Note: Descriptions are shown in the official language in which they were submitted.
~~~'~~~e
SKIN WHITENING COSMETICS
FIELD OF THE INVENTION
This invention relates to a skin whitening cosmetic
containing a specific fatty acid or a derivative thereof
which is effective to eliminate or prevent skin browning or
pigmentation in the skin, e.g., spots or freckles, due to
ultraviolet light,
BACKGROUND OF THE INVENTION
Known cosmetics for giving fairness to the facial
skin include compositions containing ascorbic acid or a
derivative thereof or a tyrosinase inhibitor, e.g., a
placenta extract and kojic acid or a derivative thereof.
Although these conventional skin whitening cosmetics exhibit
inhibitory activity against melanin production when tested in
vitro using tissue cultures, they have not succeeded in
obtaining sufficient effects on elimination of pigmentation
when actually applied to the skin.
As a result of extensive researches on various
components for their skin whitening effects, the inventors
have previously discovered that incorporation of a specific
fatty acid or a salt or an ester thereof into cosmetics
brings about excellent skin whitening effects and filed an
application for patent. It turned out, however, that such a
fatty acid or a derivative thereof is still insufficient in
- 1 -
skin whitening effect upon actual use when combined with fats
and oils generally employed in conventional cosmetics.
SUMMARY OF THE INVENTION
Accordingly, an object of this invention is to
provide a cosmetic containing a specific fatty acid or a salt
or an ester thereof in which the skin whitening effects of
the fatty acid or a salt or an ester thereof can be fully
manifested.
Other objects and effects of the present invention
will be apparent from the following description.
The present inventors have conducted extensive
investig~itions and, as a result, found that a combination of
a specific fatty acid, a salt thereof or an ester thereof
with a mono- or dihydric alcohol, and a silicone oil
unexpectedly produces excellent effects on elimination or
prevention of pigmentation in the skin, and thus reached the
present invention.
That is, the present invention relates to a skin
whitening cosmetic comprising (a) at least one compound
selected from the group consisting of a free fatty acid
having from 18 to 22 carbon atoms and containing at least two
unsaturated bonds per molecule, a salt of said fatty acid,
and an ester of said fatty acid with a monohydric or dihydric
alcohol, and (b) at least one silicone oil selected from the
- 2 -
~~~2~'r~j
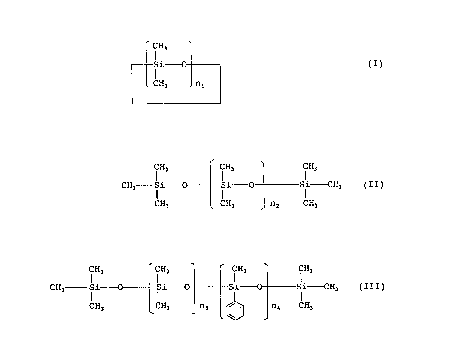
group consisting of compounds represented by formulae (I),
(II), and (III):
(I)
wherein nd represents an integer of from 4 to 6.
i Hs i Hs i Hs
CH3 Si -~%- Si Si-CH3 ( II )
CH3 CH3 n2 CH3
wherein n2 represents an integer of from 1 to 400.
CH3 CH3 CH3 CH3
CH3 Si Si Si Si~H3 ( I II )
CH3 CH3 n3 / I n4 CH3
wherein n3 and n4 each represents an integer, the sum of n3
and n4 being from l, to 400.
The present invention further relates to a skin
whitening cosmetic comprising (c) ethanol in addition to the
above-described components (a) and (b).
_ 3
DETAILED DESCRIPTION OF THE INVENTION
Fatty acids having from 18 to 22 carbon atoms and at
least two unsaturated bonds per molecule, e.g., linoleic acid
and y-linolenic acid, which can be used in the present
invention are contained in vegetable and animal fats and
oils. Rarely are these fatty acids present in a free form,
and most of them are present in the form of triglyceride.
Such tr:i.glycerides reveal no such significant effects on
inhibition of pigmentation in animal experiments as are
observed with free fatty acids or alkyl esters thereof.
Neither do saturated fatty acids, e.g., palmitic acid and
stearic acid, and in some cases, saturated fatty acids rather
accelerate melamine production. Considering that these
saturated fatty acids are present in vegetable and animal
fats and oils in large quantities, it is preferable to use a
purified unsaturated fatty acid in the preparation of the
cosmetic of the present invention.
Examples of the free unsaturated fatty acids
containing from 18 to 22 carbon atoms and at least two
unsaturated bonds per molecule include linoleic acid,
linoelaidic acid, oc-l5.nolenic acid, y-linolenic acid, dihomo-
y-linolenic acid, arachidonic acid, eicosapentaenoic acid,
and docosahexaenoic acid. These fatty acids may be used
either individually or in combination of two or more thereof.
~~~2~~~
These free unsaturated fatty acids are obtained by
saponification of natural fats and oils. Examples of the
natural fats and oils include vegetable fats and oils, e.g.,
linseed oil, safflower oil, sunflower oil, soybean oil, rape
oil, corn oil, sesame oil,, and gape seed oil; and marine
animal oils, e.g., cuttlefish oil, sardine oil, saury oily
and cod liver oil.
Examples of salts of these free fatty acids which can
be used in the present invention include metal salts, e.g., a
sodium salt and a potassium salt; amino acid salts, e.g., an
arginine salt and a lysine salt; and amine salts, e.g., a
triethanolamine salt and a monoethanolamine salt.
Examples of alkyl esters of the fatty acids which can
be used in the present invention include esters with
monohydric alcohols, e.g., methanol, ethanol, and isopropyl
alcohol; and dihydric alcohols, e.g., ethylene glycol,
propylene glycol, and 1,3-butylene glycol.
Among the above free unsaturated fatty acids and a
salt or an ester of these fatty acids, linoleic acid,
a-linolenic acid, y-linolenic acid, arachidonic acid, and
sodium linoleate are preferably used in the present
invention. Particularly, linoleic acid and a-linolenic acid
are more preferred.
The free fatty acid and a salt or an ester thereof
are preferably used :in an amount of from 0.1 to 10$ by
- 5 -
s~ ~ r
weight, and preferably from 0.1 to 5~ by weight, based on the
total amount of the cosmetic of the present invention. If
the amount is less than 0.1~ by weight, no inhibitory
activity on pigmentation tends to be exerted. If it exceeds
10~ by weight, skin irritation may be caused.
The silicone oil which is used in combination with
the above-described component (a) is selected from the
compounds represented by the above-described formulae (I),
(II), and (III).
Examples of the silicone oil of formula (I) include
KF~94 and KF995 (products of Shin-Etsu Chemical Co., Ltd.)
and SH244 and SH245 (products of Toray Silicone Co., Ltd.).
Examples of the silicone oil of formula (II) include
KF96 1 cs and KF96 2 cs (products of Shin-Etsu Chemical Co.,
Ltd.) and SH200c/1 cs, SH200c/10 cs, SH200c/100 cs, and
SH200c/500 cs (products of Toray Silicone Co., Ltd.).
Examples of the silicone oil of formula (III) include SH556
(product of Toray Silicone Co., Ltd.).
Among the above silicone oils, compounds represented
by formula (I) in which nl represents an integer of from 4 to
6 and compounds represented by formula (II) in which n2
represents an integer of from 1 to 10 are preferably used in
the present invention. Particularly, compounds represented
by formula (I) in which nl represents an integer of from 4 to
- 6 -
e~ S
and compounds represented by formula (II) in which nZ
represents an integer o.f from 1 to 7 are mare preferred.
The silicone oil is preferably used in an amount of
1~ by weight or more, more preferably 10~ by weight or more,
and particularly preferably 50~ by weight or more, based on
the total amount of the cosmetic of the present invention.
Silicone oil to be used can be arbitrarily chosen
from the above-described compounds. Two or more of these
silicone oils can be used in combination for the purpose of
improving feel on application, solubility of fatty acids, and
anti-freezing properties at low temperatures.
For example: although octamethylcyclotetrasiloxane
freezes at low temperature (10°C or lower), freezing at low
temperatures can be prevented by adding decamethylcyclopenta-
siloxane, methylphenylsiloxane or dimethylsiloxane; although
linoleic acid, a-linolenic acid and the like are not soluble
in dimethylpolysiloxane, the solubilities can be improved by
adding methylphenylsiloxane or octamethylcyclotetrasilxane;
and although octamethylcyclotetrasilxane and decamethylcyclo-
pentasiloxane have less oleophilic feel upon use and
therefore are not excellent in feel of cosmetics on
application, the feel of cosmetics can be improved by adding
dimethylsiloxane or methylphenylsiloxane.
For the same purpose, the cosmetic of the present
invention may further contain ethanol. Ethanol is preferably
- ~~~~~a~
used in an amount not more than 50.0 by weight, more
preferably from 1 to ~0~ by weight, and particularly
preferably from 3 to 25~ by weight, based on the total amount
of the cosmetic. If the amount of ethanol exceeds 50.0 by
weight, the cosmetic not only causes irritation to the skin
but feels cold on application due to volatilization of
ethanol, thus greatly deteriorating feel on use.
The cosmetics of the present invention can be
formulated into cosmetic oils, creams, and so on according to
known techniques.
If desired, the cosmetics of the present invention
may further contain other components generally employed in
cosmetics as long as the effects of the present invention are
not impaired.
The conventional compositions and its production
process as well as the conventional components added therefor
of the cosmetic compositions axe described, e.g., in
Keshohin-Gaku (Cosmetic Science), edited by T. Ikeda,
published on May 20, 1979 by Nanzando, Japan, which is
incorporated herein by reference, but the present invention
is not construed as being limited thereto.
Furthermore, if desired, the cosmetics of the present
invention can contain various conventional additives, such as
melanin production inhibitors (whitening agents) (e. g.,
vitamin C and placenta extract), anti-inflammatory agents,
g _
and antioxidants, as long as the effects of the present
invention are not impaired.
Examples of the conventional additives and their
addition amounts are shown in Table 1 below but they are not
limited thereto. The addition amounts are shown in terms of
percent by weight (except Vitamin A) based on the total
amount of the cosmetic compositions.
_ g _
n, a'' :- ,
~.~~~~~:'a-_gls:
TABLE 1
Additives Addition amount
by weight)
Whitenin~aqent
Placenta extract 0.1 to3
Kojic acid 0.1 to3
Photosensitive 0.0001 to0.002
element No. 201
Plant extract 0.01 to1
Vitamin A 500 IU to
2,500 IU
Anti-inflammatory agent
Dipotassium 0.01 to0.2
grycyrrhizic acid
Stearil 0.01 to0.2
grycyrrethinate
Allantoin 0.01 to0.2
s-Aminocaproic acid 0.01 to0.1
Methyl salicyJ.ate 0.01 to0.1
Antioxidant
Dibutylhydroxytoluene 0.01 to 1
Butylhydroxyanisole 0.01 to 1
Propyl gallate 0.01 to 0.2
Tocopherol 0.01 to 1
Erythorbic acid 0.0001 to 0.05
The cosmetics according to the present invention can be
applied to the skin by conventional manners. For example, a.
_ 10 _
lotion containing 1 wt~ of the compound of the present
invention can be applied by hands 1 to several times per day;
one or two drops of a cosmetic oil containing 3 wt~ of the
compounds can be applied to the portion at which pigmentation
occurs by fingers 1 to 3 times per day; a cream containing 5
wt~ of the compound can be applied by hands 1 to several
times per day; an emulsion containing 2 wt~ of the compound
can be applied by hands 1 to 3 times per day; and a pack can
be used in such a manner that 5 to 10 g of the pack is
applied to the facial skin other than eyes and nose, and it
allows to stand for about 30 minutes followed by being
removed 1 to 2 times per week.
Skin whitening effects according to the present invention
was tested as follows.
Test Method:
Ultraviolet light (UVB intensity: 1 J/cm2) was 4 times
(twice per week) irradiated on the shaved back of English
brown guinea pigs. One week after from the irradiation, a
pigmentation was formed.
On 6 cm2 (2 cm x 3 cm) of the back of the guinea pigs
having the pigmentation, 0.05 ml of a solution of a linoleic
acid in each of bases shown in Table 1 below was 20 times
(once per day) applied.
- 11 -
,J Li
:w
The degree of pigmentation was observed with eyes and
evaluated as follows taking that of the area where no test
compound was applied as a standard (0).
0: No reduction in pigmentation was observed.
-1: Slight reduction in pigmentation was observed.
-2: Medium reduction in pigmentation was observed.
-3: Considerable reduction in pigmentation was
observed.
The results of the evaluation are shown in Table 2
below.
- 12 -
TABLE 2
Conc,
of
Linoleic
Base Acid Evaluation
(wt~)
White petrolatum 5.0 0
Liquid paraffin 5.0 -1
" 0.1 0
Squalane 5.0 -1
Isocetyl stearate 5.0 -1
Octamethylcyclotetrasiloxane*1 0.1 -2
" 5.0 -3
Decamethylcyclopentasiloxane*Z 0.1 -2
" 5.0 -3
Dimethylpolysiloxane (500 0.1 -2
cs)
" (100 cs) 0.1 -2
" (10 cs) 0.1 -2
" (10 cs) 5.0 -3
' (1 cs) 0.1 -2
" (1 cs) 5.0 -3
Methylphenylpolysiloxane 0.1 -2
" 5.0 -3
Dimethylpolysiloxane (500 5.0 -3
cs)/methyl-
phenylpolysiloxane (1/3
by weight)
Dimethylpolysiloxane (100/deca- 5.0 -3
cs)
methylcyclopentasiloxane
(1/1 by weight)
Octamethylcyclotetrasiloxane/dimethyl- 0.1 -2
polysiloxane (500 as)
(8/12 by weight)
- 13 -
Note: *1 Formula (I)~(n=4)
*2 Formula (I) (n=5)
As is apparent from the results in Table 2, significant
reduction of pigmentation can be obtained when linoleic acid
is combined with a silicone oil or a mixture thereof as a
?case. It is also seen that the skin whitening effect
increases with an increase in concentration of linoleic acid.
Satisfactory effects can be obtained with linoleic acid of
from 0.1 to 10~k by weight, and preferably from 0.1 to 5~ by
weight. It was noted, however, that linoleic acid when used
at high concentrations is not dissolved in a silicone oil
base depending on the kind. of the silicone oil.
Solubility of linoleic acid in a silicone oil or a mixed
silicone oil is shown in Tables 3 and 4.
TABLE 3
Conc. of Linoleic Acid
Kind of Silicone Oil 1$ by wt. 5$ by wt.
Octamethylcyclotetrasiloxane soluble soluble
Decamethylcyclopentasiloxane " "
Dimethylpolysiloxane (500 cs) " insoluble
" (100 cs) " "
" (10 cs) " soluble
" (1 cs) ° "
Methylphenylpolysiloxane ~ " "
- 14 -
to
~, ,~ T3
x o ~ p w ..
a ~ - _ _ _
,
0
x ~ m
N
.~e
r-I p rl N ri N
U
.~, ~ ~ f~'~ ~',
N
N
_ _
O ~ O O C~ O i11
'
wl ~ G' ..~Li
~ , U7 r1 U7
.~
x
O
U N 'CfO T1
-t rl
I
,~ N ri N
O .~
~~i
~
~ N ~r ''I ' N '"I - N
~ A
A P
a .~ cn ..I u~
rtf
O
U
ri ~d p O
O
H N ~ x r~l ~ - r~1 ~ -
rp-I
O p O p O
'1
r t t
~i n n
o II
a ~~ ~ rol a~ ~ m
O
U -
~ U1
U 'Jy ~ ~
N
O U
+~ O
Lta ~ ~-i rl M r-i r1 M
j
\
~ ~ \ \ \ \ \ \
R~, M r-Irl M v-i rl
~
~
U7 N
U U
~o
r1 ri lrt r~
.-~
.,.I p .... O
O fli Ra
~
r1 Q1 ,-I
O
~ ~b gym
. .
o .~x .~x
U N O N O
w-1 .~, ~ r-!
~-~1
rl .-I .-i
ml .~
.~ (~ !n (.~
In
-
15
-
,~ v "~ w~
i I O
r-I .--I I
b
5C SC O O O O O O ,
,. _
N
1 O
'Jy 1
+~ ~ ~ U DC >C O O O O O O
C5
o ~ O
o a
~ Dy
c
d U
j . DC DC O O O O O O
+~ ~-1
0
o
~ f~
~
--I
r r
l i
O
rl
to
rl q
3
i
O Si y
~ U
4
, DC DC ?C?C O O O O
.
O O
W ~ ~
o
~
~ Ca
't3 a
a~ w
o
,
O ~~~~
U
O V O o ~ >C ~'"~' p O O O
N O
~
~
r-Iml In
,~
,,
N
W
0
i i
U
cd
~ N
'-' o ~ ~C >C O O O O O O
.,.i
d ~N
C7 U
.N O o
A .4 M t0 OD~ N d' l0 07
I
\ tT \ \ \ \ r1 ri .-1.--t
~
U ~ t~ c~ N o \ \
td
~ Ri -1 .~ .-1~,~,CO tD d' N
U
U
.4 .-I 1
0
O ~ rtJ
tT ~
.~ f-a
N ~
.-i
N +~ ~
!~ 'i
p O N rt
t~7
..
U ~ O O ' _ _ _ _ _ _
~ p
.-1+~ .-I
.-i
~-i
ri
~-IU U .-I
W O
.-iO ,'~y
UJ ~
u~ U
-
16
-
TABLE s
Octamethylcyclo- Linoleic
tetrasiloxane Ethanol Acid Evaluation
by wt.) (~ by wt.) (~ by wt.)
93 Z 5 X
91 4 5 X
89 6 5 X
87 8 5 0
85 10 5 O
Note: 0: Not freese at 0°C
X: Freeze at 0°C
As is apparent from the results in Table 3, linoleic acid
of high concentration lacks solubility in dimethylpolysiloxane
having a viscosity of 100 cs or more. In the case, solubility
of linoleic acid can be increased by adding an adequate amount
of octamethylcyclotetrasiloxane, decamethylcyclopentasiloxane,
methylphenylpolysiloxane, etc. as shown in Table 4.
Tables 5 and 6 shows low temperature stability of octa-
methylcyclotetrasiloxane. Although octamethylcyclotetra-
siloxan~ freezes at low temperatures because of its high
coagulation point, freezing can be prevented by a combined use
of an adequate amount of decamethylcyclopentasiloxane,
dimethylpolysiloxane, methylphenylpolysiloxane, or ethanol.
17 _
The present invention is now illustrated in greater
detail by way of the following Examples, but it should be
understood that the present invention is not deemed to be
limited thereto. All the percents are by weight unless
otherwise indicated.
EXAMPLE 1
Cosmetic Oil:
Linoleic acid 1.5~
Octamethylcyclotetrasiloxane 78.3$
Decamethylcyclopentasiloxane 20.0
Dibutylhydroxytoluene . 0.1~
Tocopherol 0.1~
Total: 100.0
EXAMPLE 2
Cosmetic Oil:
Eicosapentaenoic acid 2.0~
Dimethylpolysiloxane (2 cs) 89.8
Retinol 0.1~
Ethyl p-dimethylaminobenzoate 2.0~
Propyl p-hydroxybenzoate 0.1~
Methylphenylpolysiloxane 6.0~
Total: loo. o$
- 18 -
EXAMPLE 3
Cosmetic Oil:
y-Linolenic acid 1.5~
Octamethylcyclotetrasiloxane 79.6
Dimethylpolysiloxane (2 cs) 17.8
Soybean phospholipid 0.1~
Cinoxate 1.0~
Total: 100.0
EXAMPLE 4
Cosmetic Oil:
Ethyl linoleate 1.5~
Octamethylcyclotetrasiloxane 60.0
Decamethylcyclopentasiloxane 20.0
Dimethylpolysiloxane (100 cs) 18.3
Butylhydroxyanisole 0.2~
Total: 100.0
EXAMPLE 5
Cosmetic Oil:
y-Linolenic acid 2.0~
Octamethylcyclotetrasiloxane 85.0$
Dimethylpolysiloxane (50 cs) 12.8
Tocopherol ~ 0.2~
Total: 100.0
19 _
_ 2~~~~i~~'
EXAMPLE 6
Cosmetic Oil:
a-Linoleic acid 2.5~
Dimethylpolysiloxane (2 cs) 35.0$
Octamethylcyclotetrasiloxane 42.2
Ethanol 20.0
Stearyl glycyrrhetinate 0.1~
Ascorbyl stearate 0.1$
Dibutylhydroxytoluene 0.1$
Total: 100.0
In the above Examples 1 to 6, all the component were
uniformly mixed and dissolved with: each other to obtain
cosmetic oils.
EXAMPLE 7
Cream:
Linoleic acid 1.0$
Dibutylhydroxytoluene 0.05
,Decamethylcyclopentasiloxane 15.0
Dimethylpolysiloxane (500 cs) 2.0~
Monoglyceryl linoleate 2.0$
Monoglyceryl oleate 2.0~
Olive oil ' 2.0~
Methyl p-hydroxybenzoate 0.1~
- 20 -
~~~~~r
Citric acid 0.05%
Concentrated glycerin 15.0%
Dimethylpolysiloxane/polyethylene glycol 5.0%
copolymer '
Purified water 55.8%
Total: 100.0%
In the above Examples 1 to 6, all the component were
uniformly mixed and dissolved with each other to obtain a
cream.
The cosmetics according to the present invention, when
applied to the skin, eliminate or prevent browning or
pigmentation of the skin due to ultraviolet light and exhibit
excellent skin whitening effects.
While the invention has been described in detail with
reference to specific embodiments thereof, it will be apparent
to one skilled in the art that various changes and
modifications can be made 'therein without departing from the
spirit and scope thereof.
- 21 -