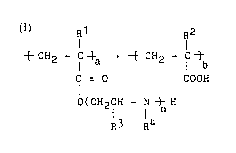Note: Descriptions are shown in the official language in which they were submitted.
2 a 22 6 0 7
ADDITIVE FOR PRODUCTION OF PAPER
This invention relates to the production of paper
employing a specific additive. More particularly, it
relates to an amphoteric polymeric additive for the
production of paper, which is capable of retaining in the
paper a water drainage-improving action and such additives
as filler and sizing agent in high yields under neutral
conditions.
Description of the Prior Art:
In recent years, the neutral paper production has
come to prevail in the place of the conventional acidic
paper production. The neutral paper production is
advantageous in (1) improving the durability of paper, (2)
decreasing the possibility of corroding machines, (3)
allowing safe use of inexpensive calcium carbonate as a
filler, and (4) permitting a paper producing machine to be
operated in a closed system, for example.
The adoption of the neutral paper production has
given rise to the problem that the drainage aid heretofore
used in the acidic paper production is either effectless or
effective insufficiently.
For the solution of this problem, the practice of
using cationized polyacrylamides obtained by the Mannich
reaction of polyacrylamides, cationized starch,
homopolymers of tertiary amino group- or quaternary
ammonium salt group-containing polymerizable monomers or
copolymers of such polymerizable monomers with nonionic
monomers, and cationic polymeric compounds such as
polyvinyl amines, polyallyl amines, and polyethylene
imines, for example, has been in vogue.
More recently, amphoteric polymeric compounds
such as Mannich reaction products of acrylic acid-
acrylamide copolymers, copolymers of tertiary amino group-
or quaternary ammonium salt group-containing polymerizable
monomers with acrylic acid, and Hofmann degradation
~ Z ~ 2 ~ 6 0 7
products of polyacrylamides have been finding utility in
this practice.
Though these cationic or amphoteric polymeric
compounds are used as water permeation-improving agents and
as yield-improving agents such as filler and sizing agent
under neutral conditions, they have the problem that they
are still short of sufficiently fulfiling their roles or
they are retained ununiformly in paper.
This invention is directed to a pulp composition
containing a novel additive for the production of paper,
particularly employing an amphoteric polymeric additive for
the production of paper, which enables the water drainage
improving action and such additives as filler and sizing
agent to be retained in high yields in paper.
In accordance with one aspect of the present
invention, there is provided a pulp composition to paper
production incorporated in the pulp composition 0.01 to 0.2
% by weight, based on its amount of pulp, of an additive
for the production of paper, comprising an amphoteric
polymeric electrolyte having as an essential component
thereof a structural unit represented by the general
formula I:
{~CH2 - IC ~a ~ ~ CH2 - C ~ (I)
C = O COOH
O(CH2CH - N ~ H
R3 R4
wherein n is an integer in the range of 1 to 5, Rl, R2, and
R3 are independently hydrogen atom or an alkyl group, R4 is
hydrogen atom, an alkyl group, or a hydroxyalkyl group, and
a and b jointly are relative numerals such that a/b is in
the range of 0.2 to 45, having at least part of the amino
~ 202~60~
group of the amphoteric polymeric electrolyte neutralized,
and possessing a cation equivalent value (Cv) in the range
of 1.0 to 15.0 meq/g, an anion equivalent value (Av) in the
range of 0.1 to 7.0 meq/g, and a Cv/Av ratio in the range
of 0.2 to 45Ø
In the general formula I mentioned above, n is an
integer in the range of 1 to 5, preferably 1 to 3, R1, R2,
and R3 are independently a hydrogen atom or an alkyl group,
providing that the number of carbon atoms of the alkyl
group is in the range of 1 to 3, preferably 1 to 2, R4 is
hydrogen atom, an alkyl group, or a hydroxyalkyl group,
providing that the number of carbon atoms of the alkyl
group or hydroxyalkyl group is in the range of 1 to 3,
preferably 1 to 2, and a and b are jointly relative
numerals such that the a/b ratio is in the range of 0.2 to
45, preferably 0.5 to 40. Viscosity of an aqueous 10 ~
solution of amphoteric polymeric electrolyte (Brookfield
viscosity at pH 3.5 and 25~C) is in the range of 50 to
100,000 cps, preferably 100 to 20,000 cps.
Though no particular method is specified for the
production of the additive for the production of paper in
the present invention, the following methods prove to be
preferable.
(1) The method which comprises causing a vinyl
type carboxylic acid monomer to react with an alkylene
imine thereby forming an aminoalkyl ester monomer and
copolymerizing a salt of the aminoalkyl ester monomer with
a vinyl group carboxylic acid monomer, (2) the method which
comprises polymerizing a vinyl type carboxylic acid monomer
thereby forming a vinyl type carboxylic acid polymer,
causing an alkylene imine to react with the polymer thereby
aminaokylating the polymer, and neutralizing the
aminoalkylation product with an acid, (3) the method which
comprises preparing an aminoalkyl ester monomer of the
reaction product of a vinyl type carboxylic acid monomer
-
.
2022~07
with an alkylene imine, subjecting a salt of the aminoalkyl
ester monomer and a vinyl type carboxylic acid monomer to
water-in-oil emulsification in the presence of water, a
surfactant, and a hydrophobic organic solvent, and then
copolymerizing the emulsification product, and (4) the
method which comprises emulsifying a vinyl type carboxylic
acid in the water-in-oil form in the presence of water, a
surfactant, and a hydrophobic organic solvent, polymerizing
the emulsification product, causing an alkylene imine to
react with the resultant vinyl type carboxylic acid polymer
emulsion thereby aminoalkylating the polymer emulsion, and
neutralizing the aminoalkylation product.
In the production of the additive of this invention
for the production of paper, copolymerization of a nonionic
monomer may be resorted to for the purpose of adjusting the
molecular weight and the ion equivalent.
The vinyl type carboxylic acid monomers which are
advantageously usable herein include acrylic acid,
methacrylic acid, and ammonium salts of such acids, for
example.
Particularly where the additive for the production
of paper is synthesized by the method of (4), the vinyl type
anionnic monomer is preferable to be an ammonium salt. In
this case, the neutralization ratio of the vinyl type
anionic monomer is in the ratio of 5 to 100 mol%, preferably
20 to 95 mol%.
The nonionic monomers which are usable herein
include (meth)acryl amides, N,N-dimethyl (meth)acryl amides,
N,N-diethyl (meth)acryl amides, hydroxyethyl
(meth)acrylates, hydroxypropyl (meth)acrylates, hydroxyethyl
(meth)acryl amides, hydroxypropyl(meth)acryl amides, and
acrylonitrile, for example.
The alkylene imines which are used preferably herein
are 1,2-alkylene imine (aziridines). Among other alkylene
imines mentioned above, 1,2-propylene imine and ethylene
imine prove to be particularly desirable on account of their
2022607
ready availability and relative inexpensiveness.
Optionally, other substituted 1,2-aziridines may be used.
The polymerization in any of the aforementioned
methods of (1), (2), (3), and (4) can be carried out by the
conventional method using a peroxide type, an azo type, or a
redox type polymerization initiator as popularly practised.
The amount of the polymerization initiator to be used in the
polymerization is in the range of 0.001 to 10 % by weight,
preferably 0.01 to 0.5 % by weight.
Ammonium persulfate, potassium persulfate, hydrogen
peroxide, and cumene hydroperoxide may be cited as examples
of the peroxide type initiator. Azobis-isobutyronitrile,
2,2'-azobis(2-amidinopropane) dihydrochloridel, 2,2'-
azobis(2,4-dimethylvaleronitrile), and 4,4'-azobis(4-
cyanopentanoic acid) may be cited as examples of the azo
type initiator. Formaldehyde sodium sulfoxylate,
thioglyconic acid, L-ascorbic acid, dimethyl
aminopropionitrile, sodium hydrogen sulfite, B-
mercaptoethanol, and combinations of divalent iron salts
with reducing agents may be cited as examples of the redox
type initiator. It is permissible to use a peroxide type
initiator or a redox type initiator in combination with an
azo type initiator.
The polymerization system may incorporate therein
any of the well-known chain transfer agents such as
isopropyl alcohol, erythruvic acid, and 2-mercaptoethanol.
The conventional nonionic surfactants may be cited
as examples of the surfactant to be used for the water-in-
oil emulsification involved in the aforementioned methods of
(3) and (4). These nonionic surfactants include sorbitan
monooleate, sorbitan monostearate, sorbitan monolaurate,
polyoxyethylene sorbitan monooleate, polyoxyethylene
nonylphenyl ether, polyoxyethylene lauryl ether, and
glycerol monooleate, for example. These nonionic
surfactants may be used either singly or in the form of a
combination of two or more members.
2022607
Such a nonionic surfactant may be used in
combination with ordinary anionic and cationic surfactants.
Hydrophobic aliphatic and aromatic hydrocarbons and
plant and animal oils and modified oils thereof may be cited
as examples of the hydrophobic organic solvent. These
hydrophobic organic solvents are represented by normal
paraffin, isoparaffin, cyclohexane, naphthene, toluene,
xylene, mineral oils, and kerosene.
The total concentration of the salt of an aminoalkyl
ester monomer, the vinyl type anionic monomer, and the
nonionic monomer in the method of (3) and the total
concentration of the vinyl type anionic monomer and the
nonionic monomer in the method of (4) are each desired to be
in the range of 20 to 80% by weight, based on the amount of
water. The concentration of the surfactant to be used
therein is preferable to be in the range of 5 to 30% by
weight, based on the amount of the hydrophobic organic
solvent. The ratio of the hydrophobic organic solvent to
water is in the range of 1 : 10 to 10 : 1, preferably 1 : 5
to 3 : 1.
The polymer concentration is preferable
approximately in the range of 5 to 80% by weight,
specifically 10 to 60 % by weight. Any polymer
concentration less than 5% by weight is undesirable because
the productivity is unduly low. Any polymer concentration
exceeding 80% by weight is undesirable because the
polymerization heat is generated in a large volume such as
to elevate the temperature of the system excessively. The
polymerization temperature is desired to be in the range of
10~ to 120~C, preferably 30~ to 90~C. The polymerization time
is approximately in the range of 10 minutes to 10 hours,
preferably 1 to 7 hours, though it is variable with the
concentration of the monomer, the polymerization
temperature, and the polymerization degree aimed at.
2022607
The aminoalkylation in the methods of (2) and (4)
can be carried out by causing an alkylene imine to react
with a vinyl type carboxylic acid polymer.
In this case, a divalent metal ion may be added,
when necessary, to the vinyl type carboxylic acid polymer to
induce partial formation of a chelate of the metal ion with
carboxylic acid before the polymer is subjected to the
aminoalkylation. There may be adopted otherwise a method of
performing the aminoalkylation by alternately adding an
alkylene imine and a neutral acid portionwise to the vinyl
type carboxylic acid copolymer.
The aminoalkylation is preferable to proceed at a
temperaturebelow 65~C, preferably in the range of 10~ to
55~C. If this reaction temperature exceeds 65~C, the
reaction solution undergoes gelation while the reaction is
in process and the reaction product is opacified with
insolubles. Conversely, if the reaction temperature is
below 10~C, the reaction time is elongated infinitely and
the reaction is consequently rendered meaningless.
The neutralization of the aminoalkyl group is
effected to an extent in the range of 50 to 150 mol%,
preferably 50 to 100 mol%, based on the amount of the added
alkylene imine. It is effected collectively or divisionally
during the course of the aminoalkylation. Though the
neutral acid is not specifically defined, it is preferable
to be hydrochloric acid, nitric acid, or sulfuric acid, for
example.
In the production of the additive of this invention
for the production of paper, the relative amounts of the
vinyl type carboxylic acid monomer and the salt of an
aminoalkyl ester monomer, the relative amounts of the vinyl
carboxylic acid polymer and the alkylene imine, and the
amount of the nonionic monomer must be fixed so that the
cation equivalent amount, Cv, will be in the range of 1.0 to
15.0 meq/g, preferably 2.0 to 12.0 meq/g, the anionic
equivalent amount, Av, in the range of 0.1 to 7.0 meq/g,
2022607
preferably 0.2 to 6.0 meq/g, and the Cv/Av ratio in the
range of 0.2 to 45.0, preferably 0.5 to 40Ø The terms
"cation equivalent amount" and "anion equivalent amount" as
used herein refer to the relevant effective components of
solids of a sample minus the amounts of the neutral acid and
the surfactant.
The additive of this invention for the production of
paper is particularly excellent in the retention of the
filler when the Cv/Av ratio is below 20. Any Cv/Av ratio
below 0.2 is undesirable because the interreactivity of the
additive with pulp is unduly weak, the drainage is poor, and
the yield of the filler is ununiform. When the Cv/Av ratio
exceeds, the additive particularly excels in water drainage
and yield of the sizing agent. Any Cv/Av ratio exceeding 45
is undesirable because the cohesive force of the filler and
the yield of the filler are both unduly low.
For the additive of this invention for the
production of paper, the compositions of the component
monomers and the conditions of polymerization are desired to
be suitably set so that the viscosity of an aqueous 10~
solution of the additive will be in the range of 50 to
100,000 cps, preferably 100 to 20,000 cps (Brookfield
viscosity measured at pH 3.5 and 25~C).
The kinds of pulp for which the additive for the
production of paper according with this invention include
ground pulp, thermomechanical pulp, sulfite pulp,
semichemical pulp, Kraft pulp, various species of synthetic
pulp, and the pulp produced by digesting used paper, for
example. It is permissible to use the additive in
combination with various adjuvants such as sizing agent,
drainage aid, retention aid, slime controlling agent,
defoaming agent, pitch-control agent, and pH adjusting
agent, for example.
A pulp composition for the production of paper is
obtained by adding to the pulp the additive for the
production of paper according with the present invention in
2022607
an amount in the range of 0.01 to 0.2% by weight, preferably
0.01 to 0.1 % by weight, based on the amount of dry pulp.
By adding the additive for the production of paper
produced as described above to pulp slurry under neutral
conditions, the drainage of the pulp slurry can be improved
and the additives such as filler and sizing agent can be
uniformly retained in the paper in high yields.
Now, the present invention will be described more
specifically below with reference to working examples. It
should be noted, however, that the present invention is not
restricted in any way by these examples.
Production of vinyl type carboxylic acid polymer
Referential Examples 1 to 4
Varying monomers indicated in Table 1 were placed in
a reaction vessel in the weight ratio indicated
correspondingly in a total amount calculated to account for
a concentration of 20% by weight, based on the finished
polymer. The monomers in the reaction vessel, with the
entrapped air displaced with nitrogen, and ammonium
persulfate and sodium hydrogen sulfite added thereto each in
an amount of 0.2% by weight, based on the total amount of
the monomers, were left polymerizing at 50~C for 4 hours, to
produce an aqueous solution of a vinyl type carboxylic acid
polymer.
Table 1
Referential Example Monomers and weight ratio
1 AA = 100
2 AA/AAm = 60/40
3 AA/AAm = 35/65
4 AA/AAm/AN = 60/35/5
AA : acrylic acid
AAm: acrylamide
AN : acrylonitrile
2022607
Production of additive for production of paper
Example A
In a reaction vessel, 167 g of the hydrochloride of
aminoethyl methacrylate obtained by the reaction of
methacrylic acid and ethylene imine and 33 g of acrylic acid
were placed in a total proportion calculated to account for
a concentration of 20 % by weight based on the finished
additive. The monomers in the reaction vessel, with the
entrapped air displaced with nitrogen, and 0.2% by weight,
based on the total amount of the monomers, of 2,2'-azobis(2-
amidinopropane) dihydrochloride added thereto were
polymerized for 4 hours, to obtain an additive A for the
production of paper according with the present invention.
Example B
In a reaction vessel, 100 g of the aqueous solution
of the vinyl type carboxylic acid polymer synthesized in
Referential Example 1 and 73 g of deionized water were
stirred at room temperature and the stirred aqueousn
solution and 6.0 g of ethylene imine added thereto were left
reacting at 50~C for 2 hours. Then, the reaction solution
and 10.1 g of 61 wt% nitric acid added thereto were stirred
for 30 minutes. The reaction was continued, with 6.0 g of
ethylene imine added thereto, for 1 hour. Then, the
reaction solution and 10.1 g of 61 wt% nitric acid added
thereto were stirred for 30 minutes, to obtain an additive B
for the production of paper according with the present
invention.
Examples C to H
The procedure of Example B was repeated, except that
the conditions indicated in Table 2 were used instead.
-10-
2022~i07
Table 2
Neutralizing acid
Weight of Final
Weight of ethylene concentration Kind of Weight of
ExamplePolymer polymer (g) im1ne (g) %) acid acid (g)
Referential
CExample 1 100 6.0 15 61% HN03 14.4
Referential
DExample 1 100 12.0 15 35% HCl 29.1
Referential
EExample 1 100 17.7 20 61% HN03 29.7
Referential
FExample 1 100 20.0 20 95~ H2S~4 16.8
Referential
GExample 2 100 20.1 20 61% HN03 33.7
Referential
HExample 4 100 20.1 20 61% HN03 33.7
2022607
Example I
In a reaction vessel, 100 g of the aqueous solution
of the vinyl carboxylic acid polymer synthesized in
Referential Example 3 and 6.6 g of an aqueous 40% calcium
chloride solution added thereto were homogenized and stirred
at room temperature. The stirred solution and 4.2 g of
ethylene amine added thereto were left reacting at 50~C for
4 hours. The resultant reaction solution and 10.2 g of 35
wt~ hydrochloric acid added thereto were stirred for 30
minutes, to obtain an additive I for the production of paper
according with the present invention.
Controls A and B
Table 3
Weight of . Neutr~1i7.ing acid
Weight of Flnal
Referential ethYlene Concentration
Ex~mple (g) acid acid (g)
A 100 1.0 15 61% HNO3 2.4
B 100 20.0 15 61% HNO3 47.9
Example J
In a four-neck flask provided with a stirrer, a
thermometer, a condenser, a dropping funnel, and a nitrogen
gas inlet, 100 g of isoparaffin solvent (produced by Exxon
Chemical K.K. and marketed under trademark designation of
"Isobar M") was placed and 11.6 g of sorbitan monooleate was
dissolved therein. The resultant solution was emulsified by
gradual addition thereto of a mixed liquid prepared as an
aqueous monomer solution by combining 80 g of acrylic acid,
20 g of acrylamide, 52.9 g of 28% aqua ammonia, and 33.9 g
of deionized water. The resultant emulsion in the flask,
with the interior of the system thoroughly displaced with
nitrogen and heated to 60~C, and 0.7 g of azobis(dimethyl
valeronitrile) added thereto as a catalyst were heated at
-12-
2022607
60~C and stirred for 4 hours, to produce a water-in-oil
vinyl type carboxylic acid polymer emulsion.
This emulsion kept at 50~C and 23.9 g of ethylene
imine added dropwise thereto were stirred for 30 minutes.
The resultant reaction solution and 57.4 g of an aqueous 61
wt% nitric acid solution added thereto were stirred for 30
minutes. The resultant mixture and 76.1 g of ethylene imine
added dropwise thereto were stirred for 30 minutes. The
mixture consequently formed and 110.7 g of an aqueous 61 wt%
nitric acid solution were stirred for 30 minutes, to produce
an additive J for the production of paper according with the
present invention.
Example K
In a four-neck flask provided with a stirrer, a
thermometer, a condenser, a dropping funnel, and a nitrogen
gas inlet, 111.6 g of isoparaffin solvent (produced by Exxon
Chemical K.K. and marketed under trademark designation of
"Isobar M") was placed and 22.3 g of sorbitan monooleate was
dissolved therein. The resultant solution was emulsified by
gradual addition thereto of a mixed solution prepared as an
aqueous monomer solution by combining 167 g of the
hydrochloride of aminoethyl methacrylate obtained by the
reaction of methacrylic acid and ethylene imine, 33 g of
acrylic acid, and 135 g of deionized waterl. The emulsion
in the flask, with the interior of the system thoroughly
displaced with nitrogen and heated to 60~C, and 0.4 g of
2,2'-azobistdimethyl valeronitrile) added thereto as a
catalyst were heated at 60~C and stirred for 8 hours, to
produce an additive M for the production of paper according
with the present invention.
Control C
In a reaction vessel, methacryloyloxyethyl trimethyl
ammonium chloride (4DAM), AAm, and AA are combined in a
4DAM/AAm/AA weight ratio of 30/60/10 were placed in a total
proportion calculated to account for a final concentration
of 20% by weight were placed. The monomers in the reaction
-13-
2022607
vessl, with the interior of the reaction vessel displaced
with nitrogen and heated to 50~C, and 0.2% by weight, based
on the total weight of the monomers, of 2,2'-azobis(2-
amidinopropane) dihydrochloride added thereto were left
polymerizing for 4 hours, to obtain an aqueous solution of
amphoteric polymeric compound C for comparison.
The physical properties of the additives for paper
production obtained in Examples A to K and Controls A to C.
-14-
2022607
Table 4
Cation Anion 10% visco~ity
equivalent value equivalent value Cv/Av (Cp3
Cv (meq/g) Av (meq/g)
Example A 5.5 2.8 2.0 550
Example B 7.9 1.9 4.2 450
Example C 4.8 5.3 0.9 440
Example D 8.0 0.5 16.0 330
Example E 9.9 3.5 2.8 700
Example F 10.4 1.5 6.9 390
Example G 10. 5 0.3 35.0 1200
Example H 10.5 0.3 35.0 3100
Example I 3.6 2.0 1.8 185
Example J 11. 6 0.5 23.2 5200
Example K 5.5 2.8 2.0 3400
Control A 1.0 12.1 0.08 1300
Control B 11.1 O. 2 55.5 740
Control C 1. 4 1.4 1.1 9500
2022607
The cation equivalent values and the anion
equivalent values indicated in Table 4 and the text of the
specification have been determined by the following methods.
(1) Cation equivalent value
In a beaker, 95 ml of distilled water was placed and
ml of an aqueous solution containing a sample in a
concentration of 1000 ppm as available components was added.
The resultant solution was adjusted to pH 3.0 by the
addition of 1% HCl or 1% NaOH, then stirred for about 1
minute, and titrated with N/4 aqueous solution of polyvinyl
potassium sulfate (PVSK) using 2 to 3 drops of a toluydine
blue indicator solution. The titration speed was fixed at 2
ml per minute and the time completing at least 10 seconds'
standing after the change of the color of the test solution
from blue to red purple was taken as the end point of
titration.
Cation equivalent amount (Cv) [meq/g]
= (amount of sample titration [ml] - amount of blank
titration [ml]) x potency of N/400 PVSK/2
The term "available components" refers to the
components of the solids of the sample minus the neutral
acid.
(2) Anion equivalent value
In a beaker, 50 ml of distilled water was placed and
about 0.3 g of an accurately weighed sample was added
thereto. The resultant solution was kept stirred and
titrated with an aqueous N/10 NaOH solution. The degree of
electroconductivity indicated on the scale was read out.
The titration amount corresponding to the last of a
plurality of points of inflection (points at which the whole
acid was neutralized) was read out.
Anion equivalent amount (Av) [meq/g]
= 0.1 x potency of N/10 NaOH x titration amount of
N/10 NaOH [ml] - amount of neutralizing acid in
accurately weighed sample [ml])/amount of available
components in accurately weighed sample [g]
-16-
2022607
Examples l to 11
Paper sheets were produced by using the additives
for paper production obtained in Examples A to K and tested
for retension of calcium carbonate and dispersibility of
calcium carbonate in paper. The results are shown in Table
5.
Controls 1 to 6
Paper sheets were produced by using respectively the
additives for paper production obtained in Controls A to C,
commercially avilable polyacrylamide Hofmann degradation
product and poly ethylene imine and were tested for
retention of calcium carbonate and dispersibility of calcium
carbonate in paper. The results are shown in Table 5.
202~607
Table 5
Calcium carbonate
Additive for Stockigt Freeness
paper Retention Dispersi- (sec) (ml)
Example
1 Example A 56 O 16 565
2 Example B 56 O 21 575
3 Example C 67 O 17 560
4 Example D 49 O 19 580
Example E 67 O 17 560
6 Example F 59 O 19 570
7 Example G 58 O 21 585
8 Example H 56 O 18 580
9 Example I 62 O 16 565
Example J 60 O 18 595
11 Example K 58 O 19 590
Control
1 Control A 19 0 8 525
2 Control B 34 X 20 580
3 Control C 52 X 16 570
4 Polyacryl 58 X 16 580
amide
5 Polyethylene 32 O 15 575
imine
6 No additive 19 O 13 520
The amount of additive was 400 ppm based on pulp slurry.
In Control 4, a commercially available Hoffman degradation product of
polyacryl amide was used.
In Control 5, polyethylene imine produced by Nippon Shokubai Kagaku
~ogyo Co., Ltd. and marketed under product code of P-1000 was used.
- 18 -
2022607
(Conditions for paper production)
The paper used for the test was produced under the
following conditions.
Pulp : NBKP, filler : heavy calcium carbonate
(commercially available), pulp/filler ratio : 100/30, pH :
9.3, sizing agent : AKD sizing agent (commercially
available) used in a concentration of 0.2% based on pulp
slurry.
AKD: Alkyl Ketene dimer
Sequence of addition: Pulp - filler - sizing agent
- additive of the invention - paper sheet.
Paper sheet: Tuppy square sheet machine.
Press: 3.5 kg/cm x 2 minutes, drying: drum drier
110~C x 150 seconds.
Basis weight: 65 g/m
(Method of evaluation)
Retention of calcium carbonate: This property was
determined by subjecting a sample paper produced under the
conditions described above to a heat treatment at 600~C x 20
minutes and consequently finding the ash content.
Dispersibility of calcium carbonate: This property
was determined by coloring calsium carbonate with a dye,
producing paper sheet containing the colored calcium
carbonate under the conditions described above, and visually
examining the produced paper sheet as to the dispersion of
the dye, and rating the result on the two-point scale,
wherein O is satisfactory dispersion and X is rejectable
dispersion.
Stockigt sizing degree: This property was determined
by measuring the sizing degree based on JIS P8122.
Freeness: This property was determined by adding a
given additive to a 0.3% NBKP pulp slurry 1(pH 7.6) and
measuring water permeation with a Canadian standard freeness
tester (JIS P8121).
-19-