Note: Descriptions are shown in the official language in which they were submitted.
2~2~3~ `
4-17653/+
Novel pyrirnidine derivatives
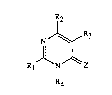
The invention relates to novel pylimidine derivatives of the forrnula
R2
NJ~R3
ll I (I),
R1 N/~Z
R4
their tautomers and salts, in which Z is 0, S or N(R) and R is hydrogen or an aliphatic
hydrocarhon radical; Rl is an unsubstituted or substituted aliphatic hydrocarbon radical, a
cycloaliphatic or araliphatic hydrocarbon radical or an aromatic radical, R2 and R3,
independently of one another, are halogen, acyl, an aromatic radical, unsubstituted or
substituted arnino, or carboxyl which, if desired, may be esterified or amidated; or R2 is
-Zl-R'2 and R3 is -Z2-R'3, Zl and Z2 independently of one another being a bond, O or
S()n and n being 0, 1 or 2, and R'2 and R'3 independently of one another being hydrogen,
an araliphatic or aliphatic hydrocarbon radical, the latter being unsubstituted or substituted
and, if desired, interrupted by -O- or -S~O)n-, n being 0, 1 or 2; or R2 and R3 together are
propylene or butylene or are the partial structure of the forrnula -CH=CH-CH=CH-, in
which, if desired, one or two of the methyne groups are replaced by -N=; R4 is a group of
the forrnula
R5
Alk~3~ (Ia)
in which Alk is a divalent aliohrtic hydrocarbon; Rs Is COOH, S03H,
'
.
2~2~37~
- 2 -
haloalkanesulfamoyl, PO2H2, PO3H2 or 5-tetrazolyl; the rings A and B or the
(hetero)aromatic ring jointly -formed by R2 and R3 are, independently of one another,
unsubstituted or substituted;
and to processes for their preparation, pharmaceutical preparations and their use.
Compounds of the forrnula I may exist as proton tautomers. If, for example, R2 is
hydroxyl, appropriate compounds may be in equilibrium with the tautomeric 4-oxo
derivatives.
The compounds of the formula I may be present as salts, in particular pharmaceutically
acceptable salts. If the compounds according to the invention have at least one basic
centre, they can thus form acid addition salts. These are formed, for example, with
inorganic acids, such as mineral acids, for example sulfuric acid, a phosphoric or
hydrohalic acid, or with organic carboxylic acids, such as (~ 4)alkanecarboxylic acids
which, for example, are unsubstituted or substituted by halogen, for example acetic acid,
such as saturated or unsaturated dicarboxylic acids, for exarnple oxalic, malonic, succinic,
maleic, fumaric, phthalic or terephthalic acid, such as hydroxycarboxylic acids, for
example ascorbic, glycolic, lactic, malic, tartaric or citric acid, such as amino acids, for
example aspar~ic or glutamic acid, benzoic acid or with organic sulfonic acids, such as
(Cl-C4)alkane or arylsulfonic acids which are unsubstituted or substituted, for example,
by halogen, for example methane- or toluenesulfonic acid. Corresponding acid addition
salts having, if desired, an additionally present basic centre can also be formed.
Furthermore, the compounds according to the invention having an acid group (Rs = for
example, COOH or 5-tetrazolyl) can form salts with bases. Suitable salts with bases are,
for example, metal salts, such as alkali metal salts or alkaline earth metal salts, for
example sodium, potassium or magnesium salts, or salts with ammonia or of substituted
organic amines, such as morpholine, thiomorpholine, piperidine, pyrrolidine, such as
mono-, di- or tri(lower allcyl)amines or mono-, di- or trihydroxy(lower alkyl)amines, for
example mono-, di- or triethanolamine. Mono(lower alkyl)amines are, for example,ethylamine or tert-butylamine. Di(lower alkyl)amines are, for example, diethylamine or
dipropylamine, and possible tri(lower alkyl)amines are, for example, triethylamine,
tributylamine or dimethylpropylamine. In addition, corresponding internal salts can be
formed. Furthermore included for pharmaceutical uses are non-suitable salts, as these can
be employed, for example, for the isolation or purification of compounds according to the
invention or their pharrnaceutically acceptable salts.
2~2~3~
- 3 -
An aliphatic hydrocarbon radical is, for example, lower alkyl, lower alkenyl andsecondarily lower al~ynyl.
A cycloaliphatic hydrocarbon radical is, for example, cycloalkyl and secondarily
cycloalkenyl.
A suitable araliphatic radical is, in particular, phenyl-lower alkyl and furtherrnore
phenyl-lower alkenyl and phenyl-lower aLkynyl.
An aromatic radical is, for example, a carbocyclic or heterocyclic aromatic radical, in
particular phenyl and furthermore naphthyl, or in particular an appropriate 5- or
6-membered and monocyclic radical which has up to 4 identical or different heteroatoms,
such as nitrogen, oxygen or sulfur atoms, preferably one, two, three or four nitrogen
atoms, an oxygen atom or a sulfur atom. Appropriate 5-membered heteroaryl radicals are,
for example, monoaza-, diaza-, triaza-, tetraaza-, monooxa- or msnothia-cyclic aryl
radicals, such as pyrrolyl, pyrazolyl, imidazolyl, triazolyl, tetrazoly, furyl and thienyl,
while s~litable appropriate ~-membered radicals are, in particular, pyridyl.
Acyl is, in par~ic~ilar, lower alkanoyl, phenyl-lower alkanoyl or unsubstituted or
substltuted benzoyl.
Substituted amino is, for example, independently of one another mono- or disubstitu~ed by
an aliphatic or araliphatic hydrocarbon radical, such as lower alkyl, lower alkenyl, lower
aLkynyl, phenyl-lower alkyl, phenyl-lowcr alkenyl or phenyl-lower alkynyl, or by a
divalent aliphatic hydrocarbon radical, such as lower alkylene or lower alkyleneoxy-lower
alkylene, disubstituted amino, for example lower alkyl-, lower alkenyl-3 lower alkynyl-,
phenyl-lower alkyl-, phenyl-lower alkenyl-, phenyl-lower a!kynyl-, di(lower alkyl)-, N
lower alkyl-N-phenyl-lower alkyl-, di-phenyl-lower alkyl-amino, lower alkyleneamino or
lower alkyleneoxy-lower alkyleneamino.
Esterified carboxyl is, for example, carboxyl which is esterified by an aliphatic alcohol,
this being derived from an aliphatic hydrocarbon radical, such as lower alkyl, lower
alkenyl and secondarily lower alkynyl and being interrupted, if desired, by -O-, such as
lower alkoxy-lower alkyl, -lower alkenyl and -lower alkynyl, for example }ower alkoxy-,
lower alkoxy-lower alkoxy- or lower alkenyloxy-carbonyl.
2~37~
- 4 -
Amidated carboxyl is, for example, carbamoyl in which the amino group, for example as
stated above, is, if desired, independently of one another mono- or disubstituted by an
aliphatic or araliphatic hydrocarbon radical or disubstituted by a divalent aliphatic
hydrocarbon radical which, if desired, is intermpted by -O-, a suitable divalent aliphatic
hydrocarbon radical which, if desired, can be interrupted by -O- being, in particular, lower
alkylene or lower alkyleneoxy-lower alkylene.
An aliphatic hydrocarbon radical which is interrupted by -O- is, in particular, lower
alkoxy-lower alkyl, -lower alkenyl or -lower alkynyl, lower alkenyloxy-lower alkyl,
-lower alkenyl or -lower aLkynyl, while the aliphatic hydrocarbon radical which is
interrupted by -S(O)n- is, in particular, lower aLtcylthio-lower aLkyl, -lower aLkenyl or
-lower alkynyl, lower alkanesulfinyl-lower alkyl or -sulfonyl-lower alkyl, loweralkenylthio-lower allcyl, -sulfinyl-lower alkyl or -sulfonyl-lower alkyl, and lower
alkynylthio-lower alkyl, -sulfinyl-lower alkyl or -sulfonyl-lower aLkyl.
A partial structure of the formula -CH=CH-CH=OEI- in which one or two of the methyne
groups ~re replaced by -N=, is, for example, -N=CH-CH=CH-, -CH=N-CH=CH-,
-CH=CH-N=CI~-, -CH=CH-CH=N-, -N=CH-N=OEI- or-CH=N-CH=N-.
, .
A divalent aliphatic hydrocarbon radical is, for example, lower alkylene or lower
alkylidene.
Haloalkanesulfamoyl is, in particular, halo-lower alkanesulfamoy}.
Substituents of an aliphatic hydrocarbon which, if desired, is interrupted by O ~r S()n
are, for example, selected from the group comprising halogen, unetheri~led or etherified
hydroxyl, S(O)n-R, unsubstituted or substituted amino or carboxyl which, if desired, is
esterified or amidated. Preferred substituents of an aliphatic hydrocarbon Rl are, for
example, halogen or hydroxyl. Appropriate substituted aliphatic hydrocarbon radicals
preferably have a substituent which is primarily located in longer-chain radicals in a
higher position than the a-position. Preferred aliphatic hydrocarbon radicals which may be
mentioned are, for example, halo-lower alkyl, and furthermore -lower aLIcenyl or -lower
alkynyl, hydroxy-lower alkyl, and furthermore -lower alkenyl or -lower alkynyl.
Etherified hydroxyl is, for exarnple, hydroxyl etheri-fied by an aliphatic alcohol, in
particular lower alkoxy or lower alkenyloxy and is also a phenyl-lower alkoxy or phenoxy
2~37~
radical.
(Hetero)aromatic radicals, including the rings A and B and, if R2 and R3 are the partial
structure of the formula -CH=CH-CH=CH- in which, if desired, one or two of the methyne
groups are replaced by -N -, the (hetero)aromatic ring forrned by this partial structure, are,
in particular, if not differently defined, in each case unsubstituted, monosubstituted or
po}ysubstituted, for example disubstituted or trisubstituted, in particular, for example, by a
substituent selected from the group comprising halogen, unetherified or etherified
hydroxyl, S(O)m-R and a hydrocarbon radical which is uninterrupted and/or interrupted by
-O- and unsubstituted or substituted, for example, by halogen or hydroxyl. Phenyl which is
unsubstituted or monosubstituted or polysubstituted, for example disubstituted or
trisubstituted, for example by a substituent selected from ~he group comprising lower
alkyl, lower alkoxy, halogen, trifluoromethyl and hydroxyl, is preferred.
Above and below, unsaturated aliphatic, cycloaliphatic and araliphatic substituents are
primarily not linked to an aromatic radical via the C atom at which a multiple bond
commences.
The general definitions used above and below have, if not defined differently, the
following meanings:
The expression "lower" means that corresponding groups and compounds in each case
contain in particular not rnore than 7, preferably not more than 4, carbon atoms.
Halogen is, in particular, halogen of atomic number not more than 35, such as fluorine,
chlorine or bromine, and furthermore includes iodine.
Lower alkyl is, in particular, Cl-C7alkyl and is, for example, methyl, ethyl, n-propyl,
isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl and furthermore includes corresponding
pentyl, hexyl and heptyl radicals. Cl-C4Alkyl is preferred.
Lower alkenyl is, in particular, C3-C7alkenyl and is, for example, 2-propenyl or 1-, 2- or
3-butenyl. C3-CsAlkenyl is preferred.
Lower alkynyl is, in particular, C3-C7alkynyl and is preferably propargyl.
..
2~2~3~
Cycloalkyl is, in particular, C3-C7cycloalkyl and is, for example, cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl and cycloheptyl. Cyclopentyl and cyclohexyl are preferred.
Cycloalkenyl is, in particular, C3-C7cycloalkenyl and is preferably cyclopent-2- or
-3-enyl, or cyclohex-2-`and -3-en-yl.
Phenyl-lower alkyl is, in particular, phenyl-C1-C4alkyl and is preferably benzyl, 1- and
2-phenethyl, while phenyl-lower alkenyl and phenyl-lower alkynyl are, in particular,
phenyl-C3-Csalkenyl and -alkynyl, in particular 3-phenylallyl and 3-phenylpropargyl.
Pyrrolyl is, for example, 2- or 3-pyrrolyl. Pyrazolyl is 3- or 4-pyrazolyl. Imidazolyl is 2- or
4-imidazolyl. TIiazolyl is, for example, 1,3,5-lH-~iazol-2-yl or 1,3,4-triazol-2-yl.
Tetrazolyl is, for example, 1,2,3,4-tetrazol-5-yl. Furyl is 2- or 3-~uryl and thienyl is 2- or
3-thienyl, while suitable pyridyl is 2-, 3- and 4-pyridyl.
Lower alkanoyl is, in pa~ticular, Cl-C7alkanoyl and is, for exarnple, formyl, acetyl,
propionyl, butyryl, isobutyryl or pivavolyl. C2-CsAlkanoyl is preferred.
Phenyl-lower alkanoyl is, in particular, phenyl-C2-C7alkanoyl and is, for example,
phenylacetyl or 2- or 3-phenylpropionyl. Phenyl-C2-C4alkanoyl is preferred.
Lower alkylene is, in particular, Cl-C7alkylene, is straight-chain or branched and is, in
particular, methylene, ethylene, propylene or butylene and also 1,2-propylene,
2-methyl-1,3-propylene and 2,2-dimethyl-1,3-propylene. C2-C5Alkylene is preferred.
Lower alkyleneoxy-lower alkylene is, in particular, C1-C4alkyleneoxy-C2-C4alkylene,
preferably ethyleneoxyethylene.
Lower alkylamino is, in particular, Cl-C7alkylamino and is, for example, methyl-, ethyl-,
n-propyl- and isopropyl-amino. C1-C4Alkylamino is preferred.
Lower alkenylamino is preferably C3-Csalkenylamino, s~lch as allyl- and methallylamino.
Lower alkynylamino ls preferably C3-Csalkynylamino, such as propargylamino.
Phenyl-lower alkylamino is preferably phenyl-C1-C4alkylamino~ in particular benzyl-, 1-
2~3~
and 2-phenylethylamino.
Pheny}-lower aLkenylamino ;s preferably phenyl-C3-Csalkenylamino, in particular
phenylallylamino and 3-phenylmethallylamino.
Phenyl-lower alkynylamino is preferably phenyl-C3-Csalkynylamino, in particular
3-phenylpropargylamino.
Di (lower aLkylamino) is, in particular, di-Cl-C4alkylamino, such as dimethyl-, diethyl-,
di-n-propyl-, methylpropyl-, methylethyl-, methylbutyl-arnino and dibutylarnino.
N-Lower alkyl-N-phenyl-lower alkylamino is, in particular, N-Cl-C4alkyl-N-
phenyl-Cl-(',4alkylamino, preferably methylbenzylamino and ethylbenzylamino.
Diphenyl-lower alkylamino is, in particular, di-phenyl-CI-C4alkylamino, preferably
dibenzylamino and diphenylethylamino.
Lower alkyleneamino is, in particular, C2-C~jalkyleneamino, such as l-a~iridinyl,
l-azetidinyl, l-pyIrolidinyl, l-piperidinyl or l-azepidinyl, while suitable lower
alkyleneoxy-lower aLkyleneamino is, in particular, 4-morpholinyl.
Lower alkoxy is, in particular, Cl-C7alkoxy and is, for example, methoxy, ethoxy,
n-propyloxy, isopropyloxy, n-butyloxy, isobutyloxy, sec-butyloxy, tert-butyloxy and
furthelmore includes corresponding pentyloxy, hexyloxy and heptyloxy radicals.
Cl-C4Alkoxy is preferred.
Lower alkenyloxy is, in particular, C3-C7alkenyloxy and is, for example, allyloxy or
but-2-en- or but-3-enyloxy. C3-CsAlkenyloxy is preferred.
Lower alkoxy-lower alkyl is, in particular, Cl-C4alkoxy-Cl-C4alkyl, such as
2-methoxyethyl, 2-ethoxyethyl, 2-n-propyloxyethyl or ethoxymethyl.
Lower alkoxy-lower alkenyl is, in particular, Cl-C4alkoxy-C3-C4alkenyl, such as
3-methoxyallyl, 3-ethoxyallyl or 2-n-propyloxyallyl, while suitable lower alkoxy-lower
alkynyl is, in paIticular, Cl-C4alkoxy-C3-C4alkynyl, such as 3-methoxypropargyl.
3 7 ~
Z1489-8039
- 8 -
Lower alkoxycarbonyl is, in particular, C2-C8alkoxycarbonyl and is, for example,methoxy-, ethoxy-, propyloxy- or pivaloyloxy-carbonyl. C2-CsAlkoxycarbonyl is
preferred.
Lower alkoxy-lower alkoxycarbonyl is, in particular, Cl-C4alkoxy-Cl-C4alkoxycarbonyl,
preferably ethoxyethoxycarbonyl, methoxyethoxycarbonyl and
isopropyloxyethoxycarbonyl.
Lower alkenyloxycarbonyl is, in particular, C3-C5alkenyloxycarbonyl, preferably
allyloxycarbonyl, while lower aLlcynyloxycarbonyl is, in particular,
C3-CsaLkynyloxycarbonyl7 such as propargyloxycarbonyl.
Lower alkenyloxy-lower allcyl is, in particular, C3-Csalkenyloxy-CI-C410wer alkyl, such
as 2-allylethyl, or 2- or 3-allylpropyl.
Lower alkenyloxy-lower alkenyl is7 in particular, C3-Csalkenyloxy-C3-Csalkenyl, such as
3-allyloxyallyl, while lower alkenyloxy-lower alkynyl is, in par~icular,
C3-Csalkenyloxy-C3-Csalkynyl, such as 3-allylpropargyl.
Lower alkylthio is, in particular7 Cl-C7alkylthio and is, for example, methyl-, ethyl-,
n-propyl-, isopropyl-, n-butyl-, isobutyl-, sec-butyl- or tert-butylthio. Cl-C4Alkylthio is
preferred.
Lower aLkanesulfinyl or -sulfonyl is, in particular, Cl-C7alkanesulfinyl or -sulfonyl and is,
for example, methane-, ethane-, n-propane- or isopropane-sulfinyl or -sulfonyl.
Cl-C4Alkanesulfinyl or -sulfonyl is preferred.
Lower alkenylthio is, in particular, C3-Csalkenylthio, such as allylthio, while lower
alkynylthio is, in particular, C3-Csalkynylthio, such as propargylthio.
Lower aLkylthio-lower alkyl is, in particular, Cl-C4alkylthio-Cl-C4alkyl, such as
ethylthiomethyl, 2-ethylthioethyl, 2-methylthioethyl, 2-isopropylthioethyl, while suitable
lower alkanesulfinyl- or lower alkanesulfonyl-lower aLkyl are, in palticular, appropriate
Cl-C4alkanesulfinyl- or Cl-C4alkanesulfonyl-C1-C4alkyl radicals.
Lower alkylthio-lower aL~enyl or -lower alkynyl is, in particular
2~37~
9 21489-8039
Cl-C4alkylthio-C3-Csalkenyl or -aL~cynyl.
Lower alkenylthio-lower alkyl is, in particular, C3-Csalkenylthio-CI-C4alkyl, such as
l-allylthioethyl or 3-allylpropyl, while suitable lower alkenylsulfinyl- or -sulffinyl-lower
alkyl are, in particular, C3-Csalkenylsulfinyl- or -sulfonyl-Cl-C4alkyl.
Lower alkynylthio-lower aL1~yl is, in particular, C3-Csalkynylthio-Cl-C4aLkyl, such as
2-propargylthioethyl or 3-propargylpropyl, while suitable lower alkynylsulfinyl- or
-sulfonyl-lower aL~cyl are, in particular, C3-Csalkynylsulfinyl- or -sul~onyl-CI-C4aL~cyl.
Lower alkylidene is, in palticular, C2-C7aLlcylidelle, such as et~lidene, lrl- or
2,2-propylidene, and furthermore 1,1- or 2,2-butylidene. C2-C3AL~cylidene is preferred.
HaloaLI~ylsulfamoyl is, in particular, halo-Cl-C7aL~canesulfamoyl, in particularhalo-CI-C4aL~cylsulfamoyl, and is, for example, trifluoromethane-, difluoromethane- or
1, 1 ,2-trifluoroethanesulfamoyl.
Halo-lower alkyl is, in particular, halo-CI-C4alkyl, such as trifluoromethyl,
1,1,2-trifluoro-2-chloroethyl or chloromethyl.
Halo-lower alkenyl is, in particular, halo-C3-Csalkenyl, such as 3-chloroallyl.
Halo-lower alkynyl is, in particular, halo-C3-Csalkynyl, such as 3-chloropropargyl.
Hydroxy-lower alkyl is, in particular, hydroxy-Cl-C4aLtcyl, such as hydroxymethyl,
2-hydroxyethyl or 3-hydroxypropyl.
Hydroxy-lower alkenyl is, in particular, hydroxy-C3-Csalkenyl, such as 3-hydroxyallyl.
Hydroxy-lower alkynyl is, in particular, hydroxy-C3-Csalkynyl, such as
3-hydroxypropargyl .
Phenyl-lower alkoxy is, in particular, phenyl-Cl-C4alkoxy, such as ~enzyloxy, 1- or
2-phenylethoxy, or 1-, 2- or 3-phenylpropyloxy.
Extensive pharrnacological investigations have shown that the compounds according to
3 7 ~
- 10-
the invention have pronounced angiotensin II antagonist properties.
As is known, angiotensin II has strong vasoconstrictor properties, additionally stimulates
aldosterone secretion and thus causes distinct sodium/water retention. The consequence of
angiotensin II activity is m~mifested in an increase in blood pressure.
The importance of angiotensin II antagonists is in suppressing the vasoconstrictor and
aldosterone secretion-stimulating effects caused by angiotensin II by competitive
inhibition of the binding of angiotensin II to the receptors.
The angiotensin II antagonist properties of the compounds according to the invention can
be detected in the angiotensin II binding test. Rat smooth muscle cells from homogenized
rat aorta are used here. The solid centrifugate is suspended in 50 rnM tris bu-ffer~ pH 7.4
using peptidase inhibitors. Samples are incubated ~or 60 minutes at 25C with
125I-angiotensin III (0.175 nM) and a varying concentration of angiotensin II or the test
substance. The incubation is then ended by addition of saline buffered with ice-cold
phosphate, and the mixture is filtered through a Whatman GF/F filter. The filters are
counted using a gatnma counter. The ICso values are determined fiom the dose-effect
curve. ICso values from about lO nM were determined for the compounds according to the
invention.
For the determination of angiotensin II-induced vasoconstriction, investigations on the
isolated guinea-pig aorta ring can be used. For this purpose, aorta rings are dissected from
each chest and fixed between two parallel clamps at an in;tial tension of 2 g. The rings are
then immersed in 20 ml of a tissue bath at 37C and aerated with 95 % 2 and 5 % C2-
The isometric reactions are measured. At 20-minute intervals, the rings are alternately
stimulated with lO nM angiotensin II (EIypertensin-CI~A) and 5 nM noradrenaline
chloride. The rings are then incubated with selected concentrations of the test substances
before treatment with the agonists. The data are analysed using a B~xco digital computer.
The concen~rations which cause a 50 % inhibition of the initial control value are given as
IC50 values. ICso values from about 5 nM were determined for the compounds according
to the invention.
The fact that the compounds according to the invention can reduce high blood pressure
induced by angiotensin II can be verified in the normotensive anaesthetized rat test model.
After calibration of the preparations with 0.9 % NaCl (l mVkg i.v.), noradrenaline (l ~lg,kg
2 3 2 ~ 3 7 ~
21489-8039
i.v.) or angiotensin II ~0.3 ~,-g/kg i.v.) in each case, increasing doses (3-6) of the test
substance are intravenously injected by bolus injection, after which angiotensin II or
noradrenaline is administered a-fter each dose at 5 m~nute intervals. The blood pressure is
measured directly in the carotid artery and recorded using an on-line data recognition
system (Buxco). The specificity of the angiotensin II antagonism is shown by the selective
inhibition of the pressure effect produced by angiotensin II, but not that peoduced by
noradrenaline. In these investigations, the compounds according to the invention showed
an inhibiting effect from a dose of about 0.3 rng/ig i.v.
The antihypertensive activity of the compounds according to the invention may also be
manifested in the renally hypertensive rat. High blood pressure is produced in male rats by
constricting a renal artery according to the Goldblatt method. Doses of the substance are
adrninstered to the rats by means of a stomach tube. Control animals receive an equivalent
volume of solvent. Blood pressure and heart beat are measured indirectly at intervals in
conscious animals by the tail clamp method of Gerold et al. (Helv. Physiol. Acta 24, 58,
1966) before administration of the substances or of the placebo and during the course of
the experiment. It was possible to detect the pronounced antihypertensive effece below a
dose of about 30 mg~cg p.o.
The comp~unds of the forrnula I can therefore be used, for example, as pharmaceutical
active ingredients, such as antihypertensives, for example for the trea~nent of high blood
pressure and cardiac insufficiency. The invention further relates to the use of the
compounds according to the invention for the production of medicarnents, in particular
angiotensin II antagonists and antihypertensives, and the use of these compounds for the
therapeutic treatmènt of high blood pressure and cardiac insufficiency. The industrial
production of the active substances is also included in the production of the
pharmaceuticals.
The invention relates in particular to compounds of the formula I, their tautomers and
salts, in which Z is O, S or N(R) and R is hydrogen or an aliphatic hydrocarbon radical, R
is an aliphatic radical which is unsubstituted or substituted by halogen or hydroxyl, a
cycloaliphatic or araliphatic hydrocarbon radical or an aromatic radical, R2 and R3,
independently of one another, are halogen, acyl, an aromatic radical, carboxyl which, if
desired, is esterified or arnidated, or R2 is -Zl-R'2 and R3 is -Z2-R'3, Zl and Z2
independently of one another being a bond or O, S()n or NH, n being 0, l or 2 and R'2
and R'3 independently of one another being hydrogen or an aliphatic hydrocarbon radical
2~37~
- 12- . 21489-8039
which is unsubstituted or substituted by halogen, hydroxyl, unsubstituted or substituted
amino, or carboxyl which, if desired, is esterified or amidated, and which, if desired, is
interrupted by -0- or -S(O)n, n being 0, l or 2, R4 being a group of the fo~nula
~2~ (Ib)
in which R5 is COOH, S03H, haloaLkanesulfamoyl, ~ H2, P03H2 or S-tetrazolyl.
The invention relates in particular to compourfds of the formula I, their tautomers and salts
in which Z is 0, S or N(R) and R is hydroge n, lower alkyl, lower aLkenyl or lower alkynyl;
Rl is lower alkyl, lower alkenyl or lower aLkynyl which in each case are unsubstituted or
substituted by substituents selected from the group comprising halogen, hydroxyl, lower
alkoxy, lower alkenyloxy, phenyl-lower aLkoxy, phenoxy, mercapto, lower alkylthio,
lower alkanesulfinyl or -sulfonyl, lower alkenylthio, lower alkenylsulfinyl or -sulfonyl,
lower alkynylthio, lower alkynylsulfinyl or -sulfonyl, amino which, if desired, is
independently of one another mono- or disubstituted by lower alkyl, lower aLkenyl, lower
alkynyl, phenyl-lower alkyl or phenyl-lower alkynyl or disubstituted by lower-aLkylene or
lower alkyleneoxy-lower alkylene, carboxyl, lower alkoxy-, lower aLkoxy-lower alkoxy-
or lower alkenyloxy-carbonyl and carbamoyl in which, if desired, the amino group is
independently of one another mono- or disubstituted by lower alkyl, lower alkenyl, lower
alkynyl, phenyl lower alkyl or phenyl-lower alkynyl or disubstituted by lower alkylene or
lower aLkyleneoxy-lower aL~cylene, or in each case 3- to 7- membered cycloaLkyl or
cycloaLkenyl, phenyl-lower aLkyl, phenyl-lower aLIcenyl, phenyl-lower aLlcynyl, phenyl,
naphthyl, pyrrolyl, pyrazolyl, imidazolyl, biazolyl, tetrazolyl, furyl, thienyl or pyridyl; R2
and R3 independently of one another are halogen, lower aLkanoyl, phenyl-lower aLkanoyl,
benzoyI phenyl, naphthyl, pyrrolyl, pyrazolyl, imidazclyl, triazolyl, te~azolyl, furyl,
thienyl, pyridyl, amino which, if desired, is independently of one another mono- or
disubstituted by lower alkyl, lower aLIcenyl, lower alkynyl, phenyl-lower aLkyl or
phenyl-lower alkynyl or disubsdtuted by lower alkylene or lower alkyleneoxy-lower
alkylene, carboxyl, lower alkoxy-, lower aLkoxy-lower aLlcoxy- or lower all~ ylo~
carbonyl or carbamoyl in which the amino group, if desired, is independently of
one another mono- or disubstituted by lower alkyl, lower alkenyl, lower alkynyl,phenyl-lower alkyl or phenyl-lower alkynyl or disubstituted by lower alkylene or lower
~ ' ' . . . .
7 ~
- 13- 21489-8039
aLkyleneoxy- lower allcylen; or R2 is -Zl-R2' and R3 is -Z2-R3', Zland Z2 independently
of one another being a bond, O or S()n and n being 0, l or 2, and R2' and R3'
independently of one another being hydrogen, phenyl-lower alkyl, phenyl-lower alkenyl or
phenyl-lower alkynyl or being lower alkyl, lower alkenyl, lower alkynyl, lower
alkoxy-lower alkyl, -lower aLkenyl or -lower aLkynyl, lower alkenyloxy-lower alkyl,
-lower alkenyl or -lower alkynyl, lower alkylthio-lower aLkyl, -lower alkenyl or -lower
alkynyl, lower alkanesulfinyl-lower alkyl or -sulfs)nyl-lower alkyl, lower
aLkenylthio-lower alkyl, -sulfinyl-lower alkyl or -sulfonyl-lower aLkyl, or lower
alkynylthio-lower alkyl, -sulfinyl-lower alkyl or -sulfonyl-lower alkyl, which are in each
case independently of one another unsubstituted or substituted by substituents selected
from the group comprising halogen, hydroxyl, lower alkoxy, lower alkenyloxy,
phenyl-lower alkoxy, phenoxy, mercapto, lower aLkylthio, lower alkanesulfinyl or-sulfonyl, lower alkenylthio, lower alkenylsulfinyl or -sulfonyl, lower alkynylthio, lower
alkynylsulfinyl or -sulfonyl, amino which is, if desired, inndependently of one another
mono- or disubstituted by lower alkyl, lower alkenyl, lower alkynyl, phenyl-lower alkyl or
phenyl-lower aLkynyl or disubstituted by lower alkylene or lower alkyleneoxy-lower
alk~lene, carboxyl, lower alkoxy-, lower aLkoxy-lower aLkoxy- or lower a~e31yloxy-
carbonyl and carb~noyl in which, if desired, the amino group is independently ofone another mono- or disubstieuted by lower alkyl, lower alkenyl, lower alkynyl,phenyl-lower alkyl or phenyl-lower aLkynyl or disubstituted by lower aLkylene or lower
alkyleneoxy-lower alkylene; or R2 and R3 together are propylene or butylene or are the
partial structure of the formula -CH=CH-CH=CH- in which, if desired, one or two of the
methyne groups (-CH=) is replaced by -N=; R4 is a group of the formula (Ia), in palticular
(Ib), in which alk is lower alkylene or lower alkylidene, in particular methylene; R5 is
COOH, SO3H, halo-lower alkanesulfamoyl, PO2H2, PO3H2 or 5-tetrazolyl; the rings A
and B and the (hetero)aromatic radicals or the (hetero)aromatic ring jointly formed by R2
and R3 are in each case independently of one another unsubstituted or substituted by
substituents selected from the group comprising halogen, hydroxyl, lower alkoxy, lower
alkenyloxy, phenyl-lower alkoxy, phenoxy, mercapto, lower alkylthio, lower
alkanesulfinyl or -sulfonyl, lower alkenylthio, lower alkenylsulfinyl or -sulfonyl, lower
alkynylthio, lower alkynylsulfinyl or -sulfonyl, or selected from lower aL~yl, lower
alkenyl, lower alkynyl, lower alkoxy-lower alkyl, -lower alkenyl or -lower aL~cynyl, or
lower alkenyloxy-lower alkyl, -lower alkenyl or -lower alkynyl, which are in each case
unsubstituted or substituted by substituents selected from the group comprising halogen,
hydroxyl, lower alkoxy, lower alkenyloxy, phenyl-lower alkoxy, phenoxy, mercapto,
lower alkylthio, lower alkanesulfinyl or -sulfonyl, lower alkenylthio, lower alkenylsulfinyl
2 ~ ~ ~ 3 7 ~
- 14- 21489-8039
or -sulfonyl, lower alkynylthio, lower alkynylsulfinyl or -sulfonyl, amino which, if
desired, is independently of one another mono- or disubstituted by lower alkyl, lower
alkenyl, lower alkynyl, phenyl-lower alkyl or phenyl-lower alkyny} or disubstituted by
lower alkylene or lower alkyleneoxy-lower alkylene, carboxyl, lower aLkoxy-, lower
alkoxy-lower alkoxy- or lower alkenyloxy-carbonyl ~d carbarnDyl in which the am~no
group, if desired, is independently of one another mDno~ or disubstituted by
lower alkyl, lower alkenyl, lower alkynyl, phenyl-lower aIkyl or phenyl-lower
a~kynyl or disubstituted by lower aLkylene or lcwer alkyleneoxy-lower aLkylene.
The invention relates in particular to compounds of the forrnula I, their tautomers and salts
in which Z is O, S or N(R) and R is hydrogen, lower aLlcyl, lower aLIcenyl or lower alkynyl,
Rl is lower alkyl, lower alkenyl or lower aL~cynyl, in each case 3- to 7-membered
cycloaL~cyl or cycloaLlcenyl, phenyl-lower alkyl, phenyl-lower alkenyl or phenyl-lower
alkynyl or phenyl, which is unsubstituted or substituted by halo~en or hydroxyl, R;! and R3
independently of one another are halogen, lower alkanoyl, phenyl or carboxyl which, if
desired, is esterified by an alcohol which is derived from lower alkyl, lower aLlcenyl, lower
alkynyl, lower alkoxy-lower aLcyl, -lower alkenyl or -lower alkynyl, or lower
alkenyloxy-lower alkyl, -lower alkenyl or -lower alkynyl, carbamoyl in whieh the arnino
group, if desired, is independently of one another mono- or disubstituted by lower alkyl,
lower aL~cenyl, lower alkynyl, phenyl-lower alkyl, -lower alkenyl or -lower alkynyl or
disubstituted by lower aLkylene or lower aL~cyleneoxy-lower aL~cylene, or R2 is -Zl-R'2 and
R3 is -Z2-R'3, Zl and Z2 independently of one another being a bond or O, S()n or NH, n
being 0, 1 or 2, and R'2 and R'3 independently of one another being hydrogen, or lower
alkyl, lower alkenyl or lower alkynyl, lower aLIcoxy-lower aLlcyl, -lower alkenyl or -lower
alkynyl, lower aLcenyloxy-lower aLkyl, -lower aLcenyl or -lower alkynyl9 lower
alkylthio-lower alkyl, -lower alkenyl or -lower aL~cynyl, lower alkanesulfinyl-lower aLkyl
or -sulfonyl-lower alkyl, lower aLkenylthio-lower alkyl, -sulfimyl-lower alkyl or
-sulfonyl-lower alkyl, lower alkynylthio-lower alkyl, -sulfinyl-lower alkyl or
-sulfonyl-lower alkyl, which is unsubstituted or substituted by halogen, hydroxyl, amino
which, if desired, is substituted as indicated above, carboxyl which, if desired, is esterified
as indicated above, or carbarnoyl which, if desired, is substituted as indicated above, R4 is
the group of the formula Ib in which Rs is COOH, SO3H, halo-lower alkanesulfamoyl,
PO2H2, PO3H2 or 5-tetrazolyl, phenyl or phenyl in radicals containing phenyl in each case
being unsubstituted or substituted by substituents selected from the group comprising
lower alkyl, lower alkoxy, halogen, trifluoromethyl and hydroxyl.
~ .
.
3 7 ~
- lS- 21489-8039
The invention relates in particwlar to compounds of the forrnula I, their tautomers and
salts, in which Z is O, S or N(R) and R is hydrogen or lower alkyl, Rl in each case is
lower alkyl or lower alkenyl which is unsubstituted or substituted by halogen or hydroxyl,
or C3-C7cycloalkyl, C3-C7cycloalkenyl, phenyl-lower alkyl, phenyl or pyridyl; R2 and R3,
independently of one another, are halogen, lower alkanoyl, phenyl-lower alkanoyl,
benzoyl, phenyl, tetrazolyl, pyridyl, amino which, if desired, is mono- or disubstituted by
lower alkyl or phenyl-lower alkyl or disubstituted by lower alkylene or lower
alkyleneoxy-lower aLlcylene, carboxyl, lower aLlcoxy-, lower alkoxy-lower alkoxy- or
lower alkenyloxy-carbonylor carb~yl in which, if desired, the amino grou~? is
independently of one another mono- or disubstituted by lower alkyl or phenyl-lower aLkyl
or disubstituted by lower aLIcylene or lower aLkyleneoxy-lower alkylene; R2 is -Zl-R2' and
R3 is -Z2-R3', Zl and Z2 independently of one another being a bond, O or S~)n and n
being 0, l or 2; and R2' and R3' independently of one another being hydrogen or
phenyl-lower alkyl or are lower alkyl, lower alkenyl, lower alkoxy-lower alkyl, lower
alkylthio-lower alkyl, or lower alkanesulfimyl-lower alkyl or -sulfonyl-lower alkyl, which
in each case are independently of one another unsubstdtuted or substituted by subsdtuents
selected from the group comprising halogen, hydroxyl, lower alkoxy, phenyl-loweralkoxy, phenoxy, amino which, if desired, is independently of one another mono- or
disubstituted by lower alkyl, lower alkenyl, lower alkynyl, phenyl~lower alkyl or
phenyl-lower alkynyl or disubstituted by lower allcylene or lower alkyleneoxy-lower
alkylene, carboxyl, lower aL~coxy- or lower aL~coxy-lower alkoxy-carbonyl as~d ca~banoyl in
which, if desired the amino group is independently of one another mono- or disubstituted
by lower alkyl or phenyl~lower alkyl or disubstituted by lower alkylene or loweralkyleneoxy-lower alkylene; or R2 and R3 together are propylene or butylene or are the
partial structure of the formula -CH=CH-CH=CH-, in which, if desired, one or twomethyne group~s) are replaced by =N-; R4 is a group of the formula Ia, primarily Ib, in
which ALk is lower alkylene or lower alkylidene, primarily methylene; R5 is carboxyl,
halo-lower alkanesulfamoyl or 5-tetrazolyl; the rings A and B and the (hetero)aromatic
radicals or the (hetero)arornatic ring formed jointly by R2 and R3 are in each case
independently of one another unsubstituted or substituted by substituents selected from the
group comprising halogen, hydroxyl, lower alkoxy, phenyl-lower alkoxy, mercapto, lower
alkylthio, lower alkanesulfinyl or -sulfonyl, or lower alkyl or lower alkoxy-lower
aLkoxy-lower alkyl which is unsubstituted or substituted by halogen, hydroxyl, lower
alkoxy, phenyl-lower aLkoxy or phenoxy.
The invention relates in particular to compounds of the formula I, their tautomers and
~037a
- 16- 21489-8039
salts, in which Z is O, S or N(R) and R is hydrogen or lower alkyl, Rl is lower alkyl,
C3-C7cycloalkyl, phenyl-lower aLkyl or phenyl, R2 and R3, independently of one another,
are halogen, phenyl, lower alkanoyl, carboxyl, lower alkoxy- or lower alkoxy-lower
alkoxy-carbonyl, carbamoyl, lower alkyl- or di(lower alkyl)-carbamoyl, lower
alkylene-carbamoyl or lower aLIcyleneoxy-lower alkylene-carbamoyl, or R2 is -Zl-R'2 and
R3 is -Z-R'3, Zl and Z2 independently of one another being a bond or O, S or NH and R'2
and R'3 independently of one another being hydrogen, lower alkyl, halo-lower aL"yl,
hydroxy-lower aL~cyl, lower aL~coxy-lower aLkyl, amino-lower aLIcyl, lower
alkylamino-lower alkyl, di(lower alkylamino)-lower alkyl, lower aLIcyleneamino-lower
alkyl, lower alkyleneoxy-lower alkyleneamino-lower alkyl, carboxy-lower alkyl, or lower
alkoxy- or lower alkoxy-lower alkoxy-carbonyl-lower alkyl, R4 is the group of the formula
Ib in which ~ is aOOH or 5-tetrazolyl, and wherein phe~yl or phenyl in
radicals containing phenyl in each case being unsubstituted or substituted
by lower alkyl~ lower alkoxy, halogen, trifluDromethyl and/or hydroxyl.
The invention relates primarily to compounds of the formula I, their tautomers and salts,
in which Z is O, Rl is lower alkyl, in particular having 3 and not more than 5 C atoms,
such as n-propyl or n-butyl, or lower alkenyl, in particular having 3 and not mor than 5 C
atoms, such as 2 propenyl, R2 and R~ independently of one another are halogen, in
particular of atomic number not more than 35, such as chlorine, tetrazolyl, such as
S-tetrazolyl, amino, which, if desired, is mono- or disubstituted by lower alkyl or
phenyl-lower alkyl, in particular having not more than 4 C atoms per aLkyl moiety, or
disubstituted by lower alkyleneoxy-lower alkylene, in particular having not more than 4 C
atoms per lower alkylene moiety, such as 4-morpholinyl, carboxyl, lower alkoxycarbonyl,
in particular having 2 and not more than S C atoms, such as methoxy- or ethoxycarbonyl,
lower allcoxy-lower alkoxycarbonyl, in particular having not more than 4 C atoms in the
alkoxy moiety, such as 2-methoxyethoxycarbonyl; or R2 is -Zl-R2' and R3 is -Z2-R3', Z
and Z2 independently of one another being a bond, O or S()n and n being 0, 1 or 2, and
R2' and R3' independently of one another being hydrogen or phenyl-lower aLlcyl, in
particular having not more than 4 C atoms in the aL~cyl moiety, such as benzyl, or lower
alkyl or lower alkoxy-lower alkyl, in particular in each case having not more than 4 C
atoms in the alkyl moiety, which in each case, if desired, is mono- or disubstituted by
halogen, in particular of atomic number not more than 35, such as chlorine, hydroxyl,
amino which, if desired, is mono- or disubstituted by lower alkyl or phenyl-lower alkyl, in
particular having not mo-re than 4 C atoms per alkyl moiety, or disubstituted by lower
alkenyloxy-lower alkylene, in particular having not more than 4 C atoms per lower alkyl
2~3~
moiety, such as methyl-, dimethyl-, benzyl- or dibenzylamino or 4-morpholinyl, carboxyl,
lower alkoxycarbonyl, in part;cular having 2 and not more than 5 C atoms, SUC}I as
methoxy- or ethoxycarbonyl; or R2 and R3 together are propylene or butylene or are the
partial structure of the formula -C~I=CH-CH=CH-, in which, if desired, one or two
methyne group(s) are replaced by =N-; R4 is a group of the formula Ia, in particular Ib, in
which Alk is lower alkylene or lower alkylidene, in particular in each case having not
more than 4 C atGms, such as methylene, ethylene or ethylidene, primarily methylene; R5
is carboxyl, halo-lower aLkanesulfamoyl, in particular of atomic nurnber not more than 35
and having not more than 4 C atoms, in particular trifluoromethanesulfamoyl, or
S-tetrazolyl; the rings A and B and the aromatic radicals or the (hetero)aromatic ring
jointly formed by R~ and R3 are in each case independently of one another unsubstituted
or substituted by hydroxyl, halogen, in pauticular of atomic number not more than 35, such
as chlorine, trifluoromethyl, lower alkyl, in particular having not more than 4 C atoms,
such as methyl, and/or lower alkoxy, in particular having not more than 4 C atoms, such as
methoxy.
.~
The invention relates primarily to compounds of the formula ~, their tautomers and salts,
in which Z is 0, Rl is lower alkyl, in particular having 3 and not more than 5 C atoms,
such as n-butyl, one of the radicals R~ and E~3 iS halogen, in particular of atomic number
not more than 35, such as chlorine, phenyl, carboxyl, lower alkoxycarbonyl, in particular
having 2 and not more than 5 C atoms, such as ethoxycarbonyl, hydrogen, hydroxyl, lower
alkylthio, in particular having not more than 4 C atoms, such as methylthio, lower
alkanesulfonyl, in particular having not more than 4 C atoms, such as methanesulfonyl,
amino, di(lower alkyl)amino, in particular having not more than 4 C atoms per lower alkyl
moiety, such as dimethylamino, morpholino, lower alkyl, in particular having not more
than 4 C atoms, such as methyl, hydroxy-lower alkyl, in particular having not more than
4 C atoms, such as hydroxymethyl, halo-lower alkyl, in particular of atomic number not
more than 35 and having not more than 4 C atoms, such as trifluoromethyl, di(lower
alkyl)amino-lower alkyl, in particular having not more 4 C atoms per lower alkyl moiety,
such as 2-dimethylarninoethyl, carboxy-lower alkyl, in particular having not more than
S C atoms, such as carboxymethyl, lower alkoxycarbonyl-lower alkyl, in particular having
not more ~han 4 C atoms per lower alkoxy or lower alkyl moiety, such as
ethoxycarbonylmethyl, lower alkoxy, in particular having not more than 4 C atoms, such
as methoxy, hydroxy-lower alkoxy, in particular having not more than 4 C atoms, such as
2-hydroxyethoxy, lower alkoxy-lower alkoxy, in particular having not more than 4 C
atoms per lower alkoxy moiety, such as 2-methoxyethoxy, di(lower alkyl)amino-lower
, .
3 ~ ~
- 18-
alkoxy, in particular having not more than 4 C atoms per lower alkyl or lower alkoxy
moiety, such as 2-dimethylaminoethyl, carboxy-lower alkoxy, in particular having not
more than 5 C atoms, such as 2-carboxyethoxy, or lower aIkoxycarbonyl-lower alkoxy, in
particular having not more than 4 C atoms per lower alkoxy moiety, such as
2-ethoxycarbonylethoxy, and the other hydrogen or lower alkyl, in particular having not
more 4 C atoms, such as methyl, and R4is the group of the forrnula Ib in which Rs is
S-tetrazolyl.
The invention relates primarily to compounds of the formula I and their salts in which Z is
O, Rl is C3-C5alkyl, such as n-propyl or n-butyl, R2 and ~3, independently l)f one another,
are halogen of atomic number not more than 35, such as chlorine, di-Cl-C4aL~cylamino,
such as dimethylamino, 4-morpholinyl, carboxyl or C2-Csalkoxycarbonyl, such as
methoxy- or ethoxycarbonyl; or R2 is -Zl-R2' and R3 is -Z2-R3', Zl and 7-2 independently
of one another being a bond, O or S()n and n being 0, 1 or 2, and R2' and R3'
independently of one another being hydrogen, phenyl-C2-C5aLkyl, such as benzyl, or
Cl-C4alkyl, such as methyl which is unsubstituted or substituted by hydroxyl or
di-Cl-C4alkylamino, such as dimethylamino, or Cl-C4alkoxy-Cl-C4alkoxy, such as
2-methoxyethoxy; or R2 and R3 together are propylene or butylene or are the partial
structure of the formula -CH=CE~-CH=CH- in which, if desired, one or two methynegroups are replaced by =N-; R4 is a group of the formula Ib in which Rs is carboxyl or
primarily S-tetrazolyl; and the rings A and B or the (hetero)aromatic ring jointly formed
by R2 and R3 are primarily unsubstituted, or furthermore substituted by hydroxyl, halogen,
in particular of atomic number no~ more than 35, such as chlorine, trifluoromethyl, lower
alkyl, in particular having not more than 4 C atoms, such as methyl, and/or lower alkoxy,
in particular having not more than 4 C atoms) such as methoxy.
;
The invention relates primarily to compounds of the formula I and their salts in which Z is
O, Rl is C3-Csalkyl, such as n-propyl or n-butyl, R2 is halogen of atomic number not more
than 35, such as chlorine, trifluoromethyl, carboxyl, C2-Csalkoxycarbonyl, such as
ethoxycarbonyl, hydrogen, hydroxyl, Cl-C4alkyl, such as methyl or n-butyl, Cl-C~alkoxy,
; such as methoxy, phenyl-Cl-C4alkoxy, such as benzyloxy, hydroxy-Cl-C4alkoxy, such as
i: 2-hydroxyethoxy, Cl-C4alkoxy-Cl-C4alkoxy, such as 2-methoxyethoxy, or
:` di-Cl-C4alkylamino-Cl-C4alkoxy,such as 2-dimethylarninoethoxy; R3 is hydrogen,
Cl-C4alkyl, such as methyl or n-butyl, carboxyl, C2-Csalkoxycarbonyl, such as
:: ethoxycarbonyl, or hydroxy-Cl-C4alkyl, such as hydroxymethyl; or R2 and R3 together are
~; propylene or butylene or are the partial structure of the formula -CH=CH-CH=CH- in
i
.~ .
.
: '.
,, ~ .
:,
:
.
3 7 ~
- 19-
which, if desired, a methyne group is replaced by =N-; R4 is a group of the formula Ib in
which Rs is carboxyl or primarily 5-tetrazolyl; and the rings A and B are prin~arily
unsubstituted, or furtherrnore substituted by hydroxyl, halogen of aton~ic number not more
than 35, such as chlorine, trifluoromethyl, Cl-C4alkyl, such as methyl, or Cl-C4alkoxy,
such as methoxy.
The invention relates primarily to compounds of the forrnula I and their salts in which Z is
0, Rl is C3-Csalkyl, such as n-propyl or n-butyl, R2 is C1-Csalkyl, such as methyl,
n-propyl or n-butyl, or hydroxyl, R3 is hydrogen or Cl-Csalkyl, such as n-propyl or
n-butyl; or R2 and R3 together are propylene or butylene or are the partial structure of the
forrnula -CH=CH-CH=CH- in which, if desired, a methyne group is replaced by =N-; R4
is a group of the forrnula Ib in which Rs is carboxyl or primarily 5-tetrazolyl; and the rings
and B are primarily unsubstituted or furthermore substituted by hydroxyl, halogen of
atomic number not more than 35, such as chlorine, trifluoromethyl, Cl-C4alkyl, such as
methyl, or Cl-C4alkoxy, such as methoxy.
The invention relates primarily to compounds of the formula I and their salts in which Z is
O, Rl is C3-Csalkyl, such as n-propyl or n-butyl, R2 and R3 together are propylene or
butylene or are the partial structure of the formula -CE~=CH-CH=CH- in which, if desired,
a methyne group is replaced by =N-; R4 is a group of the forrnula Ib in which Rs is
5-tetrazolyl; and the rings A and B are unsubstituted.
The invention relates primarily to compounds o~ the formula I and their salts in which Z is
0, R1 is C3-Csalkyl, such as n-butyl, R2 is hydrogen, halogen of atomic number not more
than 35, such as chlorine, Cl-C4alkyl, such as methyl, Cl-C4alkoxy, such as methoxy, and
R3 is hydrogen, and R4 is the group of the formula Ib in which Rs is 5-tetrazolyl.
The invention relates primarily to compounds of the formula I and their salts in which Z is
0, Rl is C3-Csalkyl, such as n-butyl, R2 is hydrogen, halogen of atomic number not more
than 35, such as chlorine or Cl-C4alkyl, such as methyl, R3 is hydrogen, and R4 is the
group of the formula Ib in which R5 is 5-tetrazolyl.
The invention relates primarily to compounds of the formula I and their salts in which Z is
O, R1 is C3-Csalkyl, such as n-butyl, R2 is Cl-C4alkyl, such as methyl or n-butyl, and R3 is
C1-C4alkyl, such as n-butyl, or R2 is hydroxy and R3 is hydrogen or methyl, and R4 is the
~roup of the formula Ib in which Rs is 5-tetrazolyl.
~"
2~2~7a
- 20 -
The invention relates in particular to the novel compounds mentioned in the examples and
to the manners of preparation described therein.
The invention further relates to processes for the preparation of the compounds according
to the invention. The preparation of compounds of the formula I takes place in a manner
known per se and is, for exampie, characteri~ed in that
a) a compound of the formula
NH
Rl--C~ (IIa)
NH-R4
or a salt thereof is reacted with a compound of the formula
I R3 Z
X~--C--CH--C ~ X2 (IIb)
a salt, a tautomer or a functionally modified derivative of the tautomer thereof, in which
Xl is the variable R2 or is etherified hydroxyl and X2 is etherified hydroxyl, or
:
b) in a compound of the formula
R2
N /~
CH2~3 (111)
or a salt thereof in which X3 is a radical which can be converted into the variable Rs~ X3 iS
converted into the variable Rs, or
c) a compound of the formula
2~37~
R2
N~R3
ll I (IVa),
R~N~Z
a tautomer or salt thereof is reacted with a compound of the formula
X4-R4 (IVb)
or a salt thereof in which X~ is reactive esterified hydroxyl and, if desired, a compound of
the formula I or a salt thereof obtainable according to the process or in another manner is
converted into another compo~md or a salt thereof accord;ng to the invent;on, a free
compound of the formula I obtainable according to the process is converted into a salt, a
salt obtainable according to the process is converted into the free compound of the formula
I or into another salt, or a mixture of isomers obtainable according to the process is
resolved and the desired compound is isolated.
Salts of starting materials which contain at least one basic centre, for example of the
forrnula IIa, are appropriate acid addition salts, while salts of starting materials which
contain an acid group are present as salts with bases, in each case as mentioned above in
connection witn corresponding salts of the formula I.
.~: .
A suitable tautomer of a cornpound of the formula IIb is, in particular, a compound of the
:: formula
,~
X--C=C--C --X2 (llb')
R3
.
in which X is hydroxyl. The enolic hydroxyl group is advantageously functionallymodified; X is accordingly, for example, reactive esterified hydroxyl, such as halogen,
etherified hydroxyl, such as lower alkoxy, unetherified or etherified mercapto, such as
lower alkylthio, or unsubstituted or substituted amino, such as lower alkyl- or di(lower
alkyl)-amino.
.
3 7 ~
Etherifled hydroxyl (Xl or X2) is, in particular, alkoxy, such as lower alkoxy, for example
methoxy or ethoxy.
X3 radicals which can be converted into the variable Rs are, for example, cyano, mercapto,
halogen, the group -N2+A- in which A- is an anion derived from an acid, amino and
different functionally modifled forms of COOH, SO3H, PO3H2 or PO2H2 and also
N-protected 5-tetrazolyl.
Reactive esterified hydroxyl, for example X4, iS, in particular, hydroxyl esterified with a
strong inorganic acid or organic sulfonic acid, for example halogen, such as chlorine,
bromine or iodine, sulfonyloxy, such as hydroxysulfonyloxy, halosulfonyloxy, forexample fluorosulfonyloxy, (Cl-C7)alkanesulfonyloxy which, if desired, is substituted, for
example by halogen, for example methane- or ~ifluoromethanesulfonyloxy,
(Cs-C7)cycloalkanesulfonyloxy, for example cyclohexanesulfonyloxy, or
benzenesulfonyloxy which, if desired, is substituted, for example by (Cl-C7)alkyl or
halogen, for example p-bromobenzene- or p-toluenesul-fonyloxy.
The reactions described in the variants above and below are carried out in a manner
known per se, for example in the absence or in the customary manner in thc presence of a
suitable solvent or diluent or a mixture thereof, the reaction being carried out, according to
need, with cooling, at room temperature or with warming, for example in a temperature
range from about -80C up to the boiling point of the reaction medium, preferably from
about -10C to about +180C, and, if necessary, in a closed vessel, under pressure, in an
inert gas atmosphere and/or under anhydrous conditions.
Vari~mt a~:
If necessary, the reaction is carried out in the presence of a base.
Suitable bases are, for example, alkali metal hydroxides, hydrides, amides, alkanolates,
carbonates, triphenylmethylides, di(lower alkyl)amides, aminoalkylamides or lower alkyl
silylarnides, or naphthaleneamines, lower alkylamines, basic heterocycles, ammoniurn
hydroxides, and also carbocyclic amines. Examples which may be rnentioned are sodium
hydroxide, sodium hydride, sodium amide, sodium (m)ethoxide, potassium tert-butoxide,
potassium carbonate, lithium triphenylmethylide, lithium diisopropylamide, potassium
3-(aminopropyl)amide, potassiumbis(trimethylsilyl)arnide, dimethylaminonaphthalene, di-
` 2~37~
- 23 -
or triethylamine, or ethyldiisopropylamine~ N-methylpiperidine, pyridine,
benzyltrimethylammonium hydroxide, 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) and
1,8-diazabicyclo[5.4.0]undec-7-ene (DBU).
If compounds of the forrnula IIb of this type are employed, in which both Xl and X2 are
etherified hydroxyl, compounds of the formula I are obtained in which R2 is hydroxyl.
Preferably, those compounds of the formula IIb are employed in which Z is O.
In order to prepare compounds of the folmula I in which Z is NH, nitriles of the formula
J Xl-CO-CH(R3)-CN (IIc) are advantageously used.
.
, In order to prepare the starting material of the formula IIa, a compound of the formula
/
~IH
`: Rl--C (IId)
.. x
or a salt thereof is used in which X2 iS etherified hydroxyl, such as lower alkoxy, for
example methoxy or ethoxy, and this is reacted with a compound of the forrnula R~,-NH2
(IIe) or a salt thereof. Compounds of the forrnula IId can be prepared, for example, from
the corresponding nitriles, while compowlds of the formula IIb and IIe are known in some
, cases or can be prepared in a manner known per se. Thus, compounds of the formula IIe
are described, for example, in EP 253,310 or can be prepared in an analogous manner.
.. ~
Functionally modified derivatives of tautomers of the formula IIb in which X is halogen
are, for example, accessible by using compounds of the forlnula IIb in which Xl is R2 and
reacting these in a manner known per se, for example with an oxalyl halide, such as oxalyl
chloride. The resulting derivative of the formula IIb' (X = halogen~ can be further
converted into other corresponding derivatives of the formula IIb', for example by reaction
with a lower alkoxide, such as sodium methoxide, with a lower alkyl thiolate, such as
sodium methylthiolate, or with ammonia or a corresponding amine, such as lower alkyl- or
di(lower alkyl)amine, for example ethylamine or diethylamine.
,, .
: Variantb):
X3 radicals which can be conver~ed into 5-lerrn:~olyl l'c5 are, ~or example, cyano or
,
.
r~
3 7 1~
- 24 -
protected ~-tetrazolyl.
In order to prepare compounds of the formula I in which Rs is 5-tetrazolyl, starting
materials of the formula III, for example, are used in which X3 iS cyano, and these are
reacted with an azide, such as HN3 or, in particular, a salt, such as an alkali metal sale
thereof, or with an organotin azide, such as tri~lower)alkyl- or tria~yltin azide. Preferred
azides are, for example, sodium azide and potassium azide and tri-~ 4alkyl-, forexample triethyl- or ~ibutyltin azide, and triphenyl azide.
Suitable protecting groups lor protected ~-tetrazolyl are the protecting groups customarily
used in tetrazole chemistry, in particular triphenylmethyl, benzyl which is unsubstituted or
substituted, for example by nitro, such as 4-nitrobenzyl, lower alkoxymethyl, such as
methoxy- and ethoxyethyl, lower aLcylthiomethyl, such as methylthiomethyl, and
2-cyanoethyl, and furthermore lower alkoxy-lower alkoxymethyl, such as
2-methoxyethoxymethyl, benzyloxymethyl and also phenacyl.
'
The protecting groups are removed following known methods, for example as described in
J. Green, Protective Groups in Organic Synthesis, Wiley-Interscience (1980). Thus, for
example, the triphenylmethyl group is customarily removed by hydrolysis, in particular in
the presence of an acid, or hydrogenolysis in the presence of a hydrogenation catalyst,
4-nitrobenzyl is removed, for example, by hydrogenolysis in the presence of a
hydrogenation catalyst, methoxy- or ethoxyethoxy is removed, for example, by treating
with a lower alkyltin bromide, such as triethyl- or tributyltin bromide, methylthiomethyl is
removed, for example, by treating with trifluoroacetic acid, 2-cyanoethyl is removed, for
example, by hydrolysis, for example with sodium hydroxide solution,
2-methoxyethoxymethyl is removed, for example, by hydrolysis, for example with
hydrochloric acid, and benzyloxymethyl and phenacyl are removed, for example, byhydrogenolysis in the presence of a hydrogenation catalyst.
A radical which can be converted into SO3H Rs is, for example, the mercapto group.
S~arting compounds of the formula I~I containing a group of this type are, for example,
oxidized by oxidation processes known per se to those compounds of the formula I in
which Rs is SO3H. Possible oxidizing agents are, for example, inorganic peMcids, such as
peracids of mineral acids, for example periodic acid or persulfuric acid, organic peracids,
such as appropriate percarboxylic or persulfonic acids, for example performic, peracetic,
trifluoroperacetic or perbenzoic acid or p-toluenepersulfonic acid, or mixtures of hydrogen
.
2~ ~37 ~
- 25 -
peroxide and acids, for example a mixture of hydrogen peroxide with acetic acid.
The oxidation is commonly carried out in the presence of suitable catalysts, suitable acids,
such as substituted or unsubstituted carboxylic acids, for example acetic acid or
trifluoroacetic acid, or transition metal oxides, such as oxides of elements of sub-group
VII, for example vandium oxide, molybdenum oxide or tungsten oxide, being mentioned
as catalysts. The oxidation is carried out under mild conditions, for example attemperatures of about -50 to about +100C.
A group which can be converted into PO3H2 R5 is to be understood as meaning, forexample, a group N2+A-, in which A- is an anion of an acid, such as a mineral acid.
Diazonium compounds of this type are, for example, reacted in a manner known per se
with a P(III) halide, such as P~13 or PBr3, and worked up by hydrolysis, those compounds
of the formula I being obtainable in which 1~5 is PO3H2.
: '
A suitable X3 radical which can be converted into haloalkylsulfamoyl Rs is, for example,
primary amino.
In order to prepare compounds of the formula I in which Rs is haloalkylsulfamoyl,
corresponding anilines, for example, are reacted with a customarily reactive esterified
haloalkylsulfonic acid, the reaction being carried out, if desired, in the presence of a base.
A suitable preferred reactive esterified halosulfonic acid is the corresponding halide, such
as the chloride or bromide.
A radical X3 which can be converted into COOH Rs is, for example, a functionallymodified carboxyl, such as cyano, esterified or amidated carboxyl, hydroxymethyl or
formyl.
Esterified carboxyl is, for example, carboxyl esterified with a substituted or unsubstituted
aliphatic, cycloaliphatic or aromatic alcohol. An aliphatic alcohol is, for example, a lower
alkanol, such as methanol, ethanol, propanol, isopropanol, n-butanol, sec- or tert-butanol,
while a suitable cycloaliphatic alcohol is, for example, a 3- to 8-membered cycloalkanol,
such as cyclopentanol, -hexanol or -heptanol. An aromatic alcohol is, for example, a
phenol or heterocyclic alcohol, which may in each case be substituted or unsubstituted, in
particular hydroxypyridine, for example 2-, 3- or 4-hydroxypyridine.
3 7
,:
- 26 -
Amidated carboxyl is, for example, carbamoyl, carbamoyl which is monosubstituted by
hydroxyl, amino or substituted o} unsubstituted phenyl, carbamoyl which is mono- or
disubstituted by lower alkyl or carbamoyl which is disubstituted by 4- to 7-membered
aLkylene or 3-aza-, 3-lower alkylaza-, 3-oxo- or 3-thiaalkylene. Examples which may be
mentioned are carbamoyl, N-mono- or N,N-di(lower alkyl)carbamoyl, such as N-methyl-,
N-ethyl-, N,N-dimethyl-, N,N-diethyl- or N,N-dipropylcarbamoyl, pyrrolidino- or
piperidinocarbonyl, morpholino-, piperazino- or 4-methylpiperazino- and also
thiomorpholinocarbonyl, anilinocarbonyl or anilinocarbonyl substituted by lower alkyl,
lower alkoxy and/or halogen.
.:
. Preferred functionally modified carboxyl is, for example, lower aL1coxycarbonyl, such as
: - methoxy- or ethoxycarbonyl, or cyano. Compounds of the formula I in which Rs is
carboxyl can be prepared by oxidation, for example starting from compounds of the
formula III in which X3 iS functionally modified carboxyl, by hydrolysis, in particular in
the presence of a base, or starting from those compounds of the formula III in which X3 iS
~;~ hydroxymeehyl or formyl, using customary oxidizing agents.
.,
As oxidizing agents, -for example in an inert solvent, such as a lower alkanecarboxylic
acid, for example acetic acid, a ketone, for example acetone, an ether, for example
tetrahydrofuran, a heterocyclic aromatic, for example pyridine, or water or a mixture
thereof, if necessary wi~h cooling or warming, for example from about 0 to about 150.
Suitable oxidizing agents are, for example, oxidizing transition metal compounds, in
particular those with elements of sub-groups I, VI or VIII. Fxamples which may be
mentioned are: silver compounds, such as silver nitrate, silver oxide or silver picolinate,
chromium compounds, such as chromium trioxide or potassium dichromate, manganesecompounds, such as potassium ferrate, tetrabutylammonium permanganate or
benzyl(triethyl)ammonium permanganate. Other oxidizing agents are, for example,
suitable compounds with elements of main group IV, such as lead dioxide, or
halogen-oxygen compounds, such as sodium iodate or potassium periodate.
;
:~ Thus, for example, hydroxymethyl and formyl are oxidized to carboxyl Rs.
This variant is preferably suitable for the preparation of those compounds of the formula I
? in which the variables have meanings which are different from unsaturated radicals.
The starting material of the formula III is, for example, accessible by starting from
:
;,j
;~
.'1 ~
;`; ~
:~
j:"
7 3
- 27 -
compo~mds of the formula IVa and reacting these in analogy to variant c) with a
compound of the formula
X4-CH2 ~ ~ 3 (r~I),
in which X3 and X4 have the abovementioned meanings.
Compounds of the formula IlI are known in some cases or can be prepared by knownmethods, for example as shown in EP 253,310 (page 52 et seq.).
Variant c:
The reaction of compounds of the formula IVa with compounds of the formula IVb is
advantageously carried out in a manner known per se in the presence of one of the
abovementioned bases~
X4 is preferably halogen, such as chlorine or bromine, or sulfonyloxy, such as methane- or
p-toluenesulfonyloxy.
.
The starting compounds of the formula IVa and IVb are known in some cases or can be
prepared in a manner known per se. Thus, for example, compounds of the formula IVa are
obtained by starting from compounds of the formula IIa in which R4 is hydrogen, in
analogy to variant a), and reacting these with a compound of ehe formula IIb. Compounds
of the forrnula IVa in which R2 and R3 are jointly the partial structure of the formula
-CH=CH-CH=CEl-, in which, if desired, one or two of the methyne groups are replaced by
=N-, can be prepared, for example, by starting frorn a corresponding compound of the
formula
R3 J~
O (IVc)
R2J~ NH~O
2~3~
- 28 -
and reacting this, if appropriate in the presence of a base, with an amidine of the formula
IIa.
The starting material of the formula IVb is known from EP 253,310 in some cases or can
be prepared using customary methods.
A compound according to the invention which is obtainable by the process can be
converted into another compound according to the invention in a manner known per se.
A compound according to the invention containing hydroxyl can be etherified by methods
known per se. The etherification can be carried out, for example, using an alcohol, sucK as
a substituted or unsubstituted lower alkanol, or a reactive ester thereof. Possible reactive
esters of the desired alcohols are, for example, those with strong inorganic or organic
acids, such as corresponding halides, sulates, lower alkanesulfonates or substituted or
unsubstituted benzenesulfonates, for example chlorides, bromides, iodides, methane-,
benzene- or p-toluenesulfonates. The etherification can be carried out, for example, in the
presence of a base, an aLIcali metal hydride, hydroxide or carbonate, or a basic amine.
Inversely, corresponding ethers, such as lower alkoxy compo-mds, can be cleaved, for
example, by means of strong acids, such as rnineral acids, for example the hydrohalic
acids, hydrobromic or hydriodic acid, which may advantageously be present in the form of
pyridinium halides, or by means of Lewis acids, for example halides of elements of main
group III or the corresponding sub-groups. These reactions can be carried out, if
necessary, with cooling or warming, for example in a temperature range of about -20 to
about 100C, in the presence or absence of a solvent or diluent, under inert gas and/or
under pressure and, if appropriate, in a closed vessel.
:,
Compounds according to the invention containing hydroxymethyl groups can be prepared,
for example, starting from corresponding compounds containing carboxyl or esterified
carboxyI, the corresponding compounds being reduced in a manner known per se, ~for
example by reduction with a hydride which may be complex, such as a hydride formed
from an element of main groups I and III of the periodic table of the elements, for example
a borohydride or aluminohydride, for example lithium borohydride, or lithium- ordiisobutylaluminium hydride (a subsequent reduction step using an alkali metal
cyanoborohyride, such as sodium cyanoborohydride, may be necessary), and furtherdiborane.
. ~
;: ~
'.;
,. .
. ,
3 7 ~
- 29 -
If an aromatic structural component is substituted by (lower)alkylthio, this can be oxidized
in the customary manner to the corresponding (lower)alkanesulfinyl or -sulfonyl. Suitable
oxidizing agents for the oxidation to the sulfoxide step are, for example, inorganic
peracids, such as peracids of mineral acids, for example periodic acid or persulfuric acid,
organic peracids, such as appropriate percarboxylic or persulfonic acids, for example
performic, peracetic, tfi~luoroperacetic or perbenzoic acid or p-toluenepersulfonic acid, or
mixtures of hydrogen peroxide and acids, for example a mixture of hydrogen peroxide
with acetic acid.
The oxidation is commonly carried out in the presence of suitable catalysts, catalysts
which can be mentioned being suitable acids, such as substituted or unsubstituted
carboxylic acids, for example acetic acid or trifluoroacetic acid, or transition metal oxides,
such as oxides of elements of sub-group VII, for example vanadium ox;de, molybdenum
oxide or tungsten oxide. The oxidation is carried out under rnild conditions, for example at
temperatures of about -50 to about +100C.
The oxidation to the sulfone step May also be carried out appropriately at low
temperatures using dinitrogen tetroxide as the catalyst in the presence of oxygen, just like
the direct oxidation of (lower)alkylthio to (lower)alkanesulfonyl. However, in this case the
oxidizing agent is customarily employed in excess.
If one of the variables (for example R2 and R3) contains amino, corresponding compounds
of the formula I, their tautorners or salts can be N-alkylated in a manner known per se;
likewise, carbamoyl or radicals (for example RV containing carbamoyl can be
N-alkylated. The ~aryl)aLkylation is carried out, for example, using a reactive ester of an
(aryl)CI-C7aLkyl halide, for example a bromide or iodide, an (aryl)C1-C7aLkylsulfonate,
for example a methanesulfonate or p-toluenesulfonate, or a di-C1-C7alkyl sulfate, for
example dimethyl sulfate, preferably under basic condisions, such as in the presence of
sodium hydroxide solution or potassium hydroxide solution, and advantageously in the
presence of a phase-transfer catalyst, such as tetrabutylammonium bromide or
benzyltrimethylammonium chloride, where, however, stronger basic condensing agents,
such as alkali metal amides, hydrides or alkoxides, for example sodium amide, sodium
hydride or sodium ethoxide, may be necessary.
In compounds of the formula (I) which contain an esterified or amidated carboxyl group
(for example R2 or R3) as a substituent, a group s)f this type can be conve-rted into a free
`
.`, `
~2~7~
- 30-
carboxyl group, for example by means of hydrolysis, for example in the presence of a
basic agent, or an acidic agent, such as a mineral acid. Tert-butyloxycarbonyl, for
example, may furtherrnore be converted into carboxyl, for example in a manner known per
se, such as by treating with trihaloacetic acid, such as trifluoroacetic acid, and
benzyloxycalbonyl may be converted into carboxyl, for example by catalytic
hydrogenation in the presence of a hydrogenation catalyst, for example in the manner
described below.
Furthermore, in compounds of the formula (I~ which contain a carboxyl group (forexample R3) as a substituent, in particular if Rs is dif~erent frvm carboxyl, this can be
converted into an esterified carboxyl group (for example R3), for exarnple, by treating with
an alcohol, such as a lower alkanol, in the presence of a suitable esterifying agent, such as
an acid reagent, for example an inorganic or organic acid or a Lewis acid, for example
zinc chloride, or a condensing agent which combines with water, for example a
carbodiimide, SUC}I as N,N'-dicyclohexylcarbodiimide, or by treating with a diazo reagent,
such as with a diazo-lower alkane, for example diazomethane. This can also be obtained if
compounds of the formula I in which the carboxyl group (for example R2) is present i
free form or in salt form, such as ammonium salt or metal salt, for example alkali rnetal
salt, such as sodium salt or potassium salt form, are treated with a reactive ester of a
(Cl-C7)alkyl halide, for example methyl or ethyl bromide or iodide, or an organic sulfonic
acid ester, such as an appropriate (C1-C7)alkyl ester, for example methyl or ethyl
methanesulfonate or p-toluenesulfonate.
Compounds of the formula (I) which contain an esterified carboxyl group (for example
R3) as a substituent can be transesterified into other ester compounds of the formula (I) by
transesterification, for example by treating with an alcohol, customarily a higher
appropriate alcohol than that of the esterified carboxyl group in the starting material, in the
presence of a suitable transesterifying agent, such as a basic agent, for example an alkali
metal (C1-C7)alkanoate, (Cl-C7)alkanolate or cyanide, such as sodium acetate, sodium
methoxide, sodiurn ethoxide, sodium tert-butoxide or sodium cyanide, or a suitable acid
agent, if appropriate with removal of the resulting alcohol, for example by distillation.
Appropriate, so-called activated esters of the forrnula (I) may also be used which contain
an activated esterified carboxyl group as a substituent (see below), and these may be
converted into another ester by treating with a (Cl-C7)alkanol.
In compounds of the formula (I) which contain the carboxyi group (for example R2) as a
2~2~37~
.
- 31 -
substituent, this can also first be converted into a reactive derivative, such as an anhydride,
incklding a mixed anhydride, such as an acid halidc, for example an acid chloride ~for
example by treating with a thionyl halide, for example thionyl chloride), or an anhydride
using a formic acid ester, for example a (Cl-C7)alkyl ester (for example by treating a salt,
such as an ammonium or alkali metal salt, with a haloformic acid ester, such as a
chloroformic acid ester, such as a (C1-C7)alkyl ester), or into an activated ester, such as a
cyanomethyl ester, a nitrophenyl ester, for example a 4-nitrophenyl ester, or a
polyhalophenyl ester, for example a pentachlorophenyl ester (for exarnple by treating with
an appropriate hydroxyl compound in the presence of a suitable condensing agent, such as
N,N'-dicyclohexylcarbodiimide), and then a reactive derivative of this type can be reacted
with an amine and in this way amide compounds of the formula (I) which contain an
amidated carboxyl group as a substituent can be obtained. In this case, these can be
obtained directly or via intermediate compounds; thus, for example, an activated ester,
such as a 4-nitrophenyl ester or a compound of the formula I containing a carboxyl group
can first be reacted with a 1-unsubstituted imidazole and the 1-imidazolylcarbonyl
compound obtained in this way brought to reaction with an amine. However, other
non-activated esters, such as (Cl-C7)alkyl esters of compounds of the formula (I), which
contain, for example, (C2-C8)alkoxycarbonyl (for example R2) as a substituent, can also be
brought to reaction with amines.
If an aromatic ring contains a hydrogen atom as a substituent, the latter can be replaced by
a halogen atom with the aid of a halogenating agent in a customary rnanner, for example
brominated with bromine, hypobromic acid, acyl hypobromite or other organic bromine
compounds, for example N-bromosuccinimide, N-bromoacetamide, N-bromophthalirnide,
pyridinium perbromide, dioxane dibromide, 1,3-dibromo-5,5-dimethylhydantoin or
2,4,4,6-tetrabromo-2,5-cyclohexadien- 1 -one, or chlorinated with elemental chlorine, for
example in a halogenated hydrocarbon, such as chloroform, and with cooling, for example
from down to about -10 to about +100C.
,, .
If an aromatic ring in the compounds according to the invention contains an amino group,
this can be diazotized in a customary manner, for example by treating with a nitrite, for
example sodium nitrite, in the presence of a suitable protonic acid, for example a mineral
acid, the reaction temperature advantageously being kept below about 5C. The diazonium
group present in the salt form which can be obtained in this way can be substituted by
analogous processes, for example as follows: through the hydroxyl group analogously to
the boiling-out of phenol in the presence of water; by an alkoxy group by treating with an
, . . .
, . ,
.
,.
.. . .
,
,.,
~2~3~
appropriate alcohol, energy having to be added; by the fluorine atom analogous to the
Schiemann react;on in the therrnolysis of corresponding diazonium tetrafluoroborates; by
the halogen atoms chloTine, bromine or iodine and also the cyano group analogously to the
Sandmeyer reaction in the reaction with corresponding Cu(I) salts, initially with cooling,
-for example to below about ~C~, and then heating, for example, to about 60 to about
150C.
If the compounds of the formula (I) contain unsaturated radicals, such as (lower)alkenyl or
(lower)alkynyl groups, these can be converted into saturated radicals in a manner known
per se. Thus, for example, multiple bonds are hydrogenated by catalytic hydrogenation in
the presence of hydrogenation catalysts, suitable for this purpose being, for example,
nickel, such as Raney nickel, and noble metals or their derivatives, for example oxides,
such as palladium or platinum oxide, which may be applied, if desired, to support
materials, for example to carbon or calcium carbonate. The hydrogenation may preferably
carried out at pressures between 1 and about 100 at and at room temperature between
about -~0 to about 20QC, in particular between room temperature and about 100C. The
reaction is advantageously carried out in a solvent, such as water, a lower alkanol, for
example ethanol, isopropanol or n-butanol, an ether, for example dioxane, or a lower
alkanecarboxylic acid, for example acetic acid.
Furthermore, in compounds of the formula I in which, for example, one of the radicals R
and/or Alk is halogen, such as chlorine, halogen can be replaced by reaction with an
appropriate substituted or unsubstituted amine, an alcohol, in particular a salt thereof, or a
mercaptan, in particular a salt thereof.
The invention relates in particular to the processes described in the examples.
Salts of compounds of the formula a) or their tautomers can be prepared in a manner
known per se. Thus, for example, acid addition salts of compounds of the formula (I) or a
tautomer thereof are obtained by treating with an acid or a suitable ion exchange reagent.
Salts can be converted into the free compounds in a customary manner, and acid addition
salts can be converted, for example, by treating with a suitable basic agent.
.~
Depending on the procedure or reaction conditions, the compounds according to the
invention having salt-forming, in particular basic properties, can be obtained in free forrn
or preferably in the form of salts.
7 ~
In view of the close relationship between the novel compound in the free form and in the
forrn of its salts, in the preceding parts and below the free compound or its salts may
analogously and expediendy also be understood as meaning the corresponding salts or the
free compound.
The novel compounds including their salts of salt-forming compounds can also be
obtained in the form of their hydrates or can include other solvents used for crystallization.
The novel compounds may exist, depending on the choice of the starting materials and
procedures, in the form of one of the possible isomers or as rnixtures thereof, for example,
depending on the number of asymmetrical carbon atoms, as pure op~ical isomers, as
antipodes, or as isomer mixtures, such as racemates, diastereomer mixtures or racemate
mixtures.
Racemate mixtures obtained can be separated separately into the pure isomers or
racemates in a known manner on the basis of the physicochemical differences of the
components, for example by fractional crystallization. Racemates obtained may
furthermore be resolved into the optical antipodes by known methods, -for exarnple by
recrystallization from an optically active solvent, chromatography on chiral adsorbents,
with the aid of suitable microorganisms, by cleavage with specific immobilized enzymes,
via the formation of inclusion compounds, for example using chiral crown ethers, only one
enantiomer being complexed, or by conversion into diastereomeric salts, for example by
reaction of a basic final substance racemate with an optically active acid, such as a
earboxylic acid, for exarnple tartaric or malic acid, or sulfonic acid, for exampie
camphorsulfonic acid, and separation of the diastereomer mixture obtained in this manner,
for exarnple on the basis of its differing solubilities, into the diastereomers from which the
desired enantiomer c~cm be liberated by the action of a suitable agent. The more active
enantiomer is advantageously isolated.
The invention also -relates to those embodiments of the process, according to which a
compound obtainable as an intermediate in any step of the process is used and the missing
steps are carried out or a starting material in the form of a derivative or salt and/or its
racemates or antipodes is used or, in particular, formed under the reaction conditions.
.
In the process of the present invention, those starting materials are preferably used which
7 ~
- 34-
lead to the compounds described as particularly useful at the beginning. The invention
Iikewise relates to novel starting materials which have been specifically developed for the
preparation of the compounds according to the invention, to their use and to processes for
their preparation, the variables Z, Rl, R2, R3, R4 and R~; having the meanings indicated in
each case for the preferred compound groups of the formula I or tautomers thereof. In
particular, compounds of the fo1mula III, their tautomers and salts in which X3 iS cyano
are preferred as a starting material.
The invention likewise relates to the use of the compounds of the formula (I) or tautomers
thereof or of pharmaceutically acceptable salts of compounds of this type with
salt-forming properties, in particular as pharmacological, primarily angiotensin II
antagonist, active substances. In this connection, they can be used, preferably in the form
of pharmaceutically acceptable preparations, in a method for the prophylactic and/or
therapeutic treatment of the animal or human body, in particular as angiotensin II
antagonists.
The invention likewise relates to pharmaceutical preparations which contain the
compounds according to the invention or pharmaceutically acceptable salts thereof as
active ingredients, and to processes for their preparation.
,
The pharrnaceutical preparations according to the invention which contain the compound
according to the invention or pharmaceutically acceptable salts thereof are those for
enteral, such as oral, furthermore rectal, and parenteral administration to ~a)
warm-blooded animai(s), the pnarmacological active ingredient being contained alone or
together with a pharrnaceutically acceptable carrier material. The daily dose of the active
ingredient depends on the age and the individual condition and also on the manner of
administration.
The novel pharmaceutical preparations contain, for example, from about 10 % to about
80 %, preferably fron~ about 20 % to about 60 %, of the active ingredient. Pharmaceutical
preparations according to the invention for enteral or parenteral adrninistration are, for
example, those in dose unit forms, such as sugar-coated tablets, tablets, capsules or
suppositories, and furthermore ampoules. These are prepared in a manner known per se,
for example by means of conventional rnixing, granulating, sugar-coating, dissolving or
lyophilizing processes. Thus, pharmaceutical preparations for oral use can be obtained by
combining the active ingredient with solid carriers, if desired granulating a mixture
,
.,~
~ ' .
3 7 ~
- 35 -
obtained, and processing the rnixture or granules, if desired or necessary, after addition of
suitable adjuncts to give tablets or sugar-coated tablet cores.
Suitable carriers are, in particular, fillers, such as sugars, for example lactose, sucrose,
mannitol or sorbitol, cellulose preparations and/or calcium phosphates, for example
tricalcium phosphate or calcium hydrogen phosphate, furthermore binders, such as starch
paste, using, for example, corn, wheat, rice or potato starch, gelatin, tragacanth,
methylcellulose and/or polyvinylpyrrolidone, if desired, disintegrants, such as the
abovementioned starches, furthermore carboxymethyl starch, crosslinked
polyvinylpyrrolidone, agar, alginic acid or a salt thereof, such as sodium alginate,
auxiliaries are primarily glidants, flow-regulators and lubricants, for exarnple silicic acid,
talc, stearic acid or salts thereof, such as magnesium or calcium stearate, and/or
polyethylene glycol. Sugar-coated tablet cores are provided with suitable coatings which
are, if desired, resistant to gastric juice, using, inter alia, concentrated sugar solutions
which, if desired, contain gum arabic, talc, polyvinylpyrrolidone, polyethylene glycol
andlor titanium dioxide, lacquer solutions in suitable organic solvents or solvent mixtures
or, for the preparation of gastric juice-resistant coatings, solutions of suitable cellulose
preparations, such as acetylcellulose phthalate or hydroxypropylmethylcellulose phthalate.
Colorants or pigments, for example to identify or to indicate different doses of active
ingredient, may additionally be added to the tablets or sugar-coated tablet coatings.
Other orally utilizable pharmaceutical preparations are hard gelatin capsules, and also soft
closed capsules made of gelatin and a plasticizer, such as glycerol or sorbitol. The hard
capsules may contain the active ingredient in the -form of granules, for example in a
mixture with fillers, such as lactose, binders, such as starches, and/or lubricants, such as
talc or magnesium stearate, and, if desired, stabilizers. In soft capsules, the active
ingredient is preferably dissolved or suspended in suitable liquids, such as fatty oils,
paraffin oils or liquid polyethylene glycols, it also being possible to add stabilizers.
Possible rectally utilizable pharmaceutical preparations are, for example, suppositories,
which consist of a combination of the active ingredient with a suppository base. Suitable
suppository bases are, for example, natural or synthetic triglycerides, paraffinhydrocarbons, polyethylene glycols or higher alkanols. Furthermore, gelatin rectal
capsules which contain a combination of the active ingredient with a ~ase substance may
also be used. Suitable base substances are, for example, liquid triglycerides, polyethylene
glycols or paraffin hydrocarbons.
2~37~
- 36-
Suitable preparations for parenteral administration are primarily aqueous solutions of an
active ingredient in water-soluble form, for example a water-soluble salt, and -furthermore
suspensions of the active ingredient, such as appropriate oily injection suspensions, using
suitable lipophilic solvents or vehicles, such as fatty oils, for example sesame oil, or
synthetic fatty acid esters, for example ethyl oleate or triglycerides, or aqueous injection
suspensions which contain viscosity-increasing substances, for example sodium
carboxymethylcellulose, surbitol and/or dextran, and, if necessary, also stabilizers.
The dose of the active ingredient depends on the warm-blooded animal species, ~he age
and the individual condition and on the manner of administration. In the normal case, an
approximate daily dose of about 10 mg to about 250 mg is to be estimated in the case of
oral administration for an approximately 75 kg patient.
`~:
The following examples illustrate the invention described above; however, they are not
intended to limit its extent in any manner.
Example 1: 2-n-Butyl-1,6-dihydro~ (2'-cyanobiphenyl-4-yl)methyl~-6-oxo pyrimidine
(2.0 g, 5.82 mmol) and tributyltin azide [Synthesis 1976, 329] (3.86 g, l l .G4 mrnol) in
o-xylene (50 ml) are stirred under reflux for 24 hours. The reaction mixture is evaporated
in vacuo and stirred into a mixture of CH2C12/CH3OH/NH3 (5:3:1, 100 ml) during the
~ course of 30 minutes. After evaporating again in vacuo, the residue is separated by means
; of flash chromatography (silica gel 60, 40-63 ~lm, CH2CI2/CH30H~I3 = 80:10:1) and the
product is recrystallized from ethanol-H2O. White crystals of 2-n-butyl-1,6-dihydro-1-
[(2'-(lH-tetrazolyl-5-yl~biphenyl-4-y l)methyl]-6-oxopyrimidine are obtained in this way.
`~ M.p.: 180-182C.
` ~ N=N
N~ N~NH
CH3 (ctl2 3 N ~3
The starting material can be prepared as follows:
a) NaH (80 % ;n white oil, 0.92 g, 30.5 mmol) is added in portions at room temperature to
a solution of 5.7 g (3.05 mmol) of 2-n-butyl-4-chloro-6-hydroxypyrimidine [J. Chem. Soc.
r
., '
'
.~ ~
3 7 ~
1964, 3204] in 60 ml of ~MF. After addition is complete, the mixture is stirred for a
further 30 minutes at room temperature and a solution of 8.3 g (30.S mmol) of
4-bromomethyl-2'-cyanobiphenyl [EP 253,310] in 50 ml of DMF is then added dropwise.
The reaction mixture is stirred at room temperature for 12 hours and then evaporated in
vacuo. Ethyl acetate is added to the residue and the mixture is washed twice with H2O,
dried (Na2soa~) and evaporated in vacuo. Flash chromatography (silica gel 60, 40-63 ,~m,
hexane-ethy} acetate = 2:1) gives 2-n-butyl-4-chloro-1,6-dihydro-[(2'-cyanobiphenyl-4-
yl)methyl]-6-oxopyrimidine which is recrystallized from diethyl ether. White crystals
remain. M.p. 96-98C.
b) A mixture of 5.5 g (14.5 mmol) of 2-n-butyl-4-chloro-1,6-dihydro-~(2'-
cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine in 8û ml of methanol and 1.62 g (16 mmol)
of triethylamine is hydrogenated at room temperature and 1 atm of H2 in the course of 1
hour in the presence of 5 % Pd-C (O.S g). The catalyst is filtered off and the filtrate is
evaporated in vacuo. Ethyl acetate is added to the residue and the mixture is washed twice
with H2O, dried (Na2SO4) and evaporated in vacuo. 2-n-Butyl-1,6-dihydro-1-[(2'-
cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine is obtained in this way, and is reacted
without further purification.
xample 2: Starting from 2-n-butyl-4-chloro-1,6-dihydro-1-[(2'-cyanobiphenyl-4-
yl)methyl]-6-oxopyrimidine (Example la) and tributyl~in azide, 2-n-butyl-4-chloro-1,6-
dihydro-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxopyrimidine is obtained in the
manner described, for example, in Example 1. M.p.: 127C (dec.).
Example 3: Starting from 2-n-butyl-1,6-dihydro-4-dimethylamino-1-[(~'-
cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine and ttibutyltin azide, 2-n-butyl-1,6-dihydro-4-dimethylamino-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxo-
pyrimidine is obtained in the manner described, for example, in Example 1. M.p.: ~46C
(decomposition) .
The starting material can be prepared, for example, as follows:
2.0 g (5.36 mmol) of 2-n-butyl-4-chloro-1,6-dihydro-1-[(2'-cyanobiphenyl-4-yl)methyl]-6-
oxopyrimidine and 33 % dimethylamine in ethanol (3.8 ml, 21.4 mmol~ in 10 ml of
ethanol are stirred under reflux for 12 hours. After evaporating in vacuo, ethyl acetate is
added to the residue and the mixture is washed twice with H20, dried (Na2SO4) and
3 7 ~
- 38 -
evaporated in vacuo. Flash chromatog~aphy (silica gel 60, 40-63 ~lm, hexane/ethyl acetate
1:2) gives 2-n-butyl-1,6-dihydro-4-dimethylamino-1-[(2'-cyanobiphenyl-4-yl)methyl]-6-
oxopyrimidine as a white foam which is immediately further processed.
Example 4: Starting from 2-n-butyl-1,6-dihydro-4-methyl-1-[(2'-cyanobiphenyl-4-
yl)methyl]-6-oxopyrimidine and triblJtyltin azide, 2-n-butyl-1,6-dihydro-4-methyl-1-[(2'-
tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxopyIimidine is obtained, for example, in the
manner described in E~xample 1. M.p. 213-216C.
The starting material can be prepared, for example, as follows:
2-n-Butyl-1,6-dihydro-4-methyl-1-[(2'-cyanobiphenyl-4-yl)methyl]-6-oxopyrirnidine is
obtained by alkylation of 2-n-butyl-4-hydroxy-6-methylpyrimidine [J. Chern. Soc. 1963,
5642] with 4-bromomethyl-2'-cyanobiphenyl in the manner described in Example la. The
product- obtained by flash chromatography (silica gel 60, 40-63 llm, C~I2Cl~/CH301~I =
98:2) is immediately further processed.
Example 5: Starting from 2-n-butyl-1,6-dihydro-4-methoxy-1-[(2'-cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine and tributyltin azide, 2-n-butyl-1,6-dihydro-4-methoxy-1-
[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxopyrimidine is obtained, for example, in
the manner described in ~xample 1~ M.p. 223-225C.
The starting material can be prepared, for example, as follows:
, .
1.0 g (2.65 mmol) o~ 2-n-butyl-4-chloro-1,6-dihydro-~2'-cyanobiphenyl-4-yl)methyl]-6-
oxopyrimidine is added to a solution of 343 mg (6.35 mmol) of sodium methoxide in
15 ml of rnethanol. The reaction mixture is stirred at room temperature for 12 hours and
then evaporated in vacuo. Ethyl acetate is added to the residue and the mixture is washed
twice with H2O, dried (Na2SO4) and evaporated in vacuo. Flash chromatography (silica
gel 60, 40-63 ~lm, hexane/ethyl acetate = 1:1) gives 2-n-butyl-1,6-dihydro-4-methoxy-1-
[(2'-cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine, which is immediately further
processed.
Example 6: Starting from 2-n-butyl-1,6-dihydro-4-morpholino-1-[(2'-cyanobiphenyl-4-
yl)methyl]-6-oxopyrimidine and tributyltin azide, 2-n-butyl-1,6-dihydro-4-morpholino-1-
~ [2'-(lH-tetrazol-S-yl)-biphenyl-4-yl)methyl]-6-oxopyrimidine is obtained, for example, in
'~:
3 7 ~
- 39 -
the manner described in Example 1. M.p. 278C (from ethyl acetate).
The starting material can be prepared, for example, as follows:
1.0 g (2.65 mmol~ of 2-n-botyl-4-chloro-1,6-dihydro-1-[(2'-cyanobiphenyl-4-yl)me~hyl]-6-
oxopyrimidine and 0.92 ml (10.6 mmol) of morpholine in 20 ml of n-butanol are stirred
under reflux for 12 hours. After evaporating in vacuo, ethyl acetate is added to the residue
and the mixture is washed twice with H20, dried (Na2SO4) and evaporated in vacuo. The
residue is recrystallized from ethyl acetate/hexane. 2-n-Butyl-1,6-dihydro-4-
morpholino-1-[(2'-cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine is obtained in this way.
M.p. 124-126C.
,
Example 7: Starting from 2-n-butyl-1,6-dihydro-4-methylthio-1-[(2'-cyanobiphenyl-4-
yl)methyl]-6-oxopyrimidine and ~ibutyltin azide, 2-n-butyl-1,6-dihydro-4-methylthio-1-
[2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxopyrimidine is obtained, ~or example, in
the manner described in Example 1. M.p. 205C (from ethyl acetate).
The starting material can be prepared, for exarnple, as follows:
.~
870 mg (2.30 mmol) of 2-n-butyl-4-chloro-1,6-dihydro-1-[(2'-cyanobiphenyl-4-
yl)methyl]-6-oxopyrimidine and 180 mg (2.53 mmol) of sodium methanethiolate in 10 ml
of 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone are stirred at room temperature for
30 minutes. After addition of H2O, the mixture is extracted with ethyl acetate, and the
organic phase is washed with H2O, dried (Na2SO4) and evaporated in vacuo. Flash
chromatography (silica gel ~0, 40-63 ~lm, hexane/ethyl acetate = 1:1) gives
2-n-butyl-1,6-dihydro-4-methylthio-1-[(2'-cyanobiphenyl-4-yl)methyl]-6-oxopyrirnidine
as a resin which is immediately further processed.
Example B: 240 mg (0.55 mmol) of 2-n-butyl-1,6-dihydro-4-methylthio-1-[2'-(lH-
tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxopyrimidine and 500 mg (1.45 mmol) of ~0 %
3-chloroperbenzoic acid in 10 ml of CH2Cl2 are stirred at room temperature for 12 hours.
After evaporating in vacuo, ethyl acetate is added to the residue and the mixture is washed
twice with H20, dried (Na2SO4) and evaporated in vacuo. Flash chromatography (silica
gel 60, 40-63 ~,lm, OEl2Cl2/CH3OH/NH3 = 80:10:1) gives 2-n-butyl-1,6-dihydro-4-
methanesulfonyl- 1 - [(2 ' -( lH-tetrazol-5-yl)biphenyl-4-yl)methyl] - 6-oxopyrimidine as an
amorphous solid.
. ' ' .
~3~37~
- 40 -
Example 9: Starting from 2-n-butyl-1,6-dihydro-4-(2-hydroxyethoxy)-1-[(2'-
cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine and tributyltin azide, 2-n-butyl-1,6-dihydro 4-(2-hydroxyethoxy)-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-
oxopyrimidine is obtained, for example, in the manner described in Example 1. M.p.
150C (frdm isopropano~lethyl acetate).
The starting material can be prepared, for example, as follows:
1 g of (2.65 mmol) 2-n-butyl-4-chloro-1,6-dihydro-1-[2'-(lH-tetrazol-~-yl)biphenyl-4-yl~-
methyl]-6-oxopyridine is added to a solution of 122 mg (5.3 mmol) of sodium in 20 ml of
ethan- 1,2-diol and the reaction mixture is stirred at 70 for one hour. After evaporating in
vacuo, ethyl acetate is added to the residue and the mixture is washed with ~I2O, dried
(Na2SO4) and evaporated in vacuo. 2-n-Butyl- 1,6-dihydro-4-(2-hydroxyethoxy)-1-[t2'-
(lH-tetrazol-5-yl)biphenyl-4-yl)methyl ]-6Oxopyrimidine is obtained. M.p. l 19-121
(from ethyl acetate/hexane).
.~
Example 10: Starting~rom2-n-butyl-1,6-dihydro-4-(2-methoxyethoxy)-1-[(2'-
cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine and tributyltin azide, 2-n-butyl- 1,6-
~ dihydro-2-(2-methoxyethoxy)-1-[2'-(lH-tetra~zol-5-yl)biphenyl-4-yl)methyl]-6-
oxopyrimidine is obtained, for example, in the manner described in ~xample 1. M.p.
195-197C (from ethyl acetate).
The starting ma~erial can be prepared, for example, as follows:
1.0 g (2.65 mmol) of 2-n-butyl-4-chloro-1,6-dihydro-1-[(2'-cyanobiphenyl-4-
yl)methyl]-6-oxopyrimidine is added to a solution of 122 mg (5.2 mmol) of sodium in
20 ml of 2-methoxyethanol and the reaction mixture is stirred at room temperature for
12 hours. After evaporating in vacuo, ethyl acetate is added to the residue and the mixture
is washed with H20, dried (~a2SO~) and evaporated in vacuo. Flash chromatography(silica gel 60, 40-63 ~1m, ethyl acetate/hexane = 1:1) gives 2-n-butyl-1,6-dihydro-4-(2-
methoxyethoxy)-1-[(2'-cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine as a viscous oil
which is immediately further processed.
Example 11: Startingfrom2-n-butyl-1,6-dihydro-4-(2-dimethylaminoethoxy)-1-
[(2'-cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine and tributyltin azide,
~$2~7~
- 41 -
2-n-butyl-1,6-dihydro-4-(2-dimethylaminoethoxy)-1-[(2'-(lH-tet razol-5-yl)-
biphenyl-4-yl)methyl]-6-oxopyrimidine is obtained, for example, in the manner described
in Example 1 as an amorphous solid.
The starting material can be prepared, for example, as follows:
1.0 g (2.65 mmol) of 2-n-butyl-4-chloro-1,6-dihydro-1-[(2'-cyanobip}lenyl-4-yl)methyll-6-
oxopyrimidine is added to a solution of 142 mg (5.92 mmol) of sodium in 20 ml of2-dimethylaminoethanol and the reaction mixture is stirred at room temperature for
12 hours. After evaporating in vacuo, ethyl acetate is added to the residue and the mixture
is washed with H20, dried (Na2SO4) and evaporated in ~acuo. Flash chromatography(silica gel 60, 4û-63 ~m, C~I2CI2/CH30H = 95:5) gives 2-n-butyl-1,6-dihydro-4-(2-
dimethylaminoethoxy)-1-[(2'-cyanobiphenyl-4-yl)methyl3-6-oxopyrimidine as an oilwhich is immediately further processed.
Example 12: Startingfrom4-benzyloxy-2-n-bu~yl-1,6-dihydro-1-[(2'-cyanobiphenyl-4-
yl)methyl]-6-oxopyrimidine and tributyltin azide, 4-benzyloxy-2-n-butyl-1,6-dihydro-l-
[(2'-(lH-tetrazol-5-yl)bipherlyl-4-yl)methyl]-6-oxopyrimidine is obtained, for example, in
the manner described in Example 1. M.p. 240C (from N,N-dimethylformamide/H2O).
The starting material can be prepared, for example, as follows:
1.0 g (2.65 mmol) of 2-n-butyl-4-chloro-1,6-dihydro-1-[(2'-cyanobiphenyl-4-yl)methyl]-
6-oxopyrimidine is added to a solution of 122 mg (5.3 mmol) of sodium in 10 ml of benzyl
alcohol and the reaction mixture is stirred a~ 60C for 3 hours. After evaporating in vacuo,
ethyl acetate is added to the residue and the mixture is washed with H20, dried (Na2SO4)
and evaporated in vacuo. Flash chromatography (silica gel 60, 40-63 ~m, hexane/ethyl
acetate = 3:1) gives 4-benzyloxy-2-n-butyl-1,6-dihydro-1-[(2'-cyanobiphenyl-4-
yl)methyl]-6-oxopyrimidine as an oil which is immediately further processed.
Example 13: Starting from 2,4-di-n-butyl-1,6-dihydro-1-L(2'-cyanobiphenyl-4-
yl)methyl]-6-oxopyrimidine and tributyltin azide, 2,4-di-n-butyl-1,6-dihydro-1-[(2'-
(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxopy~imidine is obtained, for example, in the
manner described in Example 1. M.p. 167-169C (from acetic acid/H20).
The starting material can be prepared, fo; example, as follows:
.
2~2~37~
- 42 -
a) 2.73 g (20 mmol) of n-valeroylamidine.HCl are added with ice-cooling to a solution of
920 ml (40 mmol) of sodium in 40 ml of absolute ethanol. After addition is complete, the
mixture is stirred for 30 minutes and a solution of 3.44 g (20 mmol) of ethyl
3-oxohexanoate in 5 ml of absolute ethanol is then added dropwise. The reaction mixture
is stirred at room temperature for 12 hours and then evaporated in vacuo. The residue is
dissolved ;n H2O and acidi~led by addition of acetic acid. The mixture is extracted with
ethyl acetate, washed with H2O, dried (Na2SO4) and evaporated in vacuo. Flash
chromatography (silica gel 60, 40-63 ~lm, hexane/ethyl acetate = 1:1) gives
2,4-di-n-butyl-6-hydroxypyrimidine which is immediately further processed.
b) By alkylation of 2,4-di-n-butyl-6-hydroxypyrimidine with 4-bromomethyl-2'-
cyanobiphenyl in the manner described in Example la) and flash chromatography (silica
gel 60, 40-63 ~1m, hexane/ethyl acetate = 2:1), 2,4-di-n-butyl-1,6-dihydro-1-[(2'-
cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine is obtained as an oil which is immediately
filrther processed.
Example 14: Starting from 2-n-propyl-1,6-dihydro-4-methyl-1-~(2'-cyanobiphenyl-4-
yl)methyl~-6-oxopyrimidine and tributyltin azide, 2-n-propyl-1,6-dihydro-4-methyl-1-
~2'-(lH-tetrazol-S-yl)biphenyl-4-yl)methyll-6-oxopyrimidine is obtained, for example, in
the manner described in Exarnple 1. M.p. 203-205C (from ethyl acetate).
The starting material can be prepared, for example, as follows:
a) By reaction of ethyl 3-oxopentanoate with acetamidine.HS:~l and sodium in absolute
ethanol in the manner described in Example 13a), 2-n-propyl-4-methyl-6-
hydroxypyrimidine is obtained and is immediately further processed,
b) By alkylation of 2-n-propyl-4-methyl-6-hydroxypyrimidine with 4-bromomethyl-2'-
cyanobiphenyl in the rnanner described in Example 1 a~ and flash chromatography (silica
gel 60,40-63 ~lm, hexane/ethyl acetate 3:1), 2-n-propyl-1,6-dihydro-4-methyl-1-[(2'-
cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine is obtained. M.p. 101-103C (from diethyl
ether/hexane).
Example 15: Starting from 2-n-butyl-1,6-dihydro-4,5-dimethyl-1-[(2'-cyanobiphenyl-4-
yl)methyll-6-oxopyrimidine and tributyltin azide, 2-n-butyl-1,6-dihydro-4,5-dimethyl-1-
2~2~37~
- 43 -
[2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxopyrimidine is obtained, for example, in
the manner described in Example 1. M.p. 198-200C (from acetic acid/EI20).
The starting material can be prepared, for example, as follows:
a) By reaction of ethyl 2-methyl-acetylacetate with n-valeroylamidine.HCl and sodium in
absolute ethanol in the manner described in Example 13a), 2-n-butyl-4,5-dimethyl-6-
hydroxypyrirnidine is obtained and is immediately further processed.
b) By alkylation of 2-n-butyl-4,5-dimethyl-6-hydroxypyrimidine with 4-bromomethyl-2'-
cyanobiphenyl in the manner described in Example la) and flash chrornatography (silica
gel 60, 40-63 ~lm, hexane/ethyl acetate = 2:1), 2-n-butyl-1,6-dihydro-4,5-dimethyl-1-
[(2'-cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine is obtained and is immediately further
processed.
Example 16: Starting from 2-n-butyl-1,6-dihydro-4-trifluoromethyl-1-E(2'-
cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine and tributyltin azide, 2-n-butyl-1,6-dihydro-4-trifluoromethyl-1-[2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxopyrimidine
is obtained, for example, in the manner described in Example 1. M.p. 13~-140C (-~rom
diethyl ether).
The starting material can be prepared, for example, as follows:
a) By reaction of ethyl trifluoroacetate with n-valeroylamidine.HCl and sodium in
absolute ethanol in the manner described in Example 13a), 2-n-bu~yl-4-trifluoromethyl-6-
hydroxypyrimidine is obtained and is immediately further processed.
b) By alkylation of 2-n-butyl-4-trifluoromethyl-6-hydroxypyrimidine with
4-bromomethyl-2'-cyanobiphenyl in the manner described in Example la) and flash
chromatography (silica gel 60, 40-63 ~,-m, hexane/ethyl acetate = 4:1), 2-n-butyl-1,6-
dihydro-4-trifluoromethyl-1-[(2'-cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine is obtained
and is immediately further processed.
Example 17: Starting from 2-n-butyl-1,6-dihydlo-S-ethyl-4-methyl-1-[(2'-
cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine and tributyltin azide, 2-n-butyl-1,6-dihydro-5-ethyl-4-methyl- 1 -[(2'-(lH-tetrazol-S-yl)biphenyl-4-yl)methyl]-6-oxopyrimidine
~2~37~
- 44 -
is obtained, for exarnple, in the manner described in Example 1. M.p. 162-163C (from
acetonitrile) .
The starting matelial can be prepared, for example, as follows:
a) By reaction of ethyl 2-ethyl-acetylacetate with n-valeroylamidine.HCI and sodiurn in
absolute ethanol in the manner described in Example 13a), 2-n-butyl-5-e~hyl-4-
methyl-5-hydroxypyrimidine is obtained and is immediately further processed.
b) By alkylation of 2-n-butyl-5-ethyl-4-methyl-6-hydroxypyrimidine with -
4-bromomethyl-2'-cyanobiphenyl in the manner described in Example la) and flash
chromatography (silica gel 60, 40-63 llm, hexane/ethyl acetate = 4:1), 2-n-butyl-1,6-
dihydro-S-ethyl-4-methyl-1-[(2'-cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine is obtained
and is immediately further processed.
~xample 18: Starting from 2-n-butyl-1,6-dihydro-5-isopropyl-4-methyl-1-[(2'-
cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine and tributyltin azide, 2-n-butyl-1,6-dihydro-5-isopropyl-4-methyl-1-[(2'-(lH tetrazol-S-yl)biphenyl-4-yl)-methyl]-6-
oxopyrirnidine is obtained, for example, in the manner described in Example 1. M.p.
1 82C (from diethyl ether).
The starting material can be prepared, for example, as follows:
a) By reaction of ethyl 2-isopropyl-acetylacetate with n-valeroylarr~dine.HCI and sodium
in absolute ethanol in the manner described in Example 13a), 2-n-butyl-5-isopropyl-4-
methyl-6-hydroxypyrimidine is obtained and is immediately further processed.
.
b) By alkylation of 2-n-butyl-5-isopropyl-4-methyl-6-hydroxypyrimidine with
4-bromomethyl-~'-cyanobiphenyl in the manner described in Example la) and flash
chromatography (silica gel 60, 40-63 ~lm, hexanetethyl acetate = 4:1), 2-n-butyl-1,6-
dihydro-S-isopropyl-4-methyl-1-[(2'-cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine is
obtained and is immediately further processed.
Example 19: Starting ~rom 2-n-butyl-1,6-dihydro-4-ethyl-1-[(2'-cyanobiphenyl-4-
yl)rnethyl]-6-oxopyrimidine and tributyltin azide, 2-n-butyl-1,6-dihydro-4-ethyl-1-[(2'-
(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxopyrimidine is obtained, for example, in the
.
.' ~.
,. ~
2 ~ 7 ~
- 45
manner described in Example 1. M.p. 178-180C (from acetonitrile).
The starting material can be prepared, for example, as follows:
a) By reaction of ethyl 3-oxovalerate with n-valeroylamidine.HCl and sodiurn in absolute
ethanol in the manner described in Example 13a), 2-n-butyl-4-ethyl-6-hydroxypyrimidine
is obtained and is imrnediately further processed.
b) By alkylation of ~-n-butyl-4-ethyl-6-hydroxypyrimidine with 4-bromomethyl-2'-cyanobiphenyl in the manner described in Example la) and flash chromatography (silica
gel 60, 40-63 ~lm, hexane/ethyl acetate = 2:1), 2-n-butyl-1,6-dihydro-4-ethyl-1-[(2'-
cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine is obtained and is immediately fulrther
processed.
Example 20: Starting from 2-n-butyl-1,6-dihydro-4-n-propyl-1-~(2'-cyanobiphenyl-4-
yl)methyl]-6-oxopyrimidine and tributyltin azide, 2-n-bu.hyl-1,6-dihydro-4-n-propyl-1-[(2'-
(lH-tetrazol-S-yl)biphenyl-4-yl)methyl~-6-oxopyrimidine is obtained, for example, in the
manner described in Exarnple 1. M.p. 177-178C (from ethyl acetate/hexane).
The starting material can be prepared, for example, as follows:
a) By reaction of ethyl butyrylacetate with n-valeroylamidine.HCl and sodium in absolute
ethanol in the manner described in Example 13a), 2-n-butyl-4-n-propyl-6-
hydroxypyrimidine is obtained and is immediately further processed.
b) By alkylation of 2-n-butyl-4-n-propyl-6-hydroxypyrimidine with 4-bromomethyl-2'-
cyanobiphenyl in the manner described in Example la) and flash chromatography (silica
gel 60, 40-63 llm, hexane/ethyl acetate = 4:1), 2-n-butyl-1,6-dihydro-4-isopropyl-1 [(2'-
cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine is obtained and is immediately further
processed.
Example 21: Starting from 2-n-butyl-1,6-dihydro-4-rnethyl-5-n-propyl-1-[(2'-
cyanobiphenyl-4-yl)methyll-6-oxopyrimidine and tributyltin azide, 2-n-butyl-1,6-dihydro-4-methyl-5-n-propyl-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-
oxopyrimidine is obtained, for example, in the manner described in Exarnple 1 as an
amorphous solid.
:~ .
7 ~
- ~6 -
The starting material can be prepared, for example, as follows:
a) By reaction of ethyl 2-n-propyl-2-acetylacetate with n-valeroylamidine.HCl and sodium
in absolute ethanol in the manner described in Example 13a), 2-n-butyl-~-methyl-5-
n-propyl-6-hydroxypyrimidine is obtained and is immediately further processed.
b) By alkylation of 2-n-butyl-4-methyl-5-n-propyl~6-hydroxypyrimidine with
4-bromomethyl-2'-cyanobiphenyl in the manner described in Example la) and flash
chromatography (silica gel 60, 40-63 ~lm, hexane/ethyl acetate = 4:1), 2-n-butyl-1,6-
dihydro-4-methyl-5-n-propyl-1-~(2'-cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine isobtained and is immediately further processed.
,~;
Example 22: Stalting from 2-n-butyl-1,6-dihydro-5-ethoxycarbonyl-1-[(2'-
cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine and tributyltin azide, 2-n-butyl-1,6-dihydro-5-ethoxycarbonyl- 1 -[(2'-(lH-tetrazol-S-yl)biphenyl-4-yl)methyl]-6-
oxopyrimidine is obtained, for example, in the manner described in Example 1. M.p.
175-177C (from ethyl acetate).
.
The starting material caD be prepared, for example, as follows:
a) By reaction of diethyl ethoxymethylenemalonate with n-valeroylamidine.HCl andsodium in absolute ethanol in the manner described in Example 13a), 2-n-butyl-5-ethoxycarbonyl-6-hydroxypyrimidine is obtained and is immediately further processed.
b) By aLkylation of 2-n-butyl-5-ethoxycarbonyl-6-hydroxypyrimidine with
4-bromomethyl-2'-cyanobiphenyl in the manner described in Example la) and flash
chromatography (silica gel 60,40-63 I-m, hexane/ethyl acetate = 3:1), 2-n-butyl-1,6-
dihydro-5-ethoxycarbonyl-1-[(2'-cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine is obtained
and is immediately further processed.
Example 23: 1.1 ml of lN NaOH are added to a solution of 245 mg (0.534 mmol) of
2-n-butyl-1,6-dihydro-5-ethoxycarbonyl-1-~(2'-(lH-tetrazol-S-yl)biphenyl-4-yl)methyl]-6-
oxopyrimidine in 8 ml of ethanol. The reaction mixture is stirred at room temperature for
2 hours and then evaporated in vacuo. After addition of H2O, it is acidifled with lN HCl
(pH = 3) and extracted with CH2Cl2~ The organic phases are dried (Na2SO4) and
,,,
',':
~:,
i,
3 7 ~
evaporated in vacuo. Flash chromatography (silica ge} 60, 40-63 ~lm, ~H2CI2/CH30H/NH3
= 5:3:1) gives 2-n-butyl-5-carboxy-1,6-dihydro-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-
yl)methyl]-6-oxopyrimidine as an amorphous solid.
Example 24: Starting from 2-n-butyl-1,6-dihydro-4-methoxymethyl-1-[(2'-cyanobiphenyl-4-yl)rnethyl]-6-oxopyrimidine and tributyltin azide, 2-n-butyl-1,6-
dihydro-4-methoxymethyl-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-
oxopyrimidine is obtained, for example, in the manner described in Example 1. M.p.
193C (from ethyl acetate)
-
The starting material can be prepared, for example, as follows:
a) By reaction of methyl 2-methoxyacetylacetate with n-valeroylamidine.HCI and sodium
in absolute ethanol in the manner described in Example 13a~, 2-n-butyl-4-
methoxymethyl-6-hydroxypyrimidine is obtained and is immediately further processed.
b) By aL~cylation of 2-n-butyl-4-methoxymethyl-6-hydroxypyrimidine with
4-bromomethyl-2'-cyanobiphenyl in the manner described in Example la) and flash
chromatography (silica gel 60, 40-63 ,-m, hexane/ethyl acetate = 1:1), 2-n-butyl-1,6-
dihydro-4 methoxymethyl-1-~(2'-cyanobiphenyl-4-yl)methyl]-6-oxopyli~udine is
obtained and is directly further processed.
.
Example 25: Starting from 2-n-butyl-1,6-dihydro-5-hydroxymethyl-1-[(2'-
cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine and tributyltin azide, 2-n-butyl-1,6-dihydro-S-hydroxymethyl-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-
oxopyrimidine is obtained, for example, in the manner described in Example 1. M.p.
147C (from ethyl acetate).
. ~
The starting material can be prepared, for example, as follows:
a) A solution of 2.6 g (11.6 mmol) of 2-n-butyl-5-ethoxycarbonyl-6-hydroxypyrimidine in
50 ml of absolute tetrahydrofilran is added dropwise at room temperature to a suspension
of 440 mg (11.6 mmol) of lithium aluminium hydride in 20 ml of absolute
tetrahydrofuran. After completion of the addition, the mixture is stirred at room
temperature for 1 hour and then hydrolysed by metering in S0 ml of H~O. After acidifying
with acetic acid ~pH = 4), the mixture is extracted with ethyl acetate. The organic phases
: , .
, :
, .
i'~
~2~37~
- 48 -
are dried tNa2SO4) and evaporated in vacuo. After addition of diethyl ether, the crystals
are filtered off and dried in vacuo. 2-n-Butyl-5-hydroxymethyl-6-hydroxypyrimidine is
obtained in this way. M.p. 107-109C.
b) 13.6 g (49.4 mmol) of potassium carbonate are added to a solution of 9.0 g (49.4 mmol)
of 2-n-butyl-S-hydroxymethyl-6-hydroxypyrimidine in 200 ml of
N,N-dimethylf~rmamide. The mixture is stirred at room temperature for 20 minutes and
13.4 g (49.4 mmol) of 4-bromomethyl-2'-cyanobiphenyl is then added in portions. After
completion of the addition, the mixture is stirred at room temperature for 12 hours and
then evaporated in vacuo Ethyl acetate is added to the residue, and the mixture is washed
with H20, dried (Na2SO4) and evaporated in vacuo. Flash chromatography (silica gel Ç0,
40-63 ~1m, hexane/ethyl acetate = 1:1) giYes 2-n-butyl-S-hydroxymethyl-1-[(2'-
cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine. M.p. 96-98C.
Example 26: By reaction of 2-n-butyl-1,6-dihydro-4-ethoxycarbonyl-1-~(2'-cyano-
biphenyl-4-yl)methyl]-6-oxopyrimidine and tributyltin azide, 2-n-butyl-1,6-dihydro-4-
ethoxycarbonyl- 1 -[(2'-( lH-tetrazol-S-yl)biphenyl-4-yl)methyl]-6-oxopyrimidine is
obtained, for example, in the manner described in Example 1 as an amorphous solid.
The starting material can be prepared, for exarnple, as follows:
a) By reaction of the sodium salt of diethyl oxalylacetate with n-valeroylamidine.HCl and
sodium in absolute ethanol in the manner described in Example 13a),
2-n-butyl-4-ethoxycarbonyl-6-hydroxypyrimidine is obtained. M.p. 127-128C (fromdiethyl ether/hexane).
b) By alkylation of 2-n-butyl-4-ethoxycarbonyl-6-hydroxypyrimidine with
4-bromomethyl-2'-cyanobiphenyl in the manner described in Example la) and flash
chromatography (silica gel 60, 40-63 ~m, hexane/ethyl acetate = 1:1), 2-n-butyl-1,6-
dihydro-4-ethoxycarbonyl-1-[(2'-cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine is obtained
and is immediately further processed.
Example 27: By hydrolysis of 2-n-butyl-1,6-dihydro-4-ethoxycarbonyl-1-[(2'-(lH-
tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxopyrimidine in the manner described in
Example 23, 2-n-butyl- 1,6-dihydro-4-carboxy- 1 - [(2 '-( lH-tetra~ol-S-yl)biphenyl-4-
yl)methyl]-6-oxopyrimidine is obtained. M.p. 160-162C (from acetic acid~H2O).
7 ~
- 49 -
Example 28: A solution of 400 mg (0.81 mmol) of 2-n-butyl-4-benzyloxy-1,6-dihydro-1-
[(2'-(lH-tetrazol-S-yl)biphenyl-4-yl)methyl]-6-oxopyrimidine in 75 ml of
methanoUCH2Cl2 (1:1) is shaken at room temperature and 1 atm of H2 in the presence of
100 mg of 5 % Pd-C. After the uptake of hydrogen is complete, ~he catalyst is filtered off
and washed with methanol and the ~lltrate is evaporated in vacuo. 2-n-Butyl- 1,6-
dihydro-4-hydroxy-1-[(2'-(lH-tetrazol-S-yl)biphenyl-4-yl)methyl]-6-oxopyrimidine is
obtained in this way. M.p. 153C (from isopropanol/ethyl acetate).
Example 29: By reaction of 2-n-butyl-1,6-dihydro-4-hydroxy-5-methyl-1-[~2'-
cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine and tributyltin azide, 2-n-butyl-~,6-dihydro-4-hydroxy-S-methyl- 1 -[(2' -(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-
oxopyrimidine is obtained, for example, in the manner described in Example 1. M.p.
221-223C (from acetonitrile).
The starting material can be prepared, for example, as follows:
a) A mixture of 28.1 g (0.2 mol) of n-valeroylamidine.HCl and 42.8 g (0.2 mol) of
4-aminomethyl-2'-cyanobiphenyl (EP 253,310) in 50 ml of E~OH is stirred under reflux
for 12 hours. After evaporating in vacuo and flash chromatography (silica gel 60, 40-63
m, CH2Cl2/CH30H = 4:1), N-[(2'-cyanobiphenyl-4-yl)methyl]-n-valeroylamidine.HCI is
obtained as an amorphous solid, which is immediately further processed.
b) A solution of 1.97 g (6 mmol) of N-[(2'-cyanobiphenyl-4-yl)methyl]-n-
valeroylamidine.HCl in S ml of absolute ethanol is added dropwise at room temperature to
a solution of 280 mg (12 mmol) of sodium in 5 ml of absolute ethanol. After 10 minutes,
1.05 ml (6 mmol) of diethyl methylmalonate are added and the suspension is stirred under
reflux for 24 hours. The reaction mixture is evaporated in vacuo, ~I2O is added and the
mixt!lre is acidified with 2N HCl (pH = 3). It is extracted with ethyl acetate, washed with
H2O, dried (Na2SO4) and evaporated in vacuo. Flash chromatography (silica gel 60, 40-63
llm, CH2Cl2/CH30H = 9S:S) gives 2-n-butyl-1,6-dihydro-4-hydroxy-S-methyl-1-[(2'-cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine. M.p. 204-206C (from diethyl ether).
Example 30: Starting from 2-n-butyl-1,6-dihydro-S-ethyl-4-hydroxy-1-[(2'-
cyanobiphenyl-4-yl)methyl]-6-oxopyrimidine and tributyltin azide, 2-n-butyl-1,6-dihydro-S-ethyl-4-hydroxy-1-[(2'-(lH-tetrazol-S-yl)biphenyl-4-yl~methyl]-6-
3 7 ~
- so
oxopyrimidine is obtained, for example, in the manner described in Example 1. M.p.
248-250C (from ethanol/H2O).
The starting material can be prepared, for example, as follows:
a) By reaction of diethyl ethylmalonate with N-[(2'-cyanobiphenyl-4-yl)methyl]-n-
valeroylamidine.HCl and sodium in absolute EtOH in the manner described in Example
29b), 2-n-butyl- 1,6-dihydro-S-ethyl-4-hydroxy- 1 - [(2' -cyanobiphenyl-4-yl)methyl]-6-
oxopyrimidine is obtained. M.p. 157-158C (from isopropanol).
Example 31: Starting from 2,5-di-n-butyl-1,6-dihydro-4-hydroxy-1-[(2'-cyanobiphenyl-4-
yl)methyl}-6-oxopyrimidine and tributyltin azide, 2,5-di-n-butyl-1,6-dihydro-4-hydroxy-1-
[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxopyrimidine is obtained, for example, in
the manner described in Example 1. M.p. 245-247C (from acetic acid/H20).
The starting material can be prepared, for example, as follows:
a) By reaction of diethyl n-butylmalonate with N-[(2'-cyanobiphenyl-4-yl)methyl]-n-
valeroylamidine.HCl and sodium in absolute ethanol in the manner described in Example
29b), 2,5-di-n-butyl-1,6-dihydro-4-hydroxy-1-[(2'-cyanobiphenyl-4-yl)methyl]-6-
oxopyrimidine is obtained and is immediately further processed.
Example 32: A solution of 1.96 g (5.02 mmol) of 2-n-butyl-1,6-dihydro-4-methyl-1-[(2'-
methoxycarbonylbiphenyl-~-yl)methyl]-6-oxopyrimidine in 15 ml of methanol and 5 ml of
2N NaOH is stirred at 50C for 12 hours. After evaporating the methanol in vacuo, the
mixture is acidified with 2N HCl (pH = 1) and extracted with CH2Cl2. The organic phase
is dried (Na2SO4) and evaporated. 2-n-Butyl-1,6-dihydro-4-methyl-1-[(2'-
carboxybiphenyl-4-yl)rnethyl]-6-oxopyrimidine is obtained in this way. M.p. 190-192C
(from CH2CI2/hexane).
The starting material can be prepared, for example, as follows:
By alkylation of 2-n-butyl-4-hydroxy-6-methylpyrimidinebiphenyl with
4-bromo-2'-methoxycarbonyl-biphenyl (EP 253,310) in the manner described in Example
la) and flash chromatography (silica gel 60,40-63 ~lm, hexane/ethyl acetate = 1:1),
2-n-butyl- 1,6-dihydro-4-methyl- 1 - [(2 ' -methoxycarbonyl-
3 ~ ~
- 51 -
biphenyl-4-yl)methyl]-6-oxopyrimidine is obtained and is immediately filrther processed.
Example 33: Starting from 2-n-butyl-3-~(2'-cyanobiphenyl-4-yl)methyl]-3,5,6,7-tetra-
hydro-4H-cyclopentapyrimidin-4-one and tributyltin azide, 2-n-butyl-3-[(2'-(lHtetrazol-
5-yl)biphenyl-4-yl)methyll-3,5,6,7-tetrahydro-4H-cyclopentapyrimidin-4-one is obtained,
for example, as described in Example 1. M.p. 214-216C (from acetonitrile)
N~
H3C ~~l!~ N ~O N ~ N
~3~ N~NH
The starting material can be prepared, for example, as follows:
a) By reaction of ethyl cyclopentanone-2-carboxylate with n-valeroylamidine.HCl and
sodium in absolute ethanol in the manner described in Exarnple 13a), 2-n-butyl-3,5,6,7-
tetrahydro-4H-cyclopentapyrimidin-4-one is obtained and is immediately further
processed.
b) By alkylation of 2-n-butyl-3,5,6,7-tetrahydrocy-4H-clopentapyrimidin-4-one with
4-bromomethyl-2'-cyanobiphenyl in the manner described in Example la) and flash
chromatography (silica gel 60, 40-63 ~-m, hexanelethyl acetate = 4:1), 2-n-butyl-3-
[(2'-cyanobiphenyl-4-yl)methyll-3,5,6,7-tetrahydro-4H-cyclopentapyrimidin- 4-one is
obtained and is immediately further processed.
Example 34- Starting from 2-n-butyl-3-[(2'-cyanobiphenyl-4-yl)methyl~-5~6,7,8tetra-
hydro-3H-quinazolin-4-one and tributyltin azide, 2-n-butyl-3-[(2'-(lH-tetrazol-Syl)bi-
phenyl-4-yl)methyl~-5,6,7,8-tetrahydro-3H-quinazolin-4-one is obtained, for example, in
the manner described in Example 1. M.p. 240C (from acetonitrile).
~2~3~`~
- 52 -
The starting material can be prepared, for example, as follows:
a) By reaction of ethyl cyclohexanone-2-carboxylate with n-valeroylamidine.HCl and
sodium in absolute ethanol in the manner described in Example 13a), 2-n-butyl-5,6,7,8-
tetrahydro-3H-quinazolin-4-one is obtained and is immediately further processed.
b) By alkylation of 2-n-butyl-5,6,7,8-tetrahydro-3~-quinazolin-4-one with
4-bromomethyl-2'-cyanobiphenyl in the manner described in Example la) and flash
chromatography (silica gel 60, 40-631lm, hexane/ethyl acetate = 2:1~, 2-n-butyl-3-~(2'-
cyanobiphenyl-4-yl)rnethyl]-5,6,7,8-tetrahydro-3H-quinazolin-4-one is obtained and is
immediately further processed.
Example 35: Starting from 2-n-butyl-3-[(2'-cyanobiphenyl-4-yl)methyl]-3H-
quinazolin-4-one and tributyltin azide, 2-n-butyl-3-[(2'-(lH-tetrazol-5-yl)biphenyl-4-
yl)methyl]-3H-qu;nazolin-4-one is obtained, for example, in ~he manner described in
Example 1. M.p. 179-180C (from ethyl acetate).
' .
The starting material can be prepared, for example, as ~ollows:
a) A solution of 5.0 g (36.6 mmol) of n-valeroylamidine.HCI and 5.97 g (36.6 mmol) of
isatoic anhydride in 200 ml of pyridine is stirred at 100C for 24 hours. After evaporating
in vacuo, the residue is suspended in H20, filtered off and dried at 50C in ~acuo. In this
manner, 2-n-butyl-3H-quinazolin-4-one is obtained and is immediately further processed.
b) By aL~ylation of 2-n-butyl-3H-quinazolin-4-one with 4-bromomethyl-2'-cyanobiphenyl
in the manner described in Example la) and flash chromatography (silica gel 60,
40-63 ,-m, hexane/ethyl acetate = 2:1), 2-n-butyl-3-~(2'-cyanobiphenyl-4-yl)methyl]-3H-
quinazolin-4-one is obtained and is immediately further processed.
Example 36: Starting ~rom 2-n-butyl-3-[(2'-cyanobiphenyl-4-yl)methyl]-3H-pyrido[2,3-
d]pyrimidin-4-one and tributyltin azide, 2-n-butyl-3-[(2'-(lH-tetrazol-S-yl)biphenyl-4-
yl)methyl]-3H-pyrido[2,3-d]pyrimidin-4-one is obtained, for example, in the manner
described in Example 1. M.p. 135-137C (from methanol/ethyl acetate).
The starting material can be prepared, for example, as follows:
3 7 ~
- 53 -
a) A solution of 5.0 g (3~.6 mmol) of n-valeroylamidine.HCI and 6.0 g (36.6 mmol) of
2H-pyrido[2,3-d][1,3}oxazine-2,4(1H)-dione in 200 ml of pyricline is stirred at 100C for
24 hours. After evaporating in vacuo, ethyl acetate is added to the residue, and the mixture
is washed with H20, dried (Na2SO4) and evaporated in vacuo. Flash chromatography(silica gel 60, 40-63 ~-m, CH2ClJCH30H = 98:2) gives 2-n-butyl-3H-pyrido[2,3-
d]pyrimidin-4-one which is immediately further processed.
b) By alkyation of 2-n-butyl-3H-pyrido[2,3-d]pyrimidin-4-one with 4-bromomethyl-2'-
cyanobiphenyl in the manner described in Example la and flash chromatography (silica
gel 60, 40-63 ~lm, CH2Cl2/CH3OH = 98:2), 2-n-butyl-3-[(2'-cyanobiphenyl-4-
yl)methyl]-3H-pyrido[2,3-d]pyrimidin-4-one is obtained and is immediately further
processed.
Example 37: Starting from 2-n-butyl-3-[(2'-cyanobiphenyl-4-yl)methyl]-3H-pyrido[3,2-
d]pyrimidin-4-one and tributyltin azide, 2-n-butyl-3-[(2'-(lH-tetrazol-5-yl)biphenyl-4-
yl)methyl]-3H-pyrido[3,2-d]pyrimidin-4-one is obtained, for example, in the manner
described in Example 1. M.p. 245C (from ethyl acetate).
The starting material can be prepared, for example, as follows:
a) A solution of 5.0 g (36.6 rnmol) of n-valeroylarnidine.HCl and 6.0 g (36.6 mmol) of
2H-pyrido[3,2-d][1,3]oxazine-2,4(1H)-dione in 200 ml of pyridine is stirred at 100C for
24 hours. After evaporating in vacuo, ethyl acetate is added to the residue, and the mixture
is washed with H2~, dned \Na2SO4) and evaporated in vacuo. Flash chromatography
(silica gel 60, 40-63 ~,-m, CH2Cl2/CH30H = 98:2) gives 2-n-butyl-3H-pyrido[3,2-
d]pyrimidin-4-one, which is immediately further processed.
b) By alkylation of 2-n-butyl-3H-pyrido[3,2-d]pyrimidin-4-one with 4-bromomethyl-2'-
cyanobiphenyl in the manner described in Example la) and flash chromatography (silica
gel 60, 40-63 ~Lm, CH2Cl2/CH30H = 98:2), 2-n-butyl-3-[(2'-cyanobiphenyl-4-
yl)methyl]-3H-pyrido[3,2-d]pyrimidin-4-one is obtained and is immediately further
processed.
Example 38: The following can be prepared in an analogous manner, for example asdescribed in one of the preceding Examples 1 to 5:
2-n-butyl- 1,~dihydro-4-ethyl- 1 -[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxo-
. .
2~3~3~
- 5~ -
pyrimidine,
2-n-butyl-1,6-dihydro-4-propyl-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxo-
pyrimidine,
2-n-butyl- 1 ,6-dihydro-4-isopropyl- 1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxo-
pyrimidine,
2,4-di-n-butyl-1,6-dibydro-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxo-pyrimidine,
2-n-butyl-4-t-butyl- 1 ,6-dihydro- 1-[(2'-( lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxo-
pyrimidine,
2-n-butyl-1,6-dihydro-4-trifluoromethyl-1-~(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-
oxopyrimidine,
2-n-butyl- 1 ,6-dihydro-4-phenyl- 1-[(2'-( lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxo-
pyrimidine,
2-n-butyl- 1 ,6-dihydro-4-ethoxycarbonyl- 1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-
oxopyrimidine,
2-n-butyl-4-carboxy-1 ,6-dihydro- 1 -[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxo-
pyrimidine,
2-n-butyl-1,6-dihydro-4-hydroxymethyl-1-[(2'-(lH-tetrazol-S-yl)b;phenyl-4-yl)methyl]-6-
oxopyrimidine,
2-n-butyl-1,6-dihydro-4-methoxymethyl-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl~-6-
oxopyrimidine,
2-n-butyl-1,6-dihydro-4-dimethylaminomethyl-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)-
methyl]-6-oxopyrimidine,
2-n-butyl-1,6-dihydro-3-ethoxycarbonylmethyl-1-[(2'-(lH~-tetrazol-5-yl)biphenyl-4-yl)-
methyl]-6-oxopyrimidine,
2-n-butyl-4-carboxymethyl-1,6-dihydro-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-
oxopyrimidine,
2-n-butyl- 1 ,6-dihydro-4-(2-hydroxyethyl) 1- [(2' -( lH-tetrazol-S-yl)biphenyl-4-yl~methyl]-
6-oxopyrimidine,
2-n-butyl-1,6-dihydro-4-(2-methoxyethyl)-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-
6-oxopyrimidine,
2-n-butyl- 1 ,6-dihydro-4-(2-dimethylaminoethyl)-1-[(2'-(lH-tetrazol-S-yl)biphenyl-4-yl)-
methyl] -6-oxopyrimidine,
2-n-butyl-1,6-dihydro-4-hydroxy-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxo-
pyrimidine,
2-n-butyl-1,6-dihydro-4-methylthio-1-[(2'-(lH-tetrazol-S-yl)biphenyl-4-yl)methyl]-6-oxo
2~37~
.
pyrlmldlne,
2-n-butyl- 1 ,6-dihydro-4-methylsulfonyl- 1 -[(2'-(lH-tetrazol-S-yl)biphenyl-4-yl)methyl]-6-
oxopyrimidine,
2-n-butyl-1,6-dihydro-4-morpholino-1-[(2'-(lH-tetrazol-S-yl)biphenyl-4-yl)meshyl]-6-oxo
pyrimidine,
2-n-butyl-1 ,6-dihydro-4-~2-methoxyethoxy)- 1-~(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)-
methyl]-6-oxopyrimidine,
2-n-butyl-1 ,6-dihydro-4-(2-dimethylaminoethoxy)-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)
methyl] -6-oxopyrimidine,
2-n-butyl-1,6-dihydro-5-methoxy-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl~-6-oxo-
pyrimidine,
2-n-butyl-1,6-dihydro-S-ethoxycarbonyl-1-[(2'-(l~I-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-
oxopyrimidine,
2-n-butyl-S-carboxy-1,6-dihydro-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl~-6-oxo-
pyrimidine,
2-n-butyl- 1 ,6-dihydro-5-hydroxymethyl- 1-[(2'-(lH-tetrazol-S-yl)biphenyl-4-yl)methyl]-6-
oxopyrimidine,
2-n-butyl- 1 ,6-dihydro-S-methoxymethyl-1-[(2'-(lH-tetrazol-S-yl)biphenyl-~-yl)methyl]-6-
oxopyrimidine,
2-n-butyl-1,6-dihydro-S-dimethylaminomethyl-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)-
methyl] -6-oxopyrimidine,
2-n-butyl-1,6-dihydro-5-methyl-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxo-
pyrimidine,
2-n-butyl-1,6-dihydro-4,5-dimethyl-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxo-
. . .
pyrlmldme,
2-n-butyl-1,6-dihydro-5-ethyl-4-methyl-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-
oxopyrimidine,
2-n-butyl-1,6-dihydro-4-methyl-5-propyl-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-
6-oxopyrimidine,
2-n-butyl-1,6-dihydro-5-isopropyl-4-methyl-1-~(2'-(lH-tetrazol-S-yl)biphenyl-4-yl)-
methyl] -6-oxopyrimidine,
2,5-di-n-butyl-1,6-dihydro-4-methyl-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-
oxopyrimidine,
2-n-butyl- 1 ,6-dihydro-S-methyl-4-phenyl- 1-[(2 '-( lH-tetrazol-S-yl)biphenyl-4-yl)methyl]-
6-oxopyrimidine,
2-n-butyl-1,6-dihydro-S-hydroxyethyl-4-methyl-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)-
~` 2~37~
- 56 -
methyl]-6-oxopyrimidine,
2-n-butyl-1,6-dihydro-5-methoxyethyl-4-methyl-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)-
methyl] -6-oxopyrirnidine,
2-n-butyl- 1 ,6-dihydro-5-dimethylaminoethyl-4-methyl- 1 -[(2'-(lH-tetrazol-5-yl)biphenyl-
4-yl)methyl]-6-oxopyrimidine,
2-n-butyl-1,6-dihydro-5-ethoxycarbonylmethyl-4-methyl-1-[(2'-(lH-tetrazol-5-
yl)biphenyl-4-yl)methyl]-6-oxopyrimidine,
2-n-butyl-5-carboxymethyl-1 ,6-dihydro-4-methyl- 1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)-
, methyll-6-oxopyrimidine,
2-n-propyl-1,6-dihydro-4-methyl-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)me~hyl]-6-oxo-
pyrimidine,
2-n-pentyl-1,6-dihydro-4-methyl-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyll-6-oxo-
pyrimidine.
"~
Example 39: Tablets, each containing 50 mg of active ingredient, for example
2-n-butyl-4-chloro-1,6-dihydro-1-[(2'-(lH-tetrazol-5-yl)biphenyl-4-yl)methyl]-6-oxopyri
midine, can be prepared as follows.
,, .
Composition ~lO,OOQ tablets)
Active ingredient 500.0 g
Lactose 500-0 g
Potatostarch 352.0g
Gelatin 8.0 g
Talc 60.0 g
Magnesium stearate 10.0 g
Silica ~highly-disperse~ 20.0 g
Ethanol q.s.
.~
The active ingredient is mixed with the lactose and 292 g of potato starch, and the mixture
is moistened with an alcoholic solution of the gelatin and granulated through a sieve. After
drying, the remainder of the potato starch, the talc, the magnesium stearate and the highly-
disperse silica are admixed and the mixture is compressed to give tablets of 145.0 mg
weight each and 50.0 mg of active ingredient content which, if desired, can be provided
with dividing notches for fimer adjustment of the dose.
:
Example 40: Coated ~ablets, each containing 100 mg of active ingredient, for example
,:~
. .
,'
,. .
3 7 ~
2-n-butyl-1,6-dihydro-1-[(2'-(lH-tetra~ol-5-yl)biphenyl-4-yl)methyl]-6-oxopyrimidine,
can be prepared as follows:
Composition (10,000 tablets)
Active ingredient 100.0 g
Lactose lOQ.0 g
CoTn starch 70.0 g
Talc 8.50 g
Calcium stearate 1.50 g
Hydroxypropylmethylcellulose 2.36 g
Shellac 0.64 g
Water q.s.
Methylene chloride q.s.
The active ingredient, the lactose and 40 g of the corn starch are mixed and moistened
with a paste, prepared from 15 g of corn starch and water (with warming), and granulated.
The granules are dried, and the remainder of the corn starch, the talc and the calcium
stearate are added and mixed with the granules. The mixture is compressed to give tablets
(weight: 280 mg) and these are coated with a solution of the hydroxypropylmethyl-
cellulose and the shellac in methylene chloAde; final weight of the coated tablet. 283 mg.
Example 41: Tablets and coated tablets, containing a compound according to the
invention, for example according to Examples 1-38, can be prepared in an analogous
manner to that described in Examples 39 and 40.