in the order of 600C, suitable
annealing conditions can be maintained provided that the ratio of the
partial pressures of hydrogen to water vapour in the annealing atmosphere
does not fall below 1 x 10-6.
The catalytic reaction preferably takes place over a platinum or palladium
catalyst. Alternatively, copper or nickel catalysts may be used. The
catalyst is typically heated by the reaction between hydrogen and oxygen to
a temperature of up to 200C.
The method and apparatus according to invention will now be described by
way of example with reference to the accompanying drawings, in which
Figure 1 is a schematic diagram of apparatus for the bright annealing of
,~,
MW/~M/89B117
8 ~
copper, and
Figure 2 is a schematic drawing illustrating apparatus according to theinvention including a continuous mesh belt furnace.
In Figure 1 of the drawings, there is shown a plant 2 for separating
nitrogen from air by pressure-swing adsorption. Suitable plants and
apparatus for this purpose are for example disclosed in UK Patent
Specifications 2073043A and UK 2195097A. The resulting nitrogen typically
contains from 0.5 to 3~ by volume of oxygen impurity. The nitrogen stream
1s passed through a catalytic reactor 4 in which its oxygen impurity is ;~
reacted with a small stoichiometric excess of hydrogen over a palladium or
platinum catalyst at a reaction temperature. The resulting gas mixture ~ ~ ;
comprises nitrogen, hydrogen and water vapour. It is admitted to the heat
treatment furnace 6 which may be of a batch or continuous kind in which an
article made of copper or an alloy of copper such as bronze, copper-nickel,
or brass containing up to 15% by weight of zinc, is annealed by immersion
in an annealing atmosphere at a temperature of 400-800C for a period of
tlme typically in the order of 10 minutes to 2 hours. By maintaining the ;
ratio of the partial pressure of hydrogen to the par~ial pressure of water
vapour in the atmosphere and hence in the nitrogen supplied from the ;- ;~catalytic reactor 4 at a value not less than 1 x 10 6 it is possible to
maintain conditions in the atmosphere in which the copper is not
deoxidised. Therefore its bright surface is maintained during the
annealing.
:
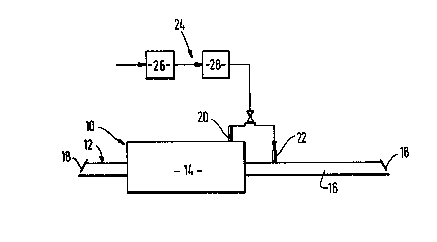
Referring to Figure 2 of the drawings, there is shown a conventional
continuous mesh belt furnace 10 having an inlet zone 12, 1 metre in length,
a hot or heated zone 14, 5.67 metres in length, and a cooling zone 16, 6.86
metres in length. The furnace 10 is provided at its respective ends with ;
baffles 18 or the like to impede the ingress of air from outside the
furnace into its interior. The hot zone 14 and the cooling zone 16 have
respectively inlets 20 and 22 for gas.
The inlets 20 and 22 are each alternatively able to be placed in :
communication for source 24 of gas mixture comprising hydrogen, water
vapour and nitrogen. The source 24 comprises a plant 26 for separating
nitrogen from air by pressure swing adsorption, and a catalytic reactor 28
MW/HM/89B117
_ 5 _ ~ $ ~
for reacting oxygen impurity in the nitrogen with hydrogen. A commercially
available catalyst comprising palladium supported on alumina is used.
Typically, the catalyst is heated by the reaction between hydrogen and
oxygen and the gas mixture leaves the reactor 28 at a temperature in the
range of 50 to 150C.
In operation, a gas mixture comprising nitrogen, water vapour and hydrogen
from the catalytic reactor 28 is passed into the furnace 10 through either
or both the inlets 20 and 22. The hot zone 14 is heated to a chosen
temperature typically in the range 500 to 800C. Copper work to be bright
annealed is loaded onto the belt (not shown) of the furnace and the belt is
then advanced slowly through the furnace at a speed such that the work
typically has a residence time in the furnace of from 30 minutes to 1 hour.
As the work passes through the hot zone 14 so it is raised approximately to
the temperature of the hot zone 14. On leaving the hot zone 14 the work
passes into the cooling zone 16 in which it is cooled by contact with the
relatively cold atmosphere therein. Typically, the work is at a
temperature between ambient and 50C when it leaves the furnace 10. By
employing an atmosphere in accordance with the invention, it can be ensured
that bright annealed work leaves the furnace 10.
A number of experimental tests of the apparatus shown in Figure 2 were
performed. The results of the tests are set out in Tables 1 to 4 below.
Table 1 shows the results of comparative experiments in which various
different proportions of hydrogen were mixed with nitrogen from a PSA plant
(0.5% oxygen impurity) and the resulting mixture passed into the furnace
without a catalytic reaction between hydrogen and oxygen being performed.
It was a feature of the furnace used that entry to the hot zone was by way ~ ;
of a muffle made of an alloy with high nickel and chrome content which had ~ -;
the property of acting as a getter for free oxygen. Accordingly, it was
found that substantially all the oxygen entering the furnace with the gas
mixture was removed therefrom. It was therefore possible to obtain bright
work (at 750C) even when the oxygen level was nominally in excess of the
stoichiometric requirement for reaction with hydrogen. Results are given
in Table 1 for the oxygen concentration, hydrogen concentration and dew
point in both the hot zone 14 and the cooling zone 16 of the furnace 10 at
the different hydrogen levels.
, ~ . .
' ~
MW/HM/89B117
The results set out in Table 2 relate to a set of experiments similar to
those of Table 1 save that the mixture of nitrogen, oxygen and hydrogen was ~ .
reacted in the catalytic reactor 28 upstream of being admitted to the hot ; :
zone of the furnace through the inlet 20. :.
The Experiments illustrated in Table 3 and 4 are respectively comparable to
those set out in Tables 1 and 2 with the exception that the gas mixture is :
not introduced directly into the hot zone 14 through the inlet 20. Rather,
it is introduced directly into the cooling zone 16 through the inlet 22. . :
Table 3 shows experiments in which the gas mixture by-passed the catalytic
reactor 28, whereas Table 4 relates to experiments in which the catalytic
reactor was used. The low dew points obtained in the experiments ~: .
illustrated by Table 3 indicate that when the gas mixture is supplied
directly to the cooling zone, a failure to react the oxygen with at least a
stoichiometric quantity of hydrogen in the catalytic reactor 28 will~tend
to result in work that is not bright even if the hot zone is operated at a ::
temperature as high as 750C.
It must be further borne in mind that if there is used a furnace 10 which
does not have a muffle that acts as a getter for oxygen and if the hot zone
14 is operated at a temperature substantially lower than 750C, there is : :~
a substantial risk that copper work will not be given a bright finish. ~ :
''.~
MW/HM/89B117 ~0~9~6~ :
TABLE 1 - H2/02/N2 MIXTURE ADMITTED DIRECTLY INTO HOT ZONE
H2 ADDITION ZONE ATMOSPHERE COMPOSITION
(Z BY VOLUME) (H = HOT; 2 H2 DEW POINT (C)
C = COOLING)
0.7 H 910mV 0% -6
C 875mV 0% -26
1.45% H 880mV 0.07% +6
C 880mV 0.1~ -6
2.05 H 915mV 0.7 10
C 930mV 0.65% +6
2.6 H 935mV 1.25% +9
C 945mV 1.3% +9
....
3.15 H 955mV 2.2% +10~ ~ ;
C 955mV 2.1 9~ ~ ;
: ~;
3.9 H 965mV 2.5% +lO ;
C 965mV 2.6% +10
4.25 H 975mV 3.1% +10
C 970mV 3.1% +11
... .
: ,;,j :. :,;
::". ,,'.~' ,, '.,. '
..: :::,:, .
- ',''',",'. ' '",
-:. ., : ;:;
: .:: . : ': . :~
'::~",,
,'' ~''"' ;'
~ , .. ....
: . ' . '. ,' ~;
MW/HM/89Bil7
- 8 - 2 ~ 6 ~.~
. . . :,,
TABLE 2 - H2/02/N2 MIXTURE CATALYTICALLY REACTED UPSTREAM
OF ENTRY INTO HOT ZONE
H2 ADDITION ZONE ATMOSPHERE COMPOSITION
(% BY VOLUME) (H = HOT; 2 ~2 DEW POINT (C~
C = COOLING)
;: ',
- H 715mV - -21~
- C 40ppm - -29 ~;
0.7 H 865mV 0% +3
C 845mV 0% -27
1.45 H 845mV 0% +10
C 860mV 0% 0
2.05 H 845mV 0.15% +15
C 860mV 0.2% +12
2.6 H 910mV 0.95% +14 ;~
C 920mV 1.2% +10
3.15 H 950mV 1.9% +11
C 950mV 2.05% +12
3.9 H 965mV 2.5Z +12
C 960mV 2.6% +12
4.25 H 970mV 3.15% +12 ;
C 970mV 3.1% +11~ ,-
MW/HM/89B117
9~
TABLE 3 - H2/02/N2 MIXTURE ADMITTED DIRECTLY INTO COOLING ZONE
H2 ADDITION ZONE ATMOSPHERE COMPOSITION
(% BY VOLUME) (H = HOT; 2 H2 DEW POINT (C)
C = COOLING)
' '
- H 4ppm 0% -21
- C 4000ppm OX -31
0.7 H 780mV 0% +3
C 75ppm 0.45% -29
1.45 H 850mV 0.15% +9
C 560mV 1.05% -24
'', ,:';~
2.05 H 930mV 0.75% +6~ i
C 900mV 1.55% -16 ~ ~ ;
.,, : ~.:: :,.
2.6 H 945mV 1.4% +7
C 930mV 2.55% -12
:. ~,: ,~":
3.9 H 975mV 2.65% +7 ;
C 955mV 3.4Z 12~
",.. ;,.. ..
. . :. :: :~.:
4.25 H 980mV 3.3% +6 ,
C 960mV - 4.2% -12~
.
,',''''.' ,',~: ,.'"',,'
' ~ ` ',""' " "~ ,''',
'' '"'',,','~"'',''",
: ~ . '; ' ~ ' :' ;.
~..:;:.,::.: ~
~ : ',' .-.';,'
', ,:' ';. ,`', '~:
,~, . , . ` : . .
.,, .'.'`:; '"'
., . . ~, '`,
.:,' .. .. ..
MW/HM/89B117
lo - 2 ~
TABLE 4 - H2/02/N2 MIXTURE CATALYTICALLY REACTED UPSTREAM
OF ENTRY INTO COOLING ZONE
H2 ADDITION ZONE ATMOSPHERE COMPOSITION
(% BY VOLUME) (H = HOT; 2 H2 DEW POINT (C)
C = COOLING) ~ -
- H 4ppm - -24
- C 4500 ppm - -36
0.7 H 815mV 0% +6 ~-
C 950ppm 0% -32~ ;~
1.45 H 825mV 0% +13
C 880mV 0.2% 0
. .
2.05 H 895mV 0.55% +11
C 920mV 0.7% +7
2.6 H 935mV 1.5X +10~ ;
C 945mV 1.65% +8
3.15 H 960mV 2.3% +10
C 960mV 2.2~ +8
3.9 H 970mV 3.1% +10
C 970mV 3.2% +8
. ~.
4.25 H 980mV 3.65% +8
C 980mV 3.7% +8
MW/HM/89B117
1l- 201956a
NOTE
Oxygen concentrations were measured using an oxygen probe. The temperature
of the gas from the hot zone at the sampling point was 750C: the
temperature of the gas from the cooling zone at the sampling point was
200C. Most results are shown in Millivolts (mV). The actual oxygen
concentration can be calculated using the formula
.,.., .:
E . 0.0215.T. loge (20.95/% 2)
.,.,.. ~.:
where
.'~, .' ~ ,`"
E is the oxygen probe reading in mV ~
: . .~ .
T is the temperature in Kelvin at the oxygen probe location.
: ::: . .::
%2 is the concentration of oxygen expressed as a percentage by volume.
;;''';' ,' ,' '
:: :: -.: :.:.:~:;:
''',','.''.,''','',
: ,:.~, . :-: ,;
, :"" ,:,, ~,;, ,.
,:'~','"''' ~- :,
.""~','~'':''
:,.,',.,'' ' :,
,., :: :
;.: :' ''',.
....
,:
..~.,:..~
.:: . . . - .
`. ,~;',
'~``'': "` '