Note: Descriptions are shown in the official language in which they were submitted.
20228~
The present invention relates to steel wires for
reinforcing rubber, and also to a method of manufac-
turing the same.
Steel radial tires, steel-wire reinforced conveyer
belts, steel-wire reinforced timing belts, steel-wire
reinforced hoses, steel-wire reinforced handrails, and
the like each comprise a rubber member and steel wires
or steel cords (made by twisting the steel wires)
embedded in the rubber member. The surfaces of the
steel wires must be kept clean before they are embedded
in the rubber member, otherwise they will greatly impair
the corrosion resistance of the wires and the wire-
rubber adhesion strength.
To have their surfaces kept clean, the wires are
sealed within polyethylene bags, along with dehydrator
and nitrogen gas, before they are delivered to the
manufacturers of steel radial tires and other steel-wire
reinforced products. It is relatively expensive to
package the steel wires in this way. Further, the
steel wires, thus packaged, can rarely remain suffici-
ently clean up until the time they are embedded in the
rubber members. Consequently, the rubber products
reinforced with these steel wires, such as steel radial
tires, fail to have adequate wire-rubber adhesion
strength, heat resistance, oil resistance, or water
resistance.
As is known in the art, once it is surface-treated
~ ~ ~ 28 ~ O
with triazine-thiol derivatives, a metal member has good
corrosion resistance and can adhere firmly to rubber.
Various methods of treating the surfaces of wires with
triazine-thiol derivatives are disclosed in, for example,
Kunio Mori, Practice of Metal Surface Treatment, Vol. 37,
p. 373 (1989), Examined Japanese Patent Application No.
60-41084 published September 13, 1985 and Unexamined
Japanese Patent Application No. 58-87034 published May
24, 1983. In these methods, the wires are immersed in a
solution prepared by dissolving a triazine-thiol
derivative in either water or an organic solvent, thereby
adsorbing the triazine-thiol derivative on the surface of
each wire.
With the conventional surface-treating methods
described above, however, it is necessary to immerse the
wires in the solution at a relatively high temperature
for a long period of time. Further, the layer of
triazine-thiol derivative formed on the surface of each
wire is not sufficiently dense. Hence, the rubber
products reinforced by the wires, thus treated, cannot be
said to be adequately resistant to heat, water, steam, or
fatigue. All the conventional methods are batch
processes, and not suitable for mass-producing steel
wires for reinforcing rubber products.
The present invention seeks to avoid the
disadvantage of the prior art.
Accordingly in a first aspect the invention is a
method of manufacturing a rubber-reinforcing steel wire
coated with a polymer derived from a triazine-thiol
compound comprising the step of drawing a steel wire
through a die in a bath of a lubricant containing a
triazine-thiol derivative represented by the following
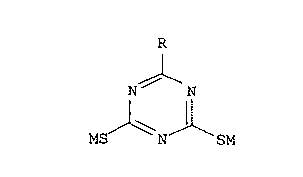
general formula:
. -- 3
where R is -OR', -SR', -NHR', or -N(R' )2; R' is H, alkyl
group, alkenyl group, phenyl group, phenylalkyl group,
alkylphenyl group, or a cycloalkyl group, and M is H, Na,
Li, K, 1/2Mg, 1/2Ba, 1/2Ca, primary, secondary or
tertiary aliphatic amine, quaternary ammonium salt, or
phosphonium salt.
In a second aspect the invention is a method of
manufacturing rubber-reinforcing steel wire coated with a
polymer derived from a triazine-thiol compound,
comprising the steps of:
immersing a steel wire and an electrode in a
solution of a triazine-thiol derivative such that said
electrode opposes said wire; and
applying a voltage between the steel wire and the
electrode,
wherein said triazine-thiol derivative is one
represented by the following general formula:
R
N ~ N
MS N SM
where R is -OR', -SR', -NHR', or -N(R' )2; R' is H, alkyl
group, alkenyl group, phenyl group, phenylalkyl group,
alkylphenyl group, or a cycloalkyl group, and M is H, Na,
Li, K, 1/2Mg, 1/2Ba, 1/2Ca, primary, secondary or
tertiary aliphatic amine, quaternary ammonium salt, or
phosphonium salt.
The rubber-reinforcing steel wires produced
according to the present invention can firmly adhere to
rubber. Hence, the composite members, each comprising a
rubber member and the steel wires of the invention, are
sufficiently resistant to heat, water, steam and fatigue.
., .~
~ o ~
-- 4
This invention can be more fully understood from the
following detailed description when taken in conjunction
with the accompanying drawings, in which:
Fig. 1 is a diagram schematically showing the wire-
drawing machine used for manufacturing several examples
of steel wires according to the invention;
Fig. 2 is a sectional view of the die incorporated
in the machine shown in Fig.l;
Fig. 3 is a gel permeation chromatograph of the
coating formed on the steel wire which has been surface-
treated with 1, 3, 5-triazine-2, 4, 6-trithiol;
Fig. 4 is a gel permeation chromatograph of the
coating formed on the steel wire which has been surface-
treated with 6-dibutylamino-1, 3, 5-triazine-2, 4-
dithiol;
.~
2022~0
Fig. 5 is an infrared spectrum of the coating
formed on the steel wire which has been surface-treated
with 1,3,5-triazine-2,4,6-trithiol;
Fig. 6 is an infrared spectrum of the coating
formed on the steel wire which has been surface-treated
with 6-dibutylamino-1,3,5-triazine-2,4-dithiol;
Fig. 7 is a diagram schematically illustrating
the electrodeposition apparatus used in manufacturing
a steel wire according to an example of the present
invention; and
Fig. 8 is a diagram representing the sizes of the
components of the electrodeposition apparatus shown in
Fig. 7.
The steel wires used as starting materials in the
present invention are: bare steel wires, copper-plated
steel wires, bronze-plated steel wires, nickel-plated
steel wires, tin-plated steel wires, zinc-plated steel
wires, copper-tin plated steel wires, cobalt-plated
steel wires, and the like.
Among the triazine-thiol derivatives, which are
used in the present invention, are:
1,3,5-triazine-2,4,6-trithiol (F),
1,3,5-triazine-2,4,6-trithiol monosodium (FN),
1,3,5-triazine-2,4,6-trithiol monopotassium,
1,3,5-triazine-2,4,6-trithiol monoethanolamine
(FME),
2022~
1,3,5-triazine-2,4,6-trithiol diethanolamine
(FDE),
1~3~5-triazine-2~4~6-trithiol triethylamine
(F.TEA),
1,3,5-triazine-2,4,6-trithiol octylamine,
1.3.5-triazine-2,4,6-trithiol tetrabutylammonium,
1,3,5-triazine-2,4,6-trithiol bis(tetrabutyl-
ammonium) (F2A),
6-anilino-1,3,5-triazine-2,4-dithiol (AF),
6-anilino-1,3,5-triazine-2,4-dithiol monosodium
(AN),
6-anilino-1,3,5-triazine-2,4-dithiol triethylamine,
6-dibutylamino-1,3,5-triazine-2,4-dithiol (DB),
6-dibutylamino-1,3,5-triazine-2,4-dithiol
monosodium (DBMN),
6-dibutylamino-1,3,5-triazine-2,4-dithiol
monoethanolamine (DBME),
6-dibutylamino-1,3,5-triazine-2,4-dithiol
ethylamine,
6-dibutylamino-1,3,5-triazine-2,4-dithiol
triethylamine,
6-dibutylamino-1,3,5-triazine-2,4-dithiol
butylamine (DBB),
6-dibutylamino-1,3,5-triazine-2,4-dithiol
tetrabutylammonium (DBA),
6-dibutylamino-1,3,5-triazine-2,4-dithiol
tetrabutylphosphonium,
2Q~
- 7 -
6-diallylamino-1,3,5-triazine-2,4-dithiol,
6-diallylamino-1,3,5-triazine-2,4-dithiol
monosodium ( DAN ),
6-diallylamino-1,3,5-triazine-2,4-dithiol
monoethanolamine ( DAME ),
6-diallylamino-1,3,5-triazine-2,4-dithiol
butylamine,
6-diallylamino-1,3,5-triazine-2,4-dithiol
ethylenediamine,
6-diallylamino-1,3,5-triazine-2,4-dithiol
ethylenetriamine,
6-octylamino-1,3,5-triazine-2,4-dithiol,
6-octylamino-1,3,5-triazine-2,4-dithiol
monosodium.
These triazine-thiol derivatives are used either
singly or in combination.
The steel wires according to the invention are
coated with a layer containing a polymer of any one of
triazine-thiol derivatives specified above. Of the
triazine-thiol derivatives listed above, F and DAN help
to improve the adhesion strength of the wires to rubber,
and DB serves to enhance the corrosion resistance of the
wire. Hence, these triazine-thiol derivatives and the
aforementioned other derivatives can be used in various
combinations.
It will now be explained a method of manufacturing
the steel wires according to this invention.
2022890
First, a method of drawing a steel wire in a bath
of lubricant containing a triazine-thiol derivative will
be described.
The steel wires, which are to be drawn, are not
limited to a specific type. When the wires are electro-
plated ones, it would be desired that the plated layer
be 100 to 10,000 A thick, more preferably 1,500 to
4,000 A thick, and amount to 0.1 to 40 g/kg, more
preferably 0.5 to 10 g/kg in terms of the weight ratio
of the plated metal to the steel.
The lubricant used in this method is a so-called
"emulsion type," which is an emulsion comprising a
triazine-thiol derivative, an extreme pressure lubri-
cant, an oiliness improver, an emulsifier, a defoaming
agent, and the like -- all dispersed in a solvent. The
solvent is, for example, neutral or alkaline water,
glycol (e.g., ethylene glycol derivatives), polyethylene
glycol, or diglyme. The lubricant may further contain a
rust preventive, and an antiseptic-mildewproof agent.
The lubricant may be applied, either not diluted, or
diluted 20 times or less, preferably diluted 5 to
lb times.
The content of the triazine-thiol derivative
in the lubricant (not diluted) usually ranges from
0.001 to 20% by weight, preferably 0.01 to 5% by
weight.
The extreme pressure lubricant prevents the wires
20~2~!~0
g
from being sticked while they are being drawn in the
lubricant. The extreme pressure lubricant is either
one of the following substances or a combination there
of:
ethylenediamine phosphate,
ethylenetriamine phosphate,
pentaethylenetetramine phosphate,
propylenediamine phosphate,
butylenediamine phosphate,
butylamine phosphate,
octylamine phosphate,
oleylamine phosphate,
fatty acid ester-ethyleneoxide adduct,
methylphosphate-propyleneoxide adduct,
butylphosphate-propyleneoxide adduct,
octlylphosphate-propyleneoxide adduct,
oleylephosphate-propyleneoxide adduct.
The content of the extreme pressure lubricant in
the lubricant (not diluted) ranges from 0.1 to 15% by
weight, preferably 1 to 10% by weight.
The oiliness improver is used not only to prevent
the steel wires from being sticked while they are being
drawn, but also to increase the wettability of the wires
to the lubricant. The oiliness improver is usually
amine salt of fatty acid such as:
octylamine acetate,
ethanolamine stearate,
2022~9~
-- 10 --
diethanolamine stearate,
diethanolamine octanoate,
diethanolamine linoleate,
diethanolamine oleate,
butylamine oleate.
Alternatively, the oiliness improver can be a
product of reaction between fatty acid and epoxide,
such as:
tetraethylene glycol oleate,
pentaethylene glycol octanoate,
nonaethyleneglycol stearate,
decaethylene glycol erucate,
decaethylene glycol linoleate.
Further, the oiliness improver can be a product
15 of reaction between fatty acid ester and epoxide, such
as:
butanedioloctanoate tetraethylene glycol,
butanediololeate hexaethylene glycol,
butanediolstearate pentaethylene glycol,
butanediolcaproate pentaethylene glycol,
hexanediolcaproate pentaethylene glycol.
The oiliness improvers specified above are used,
either singly or in combination. The content of the
oiliness improver in the lubricant (not diluted) ranges
25 from 0.1 to 20% by weight, preferably 1 to 15% by
weight.
The emulsifier emulsifies the extreme pressure
2022~0
lubricant, the oiliness improver, the defoaming agent,
and the like. The emulsifier is usually a product of
reaction between alkyl amine and an epoxy compound, such
as:
octylamine tetraethylene glycol,
dodecylamine decaethylene glycol,
oleylamine decaethylene glycol,
stearylamine octaethylene glycol.
The content of the emulsifier in the lubricant (not
diluted) is 0.1 to 10% by weight, preferably 0.5 to 5%
by weight.
The defoaming agent suppresses the foaming of the
emulsion. It is, for example, mineral spirit such as
decane, octane, hexadecane, heptadecane, nonadecane.
The content of the defoaming agent in the lubricant (not
diluted) ranges from 0.1 to 10% by weight, prefer ably
0.5 to 5% by weight.
The rust preventive is used to prevent the corrosion
of the iron or bronze components in the steel wires. It
is one selected from the following group:
methyl p-hydroxylbenzoate,
bisphenol A,
benzotriazole,
methylbenzotriazole.
The content of the rust preventive in the lubricant
(not diluted) is 0.01 to 5% by weight, preferably 0.1 to
1% by weight. The rust preventive need not be used,
2022~
- 12 -
since the triazine-thiol derivative used also prevents
the corrosion of the iron or bronze components in the
steel wires.
The antiseptic-mildewproof agent prevents the
lubricant from being contaminated by microorganisms.
It is one selected from the following group:
1,2-benzisothiazol-3-on,
chlorinated phenol,
formaldehyde,
formaldehyde-emitting agent.
The content of the antiseptic-mildewproof agent in
the lubricant (not diluted) ranges from 0.01 to 5% by
weight, preferably 0.1 to 1% by weight. This agent need
not be used, since the triazine-thiol derivative used
can also prevent the lubricant from being contaminated
by microorganisms.
In this invention, the steel wires are drawn in
the lubricant by means of a wet-type wire-drawing
machine. More specifically, the die of the machine
is placed in a tank filled with the lubricant, and a
steel wire having a diameter of, for example, 0.1 to
10 mm, preferably 1 to 4 mm, is passed through the
die at a speed of 1 to 200 m/min and thereby elon-
gated such that its diameter reduces to 0.1 to 1 mm.
When the wire is not plated one or a nickel-plated
one which is relatively hard, it is passed through
the die at a low speed and elongated to a low
2022~
- 13 -
degree. By contrast, when the wire is a copper-
plated one or a bronze-plated one which is relatively
soft, it is passed through the die at a high speed
and elongated to a high degree. In other words,
an optimal value for the speed of passing the wire
through the die, and an optimal value for the degree
of wire-elongation are determined by the type of the
steel wire.
As the steel wire is drawn through the die in the
bath of the lubricant, it is coated with a layer con-
taining a polymer of the triazine-thiol derivative.
It can be ascertained, by means of gel permeation
chromatography, that the layer thus coated on the wire
contains the polymer. Also can it be ascertained, by
means of infrared spectroscopy, that the layer contains
disulfide group, thiol group, unsaturated group, or
the like.
It can be assumed that the layer is coated on the
steel wire by virtue of the following mechanism. When
the wire contacts the lubricant, the triazine-thiol
derivative is adsorbed into the surface of the steel
wire, probably in the same way as in the conventional
surface-treating methods. As the wire is then passed
through the die and elongated, the triazine-thiol
derivative adsorbed on the wire is put under a high
pressure at a high temperature, though for an ex-
tremely short period of time. The surface temperature
2022~~
- 14 -
of a bronze-plated steel wire, for example, is said
to rise to several hundred degrees centigrade as
the wire is drawn under 100 kgf/mm2. As a result,
a layer containing a polymer of the triazine-thiol
derivative is formed on the surface of the steel
wire.
The layer, thus formed on the steel wire, is not
only dense, but also strong, and therefore protects the
wire from corrosion. In addition, since it contains
disulfide group, it can react with some component of
rubber. Hence, when the steel wire, thus drawn and
surface-treated, is embedded in a rubber member, adheres
firmly to the rubber.
It will now be explained a method of applying a
voltage between the steel wire and the electrode,
both immersed in a solution of a triazine-thiol
derivative.
This method is applied to a steel wire having
good electrical conductivity, such as a bronze-plated
steel wire, preferably a wire plated with a bronze
layer containing at least 60% by weight of copper.
The steel wire can be either one not drawn yet or
one already drawn. Further, it can be either one
made of a single wire, or one made of two or more
wires twisted together. In this method, the steel
wire functions as anode for achieving the electrode-
position of the triazine-thiol derivative, and
2022~0
an electrode made of electro-chemically inactive
material, such as platinum or carbon, is used as
cathode for accomplishing the electrodeposition of the
triazine-thiol derivative.
In this method, a solution is used in which
triazine-thiol derivative is dissolved in water or an
organic solvent. The organic solvent is one selected
from the group consisting of methanol, ethanol, isopro-
panol, ethylene glycol derivative, N,N-dimethylformamide
(DMF), dimethyl sulfoxide (DMSO), acetone, benzene,
toluene, acetonitrile, propylene carbonate, ethylene
carbonate, and the like. Of the triazine-thiol deriva-
tives used in the invention, a metal salt or an amine
salt is dissolved in water or alcohol, thus forming an
aqueous solution or an alcohol solution, and a free-
type derivative or an ammonium salt is dissolved in
an appropriate solvent, thus forming a solution.
The content of the triazine-thiol derivative in the
solution ranges from 0.001 to 10% by weight, pre-
ferably 0.05 to 2% by weight. In the case where the
solution has a low content of the triazine-thiol
derivative or has insufficient electrical conductivity,
a salt such as NaC~O3, Na2SO4, Na2HPO3, Na3BO3 or
the like may be added to the solution. In use, the
solution of triazine-thiol derivative is maintained at
0 to 80~C.
The voltage applied between the steel wire and
2022&90
the electrode opposing the wire is 100 v or less,
preferably 0.1 to 20 V. When the voltage is applied
between the wire and the electrode, a current flows
between them. This current ranges from 1 mA to
10 A, depending on the electrical conductivity of
the solution in which the wire and the electrode
are immersed. Preferably, this current is 5 to
100 mA. By virtue of this current, the triazine-
thiol derivative is adsorbed onto the surface of
the steel wire, forming a compound layer thereon.
It suffices to apply the voltage for 0.1 seconds to
10 minutes.
In order to treat a steel wire continuously by
the method according to the present invention, use
is made of an electrodeposition apparatus which com-
prises a tank filled with the solution of a triazine-
thiol derivative, an electrode located in the tank,
a power supply circuit for applying a voltage between
the electrode and a steel wire immersed in the bath
of the solution, and a wire-feeding mechanism having
a supply stand (e.g., a wire take-up device, reels,
etc.) for continuously feeding the wire through the
bath of the solution. The electrodeposition apparatus
may further comprises pre-treatment device connected to
the input side of the tank, and post-treatment devices
connected to the output side of the tank. The pre-
treatment devices is, for example, a wire-degreasing
20~2~0
- 17 -
device, and the post-treatment devices are, for
example, a wire-washing device and a wire-drying
device. It is prefer able that the electrode be a
hollow cylinder, in which case the steel wire is fed
through the electrode.
As the electrodeposition proceeds, the triazine-
thiol derivative undergoes the electro-chemical
reaction induced by the voltage applied between the
steel wire and the electrode, whereby a layer con-
taining a polymer of the derivative is formed onthe surface of the steel wire. This layer has
substantially the same properties as the layer formed
when the wire is treated by the wire drawing method
above-mentioned.
The steel wires, thus surface-treated either by
wire drawing or electrodeposition and now coated with
a layer containing the polymer of the triazine-thiol
derivative, is used by itself. Alternatively, the steel
wires, thus surface-treated, are twisted together into
a cord for practical use. The wires can be twisted
together into a cord, without no troubles, by any method
commonly used at present, such as those disclosed in
Setsuo Fukuhara, Fibers and Industry, Vol. 40, No. 11,
p. 627 (1984).
The steel wires or the steel cord are embedded in a
member made of a rubber compound, thereby manufacturing
various products such as steel radial tires, steel-wire
2~2~90
- 18 -
reinforced conveyer belts, steel-wire reinforced timing
belts, steel-wire reinforced hoses, and steel-wire rein-
forced handrails.
The composition of the rubber compound is not
limited particularly. The rubber compound comprises
rubber, a filler, a softener, a vulcanizer, a vulcaniza-
tion accelerator, and a vulcanization co-accelerator.
The rubber compound may further comprise a lubricant,
a stabilizer, an adhesion improver (i.e., an adhesion
accelerator).
The rubber is one selected from the group con-
sisting of:
natural rubbers (NR),
isoprene rubber,
butadiene rubber (BR),
solution polymerized butadiene rubber,
solution polymerized styrene-butadiene rubber
(SBR),
acrylonitrile-butadiene rubber ( NBR),
ethylene-propylene rubber,
ethylene-propylene-diene-methylene rubber (EPDM),
silicone rubber,
butyl rubber,
chlorinated butyl rubber,
brominated butyl rubber,
chloroprene rubber,
fluorocarbon rubber,
2022~91~
-- 19 --
hydrine rubber,
epichlorohydrine-ethylene oxide rubber,
epichlorohydrine-ethylene oxide-allyl glycidyl
ether rubber,
epichlorohydrine-propylene oxide-allyl glycidyl
ether rubber,
acrylic rubber and its copolymer (C~-based,
epoxy-based, or unsaturated one),
ethylene-vinylacetate-acrylate rubber,
urethane rubber.
The filler is used to increase the amount of the
rubber compound or to reinforce the rubber compound.
The filler is, for example, carbon black, rubber-rein
forcing carbon black, white carbon, hard clay, calcium
carbonate, silica, or the like. It is used in an amount
of 5 to 200 parts by weight, preferably 30 to 100 parts
by weight, to 100 parts by weight of the rubber.
The softener is added in order to improve the
workability or moldability of the rubber compound.
It is a phthalate-type plasticizer such as dioctyl
phthalate (DOP) or dibutyl phthalate; a fatty acid
ester-type plasticizer such as dioctyl adipate or
dioctyl sebacate; a phosphate-type plasticizer such as
triphenyl phosphate or tricresyl phosphate; chlorinated
paraffin; process oil; or naphthene oil. The softener
is used in an amount of 100 parts by weight or less,
preferably 5 to 50 parts by weight, to 100 parts by
2~22g~
- 20 -
weight of the rubber.
The vulcanizer is added to enhance the elasticity
of the rubber. It is selected from the group consisting
of sulfur, dicumyl peroxide, 1,1-bis(t-butylperoxy)-
3,3,5-trimethylcyclohexane, a,a'-bis(t-butylperoxy)
isopropyl benzene, 2,5-dimethyl-2,5-di(t-butylperoxy)
hexane, 2,5-dimethyl-2,5-di(t-butylperoxy)hexyne-3,
1,3,5-triazine-2,4,6-trithiol, 6-butylamino-1,3,5-
triazine-2,4-dithiol, ethylene triurea, hexamethylene
diamine, ammonium benzoate, and bisphenol A. The amount
of the vulcanizer is rather arbitrary, depending on
the type of the rubber used. However, when sulfur is
used as vulcanizer, it is used in an amount of 0.5 to
lO parts by weight, preferably 2 to 6 parts by weight,
to 100 parts by weight of the rubber. If the amount
of sulfur used is less than 0.5 parts by weight,
the rubber will not be vulcanized sufficiently, and
the rubber compound will not adhere firmly to the steel
wires; if the amount of sulfur exceeds lO parts of
weight, the rubber compound will be much less resistant
to heat than desired, and the resultant rubber-wire
composite will be far less resistant to water than
required.
The vulcanization accelerator and the vulcanization
co-accelerator are added for the purpose of promoting
the function of the vulcanizer. The vulcanization
accelerator can be: a thiazole-type accelerator such
21322~9U
as 2-mercaptobenzothiazole (M), 2-(4-morpholinyldithio)
benzothiazole, or dibenzothiazyldisulfide (DM); a
sulfenamide-type accelerator such as N-cyclohexyl-2-
benzothiazylsulfenamide (CBS), N-oxydiethylen-2-benzo-
thiazylsulfenamide, N,N-dicyclohexyl-2-benzothiazyl-
sulfenamide, or N-t-butyl-2-benzothiazylsulfenamide;
a thiuram-type accelerator such as tetramethylthiuram-
monosulfide, tetramethylthiuramdisulfide, or tetrabutyl-
thiuramdisulfide; or polyfunctional monomer such as fatty
acid amine salt, quarternary ammonium salt, organic
phosphonium salt, triallyl isocyanurate, trimethylol-
propane triacrylate, or diallyl phthalate. The vulcani-
zation co-accelerator can be at least one selected from
the group consisting of ZnO, MgO, BaO, and Ca(OH)2.
The amount of the vulcanization accelerator and
that of the vulcanization co-accelerator are arbitrary,
depending on the type of the rubber used and also the
type of the vulcanizer used. However, it is generally
appropriate to add them in an amount of 0.1 to 20 parts
by weight to 100 parts by weight of the rubber.
In order to manufacture wire-rubber composite pro-
ducts, it is advisable that the rubber compound contain
those components specified above. The rubber compound
need not contain such a lubricant, a stabilizer, an
adhesion improver as will be mentioned below. However,
if the compound contains these substances, the resultant
wire-rubber composite product will be more resistant to
2~22~Q
- 22 -
water, heat, steam and fatigue than other wise.
The lubricant may be added to improve the fluidity
of the rubber compound, thereby to render it easier to
manufacture wire-rubber composite products. It can be
stearic acid, metal (Na, Mg, Ca, Ba, Zn) stearate,
ethylene bisstearamide, ethylene biserucamide, paraffin
wax, or the like. The lubricant is used in an amount
of 0.1 to 5 parts by weight to 100 parts by weight of
rubber.
The stabilizer may be added to prevent deter-
ioration of the wire-rubber composite products. It
can be phenylenediamine-type antioxidant, phenol-type
antioxidant, nickel dithiocarbamate, benzophenone, or
the like. The stabilizer is used in an amount of 0.1 to
5 parts by weight to 100 parts by weight of rubber.
The adhesion improver, which the rubber compound
may contain, is, for example, 1,3,5-triazine-2,4,6-
trithiol, 6-dibutylamino-1,3,5-triazine-2,4-dithiol,
6-diallylamino-1,3,5-triazine-2,4-dithiol, cobalt
naphtenoate, cobalt stearate, metal (Co, Mn, Zn, Mo,
Cr) aminobenzoate, resorcin, cresol, resorcin-formalin
latex, resol-type phenolic resin (including uncured
ones)~ formalin-alkylphenol resin, formalin-cresol
resin (including uncured ones), monomethylol melamine,
dimethylol melamine, trimethylol melamine, hexamethylol
melamine, monoethoxymethylol melamine, tetramethoxy-
methylol melamine, pentamethoxymethylol melamine,
2022g90
monomethylol urea, trimethylol urea, trimethoxymethylol
urea, ethylene maleimide, butylene maleimide, phenylene
maleimide, metal (Co, Ni, Fe, Mn) abietate, or the like.
The adhesion improver is used in an amount of 0.1 to
20 parts by weight, preferably 0.5 to 5 parts by weight,
to 100 parts by weight of the rubber.
The steel wires thus surface-treated, or the cords
made of these wires are embedded in a member made of the
rubber compound specified above, thus forming a com-
posite member. The composite member is heated by means
of either hot-pressing or steam-heating, whereby the
rubber is vulcanized, whilst the wires or cords adhere
firmly to the the rubber member. This process is
performed, usually at 80 to 230~C, preferably 130 to
180~C, for 5 to 10 minutes, preferably 10 to 60 minutes.
The wire-rubber composite member is further subjected to
aftercure, if necessary depending on the type of the
rubber used or the vulcanizer used. As a result of
this, a wire-rubber composite product is manufactured.
The present invention will now be described, with
reference to several examples.
Examples 1 to 5
First, the wet-type wire-drawing apparatus used in
manufacturing Examples 1 to 5 of steel wires according
to the present invention.
As is illustrated in Fig. 1, the wire-drawing
apparatus has a lubricant tank 20 filled with lubricant
2022~
- 24 -
21. A steel wire 22 is fed from a supply bobbin 1,
guided by a guide roller 2, and enters the lubricant
tank 20. In the tank 20, the wire 22 is wound around
a free roller 3 and a driving roll 5, passing through a
die unit 4. Also in the lubricant tank 20, the wire 22
is further wound around a free roller 6 and a driving
roller 8, passing a die unit 7. Then, the wire 22 is
fed out of the tank 20 through a die 9. Outside the
lubricant tank 20, the steel wire 22 is guided by a
capstan 10 and a guide roller 11, and is finally taken
up around a take-up bobbin 12.
The dies 4 and 7 are each a combination of several
dies, mounted one upon another. As is shown in Fig. 2,
each die constituting either die unit comprises a die
case 13 and an extremely hard chip 14 fitted in the case
13.
Example 1
Two emulsion-type lubricants were prepared.
The first emulsion-type lubricant comprised 4 parts
by weight of ethylenediamine phosphate, 8 parts by
weight of triethanolamine oleate, 4 parts by weight of
laurylamine octanethyleneglycol, 3 parts by weight of
octadecane, 2 parts by weight of tetraethylene glycol
octate, 5 parts by weight of butanediol dodecylphosphate
pentapropylene glycol, 0.5 parts by weight of methyl
p-hydroxybenzoate, 1 part by weight of methylbenzotri-
azole, 0.5 parts by weight of 1,2-benzoisothiazol-3-on,
20~2~39~
- 25 -
72.5 parts by weight of water, and 0.5 parts by weight
of 1,3,5-triazine-2,4,6-trithiol (F). The second
emulsion-type lubricant was different only in that
0.5 parts by weight of 6-dibutylamino-1,3-5-triazine-
2,4-dithiol (Ds) is used in place of 0.5 parts by
weight of 1,3,5-triazine-2,4,6-trithiol (F).
These two emulsion-type lubricants thus prepared
were diluted seven times. Each diluted lubricant was
filled in the lubricant tank of the apparatus illustrated
in Fig. 1. A bronze-plated wire (a plated amount: 4.1 g
per 1 kg of steel, Cu content in the plate: 65%) was
drawn in the bath of each lubricant at rate of 30 m/min.
Thus two bronze-plated steel wires were formed, either
having a diameter of 1.20 mm (hereinafter referred to as
"Example 1-1" and "Example 1-2").
Each surface-treated wire was immersed in lN
hydrochloric acid solution so as to remove the coating
from the wire. The solution was evaporated with a
rotary evaporator, thus the coating material is
obtained. The coating material was dissolved in THF,
and was subjected to gel permeation chromatography.
Also, the coating material was subjected to infrared-
absorption spectrum analysis by KBr method.
Fig. 3 is a gel permeation chromatograph of the
coating of Example 1-1, also showing that of F monomer
(broken-line curve). Fig. 4 is a gel permeation
chromatograph of the coating of Example 1-2, also
~22~
- 26 -
showing that of DB monomer (broken-line curve).
Fig. 5 is the infrared (IR) spectrum of the coating
formed on Example 1-1, also showing those of F monomer
and F polymer. Fig. 6 is the IR spectrum of the coating
formed on Example 1-2, also showing those of DB monomer
and DB polymer. Both the F polymer and the DB polymer
had been synthesized by means of thermal polymerization.
As is evident from Figs. 3 and 4, the gel-
permeation chromatograph curves of both the coating of
Example 1-1 and that of Example 1-2 have low peaks at
retention time of about 5 minutes. Obviously, the
coatings were compounds having molecular weights greater
than that of F monomer.
As can be understood from Fig. 5, the IR spectrum
of F monomer had a strong peak at wavelength of about
1360 cm-l and a weak peak at wavelength of about
1250 cm-l. By contrast, the IR spectrum of F polymer
had no peaks at wavelength of about 1360 cm-l and a
strong peak at wavelength of about 1250 cm-l. In
view of Fig. 5, the coating of Example 1-1 had an
IR spectrum similar to that of F polymer.
As is shown in Fig. 6, the IR spectra of DB monomer
and DB polymer are different for wavelengths ranging
from 1200 to 1600 cm-l. The IR spectrum of the coating
of Example 1-2 is similar to that of the DB polymer.
In view of Figs. 2, 3, 4, and 5, the coatings of
Examples 1-1 and 1-2 are considered to contain both
2022~90
- 27 -
polymer and monomer of a triazine-thiol derivative.
Example 2
Five emulsion-type lubricants were prepared which
were identical in that each comprised the following
components, but different in that they contained the
five different triazine-thiol derivatives specified in
Table 1.
Common Components of the Five Emulsions
(~1) 4 parts by weight of ethylenediamine phosphate
(2) 8 parts by weight of triethanolamine oleate
(3) 4 parts by weight of lauryl amine
octaethyleneglycol
(4) 3 parts by weight of octadecane
(5) 2 parts by weight of tetraethylene glycol
octate
(6) 5 parts by weight of butanediol
dodecylphosphate pentapropylene glycol
(7) 0.5 parts by weight of methyl
p-hydroxybensoate
(8) 1 part by weight of methylbenzotriazole
(9) 0.5 parts by weight of 1,2-benzoisothiazol-3-on
(10) 72.5 parts by weight of water
Further, for purpose of comparison, an emulsion-type
lubricant was prepared which was identical to the five
emulsion-type lubricants specified above, except that
it contained no triazine-thiol derivatives.
The six lubricants, thus prepared, were diluted
~û2289~
- 28 -
seven times. Each diluted lubricant was filled in the
lubricant tank of wire-drawing apparatus illustrated
in Fig. 1. A bronze-plated wire (a plated amount:
4.1 g per 1 kg of steel, Cu content in the plate: 65%)
was drawn in the bath of each lubricant at rate of
850 m/min, thereby obtaining a bronze-plated wire having
a diameter of 0.30 mm. Two wires of each type is
twisted together, into a steel cord. Hence, six steel
cords were manufactured, one of which will be referred
as "Comparative Example 2-1," and the remaining five of
which will be referred to as "Examples 2-1 to 2-5."
In the meantime, an natural-rubber (NR) compound
was prepared which comprised:
(a) 100 parts by weight of natural rubber (NR)
(b) 50 parts by weight of carbon black (HAF)
(c) 5 parts by weight of process oil
(d) 5 parts by weight of sulfur
(e) 0.8 parts by weight of N-cyclohexyl-2-
benzothiazylsulfenamide (CBS)
(f) 10 parts by weight of zinc oxide
(g) 2 parts by weight of cobalt naphthenoate
(h) 1 part by weight of 6-ethoxy-2,2,4-trimethyl-
1,2-dihydroquinoline
(i) 3 parts by weight of resorcin
(j) 4 parts by weight of hexamethylol melamine
Each test piece of cord of Comparative Example 2-1
and Examples 2-1 to 2-5 was embedded its one end by 1.6 cm
2~22~390
- 29 -
into mass of the NR compound thus prepared, and then the
rubber mass was vulcanized at 140~C for 30 minutes.
Thereby six sets of cord-rubber composite members were
formed, each set consisting of several members.
Each cord-rubber composite member of every set was
subjected to pull-out test carried out by an automatic
tensile tester. More specifically, the test piece of
cord was pulled out of the rubber member at 20~C at the
rate of 50 mm/min, and the pull-out strength was measur-
ed. Also, the ratio of the cord surface area covered
with the rubber was measured. The measurements were
performed immediately after the composite had been formed
(i.e. "initial"), and after the composite had been
steam-deteriorated. The steam-deterioration was per-
formed in a water-vapor atmosphere, at humidity of 100%
and temperature of 120~C, for 10 to 25 hours as is spe-
cified in Table 1. The results of the pull-out test
were as is represented in Table 1.
As can be understood from Table 1, although
Examples 2-1 to 2-5 had an initial pull-out strength
similar to that of Comparative Example 2-1, they had a
pull-out strength far greater than that of Comparative
Example 2-1 after they had been steam-deteriorated. In
addition, as is also evident from Table 1, the wire-
rubber adhesion in Examples 2-1 to 2-5 was stronger than
that in Comparative Example 2-1.
~2~9Q
- 30 -
~ ~ r4
3 3 ~ o o m o u~ o a~ ~ ~o o
O O ~ ~ ~ ~ ~ ~ ~ ~ ~,
P~ ~; U) ~
4 0
:~ '4
:~ ~ ~ In ~ u~ o o Ln ~ o ~ o
o
~~ .
,Y d~ '4
---- ~ ~ o o ~ u
In
-
1) .
_1 4
~K I ao o <~1 0 0 0 ~1 0 ~ O
~ O
o
~1
E~~, .
.,
r ~c
a
I ~
m
m :~: m
N ::>
~ 14
'4 ~U
E~ ~
I I I I I
~s a) a) o a) a)
o X X X X X
V~ ~ ~ ~ ~
2~22t~
3 3 ~ u~ o ¢1
O O ~ ~ cl
I t~
¢~ ~
C' C
S-J ~1 _
~- ~ d' O ~';
O ~
a) ~, ~ =
C'
~ Q ~ ¢~
,~ o\~ ~ ~ . .
__ ~ O U~
d' I' C C O O
Il - C
rC t~
~ I . r ~,1~ ~
'V ~ ~ ~) ~ ~ >
_l ~ I C
~ ~ C d' d
O
I ~ I I ¢~
~ P; ~1 ~ ~1 ¢ a~ ~c
O O C O C C ~
~ h
C' ~ ~ ~ ~ ~ o
H I I I I 1 ~1
In V~
O ' ' ' ' ' C
IIIII
a a) o tQ
I ~ C Cl C C' C
C ~
~ ~ U
E~ ~ Il) 5~ m Q Ir ~-
' I ' I I O
a~ .. .. .. .. .. ~c
m ~
m :~ m ~:
20228~
- 32 -
Example 3
Five emulsion-type lubricants were prepared which
were identical in that each comprised the following
components, but different in that they contained the
6-dibutylamino-1,3,5-triazine-2,4-trithiol monoethanol
amine (DBME) in different amounts ranging from 0.15 to
3 g per 100 m~, as is specified in Table 2.
Common Components of the Five Emulsions
(1) 4 parts by weight of ethylenediamine phosphate
(2) 8 parts by weight of triethanolamine oleate
(3) 4 parts by weight of lauryl amine octaethy-
leneglycol
4) 3 parts by weight of octadecane
(5) 2 parts by weight of tetraethylene glycol
octate
(6) 5 parts by weight of butanediol
dodecylphosphate pentapropylene glycol
(7) 0.5 parts by weight of methyl p-hydroxybencoate
(8) 1 part by weight of methylbenzotriazole
(9) 72.5 parts by weight of water
Further, for purpose of comparison, an emulsion-
type lubricant was prepared which was identical to the
five emulsion-type lubricants specified above, except
that it contained no DBME.
The six lubricants, thus prepared, were diluted
seven times. Each diluted lubricant was filled in the
lubricant tank of wire drawing apparatus illustrated in
2f~2~9~
Fig. 1. A bare steel wire or a bronze-plated wire
~plate thickness: 5800 ~, Cu content in the plate: 65%~,
each having a diameter of 1.68 mm, was drawn in the bath
of each lubricant at rate of 0.5 to 100 m/min as is
shown in Table 2. Thereby six bare steel wires and
one bronze-plated wire were formed, each having a
diameter of 1.50 mm. The former will be referred to
as "Example 3-1 to 3-5" and "Comparative Example 3-1,"
and the latter will be referred to as "Comparative
Example 3-2."
In the meantime, an natural-rubber (NR) compound
was prepared which comprised:
(a) 100 parts by weight of natural rubber (NR)
(b) 50 parts by weight of carbon black (HAF)
(c) 5 parts by weight of process oil
(d) 4 parts by weight of sulfur
(e) 0.8 parts by weight of N-cyclohexyl-2-
benzothiazylsulfenamide (CBS)
(f) 10 parts by weight of zinc oxide
(g) 1 part by weight of 6-ethoxy-2,2,4-trimethyl-
1,2-dihydroquinoline
(h) 3 parts by weight of resorcin
(i) 4 parts by weight of hexamethylol melamine
Each test piece of wire of Examples 3-1 to 3-5 and
Comparative Examples 3-1 and 3-2 was embedded its one
end by 2.54 cm into mass of the NR compound thus pre-
pared, and then the rubber mass was vulcanized at 153~C
2~22g9~
- 34 -
for 30 minutes. Thereby seven sets of wire-rubber
composite members were formed, each set consisting of
several members.
Each wire-rubber composite member of every set was
subjected to pull-out test carried out by the automatic
tensile tester. More specifically, the test piece of
wire was pulled out of the rubber member at 20~C at
the rate of 50 mm/min, and the pull-out strength was
measured. Also, it was examined how much rubber
remained on the surface of each wire pulled out of
the rubber member. The measurements were performed
immediately after the composite had been formed, and
after the composite had been steam-deteriorated. The
steam-deterioration was performed in a water-vapor
atmosphere, at humidity of 100% and temperature of
120~C, for 10 to 25 hours as is specified in Table 2.
The results of the pull-out test were as is represented
in Table 2.
As is evident from Table 2, the bare steel wire
of Comparative Example 3-1, which had been treated with
the lubricant containing DBME and drawn at an extremely
low speed was easily pulled out of the rubber member.
Its pull-out strength was just as small as those of bare
steel wires not surface-treated by wire-drawing at all.
Though not shown in Table 2, almost no rubber compound
remained on the surface of the wire of Comparative
Example 3-1 which had been pulled out of the rubber
2~228g~
- 35 -
member. This reveals that the pull-out strength of
the wire of Comparative Example 3-1 resulted from the
friction between the wire and the rubber member.
The bronze-plated wire of Comparative Example 3-2,
which had been drawn in a bath of the lubricant con-
taining no triazine-thiol derivatives exhibited a great
initial pull-out strength, but an insufficient pull-out
strength once they had been steam-deteriorated.
By contrast, the bare steel wires of Examples 3-1
to 3-5 had an initial pull-out strength far greater than
that of Comparative Example 3-1. Moreover, even after
they had been steam-deteriorated, they exhibited a pull-
out strength much greater than those of Comparative
Examples 3-1 and 3-2.
Table 2
Concen- Type of Drawing Pull-out Strength (kgf)
tration wire * Speed
(g/100 m~) (m/min)Initial 10 Hr. 15 Hr.20 Hr. 25 Hr.
Comparative
Example 3-10.2 Bare0.5 40 35 30 30 30
Comparative
Example 3-2 - Bronze20 230 210 100 40 40
Example 3-10.15 Bare 20 200 190 180 160 140
Example 3-20.2 Bare 20 210 200 190 180 160
Example 3-31.0 Bare 50 230 220 210 200 170
Example 3-41.5 Bare100 240 230 210 200 190
Example 3-53.0 Bare 20 240 230 220 210 190
* Note: "Bare" means "unplated," and "Bronze" means "a bronze-plated."
;r~
2~289~
Example 4
One emulsion-type lubricants was prepared which
comprised the following components:
(1) 3 parts by weight of ethylenediamine phosphate
(2) 8 parts by weight of triethanolamine oleate
(3) 4 parts by weight of laurylamine octaethyl-
eneglycol
(4) 3 parts by weight of octadecane
(5) 2 parts by weight of tetraethylene glycol
laurate
(6) 5 parts by weight of butanediol
dodecylphosphate pentapropylene glycol
(7) 0.5 parts by weight of methyl
p-hydroxybenzoate
(8) 1 part by weight of methylbenzotriazole
(9) 72.5 parts by weight of water
(10) 1 part by weight of 1,3,5-triazine-2,4,6-
trithiol diethanolamine (FDE)
Further, for purpose of comparison, an emulsion-
type lubricant was prepared which was identical to the
emulsion-type lubricant whose composition is specified
above, except that it contained no FDE.
The two lubricants, thus prepared, were diluted
seven times. Each diluted lubricant was filled in the
lubricant tank of wire-drawing apparatus illustrated
in Fig. 1. Two bronze-plated wires (a plated amount:
of 4.1 g per 1 kg of steel, Cu content in the plate:
2~22~9~
65%) and having diameters of 1.60 mm and 1.00 mm,
respectively, were drawn in the bath of each lubricant
at rate of 800 m/min. Thereby two bronze-plated wires
were obtained, one having a diameter of 0.38 mm, and
the other having a diameter of 0.20 mm. Three wires
having the diameter of 0.20 mm were twisted together,
thus forming a core. Six wires having the diameter of
0.38 mm were twisted around the core, thus forming a
sheath, whereby a steel cord was made. As a result,
two types of steel cords were obtained.
Meanwhile, five different NR compounds were
prepared which were identical in that they comprised the
following components, but they were different in the
content of sulfur. Sheets were made of each NR com-
pound, which had a size of 1.5 mm x 12 mm x 10 cm.
(a) 100 parts by weight of natural rubber (NR)
(b) 50 parts by weight of carbon black (HAF)
(c) 5 parts by weight of process oil
(d) 1 to 8 parts by weight of sulfur (specified in
Table 3)
(e) 0.8 parts by weight of N-cyclohexyl-2-
benzothiazylsulfenamide (CBS)
(f) 10 parts by weight of zinc oxide
(g) 1 part by weight of N,N-dioctylphenylene-
diamine
Ten pieces of steel cord of the first type were
laid, side by side, on every sheet made of any NR
2~2~9a
- 39 -
compound, and then the rubber sheet was vulcanized at
153~C for 30 minutes. As a result, five types of cord-
rubber composite members were made. Both end portions
of each cord-rubber composite member, either having a
cord, were cut off. As a result of this, five groups of
composite members, each member having eight cords, were
obtained. These five groups of composite members will
be referred to as "Examples 4-1" to ~Example 4-5.~
Similarly, ten pieces of steel cord of the second
type were laid, side by side, on every sheet made of any
NR compound, and then the rubber sheet was vulcanized
at 153~C for 30 minutes. As a result, five types of
cord-rubber composite members were made. soth end
portions of each cord-rubber composite member, either
having a cord, were cut off. As a result of this, five
groups of composite members, each member having eight
cords, were obtained. These five groups of composite
members will be referred to as "Comparative Examples
4-1" to "Comparative Example 4-5."
The cord-rubber composite members of Examples 4-1
to 4-5, and also those of Comparative Examples 4-1 to
4-5 were subjected to peeling-strength test carried out
by an automatic tensile tester. More specifically, some
of the composite members of Examples 4-1 to 4-5 were
tested without being further treated; some other of the
composite members were tested after immersed in hot
water of 95~C for five days, or water-deteriorated; and
2i~2~9~
-- 40 --
the remaining composite members were tested after heated
in a test tube of 100~C for three days, or heat-
deteriorated. Thereafter, the rubber sheet was peeled
from each cord-rubber composite member at 20 ~C at the
5 rate of 50 mm/min. The cord-rubber composite members of
Comparative Examples 4-1 to 4-5 were tested in the same
way. The results of the peeling-strength test were as
is shown in Table 3.
As can be understood from Table 3, the cord-rubber
composite members of Comparative Example 4-1 to 4-5
(having cords made of steel wires drawn while passed
through a bath of the lubricant containing no FDE)
exhibited peeling strength increasing with the sulfur
content of the rubber compound. As is evident from
15 Table 3, too, Comparative Example 4-2 whose rubber
member contained 2 parts by weight of sulfur and which
was either water-deteriorated or heat-deteriorated
exhibited maximum peeling strength, and Comparative
Examples 4-3, 4-4, and 4-5 whose rubber members con-
20 tained more sulfur exhibited less peeling strength.Hence, with regard to those cord-rubber composite
members of Comparative Examples 4-1 to 4-5, no rubber
composite having optimal composition exists which
exhibits a sufficient peeling strength, both immediately
25 after they had been made and after they had been water-
or heat-deteriorated.
As can be understood from Table 3, also the
2~228~
- 41 -
cord-rubber composite members of Examples 4-1 to 4-5
(having cords made of steel wires drawn while passed
through a bath of the lubricant containing FDE) exhi-
bited peeling strength increasing with the sulfur con-
tent of the rubber compound, as those of Comparative
Examples 4-1 to 4-5. In particular, the composite
members, whose rubber members had a low sulfur content
and without being further treated, exhibited great
peeling strength; the composite members, whose rubber
members had a high sulfur content and which were either
water-deteriorated or heat-deteriorated, exhibited
great peeling strength. Hence, Examples 4-1 to 4-5
includes cord-rubber composite members which had a
sufficient peeling strength, both immediately after
they had been made and after they had been water- or
heat-deteriorated.
Table 3
Triazine-Thiol Sulfur Content Peeling Strength (kN/m)
Derivative in Rubber Compound
(phr) Initial WDed* HDed*
Comparative
Example 4-1 - 1.0 1.0 0 3.5
Comparative
Example 4-2 - 2.0 7.0 4.3 6.5
Comparative
Example 4-3 - 3.5 9.8 3.0 5.7
Comparative
Example 4-4 - 5.0 10.0 2.1 3.3
Comparative
Example 4-5 - 8.0 10.5 1.0 2.4
Example 4-1 FDE 1.0 4.8 4.3 5.5
Example 4-2 FDE 2.0 8.9 6.6 7.6
Example 4-3 FDE 3.5 10.5 6.5 6.3
Example 4-4 FDE 5.0 10.6 4.6 4.3
Example 4-5 FDE 8.0 10.4 4.2 3.2
* Note: "WDed" stands for "water-deteriorated,~' and "HDed" stands
for "heat-deteriorated."
G~
~C22g90
- 43 -
Example 5
One emulsion-type lubricant was prepared which
comprised the following components:
(1) 3 parts by weight of ethylenediamine phosphate
(2) 8 parts by weight of triethanolamine oleate
(3) 4 parts by weight of laurylamine octaethy
leneglycol
(4) 3 parts by weight of octadecane
(5) 2 parts by weight of butanediol linolenate
tetraethylene glycol
(6) 5 parts by weight of butanediol
dodecylphosphate pentapropylene glycol
(7) 0.5 parts by weight of methyl p-hydroxy-
benzoate
(8) 1 part by weight of methylbenzotriazole
(9) 72.5 parts by weight of water
(10) 1 part by weight of 6-dibutylamino-1,3,5-
triazine-2,4-dithiol monoethylenediamine
(DBME)
Further, for purpose of comparison, an emulsion-
type lubricant was prepared, which was identical to the
emulsion-type lubricant whose composition is specified
above, except that it contained no triazine-thiol
derivatives.
The two lubricants, thus prepared, were diluted
seven times. Each diluted lubricants was filled in the
lubricant tank of wire-drawing apparatus illustrated in
2~22~
Fig. 1. A bronze-plated wire (a plated amount: 4.1 g
per 1 kg of steel, Cu content in the plate: 65%) having
the diameter of 1.25 mm were drawn in the bath of each
lubricant, at rate of 800 m/min. Thereby two bronze-
plated wires were obtained, both having a diameter of0.25 mm. Five wires were twisted together, thus forming
a steel cord. As a result of this, two types of steel
cords were obtained, which will be referred to as
~Example 5-1" and "Comparative Example 5-1."
Both Example 5-1 and Comparative Example 5-1 were
left to stand for three days in an atmosphere at tem-
perature of 70~C and humidity of 90%. Then, Example
5-1 and Comparative Example 5-1 were subjected to
rapture test conducted in the Staircase method by means
of a Hunter's fatigue-testing machine, thereby deter-
mining the rapture strengths of the steel wire cords.
Example 5-1 had rapture strength of 107 kgf/mm2,
whereas Comparative Example 5-1 had rapture strength
of 81 kgf/mm2. This reveals that Example 5-1, i.e.,
the cord made of wires surface-treated with a lubricant
containing a triazine-thiol derivative, was more
corrosion-resistant than Comparative Example 5-1, i.e.,
the cord made of wires surface-treated with a lubricant
containing no triazine-thiol derivatives.
In the meantime, an NR-BR compound was prepared,
the composition of which was as follows:
(a) 70 parts by weight of natural rubber (NR)
2~2~890
(b) 30 parts by weight of butadiene rubber (BR)
c) 50 parts by weight of carbon black (HAF)
(d) 5 parts by weight of process oil
(e) 5 parts by weight of sulfur
(f) 0.8 parts by weight of N-cyclohexyl-2-
benzothiazylsulfenamide (CBS)
(g) 10 parts by weight of zinc oxide
(h) 1 part by weight of N-(1,3-dimethylbuthyl)-
N'-phenyl-p-phenylenediamine
The cord of Example 5-1 was embedded in a mass of
the NR-BR compound, followed by being set into a mold and
vulcanized at 70~C for 30 minutes. The vulcanized mass
was removed from the mold, thereby forming cord-rubber
composite member, having a diameter of 3 mm and a length
of 1 m, which will be referred to as "Example 5-2."
Similarly, the cord of Comparative Example 5-1 was
embedded in a mass of the NR-BR compound, followed
by being set into a mold and vulcanized at 70~C for
30 minutes. The vulcanized mass was removed from the
mold, thereby obtaining cord-rubber composite member,
also having a diameter of 3 mm and a length of 1 m,
which will be referred to as "Comparative Example 5-2."
The composite members of Example 5-2 and Comparative
Example 5-2 were left to stand for three days in an
atmosphere at temperature of 70~C and humidity of 90%.
Then, Example 5-2 and Comparative Example 5-2 were
subjected to rapture test conducted in the Staircase
~228~0
- 46 -
method by means of the Hunter's fatigue-testing machine,
thereby determining the rapture strengths of Example 5-2
and Comparative Example 5-2. Example 5-2 had rapture
strength of 103 kgf/mm2, whereas Comparative Example 5-2
had rapture strength of 68 kgf/mm2.
Generally, the cords in cord-rubber composite
members are said to have their fatigue strength much
reduced when the composite members are left to stand at
high temperatures and high humidities. This may be
proved true by the rapture strength of Comparative
Example 5-2. By contrast, Example 5-2 can remain strong
even if left to stand at high temperatures and high
humidities.
Examples 6 to 9
Fig. 7 illustrates an electrodeposition apparatus
used to manufacture Examples 6 to 9 of the present
invention. As Fig. 7 shows, the electrodeposition
apparatus comprises a wire supply device 31, a degreas-
ing device 32, an electrodeposition tank 33, a washing
device 34, a drying device 35, and a wire takeup device
36 -- all arranged sequentially. Three hollow cylindri-
cal electrodes 37 made of platinum or carbon are located
in the electrodeposition tank 33.
A wire-feeding roller 40a is located between the
wire supply device 31 and the degreasing device 32, a
wire-feeding roller 40b is provided between the device
32 and the tank 33, a wire-feeding roller 40b is
2~2289~
- 47 -
arranged between the tank 33 and the washing device 34,
a wire-feeding roller 40a is located between the washing
device 34 and the drying device 35. Of these wire-
feeding rollers, the two rollers 4Ob positioned at the
upstream and downstream of the tank 33, respectively,
are made of electrically conductive material. A steel
wire 22 is supplied from the wire supply device 31, fed
through the degreasing device 32, the electrodeposition
tank 33, the washing device 34, and the drying device
34, and taken up by the wire takeup device 36, being
guided by the rollers 40a and 40b all the way.
The electrodeposition tank 33 is filled with solu-
tion 38 of a triazine-thiol derivative. The solution 38
is stirred by a stirrer 39 while the electrodeposition
apparatus is processing the wire 22. A power supply 41
and a current-voltage controller 42 are located out side
the electrodeposition tank 33. The power supply 41 is
connected to the rollers, and the controller 42 is con-
nected to the power supply 41 and also to the electrodes
37, such that the hollow cylindrical electrodes 37
are positively charged and the rollers 40b and the steel
wire 22 are negatively charged.
Both the wire supply device 31 and the wire takeup
device 36 housed within electrically insulative boxes,
which are connected to the ground while the electrode-
position apparatus is processing the wire 22. The wire
supply device 31 may be replaced by a supply stand of
2~2289~
- 48 -
the type commonly used in the art. The rollers 40a are
coupled with electric motors (not shown)r and can feed
the steel wire 22 at the rate of 0.01 to 50 m/min. The
rollers 40a and 40b are designed to rotate smoothly
enough not to damage the surface of the wire 22. The
rollers 40a are made of soft material such as rubber,
whereas the rollers 40b are made of electrically
conductive material such as metal or conductive rubber.
The electrodeposition tank 33 can be made of any
material that is resistant to corrosion. Preferably, it
is made of plastics or a metal plate having a plastics
lining if the solution is an aqueous one, or made of a
corrosion-resistant metal such as stainless steel if the
solution is an organic one. It is desirable that the
tank 33 be shaped like a bathtub. The size of the tank
33 is determined by the desired capacity of the electro-
deposition apparatus.
The length and inside diameter of each hollow
cylindrical electrode 37 are determined in accordance
with the desired capacity of the electrodeposition
apparatus. Generally, the smaller the inside diameter,
the better, provided the length of each electrode 37
remain unchanged. This is because the current density
and, hence, the electrodeposition speed is inversely
proportional to the inside diameter of the hollow cylin-
drical electrode 37. However, if the inside diameter
of the electrode is too small, there will be two
- 49 -
undesired possibilities. First, the steel wire 22 may
touch the inner periphery of the electrode 37, causing
a short-circuit. Secondly, the triazine-thiol deriva-
tive may fail to diffuse sufficiently over the surface
of the wire 22, inevitably reducing the efficiency of
electrodeposition. In order to prevent these possi-
bilities, through holes can be made in each hollow
cylindrical electrode 37, or longitudinal grooves
may be formed in the surface of each electrode 37.
Further, each hollow cylindrical electrode 37 can be
replaced by a plurality of shorter hollow cylindrical
electrodes coaxially spaced apart from one another.
The sizes of the electrodeposition tank 33 and the
hollow cylindrical electrodes 37 will be specified, with
reference to Fig. 8. The electrodeposition tank 33 is
shaped like a bathtub, having a trapezoidal cross
section. The tank 33 is 130 cm long at the bottom,
240 cm long at the top, and 80 cm deep. The electrode
37 which extends along the bottom of the tank 33 has a
length of 100 cm and an inside diameter of 5 cm. The
remaining two electrodes 37, which extend along the
front and rear inclined inner surfaces of the tank 33,
respectively, have a length of 50 cm and an inside
diameter of 5 cm.
The power supply 41 can be a battery or a rectifier
connected to an AC power supply, which can output 0.1 mV
to 20 v. The current-voltage controller 42 may be
2~2~9~
- 50 -
a constant current generator, a constant voltage genera-
tor, or a pulse generator. A constant current generator
such as a galvanostat is used if the electrodeposition
is performed at a constant current, and a constant
voltage generator such as a potentiostat will be used
if the electrodeposition is carried out at a constant
voltage. It is advisable to use a pulse generator in
combination with a galvanostat or a potentiostat in
order to form a uniform coating of triazine-thiol
derivative.
The degreasing device 32 located at the inlet of
the electrodeposition tank 33 is designed to remove oil
from the surface of the steel wire 22. More precisely,
the device 33 sprays first trichloroethylene and then
alcohol onto the steel wire 22, thereby removing oil
from the wire 22 at high speed. In addition, the
degreasing device 32 may perform a high-frequency
washing on the wire 22, thereby to enhance the de-
greasing efficiency. After the wire 22 is made clear
of oil, a uniform coating of triazine-thiol derivative
can be formed on the surface of the steel wire 22.
The washing device 34 located at the outlet of the
electrodeposition tank 33 is designed to wash the steel
wire 22 which has been treated in the electrodeposition
tank 33. To be more precise, it applies hot water under
high pressure onto the steel wire 22 and then sprays a
solution capable of substituting for water, such as
2~2289~
- 51 -
methanol or acetone, onto the wire 22, so that the wire
22 may be more readily dried. The drying device 35 jets
hot air, hot nitrogen gas, or hot argon gas onto the
steel wire 22, thus drying the same.
Example 6
A steel cord, hereinafter referred to as "cord A,"
was made which comprised a core consisting of three
bronze-plated steel wires twisted together and having
a diameter of 0.2 mm and a sheath consisting of six
bronze-plated steel wires twisted around the core and
having a diameter of 0.38 mm. The bronze layer plated
on the wires consisted of 64.6% of copper and 35.4% of
zinc, and had a thickness of 2300 ~.
A 1% aqueous solution of 1,3,5-triazine-2,4,6-
trithiol monosodium (FN) was filled in the tank 33 of
the electrodeposition apparatus shown in Fig. 7 and was
maintained at 20~C. Four cords A, specified above, were
supplied into the tank 33 and fed through the solution
at the rates of 1 m/min, 3 m/min, 10 m/min, and
20 m/min, and thus surface-treated for 1 minute,
0.33 minutes, 0.1 minute, and 0.05 minutes, respec-
tively, while a voltage of 0.3 V was being applied on
the first three cords A, and a voltage of 1 V was being
applied to the last cord A. Also, a 1% aqueous solution
of 1,3,5-triazine-2,4,6-trithiol triethylamine (F.TEA)
was filled in the tank 33 and maintained at 20~C, and
two cords A were supplied into the tank 33 and fed at
~122~90
- 52 -
the rates of 3 m/min and 20 m/min, and thus surface-
treated for 0.33 minutes and 0.05 minutes, while being
applied with a voltage of 0.3 v and 1 v, respectively.
As a result of this, six surface-treated cords A,
hereinafter referred to as "Example 6-1" to "Example
6-6," were obtained.
For comparison purpose, a 1% aqueous solution of
FN was filled in the tank 33 of the electrodeposition
apparatus shown in Fig. 7 and was maintained at 20~C.
Two cords A, specified above, were immersed in the FN
solution for 3 minutes and 10 minutes, respectively.
Further, a 1% aqueous solution of F.TEA was filled in
the tank 33 and maintained at 20~C, and one cord A was
immersed in the F.TEA for 10 minutes. As a result,
three surface-treated cords A, hereinafter referred to
as "Comparative Example 6-1" to "Comparative Example
6-3," were obtained.
The Examples 6-1 to 6-6 and Comparative Examples
6-1 to 6-3 were cut into one-meter pieces. These pieces
of surface-treated cords were weighed by means of a
scale which can measure a minimum of 0.005 mg. Then,
the amount of the triazine-thiol derivative deposited
on each one-meter piece was calculated from the weight
of the one-meter piece. The results were as is shown in
Table 4.
As is clearly seen from Table 4, Examples 6-1 to
6-6 had triazine-thiol derivative coatings more than
~2~
those of Comparative Examples 6-1 to 6-3, despite that
the time for electrically depositing treatment is much
shorter than the time for immersing treatment. It
follows that the wire is far less likely to be corroded
while being subjected to the electrodeposition than
while being immersed in the solution.
Table 4
Triazine-Thiol Voltage Speed Time Amount of
Derivative Coatinq
(V) (m/min) (m/min) (mg/dm~)
Comparative
Example 6-1 FN Immersion 3 ~ 0.1
Comparative
Example 6-2 FN Immersion 10 0.2
Comparative
Example 6-3 F-TEA Immersion 10 0.1
Example 6-1 FN 0.3 1 1 0.5
Example 6-2 FN 0.3 3 0.33 1.3
Example 6-3 FN 0.3 10 0.1 1.9
Example 6-4 FN 1 20 0.05 1.1
Example 6-5 F-TEA 0.3 3 0.33 0.9
Example 6-6 F-TEA 1 20 0.05 1.0
~a
2~2~
Example 7
In order to determine the strength of adhesion
between bronze-plated steel wires having a diameter of
0.38 mm, hereinafter referred to as "wires B," and a
rubber compound later specified, the following experi-
ment was conducted. As in Example 6, the bronze layer
plated on the steel wires consisted of 64.6% of copper
and 35.4% of zinc, and had a thickness of 2300 ~.
A 1% aqueous solution of 1,3,5-triazine-2,4,6-
trithiol monosodium (FN) was filled in the tank 33 of
the electrodeposition apparatus shown in Fig. 7 and was
maintained at 20~C. Three wires B, specified above,
were supplied into the tank 33 and fed through the solu-
tion at the rates of 1 m/min, 2 m/min, and 10 m/min,
and thus surface-treated for 1 minute, 0.5 minutes, and
0.1 minute, respectively, while voltages 0.2 V, 0.5 V,
and 10 V were being applied on the three cords A,
respectively. As a result, three surface-treated
steel wires B were obtained which will be referred to
as "Example 7-1," "Example 7-2," and "Example 7-3.~
Also, a 1% aqueous solution of 6-anilino-1,3,5-
triazine-2,4-dithiol monosodium (AN) was filled in the
tank 33 and maintained at 20~C, and one steel wire B
was supplied into the tank 33 and fed at the rates of
3 m/min, and thus surface-treated for 0.33 minutes,
while being applied with a voltage of 5 V, whereby a
surface-treated steel wire s was obtained which will be
~ O ~ Q
- 56 --
referred to as "Example 7-4." Further, a 1% aqueous
solution of 6-dibutylamino-1,3,5-triazine-2,4-dithiol
monosodium (DBN) was filled in the tank 33 and main-
tained at 20~C, and one steel wire B was supplied into
5 the tank 33 and fed at the rates of 3 m/min, and thus
surface-treated for 0.33 minutes, while being applied
with a voltage of 5 v, whereby a surface-treated steel
wire B was obtained which will be referred to as
"Example 7-5." Still further a 1% aqueous solution of
6-diallylamino-1,3,5-triazine-2,4-dithiol monosodium
(DAN) was filled in the tank 33 and maintained at 20~C,
and one steel wire B was supplied into the tank 33 and
fed at the rates of 3 m/min, and thus surface-treated
for 0.33 minutes, while being applied with a voltage of
15 5 v, whereby a surface-treated steel wire B was obtained
which will be referred to as "Example 7-6."
For comparison purpose, a wire B not surface-
treated at all was used as Comparative Example 7-1.
Further, for the same purpose, a 1% aqueous solution of
20 FN was filled in the tank 33 of the electrodeposition
apparatus shown in Fig. 7 and was maintained at 20~C,
and a wire B was immersed in the FN solution for
0.5 minutes, thus obtaining a surface-treated wire,
which will be referred to as "Comparative Example 7-2."
25 Moreover, a 1% aqueous solution of FN was filled in
the tank 33 and maintained at 70~C, and a wire B was
immersed in the T.TEA for 30 minutes, thereby obtaining
~ ~ ''7J ~
- 57 -
a surface-treated steel wire, which will be referred to
as "Comparative Example 7-3."
The Examples 7-1 to 7-6 and Comparative Examples
7-1 to 7-3, i.e., nine types of surface-treated steel
wires, were cut into pieces 10 cm long. Both end por-
tions of each wire piece, thus obtained, were coated
with epoxy resin which sets at room temperature. All
pieces of surface-treated wires were left to stand for
three days in an atmosphere at temperature of 70~C and
humidity of 90%.
Meanwhile, an NR-BR compound was prepared, the
composition of which was as follows:
(a) 100 parts by weight of natural rubber (NR)
(b) 50 parts by weight of carbon black (HAF)
(c) 5 parts by weight of sulfur
(d) 0.8 parts by weight of N-cyclohexyl-2-
benzothiazylsulfenamide (CBS)
(e) 10 parts by weight of zinc oxide
Each steel wire piece, which had been left to
stand at temperature of 70~C and humidity of 90% for
three days, was embedded its one end in mass of the
NR compound specified above. Each NR compound mass
containing each piece of wire was heated at 140~C for
30 minutes, thus producing wire-rubber composite
member. Each wire-rubber composite member was subjected
to pull-out test carried out by an automatic tensile
tester (i.e., Autograph p-100 manufactured by K.K.
2 ~
- 58 -
Shimazu). More precisely, the wire was pulled out
of the rubber member at the rate of 50 mm/min, thus
measuring the pull-out strength of each composite
member. From this pull-out strength, the wire-rubber
adhesive property of each composite member was evalu-
ated. Further, the ratio of the cord surface area
covered with the rubber was measured. The results were
as is shown in Table 5.
Comparative Example 7-1, i.e., a bronze-plated
steel wire not surface-treated, rusted and did not
adhered firmly to the NB compound. Hence, its pull-out
strength was 5 kg/cm only as is shown in Table 5.
The coating of Comparative Example 7-2 was not
sufficiently much since this steel wire had been made by
immersing a wire in the FN solution for a short time,
i.e., 0.5 minutes only. Thus, Comparative Example 7-2
was not adequately resistant to corrosion, and its
drawing strength was no more than 10 kg/cm. Although
Comparative Example 7-3 had a thick coating since it had
been made by immersing a wire for a relatively long
time, i.e., 30 minutes, it did not firmly adhere to the
NR compound. This is because the steel wire rusted
whilst the coating was formed on the steel wire. It may
be economical and not harmful to immerse a steel wire in
an aqueous solution of a triazine-thiol derivative, but
this process does seem improper as a method of forming a
protective coating on a steel wire. This is why steel
~o~9o
- 59 -
wires are generally immersed in an organic solution of
a triazine-thiol derivative in order to produce steel
wires which are sufficiently resistant to corrosion.
By contrast, as is evident from Table 5, Examples
7-1 to 7-6 adhered firmly to the NR compound. This is
because they were made by subjecting steel wires to
electrodeposition for a relatively short time, while
feeding the wires through an aqueous solution of a
triazine-thiol derivative, thus forming dense,
corrosion-resistant coatings on the steel wires.
Table 5
Pull-out
Triazine-Thiol Temp. Voltage Speed Time Strength RCA*
Derivative (~C) (V) (m/min) (min)(kg/cm) (%)
Comparative
Example 7-1 - - - - - 5 0
Comparative
Example 7-2 FN 20 Immersion 0.5 10 20
Comparative
Example 7-3 FN 70 Immersion 30 10 20
Example 7-1 FN 20 0.2 1 1 30 45
Example 7-2 FN 20 0.5 2 0.5 40 85 o
Example 7-3 FN 20 10 10 0.1 30 60
Example 7-4 AN 20 5 3 0.33 45 75
Example 7-5 DBN 20 5 3 0.33 35 60
Example 7-6 DAN 20 5 3 0.33 65 100
* Note: "RCA" stands for "rubber-covered area."
~Q
C~
2Q22~0
- 61 -
Example 8
The following experiment was conducted in order to
determine how the sulfur content of a rubber composition
influences the adhesion strength between cord A speci-
fied above and the rubber compound.
A 1% aqueous solution of 1,3,5-triazine-2,4-
trithiol monosodium (FN) was filled in the tank 33 of
the electrodeposition apparatus shown in Fig. 7 and was
maintained at 20~C. Cords A were supplied into the
tank 33 and fed at the rate of 3 m/min, while applying a
constant voltage of 0.3 V on the cords A by means of
a potentiostat. Also, other cords A were supplied into
the tank 33 and fed at the rate of 3 m/min, while
supplying a constant current of 10 mA by means of a
galvanostat. As a result, surface-treated cords of two
types were prepared.
For comparison, cords A not surface-treated at
all were prepared, and cords A were immersed in the 1%
aqueous solution of FN.
In the meantime, five NR compounds were prepared
which were identical in composition, except for the con-
tent of sulfur. They comprised 100 parts by weight
of natural rubber (NR), 50 parts by weight of carbon
black (HAF), 10 parts by weight of zinc oxide, 0.8 parts
by weight of CBS (i.e.~ vulcanization accelerator), and
sulfur used in an amount ranging from 1 to 10 parts by
weight as is shown in Table 7. More precisely, the
2~22~90
- 62 -
first NR compound was prepared by mixing natural rubber,
carbon black, and zinc oxide were mixed by a Banbury
mixer, thus forming a mixture, and then adding 0.8 parts
by weight of CBS and 1 part by weight of sulfur were
added to this mixture. The other four NR compounds were
obtained in the same way, except that sulfur was added
to the mixture in different amounts, i.e., 2 parts by
weight, 3.5 parts by weight, 5 parts by weight, and
10 parts by weight, respectively, as is specified in
Table 6. Each of the five NR compounds, thus prepared,
was processed into sheets having a size of 10 cm x
1.5 cm x 1.5 mm. Hence, sheets having the same size,
but made of five different NR compounds, were obtained.
The cords A not surface-treated at all were cut
into pieces 10 cm long. Every 12 cord pieces were
sandwiched between two sheets made of each of the five
different NR compounds, thus forming a cord-rubber
composite unit. Both end portions of this composite
unit, either 2 cm long, were wrapped with aluminum
foil, and the composite unit was pressed at 140~C for
30 minutes, with a pressure of 10 kg/cm2, thereby
obtaining a cord-rubber composite member. As a result
of this, five cord-rubber composite members were made,
which will be referred to as "Comparative Example 8-1"
to "Comparative Example 8-5."
The cords A, which had been immersed in the 1%
aqueous solution of FN, were cut into pieces 10 cm long.
2~3~0
- 63 -
Every 12 cord pieces were sandwiched between two sheets
made of each of the five different NR compounds, thus
forming a cord-rubber composite unit. The composite
unit was processed and pressed in the same way as in
producing Comparative Examples 8-1 to 8-5, thereby
obtaining a cord-rubber composite member. As a result
of this, five cord-rubber composite members were made,
which will be referred to as "Comparative Example 8-6"
to "Comparative Example 8-10."
Also, the cords A, which had been been subjected
to electrodeposition, while applied with the constant
voltage, were cut into pieces 10 cm long. Every 12 cord
pieces were sandwiched between two sheets made of each
of the five different NR compounds, thus forming a cord-
rubber composite unit. The composite unit was processed
and pressed in the same way as in producing Comparative
Examples 8-1 to 8-5, thereby obtaining a cord-rubber
composite member. As a result of this, five cord-rubber
composite members were made, which will be referred to
as " Example 8-1" to "Example 8-1."
Further, the cords A, which had been been subjected
to electrodeposition, while applied with the constant
current, were cut into pieces 10 cm long. Every 12 cord
pieces were sandwiched between two sheets made of each
of the five different NR compounds, thus forming a cord-
rubber composite unit. The composite unit was processed
and pressed in the same way as in producing Comparative
2~22~0
- 64 -
Examples 8-l to 8-5, thereby obtaining a cord-rubber
composite member. As a result of this, five cord-rubber
composite members were made, which will be referred to
as " Example 8-6" to " Example 8-10."
A notch having a width of l cm was cut in each of
Examples 8-l to 8-lO and Comparative Examples 8-l to
8-lO. Then, these twenty cord-rubber composite members
were subjected to peeling-strength test carried out by
an automatic tensile tester. More specifically, one
rubber sheet was peeled from each cord-rubber composite
member at the rate of 50 mm/min, and the peeling
strength of the composite member was measured. Further,
twenty cord-rubber composite members, which were iden-
tical to Examples 8-l to 8-10 and Comparative Examples
8-l to 8-lO, were prepared and then kept immersed in
hot water at 95~C for three days, and further left to
stand in air at 20~C for one day. Peeling-strength
test was performed on the cord-rubber composite members,
thus water-deteriorated, in the same way, and peeling
strength of these composite members were measured. The
results of the peeling-strength test were as is shown in
Table 6.
As can be understood from Table 6, the peeling
strength of each cord-rubber composite member is pro-
portional to the sulfur content of the NB compound
used, whether the cords had been surface-treated or
not. Of the comparative examples, which had been
~22~90
- 65 -
water-deteriorated, rubber sheets contained 2 parts
by weight of sulfur exhibited the greatest peeling
strengths. Of the examples, which had been water-
deteriorated, rubber sheets contained 3.5 parts by
weight of sulfur exhibited the greatest peeling
strengths. Also, as is evident from Table 6, of
Examples 8-1 to 8-10 which had cords subjected to
electrodeposition, those which had not been water-
deteriorated and whose rubber sheets contained a
relatively low sulfur content exhibited a relatively
great peeling strength, and those which had been water-
deteriorated exhibited a great peeling strength,
regardless of the sulfur content of their rubber sheets.
In particular, those of Examples 8-1 to 8-10, which had
been water-deteriorated and whose rubber sheets had a
high sulfur content, exhibited a peeling strength far
greater than those of Comparative Examples 8-1 to 8-10
which had been water-deteriorated and whose rubber
sheets had a high sulfur content.
The steel cords hitherto used for reinforcing
automobile tires are those prepared by immersing steel
cords in a surface-treating solution. These steel cords
are embedded in members of rubber compounds having high
sulfur contents, in order to increase the peeling
strength which the tires may exhibits right after
they have been manufactured, inevitably decreasing
the cord-rubber adhesion as the tires gradually
~22~90
- 66 -
water-deteriorated. By contrast, the steel cord
according to the invention, which has been subjected
to electrodeposition, is rarely influenced by the
sulfur content of the rubber member in which it is
embedded. Hence, the steel cord of the invention serves
to manufacture cord-rubber composite members which have
a sufficient peeling strength, either immediately after
they are made or after they are water-deteriorated. As
is evident also from Table 6, it is recommendable to
supply a constant current, rather than to apply a
constant voltage, to the steel cords, in order to
treating the surface of the steel cords.
Table 6
Peeling Strength
Triazine-Thiol Sulfur Content in (kN/cm)
Derivative Surface Treatment Rubber Compound
(phr)Initial WDed*
Comparative
Example 8-1 - Not Treated 1 0.8 0.7
Comparative
Example 8-2 - Not Treated 2 8.5 5.5
Comparative
Example 8-3 - Not Treated 3.5 9.8 2.1
Comparative
Example 8-4 - Not Treated 5 10.5 1.0
Comparative
Example 8-5 - Not Treated 10 11.0 0.5
Comparative
Example 8-6 FN Immersion 1 2.1 1.1
Comparative
Example 8-7 FN Immersion 2 9.5 6.5
Comparative
Example 8-8 FN Immersion 3.5 9.9 3.8
Comparative
Example 8-9 FN Immersion 5 10.1 3.3
Comparative
Example 8-10 FN Immersion 10 10.0 2.1
(Continued)
C~3
e~
o
Peeling Strength
Triazine-Thiol Sulfur Content in (kN/cm)
Derivative Surface Treatment Rubber Compound
(phr)Initial WDed*
Constant
Example 8-1 FN Voltage Applied 1 3.2 2.5
Constant
Example 8-2 FN Voltage Applied 2 9.9 6.5
Constant
Example 8-3 FN Voltage Applied 3.5 10.5 7.6
Constant
Example 8-4 FN Voltage Applied 5 10.5 5.5
Constant
Example 8-5 FN Voltage Applied 10 10.0 3.4
Constant
Example 8-6 FN Current Applied 1 4.5 3.5
Constant
Example 8-7 FN Current Applied 2 10.5 7.0
Constant
Example 8-8 FN Current Applied 3.5 10.4 7.8
Constant
Example 8-9 FN Current Applied 5 10.7 6.5
Constant
Example 8-10 FN Current Applied 10 10.4 3.8
* Note: "WDed" stands for "water-deteriorated."
o
2~22~
- 69 -
Example 9
The following experiment was conducted, thereby
to determine how the type of vulcanizer in a rubber
compound influences the adhesion strength between cord A
specified above and the rubber compound.
A 1% aqueous solution of 1,3,5-triazine-2,4-
trithiol monosodium (FN) was filled in the tank 33 of
the electrodeposition apparatus shown in Fig. 7 and was
maintained at 20~C. Cords A were supplied into the
tank 33 and fed at the rate of 3 m/min, while supplying
a constant current of 10 mA by means of a galvanostat.
Further, other cords A were supplied into the tank 33
and fed at the rate of 3 m/min, while applying a
constant voltage of 0.3 v on the cords A by means of
a potentiostat. As a result, surface-treated cords of
two types were prepared.
For comparison, cords A were immersed in the 1%
aqueous solution of FA.
Meanwhile, four rubber compounds were prepared.
The first rubber compound comprised 100 parts by weight
of butadiene rubber (BR), 0.5 parts by weight of tetra-
butylthiuramdifulfide (TT, vulcanization accelerator),
4 parts by weight of sulfur, 5 parts by weight of zinc
oxide, and 1 part by weight of isopropylphenylenediamine
(IPPD). The second rubber compound comprised 100 parts
by weight of styrene-butadiene rubber (SBR), 5 parts by
weight of a,a'-bis(t-butylperoxy)isopropylbenzene (PKD),
~2~
- 70 -
and 1 part by weight of IPPD. The third rubber compound
comprised 100 parts by weight of acrylonitrile-butadiene
rubber (NBR), 6 parts by weight of PDK, and 1 part by
weight of IPPD. The fourth rubber compound comprised
100 parts by weight of ethylene-propylene-diene-
methylene rubber (EPDM), 8 parts by weight of PKD, and
1 part by weight of IPPD.
Each of the four rubber compounds, thus prepared,
was processed into sheets having a size of 10 cm x
1.5 cm x 1.5 mm. Hence, sheets having the same size,
but made of four different rubber compounds, were
obtained.
The cords A, which had been immersed in the FA
solution, were cut into pieces 10 cm long. Every 12
cord pieces were sandwiched between two sheets made of
each of the four different rubber compounds, thus
forming a cord-rubber composite unit. The composite
unit was pressed, thereby obtaining a cord-rubber com-
posite member. As a result of this, four cord-rubber
composite members were made, which will be referred to
as "Comparative Example 9-1" to "Comparative Example
9-4."
Cords A, which had been subjected electrodeposition
while being supplied with a constant current, were cut
into pieces of 10 cm long. Twelve cord pieces were
sandwiched between two sheets made of the first rubber
compound, and other twelve cord pieces were sandwiched
2 Q ~
- 71 -
between two sheets of the second rubber compound. AS
a result, two cord-rubber units were made. These units
were hot-pressed, thereby obtaining two cord-rubber
composite members, which will be referred to as "Example
9-1" and "Example 9-2." Other cords A, which had been
subjected electrodeposition while being applied with a
constant voltage, were cut into pieces of 10 cm long.
Twelve cord pieces were sandwiched between two sheets
made of the third rubber compound, and other twelve
cord pieces were sandwiched between two sheets of the
fourth rubber compound. As a result, two cord-rubber
units were made. These units were hot-pressed, thereby
obtaining two cord-rubber composite members, which will
be referred to as "Example 9-3" and "Example 9-4."
A notch having a width of 1 cm was cut in each of
Examples 9-1 to 9-4 and Comparative Examples 9-1 to 9-4.
Then, these eight cord-rubber composite members were
subjected to peeling-strength test carried out by an
automatic tensile tester. More specifically, one rubber
sheet was peeled from each cord-rubber composite member
at the rate of 50 mm/min, and the peeling strength of
the composite member was measured. Further, eight cord-
rubber composite members, which were identical to
Examples 9-1 to 9-4 and Comparative Examples 9-1 to
9-4, were prepared and then kept immersed in hot water
at 95~C for three days, and further left to stand in
air at 20~C for one day. Peeling-strength test was
~2~ 0
performed on the cord-rubber composite members, thus
water-deteriorated, in the same way, and peeling
strength of these composite members were measured.
Further, other eight cord-rubber composite members,
which were identical to Examples 9-1 to 9-4 and
Comparative Examples 9-1 to 9-4, were prepared and
left to stand at 100~C in an oven for three days, thus
heat-deteriorated. Peeling-strength test was performed
on the cord-rubber composite members, thus heat-
deteriorated, in the same way, and peeling strengthof these composite members were measured. The results
of the peeling-strength test were as is shown in
Table 7.
Although not shown in Table 7, the steel cords,
which had not been surface-treated with a solution of
triazine-thiol derivative, did not adhered to the rubber
compound containing peroxide used as vulcanizer.
However, as is evident from Table 7, the cords of
Comparative Examples 9-1 to 9-4, which had been immersed
in a solution of triazine-thiol derivative, adhered well
to the rubber compounds. Further, the cords of Examples
9-1 to 9-4, which had been subjected to electrode-
position while passing through a bath of a solution of
triazine-thiol derivative, adhered very firmly to the
four rubber compounds which contained either sulfur
or peroxide used as vulcanizer. Examples 9-1 to 9-4
exhibited great peeling strength, immediately after
they had been made, water-deteriorated, and heat-
deteriorated.
Table 7
Rubber Compound Peeling Strength
Triazine- Surface (Parts by Weight) (kN/cm)
Thiol Treatment * *
Derivative Rubber PKD TT S ZnO IPPD Initial WDed HDed
Comparative Constant BR
Example 9-1 FN Voltage Applied 100 - 0.5 4 5 1 3.6 0 2.2
Comparative Constant SBR
Example 9-2 FN Voltage Applied 100 5 - - - 1 4.2 2.8 3.4
Comparative Constant NBR
Example 9-3 FN Voltage Applied 100 6 - - - 1 5.4 3.5 4.8
Comparative ConstantEPDM
Example 9-4 FN Voltage Applied 100 8 - - - 1 4.3 4.1 4.2
Constant BR
Example 9-1 FN Current Applied 100 - 0.5 4 5 1 6.8 5.6 5.5
Constant SBR
Example 9-2 FN Current Applied 100 5 - - - 1 7.5 6.9 6.6
Constant NBR
Example 9-3 FN Current Applied 100 6 - - - 1 8.2 7.7 7.6
Constant EPDM
Example 9-4 FN Current Applied 100 8 - - - 1 5.6 6.7 7.2
* Note: "WDed" stand for "water-deteriorated," and "HDed" stands for
"heat-deteriorated."