Note: Descriptions are shown in the official language in which they were submitted.
2022252
INFUSION APPARATUS
BACKGROUND OF THE INVENTION
The present invention relates to liquid dispensing
apparatus and pertains particularly to an improved infuser
apparatus for delivering intravenous drugs at a controlled
rate to a patient.
It is often necessary to intravenously supply patients
with pharmaceutically active liquids over a long period of
time at a controlled rate. It is desirable that this be
accomplished while the patient is in an ambulatory state.
A few devices have been developed in the past for
accomplishing this purpose.
The prior art devices typically comprise an elastic
bladder forming a liquid container mounted in an elongated
cylindrical housing, and having a flow control valve or
device and tubing for supply of the liquid to the patient.
The elastic walls of the bladder expand along the walls of
the cylindrical housing when filled with the liquid, and
~g
2022252
provide the pressure for expelling the liquid. These prior
art devices are typically filled b~ hand by means of a
syringe which often require an inordinate amount of force.
Another drawback to the prior art devices is that the
bladder is forced to expand into an unnatural elongated
configuration along the housing walls as the container is
filled. As a result of this unnatural configuration, the
pressure of the bladder and the flow rate of the unit
varies widely with the volume of liquid therein.
Therefore, they do not have a reasonably stable pressure
and flow rate over the infusion period.
Most of such devices either have a flow rate that
decreases with pressure, which decreases with volume, or
one that remains roughly constant until the end where it
surges. Attempts have been made to control pressure and
flow rates by means of complicated and expensive flow
control valves and devices. Other approaches have utilized
exotic and expensive elastic materials in an effort to
control the pressures and flow rates.
In our aforementioned application, we disclose an
apparatus for solving the aforementioned problems of the
prior art. However, one problem remains, namely that the
materials that provide optimum elasticity do not have
sufficient chemical inertness for medical application.
2022252
Similarly, materials that are sufficiently chemically inert
for medical or pharmaceutical use are not sufficiently
elastic to serve the function of an effective inflatable
bladder.
It is desirable that the bladder of an inflatable
bladder infuser be chemically inert in order to avoid
contamination of the medication, and that the pressure and
flow rate be reasonably constant over the infusion period.
Accordingly, it is desirable that an improved infuser
apparatus be available.
SUMMARY AND OBJECTS OF THE INVENTION
It is the primary object of the present invention to
provide an improved liquid infuser apparatus.
In accordance with a primary aspect of the present
invention, a liquid infuser apparatus comprises an elastic
reservoir mounted within a spherical chamber, and
comprising an inner inert layer and an outer elastic
capable of maintaining a substantially constant pressure
over the range of the infusion cycle.
BRIEF DESCRIPTION OF THE DRAWING
The above and other objects and advantages of the
present invention will become apparent from the following
description when read in conjunction with the accompanying
drawings wherein:
--3--
2n22252 -
Fig. 1 is a side elevation in section view of a
preferred embodiment of the invention;
Fig. 2 is a view like Fig. 1 of the embodiment of Fig.
1 with the bladder shown inflated;
Fig. 3 is an end view of the central support member
retaining means;
Fig. 4 is an enlarged detailed view of the check valve
assembly of the embodiment of Fig. l;
Fig. 5 is a view like Fig. 4 showing the valve open;
and
Fig. 6 is a section view taken generally on lines 6-6
of Fig. 4.
DETAILED DESCRIPTION OF A PREFERRED EMBODIMENT
Referring to the drawings, and particularly to Figs.
1-2, there is illustrated a preferred embodiment of the
invention, wherein the infuser pump is separate from the
charging or filler pump. Moreover, it may be filled by any
suitable means, such as a syringe or any other pressurizing
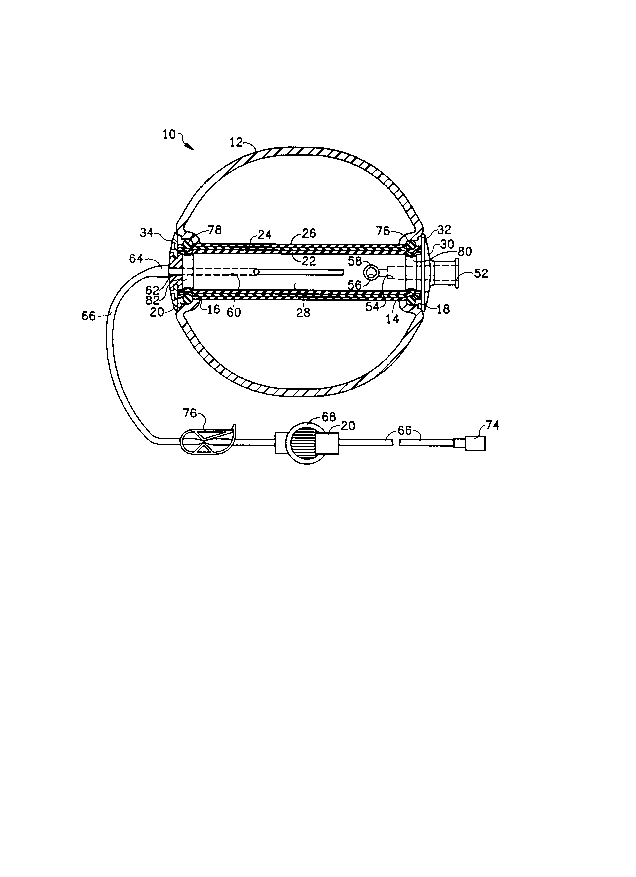
means. As illustrated in Figs. 1 and 2, an infuser pump,
substantially like the Figs. 9-11 embodiments of our prior
application, is designated generally by the numeral 10 and
comprises an outer substantially spherical housing of a
size to accommodate the necessary volume of intravenous
fluid to be pumped.
--4--
2022252
The housing 12 has a substantially spherical
configuration and is provided with coaxial, or more
particularly aligned bores or ports 14 and 16, in which is
mounted an inflatable bladder assemblv. The housing 12 may
be made of unitary construction, such as by blow molding,
or may be of two identical half shells assembled. The
ports are formed in axial recesses 18 and 20. The
inflatable bladder assembly comprises a first or inner
elongated semi-elastic sleeve 22, and a pair of outer
elongated latex rubber elastic sleeves 24 and 26 mounted on
an elongated central cylindrical support member 28. The
inner sleeve 22 is preferably made of a drug compatibility
rubber with low leach characteristics that meets USP class
6 testing standards.
A preferred rubber material for the inner sleeve 22 is
a class of thermoplastic rubber sold under the mark KRATON
by Shell Chemical Company of Houston, Texas. These
materials are available as KRATON* D and G 2000 series
rubber, and have FDA status for use in certain applications
- or ingredients of articles for food contact. These
materials have less than optimum elastic characteristics,
and are referred to herein as semi-elastic. When
stretched, they return to a position of about 75 to about
90 percent of original configuration.
*Trade-mark
2022252
The outer sleeves 24 and 26 are preferably made of a
natural latex rubber with excellent elastic
characteristics. A material with good elastic
characteristics returns from a stretched condition to its
original un-stressed or stretched condition. A good
elastic material also has a uniform elastic force over the
range stretched. Natural latex rubbers are the preferred
material for the outer sleeves membranes 24 and 26.
The central support member 28 is preferably of a
generally elongated cylindrical configuration, with an
annular radially extending retaining flange 30 on one end
for engaging a shoulder 32 on the housing 12. The opposite
end of the support member 28 includes a bayonet type
coupling with a retaining nut 34. The central support
member may be constructed of any suitable pharmaceutically
compatible material, such as metals, plastics, glass, etc.
The coupling comprises a generally rectangular
projection 36, with shoulders 38 and 40 formed by annular
slots in which the retainer nut rotates. The retainer nut
34 included a recess 42, with a rectangular opening 44 for
receiving projection 36 on the end of support member 28. A
pair of side lips 46 and 48 extend under shoulders 38 and
40 when the nut is rotated 90 degrees for retaining the nut
in place and the support member 28 in the housing bores.
2~22252
The nut 34 rests in annular recess 50 surrounding recess
20.
The support member 28 includes an inlet port 52
communicating by means of a passage 54, including a one-way
valve 56, 59 with the interior of the membrane or sleeve
22. Any suitable check valve may be used to permit
uncoupling of the filling unit without leakage of fluid
from the pressurized bladder. However, a valve as
illustrated in Figs. 4-6 is preferred. The check valve
comprises a cross throughbore 56 communicating with the end
of passage 54, and in which is slip fitted an elastic tube
58, which may be of a suitable rubber such as silicone.
The tube 58 covers the end of passage 54 to prevent back
flow from inside the bladder formed by sleeve 22. The tube
58 collapses, as shown in Fig. 5, in response to higher
pressure in passage 54 enabling flow of liquid into sleeve
22.
An outlet passage 60 in support member 28 co~municates
via an outlet port 62 and suitable coupling assembly 64,
with an outlet or intravenous feeding line comprising a
two-part tube 66, which includes a filter 68, and may
include flow control means 70 and a male luer lock
adaptor. The outlet line may be controlled by a suitable
valve assembly (not shown) or preferably by the well known
2D2~252
type clamp known as a Roberts clamp 76. The luer lock has
a valve that closes the outlet port when the feeding line
is uncoupled therefrom. The coupling is effective to open
the outlet valve when coupled to the outlet fitting. Such
luer locks are well known off-the-shelf items for I.V.
delivery systems. The delivery tubes 66 may be selected in
size and length to and aid in maintaining a predetermined
pressure and flow rate. A suitable tube size for the
particular application is 0.088 inch O.D. by 16.5 inch in
length. Orifices or other means, such as flow regulating
capillary tubes may be also used in controlling the flow.
The elastic sleeves 24 and 26 are mounted over the
sleeve 22. Sleeves 24 and/or 26 may be stretched radially
when in position over sleeve 22, e.g. 24 is stretched
radially over 22, with 26 slip fit over the assemblies of
22 and 24. The outer bladder 26 slips radially over the
assembly of 22 and 24. The composite assembly of 22, 24,
26 is slideably engaged with a slip fit over the mandrel or
support member 28. Radial stretching of the bladder 24
compensates for material 22's less than perfect
elasticity. ~ore specifical~y, the wall thickness and
amount of stretch of bladder 24 are selected to lust
compensate for bladder 22's material less than perfect
elasticity. The initial strain conditions and bladder wall
202~252
thicknesses are also chosen to minimize the non-linearity
exhibited in a bladder's stress versus strain.
It is well known that a single bladder infusion device
constrained at both ends exhiblts a highly non-linear
stress versus strain relationship. This causes a time
varying flow characteristic. The prior art required
stretching the membrane both axially and radially over a
mandrel to reduce this non-linear behavior and thus
generate a more constant flow versus time. We have
improved the state of the art by incorporating a chemically
inert inner bladder and an elastic outer bladder. Further,
we have devised a structure and method for maintaining
constant flow versus time while the device is infusing by
radially stretching an intermediate bladder over the inner
bladder.
The inner semi-elastic drug compatible tube or
membrane 22 is mounted on the cylindrical support member
28, preferably in a slightly snug but un-stretched radial
fit, and essentially relaxed elongated or non-stretched
longitudinal fit. The inner sleeve 22 preferably has what
shall be called a slip fit on the support member. This
slip fit is preferably with a clearance of on the order of
about one-thousandths of an inch of the sleeve on the
support. This provides a non-stretched fit, with
2022252
essentially zero volume of the pressure chamber when in the
non-stretched or totally relaxed state or mode.
The elastic sleeves 24 and 26 are respectively stretch
fit and snug fit radially over the inner semi-elastic
sleeve 22. The intermediate sleeve 24 is radially
stretched up to about five percent over the inner sleeve 22
for compressing it. The outer sleeve 26 is slip fitted
over the intermediate sleeve 24. All of these sleeves 22,
24, and 26 are fitted over the support member 28 and
clamped at the ends by means of a pair O r O-rings 76 and
78. These O-rings 76 and 78 bias the ends of the multiple
sleeves into annular grooves 80 and 82 in the outer surface
of the member 28. The O-rings 76 and 78 are held in place
by the walls of the housing forming the recesses 18 and
20. The multiple sleeves when being filled tend to
elongate and roll over the ends thereof as shown in Fig.
2. The support member 28 is of a fixed length and holds
the ends of the sleeves at a fixed position. The multiple
thin sleeves easily roll over the ends thereof as the
bladder made up of the multiple 1eeves fills and expands.
The pressure applied by the pressure chamber, formed
by the multiple sleeves, will be substantially a function
of the thickness of the wall of the elastic sleeve or
sleeves. For example, a typical two to three (2-3) psi may
--10--
2022252
be obtained by a wall thickness of about eighteen to
twenty-thousandths (.018 - .020) of an inch. In order to
obtain higher pressure with superior uniformity, a
multi-layered sleeve configuration as described hereinabove
has been found to be preferred.
As illustrated in Fig. 1, a plurality of sleeves
(three illustrated) 22, 23 and 24 are slip fitted
(non-stretched) on the support member. The inner sleeve 22
is slip fitted on the support member 28, and a second
sleeve 24 is slightly stretch fitted over the first sleeve
22. Thereafter, a third sleeve 26 is slip fitted over the
intermediate sleeve 24. These are shown in the fully
deflated position in Fig. 1 and in the fully inflated
condition in Fig. 2, showing the fold or roll over the
ends. These multiple layers have been found to be superior
to the use of thicker membranes or sleeves to obtain higher
and uniform pressures. The use of multiple layers also
enables the use of a semi-elastic substantially chemically
(medically) inert inner membrane or sleeve for contact with
the infusible liquid. The multiple sleeves will roll or
fold over at the ends, as illustrated in Fig. 2. Thus, to
increase the pressure, additional sleeves of substantially
the same thickness are used.
2022252
When being filled, the elastic multi sleeve membrane
has a tendency to elongate, but expands into a spherical
configuration (Figs. 10 and 11 of our prior application).
The sleeve is shown in the partially filled position in
Fig. 10 and in the fully filled position in phantom.
The elongation is accommodated in this pump
configuration by an accordion effect at the ends of the
bladder, as shown in Fig. 2, wherein the bladder rolls over
the ends thereof and outward along the support member 28 as
it expands outward to fill the housing 12. The
accommodation of the elastic membrane in the spherical
configuration enables it to expand and contract in its
- natural fashion, and to maintain a substantially constant
pressure and thereby flow rate over the intravenous
injection period.
The layered or multiple sleeve configuration has been
found to better accommodate the accordion fold and maintain
a more uniform pressure than a thicker sleeve. The tubular
elastic sleeve membranes are selected and mounted on the
support member in a manner that enables them to roll or
fold over at the ends when being filled.
In operation, an assembled infuser pump unit is
selected, and the inlet port 52 is secured to a source of
fluid under pressure. As fluid is being introduced into
2022252
the inlet, the valve 58 collapses in Fig. 5 as fluid flows
into the inner sleeve or membrane 22. As the reservoir or
bladder formed by the sleeves begins to fill, it expands
and attempts to elongate. The ends of the sleeves begin to
fold and roll over the ends thereof as in Fig. 2. The
bladder forms a substantially spherical shape as its
natural form of expansion. The roll at the ends
accommodates this expansion and aids in maintaining a
substantially constant pressure over the range of infusion.
As the bladder deflates, the outer elastic membranes
force the inner semi-elastic membrane back to substantially
its original position. This helps to evacuate the entire
volume of fluid. It also will be appreciated that any form
of pressurized filling apparatus may be used. For example,
the squeeze fill embodiment of Fig. 1 of our prior
application could be utilized with this infusion pump.
While we have illustrated and described our invention
by means of specific embodiments, it is to be understood
that numerous changes and modifications may be made therein
without departing from the spirit and scope of the
invention as defined in the appended claims.
h~ CLAIM:
-13-