Note: Descriptions are shown in the official language in which they were submitted.
_1_
IMPROVED CARBOXYLIC ACID PURIFICATION AND
CRYSTALLIZATION PROCESS
This invention generally relates to an improved
process for the purification and crystallization of
carboxylic acids. More particularly, it relates to a
novel process in which an aqueous solution of sodium
succinate is converted to a supersaturated solution of
succinic acid from which high purity succinic acid is
crystallized.
Succinic acid and its derivatives are widely used as
specialty chemicals with applications in polymers, foods,
pharmaceuticals, and cosmetics. Furthermore, succinic
acid is a valuable 4-carbon intermediate useful in
processes for the production of 1,4-butanediol,
tetrahydrofuran, arid gamma-butyrolactone. These
processes require material of high purity since the end
products are produced by chemical catalysts whioh can be
poisoned by impurities.
For a fermentation based carboxylic acid process to
be economically attractive for the production of
specialty and commodity chemicals, the development of a
low-cost fermentation must be combined with low cost and
efficient product recovery and purification methods. The
anaerobic fermentations which are most promising for the
production of organic acids usually operate optimally at
pH's where salts of the organic acids rather than the
free acids are formed. However; the free acids and their
derivatives are the articles of commercial interest. In
addition, contaminating proteins and cell by-products
CA 02022037 1999-10-26
2
need to be removed from the free carboxylic acids because of
their interference in chemical catalysis. Therefore, an
effective fermentation and recovery process must remove both
cells and proteins and subsequently convert the acid salts to
free acids of high purity.
Several possible alternatives exist for the preliminary
recovery of succinic acid salts from the fermentation broth.
For example, we have previously demonstrated that the use of
conventional electrodialysis with special membranes can be
employed to recover succinates from whole fermentation broths
and that the succinate can be converted into the free succinic
acid by water-splitting electrodialysis using the high
efficiency bipolar membranes.
It is a general aim of the present invention to disclose a
novel method of obtaining a carboxylic acid of high purity by
using water-splitting electrodialysis to convert an
undersaturated aqueous solution of a carboxylic acid salt into a
supersaturated aqueous solution of the free acid and then
crystallizing the acid from the solution.
The invention provides a process for the production and
purification of succinic acid which comprises:
(a) anaerobically growing a succinate producing
microorganism on a carbohydrate substrate to produce a
fermentation broth containing acetate and succinate;
(b) subjecting the broth to water-splitting electrodialysis
to convert the acetate to acetic acid and to produce a
supersaturated succinic acid solution; and,
(c) crystallizing the succinic acid from the supersaturated
succinic acid solution which also contains acetic acid.
In a preferred embodiment the broth from which the succinic
acid has been crystallized is concentrated and recycled to step
(a) .
CA 02022037 1999-10-26
2a
The invention further provides a process for producing
substantially pure succinic acid from an aqueous mixture of
acetate and succinate which process comprises subjecting said
aqueous mixture to water-splitting electrolysis to convert the
acetate to acetic acid and to produce a supersaturated succinic
acid solution and subsequently crystallizing succinic acid from
the supersaturated succinic acid which also contains acetic
acid.
In the inventive process of the present invention, an
undersaturated carboxylic acid salt aqueous solution is
subjected to water-splitting electrodialysis to form a
supersaturated solution of the free carboxylic acid. Free
carboxylic acid of high purity is then crystallized from the
supersaturated solution by conventional means, such as seeding
with acid crystals.
The present invention is especially useful with succinic
acid because sodium succinate is considerably more water soluble
than free succinic acid. In addition, we have unexpectedly
discovered that the water-splitting electrodialysis converts
sodium acetate which inhibits the crystallization of succinic
acid into free acetic acid which promotes such crystallization.
In the drawings:
-3-
h
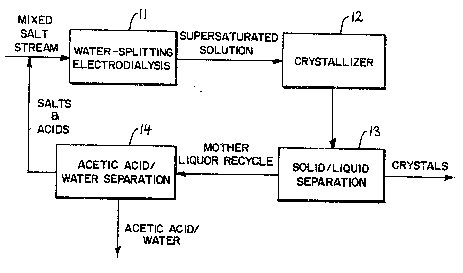
Fig. 1 is a schematic flow diagram of the process of
the present invention; and
Fig 2 is a schematic illustration of the water-
splitting electrodialysis of a salt solution to generate
a free acid and a base.
In the preferred embodiment of the present invention,
the feed stream is a fermentation broth obtained by an
anaerobic fermentation of a low cost carbohydrate
substrate by Anaerobiospirillum succiniciproducens in the
presence of sodium ions and added tryptophan. The broth
is an aqueous preparation which contains both sodium
succinate and sodium acetate.
A schematic diagram of the process of the present
invention is shown in Figure 1. A mixed salt stream
containing both sodium acetate and sodium succinate is
concentrated to about 10 to about 25% succinate by weight
prior to introduction into the water-splitting
electrodialysis unit 11. While in the preferred
embodiment conventional electrodialysis is used to
perform this task, other unit operations may also be
feasible. The mixed salt stream which is undersaturated
with sodium succinate is then treated using water-
splitting electrodialysis. The resulting stream from the
water-splitting electrodialysis unit contains some
residual sodium salts and free succinic and acetic acids;
it is supersaturated with respect to succinic acid. This
solution is then seeded with crystals of succinic acid in
a crystallizer 12 or other suitable vessel. The succinic
acid slurry from the crystallizer is then taken to a
solid/liquid separator 13, e.g. a hydrocyclone or
microfiltration unit, to separate the crystals which are
then available as product or for re-use as seeds.
The liquid from the separator 13 can be treated in a
suitable vessel 14 to remove some of the water and the
acetic acid and then recycled to the feed stream. This
may be accomplished by a number of different
operations. The appropriate use of acetic acid removal
-4_
will allow optimization of acetic acid concentration for
crystallization to be obtained.
The fundamental concept by which water-splitting
electrodialysis may be used to produce suceinic acid and
a base sodium hydroxide can be understood by reference to
Eig. 2 in which a greatly magnified portion of a bipolar
membrane 15, not drawn to scale, is shown schemati-
cally. The bipolar membrane consists of three portions,
a cation selective portion, 16, an anion selective
portion, 17, and an interface region, 18, between the
anion and cation portions. When a direct current is
passed across the bipolar membrane as shown, the
transport of ions between solutions 19 and 20 is
interrupted since anions are excluded from the cation
side 16 and cations are excluded from the anion side
17. Since little or no salt is present in the interface
region 18, the dissociation of water to H+ and OH-
provides the ions for carrying the current across the
membrane. Water at the interface is replaced by
diffusion through the anion portion 17, and cation
portion 16, from the solutions 19 and 20, respectively.
When used in conjunction with monopolar membranes (one
arrangement of which is shown in Fig. 2) the bipolar mem-
brane functions to produce the ions needed to generate
succinic acid and base from sodium succinate (MX). If
membrane 21 is an anion permeable membrane, then as H+
enters solution 20 from the bipolar membrane, 15, an
equivalent amount of X- will enter solution 19 from
compartment 22 producing a solution of succinic acid (HX)
in solution 20. Similarly, if membrane 23 is a cation
membrane, then as OH- enters solution 19 from the bipolar
membrane 15, M+ will enter solution 19 from compartment
24 to form a solution of sodium hydroxide (MOH).
In the current application both membranes 21 and 23
are cation exchange membranes. This configuration
creates compartments 20 and 24 which contain both the
free acid (e.g. succinic acid) and the salt from which it
-5-
came (e. g. sodium succinate). In order to regenerate
base, membranes 25 and 26 will be bipolar membranes. The
base streams will then be 19 and 22.
The electrical potential required to generate acid
and base by means of a bipolar membrane, as given by
electrochemical theory, should be on the order of 0.8
volts to produce 1N solutions of strong acid and base.
Some additional potential is also required to overcome
the resistance to transport of H+ and OH- through the
cation and anion portion of the membrane, respectively.
The production of bipolar membranes exhibiting a
potential drop of less than 1.2 volts in 0.5 M Na2S04 at
about 30° C. and at high current densities (e.g. 100
A/ft2)(109 mA/cm2) has been reported in the Chlanda et
al. U.S. Patent No. 4,766,161.
The invention is further illustrated by reference to
the examples.
Examples
General Procedures
Preparation of Succinate Salt
Succinate salt solutions are prepared by anaerobic
fermentations using a strain of Anaerobiospirillum
succinici producens (deposited in the American Type
Culture Collection as ATCC 29305 and redeposited under
the provision of the Budapest Treaty as ATCC 53488) at
39°C in a fermentor with an initial volume of 551 for 29
hours. The media contains approximately 35 g/1 dextrose,
10 g/1 corn steep liquor, and 25 ppm tryptophan. A 5%
inoculum is used. The pH is maintained between 6.1-6.3
by addition of sodium carbonate on a demand basis.
Agitation speed is 100 rpm.
The cells in the fermentation broth may be removed by
processing the broth through an ultrafiltration unit with
a hollow fiber cartridge of 0.2 micron pore size.
Concentration of the Succinate Solution
The sodium succinate concentration in the broth can
be adjusted to the desired concentration of about 10% to
-6-
about 25~ by weight by using a conventional electro-
dialysis unit. The electrodialysis stack consists of an
alternating series of anion and cation selective mem-
branes separated by flow distribution gaskets. The mem-
branes are bound on one end by an anolyte compartment and
an anode while on the other end by a catholyte compart-
ment and cathode. The preferred stack pack may contain
the following:
cell pairs
10 anion membrane - AMV
cation membrane - CMR
effective area - 178 cm2
electrolyte - 1 M Sodium Succinate in Water
The unit consists of three independent flow channels fed
to the electrodialyzer stack pack. The three streams
are: 1) diluting stream - feed materials, broth
2) concentrating stream - product
3) electrolyte - sodium succinate
From each reservoir, material is pumped through a valve,
rotameter, pressure gauge, the stack pack, and then back
to the reservoir.
The electrical current is supplied by a regulated nC
power supply model. It is connected to the anode and
cathode of the membrane stack and can produce 0-20
amperes and deliver 0-50 volts. A Fluke A75 multimeter
is used to measure the voltage drop across selected cell
pairs. Two platinum wires are inserted between eight
cell pairs and then connected to the voltmeter.
Conversion of Succinate to Succinic Acid
A suitably concentrated but undersaturated succinate
solution obtained by conventional electrodialysis and
sometimes evaporation also can be converted into a super-
saturated succinic acid solution by passing it through a
water-splitting electrodialysis unit 10.
The preferred unit contains bipolar membranes and is
a two compartment stack. The stack which is schemati- w
cally illustrated in Fig. 1 consists of alternating
ration permeable and bipolar membranes. The anode and
cathode compartments are bound by a Nafion membrane at
each end of the membrane stack.
The test membrane stack contains the following:
8 cell pairs
-ration membrane
°bipolar membrane
effective area 102. 4cm2
electrolyte (2.5 N NaOH)
The unit consists of three independent flow channels
fed to the electrodialyzer stack. The three streams are:
1. Acid stream (initially the sodium succinate salt
stream)
2. Base stream (becomes more concentrated as run
proceeds)
3. Electrode rinse stream (2.5 N NaOH)
Conductivity was measured using a portable
conductivity meter.
Succinate and acetate concentrations are the anion
concentration and were measured after appropriate
dilution and acidification by an HPLC method.
Total protein content was determined by Kjeldahl
apparatus and reported as nitrogen x 6.25%.
Sulfate concentration was determined by gravimetric
determination of barium sulfate precipitation. Sodium
concentration was determined using an ion selective meter
and a sodium electrode.
Crystallization of Succinic Acid
from Supersaturate Solution
The crystallization of succinic acid of high purity
from the supersaturated solution is conducted at 30°C
using 125 ml of broth obtained after water-splitting
electrodialysis. The supersaturated solution is seeded
with crystals of pure succinic acid in a crystallizer.
The crystals of succinic acid which formed are filtered
arid washed with cold water. The resulting crystals when
analyzed for succinate, acetate, protein, sodium, and
sulfate are found to be of high purity (about 99.9%).
_8_
Example 1
Effect of Impurities on Crystallization
TABLE 1
PROCESS STREAM COMPOSITIONS
(WEIGHT ~ COMPOSITION, DRY BASIS)
Fermentor After Water-Splitting After
Product ED ED Crystallization
Succinate 51.5 63.0 77.6 99.91
Acetate 13.2 8.8 18.6 -
Protein 9.7 0.8 0.6 0.07
Sodium 25.6 27.3 2.8 0.02
Sulfate 0.1 0.6 0.4 -
Table 1 shows the process stream compositions ob
tained after each step in the process. The main items to
note are the relative compositions of the solution after
water-splitting and the composition of the crystalline
material obtained from it. The extreme purity of the
crystalline material indicates that crystallization is a
viable means for product purification: .
The concentrations from the fermentor, after conven-
tional electrodialysis; and grior to the water-splitting
electrodialysis were 50.2; 146.7, and 215.9 gm dissolved
sol'ids/iiter,; respQCtively. Clearly, the stream Teaving
the conventional electrodialysis was further concentrated
by evaporation prior to water-spiitt,ing. This step is
necessary only to' the extent to create a supersaturated
s4lution after water-spli ting.
Examples 2 and 3
A separate set of crystallization experiments was
performed to determine the effect of acetic acid/sodium
acetate oa succinic acid crystallization. Compositions
were chosen to mimic those found after water-splitting:
-9-
1.5M succinic acid; 0.5M sodium acetate; and either 0.2M
sodium acetate or acetic acid.
TABLE 2
THE EFFECT OF ACETIC ACID AND SODIUM ACETATE ON
SUCCINIC ACID CRYSTALLIZATION AT 30°C FOR A
MODEL SYSTEM DESIGNED TO MIMIC BROTH CONDITIONS
Ex.2 Ex. 3
Water, gm 180 180
Sodium succinate, gm 16.2 16.2
Succinic acid, gm 35.4 35.4
Sodium acetate, gm 6.8 ---
Acetic acid, gm --- 3.2
Crystal yield, gms 1.22 5.17
The results of the acetic acid/sodium acetate
impurity studies in Table 2 show four times more succinic
acid crystals formed in the presence of acetic acid than
sodium acetate. These results indicate acetic acid has a
crystallization promotion effect.
Examples 4, 5 and 6
In a companion study, sodium acetate and acetic acid
are added to broth solutions which are supersaturated.
As demonstrated by Table 3, addition of acetic acid
greatly enhances yield while sodium acetate causes a
complete cessation of crystallization.
TABLE 3
THE EFFECT OF ADDED ACETIC ACID AND SODIUM ACETATE ON
SUCCINIC ACID CRYSTALLIZATION AT 30°C FOR THE
FERMENTATION PRODUCT
Experiment Number Ex. 4 Ex. 5 Ex. 6
Broth, ml 200 200 200
Sodium Acetate, gm 6.8 --- ---
Acetzc acid, gm --- 3.2 ---
Crystal yield, gm/1 --- 18.0 11.5
10
Example 7 and 8
The ability to remove high quality crystals from
solution produced by water-splitting has several impli-
cations. Clearly, creation of supersaturation by water-
s splitting is demonstrated. This phenomenon should occur
in any system wherein the salt is more soluble than the
acid. As can be seen in Table 4 this can be accomplished
without the formation of crystals on the membrane and
current efficiency is preserved during the process.
Finally, the crystallization step is not only feasible
for removing impurities but facilitated by their
presence.
TABLE 4
WATER-SPLITTING ELECTRODIALYSIS RECOVERY OF
SUCCINIC ACID FROM FERMENTATION PRODUCT
Ex. 7 Ex. 8
Sodium Removal, % 78.9 81.2
Salt Stream
Initial Succinate Conc., g/1 78 126
Final Succinate Conc., g/1 91 52
Initial Acetate Conc., g/1 13 29
Final Acetate Conc., g/1 15 36
Temperature, °C
Current Efficiency, ~ 78.9 76.2
Crystallization* No Yes
Membrane Fouling No No
*Supersaturated with respect to succinic acid.
The recovery per gals using water-splitting electro-
dialysis was low with only 21.8 gm/1 of crystals
produced. For this reason the process should be thought
of as a "stripping" crystallization, wherein the succinic
_. -11-
acid in excess of solubility is "stripped" from solution
by crystallization.
It will be apparent to those skilled in the art that
the relationship of impurities to crystallization is
quite complex. In the preferred embodiment of the
process of the present invention impurities, such as
amino acids and saltsr are effectively excluded from the
succinic acid crystals. In addition, we have discovered
that the crystallization of succinic acid is unexpectedly
inhibited by the presence of sodium acetate while it is
enhanced by the presence of acetic acid. This remarkable
result shows that the use of water-splitting electro-
dialysis not only creates solutions supersaturated with
respect to succinic acid, but also converts a crystal-
lization inhibiter, sodium acetate, to a crystallization
promoter, acetic acid.
It also will be apparent that the foregoing descrip-
tion has been for purposes of illustration and that the
process can be used to prepare other free carboxylic
acids, such as malefic, fumaric, citric or amino acids
such as glutamic acid.
Representative of the carboxylic acid salts which can
be used in the process of the present invention are those
having salts that are more water soluble than the free
acids. Those salts axe usually the sodium, potassium and
ammonium salts but may be other salts in some cases.
From the foregoing, it will be apparent to those
skilled in the art, that water-splitting electrodialysis
can be used to produce supersaturated carboxylic acid
solutions from undersaturated acid salt solutions; that
nucleation of crystals on the membrane surface is not
important; and that a highly purified crystalline acid
product can be obtained from a fermentation broth. Other
advantages of the process will be apparent to those
skilled in the art. Therefore. it is intended that the
invention be limited only by the claims.