Note: Descriptions are shown in the official language in which they were submitted.
2~2~2
The present invention relates to a
pharmaceutical composition capable of slowing the
progression of Parkinson's disease in a patient.
Parkinson's disease, also known as shaking
palsy, is a chronic, progressive d~sease of the nervous
system characterized by muscular weakness, tremor and
muscular rigidity. Drugs are available for symptomatic
treatment; however, there were no drugs heretofore
understood or recognized to slow the progression of the
disease.
At present, the preferred method of treating
Parkinson's disease involves administration of L-dopa,
the chemical name of which is (-)-3-(3,4-
dlhydroxyphenyl)-L-alanine. Unfortunately, such
treatment can often result in one or more of
compllcations, toxicity problems and decreased efficacy
of L-dopa upon prolonged treatment. Furthermore, L-dopa
does not appear to hinder the progression of Parkinson's
dlsease, but rather provides merely symptomatic
treatment. Thus, there would appear to be a need for a
drug which reduces or eliminates progression of the
disease, preferably without the onset of the undesirable
results of L-dopa therapy as discussed above.
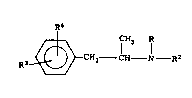
Canadian patent 871,155, issued May 18, 1971,
teaches a group of compounds, and methods for the
preparation of such compounds, the general formula of
which is as follows:
2~22~2
R3 ~ CH2 -CH - N - R2
wherein,
R3 is hydrogen, halogen, nitro or amino,
R4 is hydrogen, halogen, nitro or amino,
R is a lower alkyl group; and
R2 is a hydrogen or an unsubstituted propyl,
propenyl or propynyl group or such a group
substituted by a halogen atom or a hydroxy
group.
Deprenyl, which is the racemic mixture of the
dextrorotatcry and levorotatory optical isomers of N,~-
dimethyl-N-2-propynylbenzeneethanamine, is encompassed
by this group of chemical compounds. Selegillne is the
levorotary optical lsomer of deprenyl.
It would be desirable to have a
pharmaceutical composition which could decelerate the
progression rate of the disease while mitigating the
complications, toxicity and declining long-term effects
(discussed above) of known pharmaceutical compositions.
It is an ob~ect of the present invention to
provide a pharmaceutical composition, the functlon of
which is to decelerate the progression of Parkinson's
disease.
Accordingly, in one of its aspects, the
present invention provides an antiparkinsonian
pharmaceutical composition comprising a therapeutically
effective amount of an active ingredient of the formula:
2~2~
R3 ~ CH2- CH - N - R2
or a pharmaceutically acceptable acid addition salt
thereof,
wherein,
R3 is selected from the group comprising
hydrogen, halogen, nitro and amino,
R4 is selected from the group comprising
hydrogen, halogen, nltro or amino,
R is a lower alkyl group; and
R2 is a hydrogen or is selected from the group
comprising a propyl group, a propenyl group and a
propynyl group unsubstituted or substituted by at least
one of a halogen atom and a hydroxy group,
together with at least one pharmaceutically acceptable
excipient.
In another of its aspects, the present
invention provide~ an antiparkinsonian pharmaceutical
composition, substantially free of L-dopa, comprising an
active ingredient of the formula:
R3 ~ CH2 CH - N R2
2~22 ~2
or 2~ pharmaceutically acceptable acid addition salt
thereof,
wherein,
R3 is selected from the group comprising
hydrogen, halogen, nltro and amino,
R4 is selected from the group comprising
hydrogen, halogen, nitro or amlno,
R is a lower alkyl group; and
R2 is a hydrogen or is selected from the group
comprising a propyl group, a propenyl group and a
propynyl group unsubstituted or substituted by at least
one of a halogen atom and a hydroxy group;
together with at least one pharmaceutically acceptable
excipient.
In yet another of its aspects, the present
invention provides an antiparkinsonian pharmaceutical
composition, susbstantially free of L-dopa, comprising a
therapeutically effective amount of selegiline or a
pharmaceutically acceptable acid addition salt thereof,
together with at least one pharmaceutically acceptable
excipient.
In yet another of its aspects, the present
invention provides a pharmaceutical composition,
substantially free of L-dopa, comprising a
therapeutically effective amount of selegiline or a
pharmaceutically acceptable acid addition salt thereof,
together with at least one pharmaceutically acceptable
excipient, for the use of decelerating the progression
of Parkinson's disease in a patient.
Thus, an aspect of the present invention
resides in the discovery that seleglline may be used, as
an active ingredient, in the absence of other active
-- 4 --
2~2~
compounds such as L-dopa, to decelerate the progression
of Parkinson's disease. Accordingly, the undesirable
shortcomings (discussed above) associated with the use
of L-dopa may be obviated or mltigated by use of the
lnstant antiparkinsonian pharmaceutical composltion.
As used throughout this specification, the
term "pharmaceutical composition" refers to a regimen of
treatment as regards Parkinson's disease and not
necessarily to a single uni~ dosage of any particular
active ingredient. Such a composition may comprise one
or more units containing the same or different active
ingredient or ingredients administrable to a patient for
the purpose of treating Parkinson's disease in that
patient. As used herein, "antiparkinsonian
pharmaceutical composltion" refers to a pharmaceutical
composition administrable to a patient for the purpose
of decelerating the progression of Parkinson's disease
in that patient relative to the progression rate of the
disease in an untreated patient.
The term "therapeutically effective amount",
as used with respect to the present invention, means a
pharmaceutically acceptable amount effective to attain
deceleration of the progression of Parkinson's disease.
The term "pharmaceutically acceptable", as throughout
this specification, means non-toxic and applies to
compositions otherwise suitable for administration to a
patient.
As used throughout this specification, the
term "acid addition salt" refers to a salt formed by
reaction with an organic or inorganic acid. Non-
limiting examples of organic acids include acetic acid,
citric acid, fumaric acid and the like, while non-
limiting examples of inorganic acids include
-- 5 --
hydrochloric acid, sulphurlc acld, phosphoric acid and
the like. A preferred pharmaceutically acceptable acid
addition salt- i8 selegiline hydrochloride, which is also
known as ELDEPRYL (trade-mark).
The term "pharmaceutlcally acceptable
exclpient~ refers to those ~xclpients normally empl~yed
in the formatlon of pharmaceutlcal composltions intended
for administration by the known routes of administration
of pharmaceutlcals. For example, for composltlons
lntended for oral admlnlstratlon, sultable
pharmaceutlcal exciplents include the non-toxic
pharmaceutically acceptable carriers such as starch,
glucose, lactose, dextrose, sucrose, mannitol, sorbltol,
gelatin, malt, rlce, flour, chalk, silica gel, magnesiu~
carbonate, magnesium stearate, sodium stéarate,
glyceryl, monostearate, sodium chlorlde, talc, drled
sklm milk, glycerol, propylene glycol, water, ethanol
and the llke. Mixtures of one or more of such carriers
may also be used.
The composition o the present inventlon may
be adapted for admlnlstratlon by accepted methods of
admlnlstratlon lncludlng oral and parenteral (including
transdermal) means. Such oral composltions may take the
form of solutions, suspensions, tablets, pills,
capsules, powders, sustained release formulations and
the like.
Accordlng to a preferred embodlment of the
invention, the dosage unit ls for oral admlnlstratlon
and comprlses from about 4 tD about 8 mg of selegillne
hydrochloride. More preferably, the oral dosage unit ls
about 5 mg seleglline hydrochlorlde. However, lt will
-- 6 --
2 ~ 2
be appreciated that the invention is not limited to
unit;s of this dosage or method of administration.
Preferably, the therapeutically effective oral
daily dosage in respect of the present composition is 10
mg/day. This oral daily dosage may be varied as
required depending on the welght of the patient and the
extent to which the patient is afflicted with the
disease.
Aspects of the invention are further described
for illustrative purposes only in the following
specific, non-limiting Example. In the Example,
reference will be made to the accompanying Figure which
i8 a Kaplan-Meier survival curve for a Placebo Group and
a Selegiline Group.
Exam~le
In the ~xample, a double-blind, placebo-
controlled study of patients with early, untreated
Parkinson's disease was conducted in order to determine
the effectiveness of selegiline hydrochloride in
retarding the advancement of Parkinson's disease.
1. Patient Selection
Patients with Parkinson's disease for less
than 5 years, and having at least 2 of the 3 cardinal
features typical of the disease (i.e. rigidity,
bradykinesia and rest tremor) were considered for the
study. Further evaluation of prospective patients
included development of medical history, physical
examination, and the Mini-Mental State Examination
(MMSE). Further, prospect$ve patients were screened
using five assessment scales to measure the severity of
parkinsonism, as follows:
-- 7 --
2~2,?~2
(1) Unified Parkinson's Disease Rating Scale
(UPDRS) Motor Examination. The scoring method included
rating each of 13 different motor features on a 5-point
scale (0, normal; 4, severely affected). The ratings
for each motor feature were then summed for a total
score for each patient.
(2) Hoehn and Yahr Staging scoring method, as
described by Hoehn and Yahr in Neurology 17, 427 (1967).
The test was modified as follows: stage 1, unilateral
disease; stage 1.5, unilateral disease with neck
rigidity; stage 2, bilateral disease without impaired
balance; stage 2.5, bilateral disease with impaired
balance after being pushed, but able to regain balance
without assistance.
(3) The Webster Step-Second Test as described
by Webster in Mod. Treat. 5, 257 (1968). The scoring
method included measuring the time (in seconds) to stand
and walk a prescribed course and to sit again.
(4) UPDRS Activities of Daily Living (ADL).
The scoring method included reviewing with the patient
13 different ADL's and rating the degree of dysfunction
for each ADL on a 5-point scale (0, normal; 4, severely
disabled). The ratings were then summed for a total
score for each patlent.
(5) The Schwab and England ADL (Schwab and
England, Third Sympos~um on Parkinson's D~sease, 152-157
~1969)). The scoring method included estimating by the
patient of the percentage of normal function to the
nearest 5%.
-- 8 --
2 0 ~ 2 ~ ~ ~
Depression in the prospective patients was
also assessed. The scoring method used was the UPDRS
subscale for depression (0, none; 4, severe).
Laboratory tests included a complete blood
count, urine analysis, a standard blood chemistry panel
including liver enzymes (Sequential Method Analysis no.
18) and an electrocardiogram.
Upon completing the evaluation, 54 patients
were chosen for the study. The patients were randomly
divided into 2 groups: the Selegiline Group and the
Placebo Group. The data ~enerated from the evaluation
of prospective patients is provided in Table 1.
2. Treatment
Selegillne Group tablets (5 mg selegiline
hydrochloride together with excipient) and Placebo Group
tablets (excipient only) were prepared. A dosage of two
tablets daily per patient was selected for both Groups
in the study. Both patient and clinic personnel were
blinded as to treatment status.
3. Follow-up Evaluation
One month following initiation of treatment
(wa~h-in study), follow-up evaluations were conducted,
and continued routinely at 5-month intervals thereafter
_ g _
20~2~i
C
a O ~ o NN ~1.1
0 8 0 _ O O O 0
.1 0 ~ 0 ~ It~
O N N N N e
o
0o o ~o ~l
C C C ~ O O
~ o ~ ~ ~ r~
~ c
~0 V ~ b N Ol ~ IO V
Dl a b b 0~ O; - CD r ~
C o a _ m 0 e 0 ~ e
~ ~0~0 ~CD0 ~
~ b. O ~ ~ ~ ~ ~ ~n
~ ~ c ~ e ~ ~ b
b V b
~u~ 3~ 0 'o
~ ~ 0 0 ~O ~ 0
N 0 o V
O C ~1 0 ~1 0 O
~ ~ N N C
C N N N ~ b
3 3 ~
, o, o,
r o ~ o D C ~ ~4
c
-- 10 --
2 ~
until patients reached the "end point" defined as the
earlier of the patient requiring L-dopa therapy or 3
years of selegiline hydrochloride treatment. Threatened
1085 or significant change in employability or the
abllity to handle domestic responsibilities, or failing
postural reflexes were used as indicators of the
requirement for L-dopa therapy. Upon reaching the end
point, treatment was discontinued and a final follow-up
examination was conducted 1 month later (wash-out
study). Follow-up evaluations consisted of all five
assessment measures and a depression rating - the
results these evaluations are provided in Table 2.
A wash-out period of 1 month was chosen since
this was believed to be an adequate amount of time to
detect a transient dopaminomimetic effect, if there had
been one. This is due to the fact that selegiline
hydrochloride and lts metabolites are typically cleared
from the body wlthin 24 to 48 hours after cessatlon of
treatment. Moreover, during treatment, any effect of
selegiline hydrochloride on enzyme activity would be
substantially reversed within 2 to 3 weeks after
cessation of treatment.
As described above, half of the patients were
assigned to each arm of the study, baseline
characteristics being comparable in each Group (Table
1). A wash-in examination was performed on all 54
patients. Further analysis of 3 patients (2 in Placebo
Group (PG), 1 in Selegiline Group (DG)) was discontinued
because they were unable to remain off antiparkinsonian
medication for more than one month. Seven patlents
dropped out for the following reasons: lost to follow-
up (2 in DG); insomnia (1 in DG); euphoria-intoxicated
sensation (1 in PG); transient elevation in liver
enzymes (1 in PG); dysphagia (1 in DG); and incidental
-- 11 --
2 ~ 2
~ ~o ~ o
a N N O O .~ O O O ~ O
~: ~ 'a o O 0 0 N ~ O O
~o N N 0 0 rl ~I N r
to V 0 t` Y~ ~ ~ 0 ~ 0 o V
N N 0. 0 N ~ N N 1` Ul O
b
a
C
a ,, ,~ rl ~ ,. r~ ,. ,, r~ ,, o
a
_~ ~O ~q ~ r~ O,, ~ ,0 u~ O
N .1 O O 1.'1 1.) 0 0 ~I rl
~ a ~ 1
aa b r~ 0 0 0 ~ 0 N ~ O N _
~1 ~1 IC ~ N N N 0 0 0 0 V
a 0 1~ 0 o ,1 ~ o N 1~ N ~
a o N _~ O O ~ O O ~ O ~ ,
.c ~,4 ~ 1 -
U N N ~O I o~ o~ O
a N N N N N N N N N N
~a c O _~ O ~ au a .c
O O U U U ~ C U U O ~ _
~ ~ ~ vU~ ~ ~ a o. a ~ a ~
c~u ~ v~ eVâ ~vu .!
~ ~ ~0 D~ U 1~ a
2 r ~ 3 :~ a o
2 ~J h ~ 2
surgery (1 in PG). The remaining 44 patients reached
end point, 38 of which underwent wash-out examinations
(19 per group). Subjective complaints were almost all
transient and included insomnia [4 in PG, 14 in DG,
including 1 drop-out in DG], headache [10 in PG, 7 in
DG~, dizziness t6 in PG, 8 in DG] nausea [5 in each
Group], and euphoria [1 in each Group, including 1 drop-
out in PG].
4. Analysis of Data
Survivorship was analyzed by calculating a
Kaplan-Meier curve for each Group by using time to end
point (excluding 3 drop-outs, n=51). The 2 curves were
compared using the log rank test (see R. Miller,
Survival Analysis (Wiley, New York, 1981)). The Kaplan-
Meler survlval curves for each Group are lllustrated ln
the attached Figure and indicate that selegiline
hydrochloride delayed the time to end point (P<0.002).
The average time to end point was 312.1 days (44.47 +
SEM) for patients in the Placebo Group and 548.9 days
(61.01 + SEM) for patients ln the Selegiline Group. One
patient in the Selegiline Group reached end point by
staying on selegiline for 3 years.
The rate of disease progression was determined
by fitting a separate line for each assessment score
versus time by least squares for each patient who
reached end polnt (n-44) wlth all of the data points
available (Note: two patients ln PG had less than four
data polnts). Thereafter, the slope of the llnes were
averaged for each assessment measure in the Selegiline
Group and the Placebo Group by a two-tailed Wilcoxon
test. The wash-in (n=54) and wash-out (n=38) studies
were assessed for statistical significance by using the
Wilcoxon signed rank test (see Table 2). As illustrated
- 13 ~
2~ 2~i~2
in Table 2, only the change in the Webster Step-Second
Test in the Placebo Group at wash-out is statistically
slgnificant. The disease status was remarkably similar
at end point in both Groups, indlcating that (i) the
declslon to admlnlster L-dopa had been consistently
applied between Groups and was therefore not biased in
favour of either Group, and (ii) patients in the
Selegiline Group were taking nearly twlce as long to
reach this stage of disability as those in the Placebo
Group. The scores did not change appreciably after
treatment was stopped (wash-out) in either Group,
indicating that the delay to end point in the Selegiline
Group was not due to a transient therapeutic effect on
parkinsonian signs or symptoms or signs at wash-in.
Moreover, there was no dlfference in the depresslon
scores at wash-in or wash-out in either the Selegiline
Group or the Placebo Group, indlcatlng that an
antidepressant effect did not interfere with the
declslon to administer subse~uently L-dopa.
The rate of disease progression, on an annual
basis, for Parkinson's disease patients ln both the
Selegiline Group and the Placebo Group (n=44) was
assessed. The results of this assessment are provided
in Table 3 and illustrate that the rate of progression
was slowed by 40 to 83~ per year as measured by the five
assessment scales. Only the Webster Step-Second Test
failed to reach a statistical slgnificance (P ~ 0.07).
- 14 -
2Q2~
U
C o ~ .,. o ~
a u>
U
Cl
~ O O O O N
1~ O O O O O
1.~
O
U O . U~ U- N
_l _~1 ~ N O . i
_I N
U :1 t-- N ~ ~O N
V~ Cl--'.0 0 Il) N 11
NIt) 11-1 ~1 1.)
0~I t` O N
~1 0 1~ ri
O N
.~1 N O r') I 1~ 0
U :~ '-- I` 'O ~ N
_I b CI~ O ~1 ~ Cl.
D. Cl--~I N
~ _O U C
~ u ~ e U b a 1~
1~ C O U U ~ C 1~ C U
,~ U 0 V V ~ Q C ~ U _I
U ~o~ C U V ~ ~0 ~ ~ ~'
O~
,~ .c ,4 a ~
n P
-- 15 --