Note: Descriptions are shown in the official language in which they were submitted.
PH 35 3 9
NITROG~N COMPOUNDS
Ihis invention concerns novel nltrogen comp~unds and, more
particularly, novel quinoline derivatives which possess
pharmacologically useful properties in antagonising at least in part
one or more of the actions of the substances known as angiotensins,
and in particular of that known as angiotensin II (hereinafter
referred to as "AII"). The invention also concerns pharmaceutical
compositlons of the novel compounds for use in treating diseases or
medical conditions such as hypertension, congestive heart failure
and/or hyperaldosteronism in warm-blooded animals (including man), as
~ell as in other diseases or medical cond~tions in which the
renin-angiotensin-aldosterone system plays a significant causative
role. The invention also includes processes for the manufacture of
the novel compounds and their use in treating one cf the
afore-mentioned diseases or med~cal conditlons and for the production
of novel pharmaceuticals for use in such medlcal treatments.
The angiotensins are key mediators of the renin-angiotensin-
aldosterone system, which is involved in the control of homeostasis
and fluid~electrolyte balance in many warm-blooded animals, including
man. The angiotensin known as AII is produced by the action of
angiotensin converting enzyme (ACE) from angiotensin I, itself
produced by the action of the enzyme renin from the blood plasma
protein angiotensinogen. AII i9 a potent spasmogen especially in the
vasculature and is known to increase vascular resistance and blood
pressure. In addition, the angiotensins are known to stimulate the
release of aldosterone and hence result in vascular congestion and
hypertension via sodium and fluid retention mechanisms. aitherto
there have been a number of different approaches to pharmacological
intervention in the renin-angiotensin-aldosterone system for
therapeutic control of blood pressure and/or fluid/electrolyte
balance, including, for example, inhibiting the actions of renin or
ACE. Uowever, there remains a continuing need for an alternative
approach because of the side-effects and/or idiosyncratic reactions
associated with any particular therapeutic approach.
Certain substituted imidazoles and benzimidazoles described
in European Patent Application, publication no. 253310 A2 and U.S.
c~ ln~ 3 ~ r r~ 2 ~
t~ .9 r~ Y
Patent no. 4880804 respectively are known to inhibit the action of
angiotensin II, as too are certain substituted pyrroles, pyrazoles and
triazoles described in European Patent Application, publication no.
323841 A2. Also certain structurally related~quinoline derivatives
described in European Patent Application, publication no. 348155 A1
are known to be antagonists of leukotriene D4. In addltion, a
structurally related compound, methyl 2-[(3-methoxycarbonylquinolin-
4-yloxy)methyl]benzoate, is described in J. Chem. Soc., Pèrkin Trans.
1, 1972, 1803-8 but without indication of any useful pharmacological
properties.
We have now discovered that the compounds of the invention
~set out below) surprisingly antagonise one or more of the actions of
the substances known as angiotensins (and in particular of AII) and
thus minimise the physiological effects associated with their presence
ir warm-blooded animals (includin~ man) and this ls the basls of the
invention.
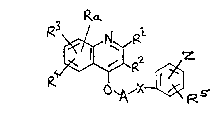
According to the invention there is provided a quinoline
derivative of the formula I ~set out hereinafter, together with the
other chamical formulae identified by Roman numerals) wherein R1 is
hydrogen, (1-8C)alkyl, (3-8C)cycloalkyl, phenyl or substituted
(1-4C)alkyl, the latter containing one or more fluoro substituents or
bearing a (3-8C)cycloalkyl, hydroxy, (1-4C~alkoxy or phenyl
substituent; R is hydrogen, (1-8C)alkyl, (3-8C)cycloalkyl,
(3-8C)cycloalkyl-(1-4C)alkyl, carboxy, (1-4C)alkoxycarbonyl, cyano,
nitro, phenyl or phenyl(l-4C)alkyl; R3 and R4 are independently
selected from hydrogen, (1-4C)alkyl, (1-4C)alkoxy, fluoro(1-4C)alkoxy,
halogeno, hydroxy, trifluoromethyl, cyano, nitro, amino,
(1-4C)alkanoylamino, alkylamino and dialkylamino of up to 6 carbon
atoms, dialkylamino-alkyl of 3 to 8 carbon atoms, (1-4C)alkanoyl,
carbamoyl, N-alkylcarbamoyl and di-(N-alkyl)carbamoyl of up to 7
carbon atoms, carboxy, (1-4C)alkoxycarbonyl, (1-6C)alkylthio,
(1-6C)alkylsulphinyl, (1-6C)alkylsulphonyl, and substituted
(1-4C)alkyl, the latter bearing an amino, hydroxy or (1~4C)alkoxy
substituent; or R3 and R4 together form (1-4C)alkylenedioxy attached
to adjacent carbon atoms of the benzene moiety of formula I; Ra and R5
are independently selected from hydrogen, (1-4C)alkyl, (1-4C)alkoxy,
halogeno, trifluoromethyl, cyano and nitro; A is methylene; ~ is
~ ~ 3 ~
phenylene optionally bearing a substituent selected from (1-4C)alkyl,
(1-4C)alkoxy, halogeno, trifluoromethyl, cyano and nitro, or X is a
direct bond between the adjacent phenyl group and moiéty A; Z is
1~-tetrazol-5-yl, -CO.N~.(lH-tetrazol-5-yl) or a group of the formula
-CO oR6 or -Co.N~.S02.R7 in which R6 is hyd~ogen or a non-toxic,
biodegradable residue of a physiologically acceptable alcohol or
phenol, and R7 is (1-6C)alkyl, (3-8C)cycloalkyl or phenyl; and
wherein any of said phenyl moieties may be unsubstituted or bear one
or two substituents independenely selected from ~1-4C)alkyl,
(1-4C)alkoxy, halogeno, cyano and trifluoromethyl; or a non-toxic salt
thereof; but excluding methyl 2-[(3-methoxycarbonylquinolin-4-yloxy)-
m~thyl]benzoate.
It will appreciated that, dependlng on the nature of the
substituents, certain of the formula I compounds may possess one or
more chiral centres and may be isolated in one or more racem~c or
optically active forms. It is to be understood that this invention
concerns any form of such a compound of formula I which possesses the
afore-mentioned useful pharmacological properties, it being well known
how to make optically active forms, for example by synthesis from
suitable chiral intermediates, and ho~ to determine their
pharmacological properties, for example by use of the standard tests
described hereinafter.
It is to be understood that generic ~erms such as "alkyl"
include both straight and branched chain variants ~hen the carbon
numbers permit. However, when a particular radical such as "propyl"
is given, it is specific to the straight chain variant, branched chain
variants such as "isopropyl" being specifically named where intended.
The same convencion applies to other radicals.
A particular value for R1 or R2 when it is alkyl is, for
example, methyl, ethyl, propyl, butyl, isobutyl, sec-butyl, pentyl or
hexyl; and when it is cycloalkyl is, for example, cyclopropyl,
cyclopentyl or cyclohexyl.
A particular value for R1 when it is alkyl bearing one or
more fluoro substitutents is, for example, fluoromethyl,
trifluoromethyl, 2,2,2-trifluoroethyl or pentafluoroethyl; and ~hen it
is alkyl bearing a hydroxy, cycloalkyl, tl-4C)alkoxy or phenyl
substituent is, for example, hydroxymethyl, 1-hydroxyethyl,
~ ~ 4 -
2-hydroxyethyl, cyclopropylmethyl, cyclopentylmethyl,
cyclohexylmeehyl, 2-methoxyethyl, Z-ethoxyethyl, benzyl, 1-phenylethyl
or 2-phenylethyl.
A part~cular value for R2 when it is cycloalkyl-alkyl is,
for example, cyclopropylmethyl, cyclopentylmethyl, cyclohexylmethyl or
2-cyclopentyl-ethyl; when it is phenylalkyl is, for example, benzyl,
l-phenylethyl or 2~phenylethyl; and when it is alkoxycarbonyl is, for
example, methoxycarbonyl, ethoxycarbonyl or propoxycarbonyl.
Appropriate values for R3, R4, R5 or Ra, or for an optional
substituent which may be present when X is phenylene, as defined
above, include by way of example:-
for alkyl: methyl and ethyl; for alkoxy: methoxy and ethoxy; for
fluoroalkoxy: trifluoromethoxy, 2-fluoroethoxy, 2,2,2-trifluoroethoxy
and 3,3,3-trifluoropropoxy; for halogeno: fluoro, chloro, bromo and
iodo; for alkanoylamino: formamido, acetamido and propanamido; for
alkylamino: methylamino, ethylamino and butylamino; for dialkylamino:
dimethylamino, diethylamino and dipropylamino; for dialkylamino-alkyl:
dimethylaminomethyl, 2-(dimethylamino)ethyl, 2-(diethylamino)ethyl and
3-(diethylamino)propyl; for alkanoyl: formyl, acetyl and butyryl; for
N-alkylcarbamoyl: N-methyl and N ethylcarbamoyl; for
di(N-alkyl)carbamoyl: N,N-dimethylcarbamoyl and N,N-diethylcarbamoyl;
for alkoxycarbonyl: methoxycarbonyl, ethoxycarbonyl and
propoxycarbon~^1; for alkylthio: methylthio, ethylthio and butylthio;
for alkylsulphinyl: methylsulphinyl, ethylsulphinyl and
butylsulphinyl; and for alkylsulphonyl: methylsulphonyl,
ethylsulphonyl and butylsulphonyl; for alkyl bearing an amino, hydroxy
or alkoxy substituent: hydroxymethyl, 1-hydroxyethyl, 2-hydroxyethyl,
aminomethyl, 2-aminoethyl, 2-methoxyethyl and 2-ethoxyethyl; and
alkylenedioxy: methylenedioxy and ethylenedioxy.
A particular value for R6 when it is a non-toxic,
biodegradable residue of a physiologically acceptable alcohol or
phenol is, for example, a residue derived from a (1-6C)alkanol such as
methanol or ethanol, or pheno-l, glycerol or the like.
A particular value for R7 when it is alkyl is, for example,
methyl, ethyl, propyl, isopropyl, butyl or pentyl; and when it is
cycloalkyl is, for example, cyclobutyl, cyclopentyl or cyclohexyl.
Particular values for optional substituents which may be
~ 3 3 ~ ~ 5 -
present on one or more phenyl moieties include, by way of example, for
halogeno: fluoro, chloro and bromo; for alkyl: methyl and ethyl; and
for alkoxy: methoxy and ethoxy.
A spe~ific value for X which is of particular interest is,
for example, ~-phenylene.
A preferred value for R6 or R5 is, for example, hydrogen and
for R1 is, for example, methyl, ethyl or propyl.
A preferred value for A is, for example, when it is
methylene.
A preferred group of compounds of the ~nvention comprise~
those compounds of the formula Ia (set out hereinafter) wherein R1,
R2, R3, R4 and R5 have any of cheir meanings as deflned above and z
is carboxy, lH-tetrazol-5-yl or benzenesulphonamido, the laeter
optionally containing one or two substituents independently selected
from halogeno (such às fluoro, chloro or bromo), (1-4C)alkyl (such as
methyl or ethyl), (1-4C)alkoxy (such as methoxy or ethoxy), cyano,
nitro and trifluoromethyl; together with the non-toxic salts thereof.
A preferred value for Z or zl is, for example, carboxy or
lH-tetrazol-5-yl, which latter is especially preferred and, in
psrtlcular, when it is attached ortho to the group X.
A particularly preferred combination of values in any of the
above definitions is wherein the quinoline moiety together ~ith the
attached substituents Rl, R2, R3 and R4, and Ra vhen present, has any
of the following values:- 2-methylquinoline, 2-ethylquinoline,
2-ethyl-6-methoxyquinoline, 6,7-dimethoxy-2-ethylquinoline) 2-ethyl-
5,6,7-trimethoxyquinoline, 2-ethyl-6-hydroxyquinoline, 2-ethyl-6-
methylthioquinoline, 2-ethyl-7-hydroxymethylquinoline, 2-ethyl-6-
(2-fluoroethoxy)quinoline, 2-ethyl-6-(2,2,2-trifluoroethoxy)-
quinoline, 2-ethyl-6-carboxamidoquinoline, 2-ethyl~6-fluoroquinoline,
2-ethyl-6-isopropoxyquinoline or 6-aminomethyl-2-ethylquinoline; and
in which the substituent O.A.X- is attached at the 4-position of the
quinoline ring.
Compounds of the invention which are of particular interest
include, for example, the specific embodiments set out hereinafter in
the accompanying Examples. Of these compounds, those described in
Examples 7, 25, 33, 36, 38 and 47 are particularly preferred and are
provided, together with their non-toxic salts, as a further feature of
3 ~ ~ ~ 6 -
the invention.
. Although all of the iormula I compounds can form salts with
suitable acids, it will be appreciated that those compounds of formula
I wherein Z is other than an ester group or in which R3 or R4 ls a
carboxy group ca~ form salts with bases as well as with acids
Particularly suitable non-toxic salts for such compounds therefore
also include, for example, salts ~ith bases affording physiologically
acceptable cations, for example, alkali metal (such as sodium and
potassium), alkaline earth metal (such as magnesium and calcium),
aluminium and ammonium salts, as well as salts with suitable organic
bases, such as with ethanolamine, methylamine, diethylamine or
triethylamine, as well as sal~s wieh acids forming physiologically
acceptable anions, such as salts with mineral acids, for example with
hydrogen halides (such as hydrogen chloride and hydrogen bromide3,
sulphuric and phosphoric acid, and with strong organic acids, for
example with p-toluenesulphonic and methanesulphonic acids.
The compounds of formula I may be obtained by standard
procedures of organic chemistry uell known ln the art for the
production of structurally analogous compounds. Such procedures are
provided as a further feature of the invention and include, by way oE
example, the following procedures in which the generic radicals have
any of the values given above, unless stated otherwise:
a) For those compounds in which Z is carboxy (that is in which
Z is a group of the formula -CO.ORfi in ~hlch RS is hydrogen), a
carboxylic acid derivative of the formula II, in which Q is a
protected carboxy group selected from (1-6C)alkoxycarbonyl (especially
methoxy-, ethoxy-, propoxy- or t-butoxy-carbonyl), phenoxycarbonyl,
benzyloxycarbonyl and carbamoyl, is converted to carboxy.
The conversion may be carried out, for example by
hydrolysis, conveniently in the presence of a suitable base such as an
alkali metal hydroxide, for example, lithium, sodium or potassium
hydroxide. The hydrolysis is generally carried out in the presence of
a suitable aqueous solvent or diluent, for example in an aqueous
(1-4C)alkanol, such as aqueous methanol or ethanol. ~owever, it may
also be performed in a mixture of an aqueous and non-aqueous solvent
such as water and toluene using a conventional quaternary ammonium
phase tranfer catalyst. The hydrolysis is generally performed at a
temperature in the range, for example, 0 - 120C, depending on the
reactivity of the group Q. In general, when a is carbamoyl,
temperatures in the range, for example, 40 - 120C are required to
effect the hydrolysis.
Alternatively, when Q ~s benzyloxycarbonyl, the conversion
may also be performed by hydrogenolysis, for example using hydrogen at
1-3 bar in the presence of a suitable catalyst, such as palladium on
charcoal or on calcium sulphate, in a suitable solvent or diluent such
as a (1-4C)alkanol (typically ethanol or 2-propanol) and at a
temperature ln the range, for example, 0 - 40C.
Further, when Q is t-butoxycarbonyl, the conversion may also
be carried out by hydrolysis at a temperature in the range, for
example, 0 - 100C, in the presence of a strong acid catalyst, such as
trifluoroacetic acid. The hydrolysis may either be performed in an
excess of the acid or in the presence of a suitable diluent such as
tetrahydrofuran, t-butyl methyl ether or 1,2-dimethoxyethane.
b) For those compounds of formula I wherein Z ls tetrazolyl, a
compound of the formula III in which L is a suitable protecting group;
such as trityl or benzhydryl, affixed to a nitrogen of the tetrazolyl
moiety, is deprotected.
The reaction conditions used to carry out the deprotection
necessarily depend on the nature of the group L. As an illustration,
when it is trityl or benzhydryl, the decomposition conditions include,
for example, acid catalysed hydrolys~s in a mineral acid (such as
aqueous hydrochloric acid), conveniently in an aqueous solvent (such
as aqueous dioxan or 2-propanol). Alternatively, a trityl or
benzhydryl group may be removed by hydrogenolysis, for example as
described in (a) above for conversion of a benzyloxycarbonyl to a
carboxy.
c) A quinolone of the formula IV wherein R1 is other than
hydrogen is alkylated with a compound of the formula V wherein ~al.
stands for a suitable leaving group such as chloro, bromo, iodo,
methanesulphonyloxy or ~-toluenesulphonyloxy.
The reaction is generally carried out in the presence of a
- 8 ~
suitable base, for example, an alkali metal alkoxide such as sodium
m~thoxide or sodium ethoxide or an alkali metal hydride such as sodium
hydride or an organic base such as diisopropylethylamine and in a
suitable solvent or diluent, for example, a (1-4C)alkanol such as
~~hanol or ethanol when an alkali metal alkoxide is used, or in a
polar solvent such as N,N-dimethylformamide and at a temperature in
the range, for example, ~0 - 100C. AlternatiYely, a quaternary
ammonium hydroxide may be used in a mlxture of an aqueous and
non-aqueous solvent such as water and dichloromethane. In carrying
10 out process (c), when in the starting material Z is an acidlc group,
about two molecular equivalents of a suitable base is generally
required, whereas when Z is a non-acidic group the presence of one
molecular equivalent of a suitable base is generally sufficient.
Procedure (c) is particularly suitable for the production of
those compounds of the formula I in which Z is a group of the formula
-CO.OR6 in which R6 is other than hydrogen, for example wherein R6 is
(1-6C)alkyl, benzyl or phenyl, which compounds are also starting
materials of formula II for the reaction described in (a) above.
Similarly, using an analogous procedure, but starting with the
appropriate halomethyl tetrazolyl derivative of the formula VI, the
starting materials of the formula III may be obtained for procedure
(b? '
The majority of the quinolones of formula IV are already
known and the remainder can be made by analogy therewith using
standard procedures of organic chemistry well known in the art, for
example as described in standard works of heterocyclic chemistry such
as that edited by Elderfield. The necessary compounds of the.formula
V (and also of formula VI) may be made by standard procedures such as
those which are illustrated in Scheme 1 for compounds in which X is
phenylene.
d~ A halogenoquinoline of the formula VII wherein yl is a
halogeno group (such as chloro, bromo or iodo) is reacted with an
alcohol of the formula VIII.
The reaction is generally carried out in the presence of a
suitable base, for example an alkali metal alkoxide such as sodium
- - 9 ~
methoxide or ethoxide or an alkali metal hydride such as sodium
hydride and in a suitable solvent or diluent, for example a
(1-4C)alkanol such as methanol or ethanol when an alkali metal
alkoxide is used, or a polar solvent such as N,N-dimethylformamide.
Alternatively, an alcohol of the formula VIII may be used in the form
of its preformed alkali metal salt (when Z is a non-acidic group) or
di-alkali metal salt (when Z is an acidic group). The reaction is
usually performed at a temperature in the range of 40 to 120C.
Alternatively, the reaction may in preference be carried out with a
formula VIII compound in the presence of an acid catalyst such as
~-toluenesulphonic acid, instead of under basic conditions, and in the
presence of an inert solvent or diluent such as toluene.
The haloquinolines of the formula VII may be obtained, Eor
example, by halogenation of th~ corresponding quinolones of formula
IV, for example, by reaction with phosphorus oxychloride in the
absence of a solvent, or in the presence of an inert solvent or
diluent such as toluene or dioxane, and at a temperature in the range
60 to 110C. The alcohols of the formula VIII are in general known or
can be prepared by standard procedures well known in the art.
Uhereafter, those compounds of formula I wherein Z is
l~-tetrazol-5-yl may be obtained by stepwise converslon of a compound
of the formula I wherein Z is a group of the formula -CO.OR6 into the
corresponding nitrile under standard conditions, followed by reaction
of the nltrile with an azlde such as an alkali metal azi~e, preferably
in the presence of an ammonium halide, and preferably in the presence
of a suitable polar solvent such as N,N-dimethylformamide and at a
temperature in the range, for exampie, 50 to 160C.
Uhereafter, those compounds of the formula I wherein Z is
-CO.NH.(lH-tetrazol-5-yl), a group of the formula -Co.N~.So2R7 or a
group of the formula -CO.OR6 in which R6 is other than hydrogen, may
be obtained, for example, by reacting a carboxylic acid of the formula
I in which Z is carboxy (or a reactive derivative of said acid) with
5-aminotetrazole, a sulphonamide of the formula N~2.So2R7 or a salt
thereof (for example, an alkali metal salt), or a hydroxy compound of
the formula ~o.R6 or with a salt thereof (for example, an alkali metal
thereof). Suitable reactive derivatives include, for example the
chloride, bromide, azide, anhydride and mixed anhydride with formic or
- lo ~ 2 ~ ~
acetic acid of the carboxylic acid of formula I as defined above.
When the free acid form is used, the reaction is generally carried out
in the presence of a suitable dehydrating agent such as
dicyclohexycarbodiimide or 3-(3-dimethylaminopropyl~-1-ethylcarbodi-
S imide in the presence of a base such as triethylam~e or pyridine.
Uhen a reactive derivative is used, either the reaction is carried out
in the presence of a base such as mentioned above, or, for the
preparation of a compound of the formula I wherein Z is a group of the
formula -Co.N~.So2R7 or a group of the formula -CO.OR6, the
sulphonamide or hydroxy compound is used in the form of a salt, such
as its alkali metal salt (in particular the lithium~ sodium or
potassium salt thereof). The reaction is generally performed in the
presence of a suitable diluent or solvent such as dioxan, t-butyl
methyl ether or tetrahydrofuran and at a temperature ln ehe range, for
example, 0 - 60C.
~ hereafter, when a non-toxic salt of a compound of formula I
is required, it may be obtained, for example, by reaction with the
appropriate base affording a physiologically acceptable cation, or
uith the appropriate acid affording a physiologically acceptable
anion, or by any other conventional salt formation procedure.
Further, when an optically active form of a compound of
formula I is required, one of the aforesaid processes may be carried
out using an optically active starting material. Alternatively, the
racemic form of a compound of formula I in which Z is an acidic group
may be resolved, for example by reaction with an optically active form
of a suitable organic base, for example, ephedrine, N,N,N-trimethyl-
(l-phenylethyl)ammonium hydroxide or 1-phenylethylamine, followed by
conventional separation of the diastereoisomeric mixture of salts thus
obtained, for example by fractional crystallisation from a suitable
solvent, for example a (1-4C)alkanol, ~hereafter the optically active
form of said compound of formula I may be liberated by treatment with
acid using a conventional procedure, for example usi~g an aqueous
mineral acid such as dilute hydrochloric acid.
Certain of the intermediates defined herein are novel, for
example the compounds of the formula II, III and IV, and are provided
as a further feature of the invention.
As stated above, ~he compounds of formula I will have
r~ 2 s~
bene~icial pharmacological effects in warm-blooded animals (including
man) in diseases and medical conditions ~here amelioration of the
vasoconstrictor and fluid retaining properties of the renin-
angiotensin-aldosterone system is desirable, at least in part by
S antagonism of one o~ more of th~ physiological actions of AII. The
compounds of the invention will thus be useful in the treatment of
diseases or medical conditions such as hypertension, congestive heart
failure and/or hyperaldosteronism in warm-blooded animals (including
man), as well as in other diseases or medical conditions in which the
renin-angiotensin-aldosterone system plays a significant causative
role.
The antagonism of one or more of the physiological act~ons
of AII and, in par~icular, the antagonism of the interaction of AII
~ith the receptors which mediate its effects on a target tissue, may
be assessed using one or more of the following, routine laboratory
procedures:
Test A: This ln vitro procedure involves the incubation of the
test compound initially at ~ concentration of 100 micromolar (or less)
in a buffered mixture containing fixed concentrations of radiol~belled
AII and a cell surface membrane fraction prepared from a suitable
angiotensin target tissue. In this test, the source of cell surface
membranes is the guinea pig adrenal gland which is well known to
respond to AII. Interaction of the radiolabelled AII with its
receptors (assessed as radiolabel bound to the particulate membrane
fraction following removal of unbound radiolabel by a rapid filtration
procedure such as is standard in such studies) is antagonized by
compounds which also bind to the membrane receptor sites and the
degree of antagonism (observed in the eest as displacement of
membrane-bound radioactivity) is determined readily by comparing the
receptor-bound radioactivity in the presence of the test compound at
the specified test concentration ~ith a control value determined in
the absence of the test compound. Using this procedure compounds
showing at least 50% displacement of radiolabelled AII binding at a
concentration of 10 4 ~ are retested at lower concentrations to
determine their potency. For determination of the IC50 (concentration
for 50% displacement of radiolabelled AII binding), concentrations of
the test compound are ordinarily chosen to allow testing over at least
- 12 - ~i& ~ ~s 2 ~ ~
four orders of magnitude centred about the predicted approximate ICSo,
which latter is subsequently determined from a plot of percentage
displacement against concentration of the test compo~nd.
In general, compounds of formula I as defined above wherein
Z is an acidic group show significant inhibitlon ln Test A at a
concentration of 50 micromolar or much less.
Test B: This in vitro test involves the measurement of the
antagonistic effects of the test compound against AII-induced
contractions of isolated rabbit aorta, maintained in a physiological
salt solution at 37DC. In order to cnsure that the effect of the
compound is specific to aneagon~sm of AII, the effect of the test
compound on noradrenaline-induced contractions may al~o be determined
in the same preparation.
In general, compounds of formula I as defined above wherein
Z is an acidic group show significant inhibition in Test B at a final
concentration of 50 micromolar or much less. ~Note: Compounds of
formula I wherein Z is a group of the formula -CO.OR6 in which R6 is
other t~an hydrogen in general show only weak activity in the in vitro
Te~t~ A o~ B.]
~0 Test C: This in vivo cest involves using terminally-anaesthetised or
conscious rats in which aA arterial catheter has been implanted under
anaesthesia for the measurement of changes in blood pressure. The AII
antagonistic effects of the test compound following oral or parenteral
administration, are assessed against angiotensin II-induced pressor
responses. To ensure that the effect is specific, the effect of the
test compound on vasopressin-induced pressor responses may also be
determined in the same preparation.
The compounds of formula I generally show specific
AII-antagonist properties in Test C at a dose of 50 mg/kg body weight
or much less, without any overt toxicological or other untoward
pharmacological effect.
Test D: This in vivo test involves the stimulation of endogenous AII
biosynthesis in a variety of species including rat, marmoset and dog
by introducing a diet of low sodium content and giving appropriate
daily doses of a saluretic known as frusemide. The test compound is
then administered orally or parenterally to the animal in which an
arterial catheter has been implanted under anaesthesia for the
- 13 - ~J ~ ~ 3 ~ ~ ~
measurement of changes in blood pressure.
In general compounds of formula I will show AII-antagonist
properties in Test D as demonstrated by a significan~-reduction in
blood pressure at a dose of S0 mg/kg body welght or much les~, ~lthout
any overt toxicological or other untoward pharmacological effect.
By way of illustration of the angiotensin II inhibitory
properties of compounds of formula I, the compound of example 7 gave
the following results in tests A, B and C described above:-
In test A: an average ~C50 of 1.7xlO 8M;
In test B an average PA2 of 8.95;
In test C: ED50 of 0.5 mg/kg (l.v. administration).
The compounds of formula I will generally be administered
for therapeutic or prophylactic purposes to warm-blooded animals
(including man) requiring such treatment in the form of a
pharmaceutical composition, as is well known in the pharmaceutical
art. According to a further feature of the invention there is
provided a pharmaceutical composition comprising a compound of formula
I, or a salt thereof as defined above, together with a
pharmaceutically acceptable diluent or carrier. Such compositions
will conveniently be in a form suitable for oral administration (e.g.
as a tablet, capsule, solution, suspension or emulsion) or parenteral
administration (e.g. as an injectable aqueous or oily solution, or
in~ectable emulsion).
The compounds of formula I may also be advantageously
administered for therapeutic or prophylactic purposes together with
another pharmacological agent kno~n in the general art to be of value
in treating one or more of the diseases or medical conditions referred
to hereinabove.
In general a compound of formula I (or a pharmaceutically
acceptable salt thereof as appropriate~ will generally be administered
to man so that, for example, a daily oral dose of up to 50 mg/kg body
ueight (and preferably of up to 10 mg/kg) or a daily parenteral dose
of up to 5 mg/kg body ~eight (and preferably of up to 1 mg/kg) is
received, given in divided doses as necessary, the precise amount of
compound (or salt) administered and the route and form of
administration depending on size, age and sex of the person being
treated and on the particular disease or medical condition being
- 14 - 2~
treated according to principles well known in the medical arts.
In addition to their aforesaid use in therapeut1c medicine
in humans, the compounds of formula I are also useful~in the
veterinary treatment of similar conditions affecting commercially
valuable uarm-blooded animals, such as dogs, cats, horses and cattle.
In general for such treatment, the compounds of the formula I will
generally be administered in an analogous amount and manner to those
described above for administration to humans. The compounds of
formula I are also of value as pharmacologlcal tools in the
development and standardisation of test systems for the evaluation of
the effects of AII in laboratory animals such as cats, dogs, rabbits,
monkeys, rats and mice, as part of the continuing search for new and
improYed therapeutic agents.
The invention will now be illustrated by the following non-
limiting Examples in which, unless otheruise stated:-
(i) concentrations and evaporations were carried out by rotary
evaporation in vacuo;
(ii) operations were carried out at room temperature, that is in
the range 18-26C;
(iii) flash column chromaeography was performed on Herck Kieselgel
60 (Art. no. 9385) obtained from E Merck, Darmstadt, Germany;
(iv) yields, where given, are intended for the assistance of the
reader only and are not necessarily the maximum attainable by diligent
process development;
(v) lH NMR spectra were normally determined at 200 M~z in CDC13
using tetramethylsilane (TMS) as an internal standard, and are
expressed as chemical shifts (delta values) in parts per million
relative to TMS using conventional abbreviations for designation of
major peaks: s, singlet; m, multiplet; t, triplet; br, broad;
d,doublet;
(vi) 13C NMR spectra were normally determined at 100 MHz in CDCl3
or d6-dimethylsulphoxide (d6-DMS0) usin~ the solvent s~gnal as
internal standard, and are expressed as chemical shifts ~delta values)
in parts per million relative to TMS;
(vii) all end-products had satisfactory microanalyses; and
(viii) the term "lH-tetrazol-5-yl" stands for
"1~-1,2,3,4-tetrazol-5-yl".
- 15 -
~xample 1
1.25M sodium hydroxide solution (2.4 ml) was added to a solution of
methyl 4'-[(2-ethylquinolin-4-yloxy)methyllbiphenyl-2- carbo~ylate (A)
(380 mg) in ethanol (5 ml). The solution was heated under reflux for
2 hours a~ then volatil~ material was removed by evaporation. The
residue was dissolved in water (30 ml) and the solution acid~fied to
p~ 4 with 2M hydrochloric acid. The precipitated solid was a
collected, dried under high vacuum and recrystallised from ethanol to
give 4'-l(2-ethylqu~nolin-4-yloxy)methyl]biphenyl-2-car~oxylic acid
10` (254 mg), as white crystals9 m.p. 204-205C;
NMR (d6-dimethylsulphoxide (d6~DMSO)): 1.34(t,3H), 2.9(q,2~),
5.42(s,2~), 7.1(s,1~), 7.38-7.78(complex m,10~), 7.88(d,1H),
8.15~dd,1~), 12.74(br,1~); mass spectrum (negative fast' atom
bombardment [-ve FAB], DMSOtglycerol (GLY): 382 (M-H) , 172;
microanalysis found: C,78.0; ~,5.4; N,4.05; C25~21N03 requires:
C,78.3; H,5.5; N,3.7X.
The starting material (A) was obtained as follows:-
(i) ACl.6M solution of butyllithium in hexane (24.0 ml) was
added dropwise to a stirred solution of 4-bromotoluene (6.0 g) in dry
tetrahydrofuran (T~F) (50 ml) at -78C under an atmosphere of argon.
The temperature was maintained at -78C for 20 minutes and then a lM
solution of anhydrous zinc chloride in ether (38.6 ml) was added. The
solution was kepe at -78C for 15 minutes, and then tetrakis
(triphenylphosphine)palladium (60 mg~ in T~F (5 ml) was added,
followed by methyl-2-iodobenzoate (6.1 g) in THF (10 ml). The
solution was allowed to reach ambient temperature over 1 hour, then
heated under reflux for 5 hours. The solvent was removed by
evaporation and the residue was dissolved in chloroform (150 ml). The
solution was washed with a solution of ethylene diaminetetracetic acid
(10 g) i~ water (100 ml) and the aqueous layer ~as re-extracted with
chloroform (100 ml). The combine'd organic extracts ~ere dried (MgS04)
and the solvent removed by evaporation. The residue was purified by
flash chromatography, eluting with ethyl acetate/hexane (1:9 vtv) to
give methyl 4'-methylbiphenyl-2-carboxylate (B) as a colourless oil
(4.4 g); NMR: 2.4(s,3~), 3.65(s,3H), 7.2(s,4~), 7.35(m,3~), 7.5(m,1~),
- 16 -
7.8(d,1~).
(ii) N-Bromosuccinimide (8.1 g) and azo(bisisobutyronitrile) (130
mg) were added to a solution of compound (B) (9.3 g) in
carbonte~rachLoride (300 ml). The mixture was heated under reflux for
4 hours and then cooled to ambient temperature. Insoluble materlal
was removed by filtration and the filtrate concentrated. The residue
was purified by flash chromatography, eluting with ethyl
acetate/hexane (1:9 vtv) to give methyl 4'-(bromomethyl)biphenyl-2-
carboxylate (C) as a solid (10.9 g), m.p. 48-50C; NMR: 3.65(s,3~,
4.55(s,2~), 7.25-7.60 complex (m,7H), 7.85(d,1H~.
(iii) Sodium hydride (60Z dispersion in mineral oil; 60 mg) was
added to a stirred solution of 2-ethyl-4-quinolone (260 mg) prepared
by the method described in ~ y~., 1955, Coll. Vol. III, p.374 and
pS93), in N,N-dimethylformamide (DMF)(2.5 ml). The mixture was
stirred until evolution of hydrogen had ceased and then a solution of
the bromomethyl compound (C) (460 mg) in DMF (1 ml) was added. The
reaction mixture was stirred for 16 hours. The solvent was removed by
eYaporation and the residue was partitioned between water (10 ml) and
ethyl acetate (2 x 5 ml). The organic phase was washed with water,
followed by saturated sodium chloride solution and dried (MgS04). The
solvent was ev-~porated and the residue purified by flash
chromatography, eluting with ethyl acetate/dichloromethane (1:4 v/v~
to give methyl 4'-l(2-ethylquinolin-4-yloxy)methyl]biphenyl-2-
carboxylate ~A) as a solid (385 mg), m.p. 132-134C; NMR: 1.41(t,3H),
2.97(q,2~), 3.68(s,3~), 5.34(s,2H), 6.77(s,1H), 7.44-7.7(complex
m,9H), 7.87(dd,1H~, 8.0(d,1H), 8.26(dd,1~); mass spectrum (positive
chemical ionisation l+ve CI~: 398 (M+~)+, 225, 174; 13C NMR: (benzylic
CH2) 69.73.
xamples 2-4
Using a similar procedure to that described in Example 1,
but starting from the appropriate ester of the formula II in which Q
is a methoxycarbonyl group the following compounds were obtained:-
(~xample Z): 4'-[(2-Methylquinolin-4-yloxy)metbyl]biphenyl-2-
carboxylic acid hydrochloride, m.p. 184-186C; NMR (d6-DMS0):
- 17 ~ 3 ~ ~ ~
2.93(s,3H), 5.67(s,2H), 7.38-7.72(m,8H), 7.77(dd,1H), 7.84(dd,1H~,
8.08(dt,1H), 8.29(d,1H), 8.33(d,1H); mass spectrum (-ve FAB,
DMS0/m-nitrobenzyl alcohol)(NBA): 368 (M-H) ; microanalysis found:
C,71.2; H,5.0, N,3.6; C1, 8.6Z, C24H1gN03.HCl requires:C,71.0; H,5.0;
N,3.5; C1,8.7%; starting from methyl 4~-[(2-methylquinolin-4-yloxy)-
methyllbiphenyl-2-carboxylate, obtained as a solid, m.p. 146 C; NMR:
2.71(s,3~), 3.67(s,3H), 5.33(s,2H), 6.74(s,1H), 7.3-7.6(complex m,8~),
7.68(dt,1H), 7.87(dd,1H), 7.98(d,1H), 8.23(dd,1~; ltself obtained
from 2-methyl-4-quinolone, using analogous procedures to those
described in Example 1;
(~xample 3): 4'-l(2-Propylquinolin-4-yloxy)methyl]biphenyl-Z-
carboxylic acid , m.p. 198-200C; NMR (d6-DMSO)o 0.97(t,3H~,
1.8(m,2H), 2.85(t,2~), 5.43(s,2H), 7.08(s,1~), 7.35-7.65(complex
m18H), 7.73(dt,2~),7.87(d?1~), 8.15(dd,1H), 12.7(br s,lH); mass
spectrum (-ve FAB, DMS0/GLY): 396 (M-H) , 186; microanalysls, found:
, ; C26H23N03Ø33C2H50H requires: C,77.8; H,6-1;
N,3,4X; starting from methyl 4'-[(2-propylquinolin-4-yloxy)methyl~-
biphenyl-2-carboxylate, obtained as a viscous oil; NMR: 1.03(t,3H),
1.87(m,2~, 2.94(t,2H), 3.68(s,3H), 5.36(s,2H), 6.75(s,1~), 7.34-
7.6(complex m,8H), 7.68(dt91H), 7.85(dd,21~), 8.03(d,1H), 8.25(dd,1~);
13C NMR (CDCl3): (benzylic CH2) 69.8; itself obtained from 2-propyl-4-
quinolone using analogous procedures to those described in Example 1;
[ Note: lM aqueous citric acid solution was used in place of 2H
hydrochloric acid in the work-up procedure~.
(~xample 4): 4'-[(2-Butylquinolin-4-yloxy)methyllbiphe~yl-2-carboxylic
acid, m.p. 148C; NMR (d6-DMS0): 0.93(t,3H), 1,38(m,2H), 1.8(m,2H),
2.95(t,2~), 5.5~s,2~), 7.25(s,1H), 7.33-7.68(complex m,8H), 7.7-
7.85(m,2H), 7.98(d,1H), 8.2(d,1~); mass spectrum (-ve FAB, DMS0/GLY):
410 (M-H) , 200; microanalysis found: C,77.1; H,6.1; N,3.1%;
Cz7H25NO3Ø5~20 requires: C,77.1; H,6.2; N,3.3%; starting from methyl
4'-l(2-butylquinolin-4-yloxy)methyl]biphenyl-2-carboxylate obtained as
viscous oil; NMR: 0.97(t,3H), 1.4S(m,2H), 1.78(m,2H), 2.94(t,2H),
3.68(s,3H), 5.35(s,2H), 6.76(s,1H), 7.35-7.6(complex m,8H),
7.69(dt,lH), 7.88(dd,1H), 8.0(d,1H), 8.25(dd,1H); mass spectrum (+ve
CI): 426 (M+H)+, 225, 202; itself obtained from 2-butyl-4-quinolone
using analogous procedures to those described in Example 1.
- 18 -
The starting materials, 2-methyl-4-quinolone and
2-propyl-4-quinolone, were obtained as described in ~. Syn., 1955
Coll. VoI. III, page 374 and page 593. 2-Butyl-4-quinolone ~as
obtained using an analogous procedure, starting from ethyl 3-oxo-
heptanoate and had the following NMR spectrum: 0.88(t,3H), l.~ (m,2H),
1.7(m,2H), 2.7(t,2H), 6.27(s,1H), 7.34(t,1H), 7.6(dt,1H)), 7.78(d,1H),
8.36(d,1~), 11.8(br s, lH).
Bxample 5
5M Aqueous sodium hydroxide solution (2 ml) was added to a
solution of methyl 4-E (2-propylquinolin-4-yloxy)methyllbenzoate (A)
(500 mg) in methanol (5 ml). the solution was allowed to stand for 16
hours. ~ater (50 ml) was added and the mixture heated to dissolve the
solid precipitate. The solution was filtered and the filtrate
acidified to pH4 with lM aqueous citric acid solution. The
precipitated solid was collected by filtration and dried under high
vacuum to give 4-1~2-propylquinolin-4-yloxy)methyl]benzoic acid (347
mg), as a white powder, m.p. 225-227C; NMR (d6-DMSO): 0.95(t,3H),
1.7-l.9(m,2H), 2.84(t,2H), 5.5(s,2H), 7.05(s,1H), 7.5(dt,1H),
7.7(m,3H), 7.88~d,1H), 8.0(djlH), 8.15(dd,1H), 12.9(br,1~); mass
spectrum (-ve FAA, DMS0/Gly~; 32) (M-~) , 186; microanalysis found:
C,73-9; H,5-9; N,4.2%; C2oHlgN03~0~25H20 requires; C,73.7; H,6.0;
N,4.3%.
The starting ester (A) was obtained from 2-propyl-
4-quinolone (561 mg) and methyl 4-(bromomethyl)benzoate (700 mg)
together with appropriate amount to the other necessary agents and
solvents, using a similar procedure to that described in Example 1,
part (iii) and purification by flash chromatography eluting with a
mixture of methanol and dichloromethane (1:9 v/v), as a solid (540
mg), m.p. 62-6SC; NMR: l.O(t,3H), 1.83(m,2H), 2.9(t,2H), 3.95(s,3H),
5.37(s,2H), 6.69(s,1H), 7.46(dt,1H), 7.58(d,2H), 7.67(dt,1H),
8.0(d,1H), 8.1(d,2H), 8.2(dd,1~).
Example 6
4-[(2-Propylquinolin-4-yloxy)methyl]benzoic acid (240 mg)
was added to a mixture of benzene sulphonamide (120 mg),
_ 19 -
4-dimethylaminopyridine (90 mg) and 1-[3-(dimethylamino)propyl3-
3-ethylcarbodiimide hydrochloride (150 mg) in dichloromethane (20 ml)
and the mixture stirred overnight. Chloroform (20 ml) was added the
mixture was washed successively with lM citric acid solution ~10 ml),
water (2 x 10 ml), saturated sodlum chloride solution (5 ml) and then
dried (MgS04). The solvent was removed by evaporation and the residue
tr1turated with methanol to give 4-1(2-pro w lquinolin-4-yloxy)methYll-
N-phenylsulphonylbenzamide as a white powder (160 mg), m.p. 140 C
(dec.); NMR (d6-DMS0): 0.95(t,3H), 1.79(m,2H), 2.88(t,2~), 5,5(s,2~),
7.14(s,1H), 7.5-7.7(m,6H), 7.78(dt,1H), 7.87-8.02(m,5H), 8.18(dd,1~);
mass spectrum (-ve FAB, DMS0/Gly): 495 (M-H) ; microanalysis found:
C,66.1; H,5.4; N,5.8X; C26H24N204SØ5H20 requires: C,66.5; ~,5-3;
N,6.0Z.
~xample 7
A mixture of 2-methyl-4-[~2~-(2-triphenylmethyl-2H-tetrazol-
5-yl)biphenyl-4-yl)methoxy~quinoline (A) (890 mg~.and a 7.5M solution
of hydrogen chloride dioxane (10 ml) and water (1 ml) was allowed to
stand for 72 hours. Volatile material was removed by evaporation and
the residue was triturated with ether (2 x 50 ml). The ether was
decanted off and the solid residue crystallised from isopropanol to
give 2-methyl-4-[(2'-(1~-tetrazol-5-yl)biphenyl-4-yl)methoxy]quinoli~e
hydrochloride (370 mg)9 as a white solid, m.p. 188-190C; NMR (d6-
DMS0): 2.92(s,3H), 5.63(s,2H), 7.21(d,2~), 7.56-7.87(m,8H),
8.07(dt,1H), 8.28(dd,1H), 8.32(dd,1H); mass spectrum l-ve FAB,
DMS0/NBA]: 392 (M-H) , 158; microanalysis found: C,66.0; H,4.6;
N,15.5%; C24H19N50.~C1Ø5R20 requires: C,65.7; H,4.8; N,16.0X.
The starting material (A) was obtained as follows:-
Sodium hydride (60% dispersion in mineral oil; 90 mg~ was
added to a stirred solution of 2-methyl-4-quinolone (340 mg) in DMF
(10 ml). The mixture was stirred until evolution of hydrogen had
ceased and a solution of 5-2-~4' bromomethylbiphenylyl)]-2-
triphenylmethyl-2H tetrazole (1.2 g) (obtained as described in
~uropean Patent 0291969) in DMF (5 ml) was added. The mixture was
stirred for 16 hours. The solvent was removed by evaporation and the
- - ~o - ~c~ 3
residue partitioned between water (20 ml) and dichloromethane (2 x 10
ml). The organic layer was washed with saturated sodium chloride
solution (5 ml) and dried (MgS04). The solvent was rémoved by
evaporation and the resultant oil was puri~ied by flash
chromatography, eluting with methanol/dichlorome~hane (1:99 v/v) to
give 2-methyl-4-12'-(2-triphenylmethyl-2~-tetrazol-5-yl)biphenyl-4-
ylmethoxy]quinoline (A) (890 mg) as a white solid m.p. 168-170 C
(dec.); NMR: 2.7(s,3~), 5.14(s,2H), 6.7(s,1~), 6.9(dd,6~), 7.15-
7.55(complex m,17~), 7.65(dt,1H), 7.95~m,2~, 8.1(dd,1H).
Bxample 8
Using an analogous procedure to that described in Example 7
but starting from 2-propyl-4-[(2'-(2-triphenylmethyl-2~-tetrazol-5-
yl)biphenyl-4-yl)methoxy]quinoline (A), there was obtained
2-propyl-4-[(2~ tetrazol-5-yl)biphenyl-4-yl)methoxy]quinoline
hydrochloride~ m.p. 178-180C; N~R (d6-DMSO): 0.98(t,3~), 1.88(m,2H),
3.08(t,2H), 5.6(s,2H), 7.2(d,2H), 7.5- 7.85(complex m,8H),
8.02(dt,1~), 8.2(d,1H), 8.28(dd,1~); mass spectrum ~-ve FAB,
DMS0/NBA): 420(M-H) ; microanalysis found: C,67.9; ~,5.2; N,14.9%;
C26~23N50.HCl requires C,68.2; ~,5.3; N,15.3X.
The starting material (A) was obtained as uhite solid, m.p.
150-152C; NMR: 1.05(t,3H), 1.88(m,2~), 2.92(t,2H), 5.2(s,2H),
6.73(s,lH), 5.94(dd,6H), 7.15-7.58(complex m,17~), 7.68(dt,1~),
8.0(m,2H), 8.12(dd,1H), 13C NMR (CDCl3): (benzylic C~2) 69.67;
starting from 2-propyl-4-quinolone using a similar procedure to that
described in Example 7.
Examples 9-29
Using an analogous procedure to that described in Example 7,
but starting from the appropriate triphenylmethyl tetrazoles (III),
t~e follouing compounds of formula I were obtained in yields of
7~-90%:-
(~xample 9): 5-Cyano-2-ethyl-4-[(2'-(1~-tetrazol-5-yl)biphenyl-4-yl)-
~ethoxy]quinoline hydrochloride, m.p. 240C (dec); NMR (d6-DMSO):
1.40(t,3~), 3.16(q,2~), 5.57(s,2~), 7.17(d,2~), 7.50-7.75(m,7~),
8.10(t,1H), a.32(d,1H), 8.62(d,1H); mass spectrum (-ve FAB, DMS0/GLY):
431 (M-H) ; microanalysis, found: C,fi5.2; H,4.5; N,16.6; H20, 2.0%;
C26H2oN60.HClØ5H20 requires: C,64.9; ~,4.8; N,16.8; H20, 1.8%;
(Example 10): 2-~thyl-6-trifluoromethylA~-[(2'-(lH-tetrazol-5-yl)-
biphenyl-4-yl~methoxy3quinoline hydrochloride, m.p. 188-190C; NMR
(d6-DMS0): 1.43(t,3H), 3.20(q,2H), 5.67(s,2H), 7.20(d,2~),
7.55-7.73(m,7H), 8.30(d,1H), 8.48(d,2H); mass spectrum (-ve FAB,
DMS0/GLY): 474 (M-H) ; microanalysis, found: C,60.6; H,3.9; N,13.3Z;
C26H20N50F3.HCl requires: C,61.0; H,4.1; N,13.7%;
(Example 11): 2-~thyl-8-trlfluoromethyl-4-l(2'-(lH-tetrazol-5-yl)-
biphenyl-4-yl)metho~y]quinoline , m.p. 110-113C; NMR (CDCl3):
1.42(t,3~), 3.05(q,2H~, 5.37(s,2H), 6.85(s,1H), 7.28-7.62(m,8H),
8.03(d,1H), 8.14(dd,1a), 8.43(d,1H); mass spectrum (-ve FAB,
DMS0/GLY): 474 (M-H) ; microanalysis, found: C,64.6; H,4.4; N,13.3Z;
C26H20N50F3. 0.67 dioxane requires C,64.5; ~,4.7; N,13.1%. *Isolated
as free base;
(Example 12): 2-Ethyl-6-methoxy-4-[(2'-(lH-tetrazol-5-yl)biphenyl-
4-yl)methoxy]quinoline hydrochloride, m.p. 213-215C; NMR (d6-DMSO):
1.41(t,3H), 3.12(q,2H), 3.95(s,3H), 5.68(s,2H), 7.20(d,2~),
7.50-7.76(m,9H), 8.21(d,1H); mass spectrum (-ve FAB, DMS0/GLY): 436
(M-H~ ; microanalysis, found: C,66.1; ~,5.0; N,14.6%; C26H23N502.HCl.
requires C,65.9; H,5.1; N,14.8%;
(Example 13): ~-Ethyl-8-metho~y-4-l(2'-(lH-tetrazol-5-yl~biphenyl-
4-yl)methoxy]quinoline hydrochloride, m.p. 125-127C; NMR (d6-DMSO):
1.40(t,3H), 3.24(q,2H), 4.14(s,3H), 5.66(s,2H), 7.21(d,2H),
7.50-7.84(m,10H); mass spectrum (-ve FAB, DMSO~GLY): 436 (M-H) ;
microanalysis, found: C,63.7; H,5.8; N,13.5X;
C26H23N502.HClØ5H20Ø5dioxane requires C,63.8; H,5.3; N,13.3%.
(Example 14): 2-Ethyl-5,7-dimethoxy-4-[(2'~ -tetrazol-5-yl)biphenyl-
4~yl)methoxy]quinoline hydrochloride, m.p. 203-205C; NMR (d6-DMSO):
1.40(t,3H), 3.07(q,2H), 3.91(s,3H), 3.94(s,3H), S.56(s,2H),
6.80(d,1H), 7.21(d,2H), 7.31(s,2H), 7.51(d,2H), 7.56-7.62(m,2H),
7.68-7.75(m,2H); mass spectrum (-ve FAB, DMS0/GLY): 466(M-a) ;
microanalysis, found: C,63.6; H,5.3; N,12.9; H20, l.OX;
C27H25N503.HClØ25H20Ø25dioxane requires C,63.5; H,5.2; N,13.2;
a2o, o.sx;
(Example 15): 2-~thyl-6,7-dimethoxy-4-1(2'-~ tetrazol-5-yl)-
- 22 ~ 3 r~ ~ ~
biphenyl-4-yl)methoxylquinoline hydr~chlorlde, m.p. 272~C (decomp);
NMR (d6DMS0~: 1.40~t,3~), 3.10(q,2~), 3.9S(s,3H), 3.98(s,3H),
5.6S~s,2H), 7.20(d,2H), 7.43(d,2H), 7.53-7.74(m,7~); mass spectrum
(-ve FAB, DMSOtGLY): 466(M-H) ; microanalysis, found: C,62.7; H,5.1;
N~13-5; H20, 2-5X; C27H25N503.HC1Ø75H20 requires C,62-7; ~5-1;
N,13.5; H20, 2.6%;
(Example 16): 2-~thyl-5,8-dimethoxy-4-t(2'-(1~-~etrazol-5-yl)-
biphenyl-4-yl)methoxylquinoline hydrochloride, m.p. 171C (decomp.);
NMR (d6-DMSO): 1.39(t,3H), 3.22~q,2~), 3.90(s,3H), 4.09(s,38),
5.62(s,2H), 7019-7.28(m,3H), 7.51-7.77(m,8~); mass spectrum (-ve FAB,
DMS0/GLY): 466 (M-H)-; microanalysis, found: C,61.3; ~,5.4; N,12.9;
H20, 5.8X; C27H25N503.HC1.1.5H20 requires C,61.1; ~,5.3; N,13.2; H20,
5.1X;
(~xample 17): 2-Ethyl-5,6,7-trimethoxy-4-[(2'-(lH-tetrazol-5-yl)-
biphenyl-4-yl)methoxylquinoline hydrochloride, m.p. 181-182C; NMR
(d6-DMS0): 1.42(t,3H), 3.10(q,2H), 3.68(s,3H), 3.87(s,3H), 4.00(s,3H),
5.55(s,2H), 7.20(d,2H), 7.42(s,1H), 7.53-7.62(m,5H), 7.68-7.74(m,2H);
mass spectrum (-ve FAB, DMS0/GLY): 496 (M-a) ; microanalysis, found:
' ; 2' 2-9%; C28~27N504 HCl ~2o requires C,60.9;
~,5.3; N,12.7; H20, 3-3%;
(Example 18): 7-Cyano 2-ethyl-4-[(2~-(lD-tetrazol-5-yl)biphenyl-4-yl)-
~ethoxylquinoline hydrochloride, m.p. 160-163C; NMR (d6-DMSO):
1.43(t,3~), 3.17(q,2H), 5.64(s,2H), 7.20(d,2H), 7.5~(d,2~),
7.58-7.72(m,5H), 8.08(d,1~), 8.40(d,1~), 8.76(s,1H); mass spectrum
(-ve FAB, DMS0/GLY): 431 (M-~) ; microanalysis, found: C,64.3; L,4.9;
N,16.6Z; C26H20N60.HCl.H20Ø1dioxane requires C,64.1; ~,4.8; N,17.0%;
(Example 19): 2-Bthyl-7-methoxy-4-l(2'-(1~-tetra2Ol-5-yl)biphe~yl-
4-yl)methoxylquinoline hydrochloride, m.p. 172-174C; NMR(d6-DMSO):
1.44(t,3H), 3.15(q,2H), 3.97(s,3H), 5.64(s,2H), 7.21(d,2~),
7.38-7.77(m,9H), 8.20(d,1H); mass spectrum (-ve FAB, DMS0/GLY):
436(M-~) ; microanalysis, found: C,63.8; H,5.5; N,13.8; H20, 3.5~;
C26H23N502.~Cl.~20 requires C,63.5; H,5.3; N,14.2; H20, 3.7%;
(Bxample 20): 6-Carbomethoxy-2-ethyl-4-[(2'-(1~-tetrazol-5-yl)-
biphenyl-4-yl)methoxylquinoline hydrochloride, m.p. 202-204C; NMR
(d6-DMS0): 1.50(t,3H), 3.28(q,2H), 4.00(s,3H), 5.74(s,2H), 7.29(d,2H),
7.56-7.82(m,7~), 8.48-8.55(m,2H), 8.82(s,1~); mass spectrum (-ve FAB,
DMS0/GLY): 464 (M-H) ; microanalysis, found: C,64.6; ~,4.7; N,13.8%;
- 23 - ~ ~ ~ 3 ~ ~ ~
C27H23N503.~Cl requires C,64.6; H,4.8; N,14.0%;
~xample 21): 2-Ethyl-5-methyl-4-[(2'-(1~-tetrazol-5-yl)biphenyl-
4-yl)- methoxylquinoline hydrochloride, m.p. 168-169C ~dec.); NMR
(d6-DMSO): 1.42(t,3H~, 2.75(s,3H), 3.17(q,2H), 5.61(s,2L), 7.20(d,2H),
7.54-7.71(~,8H), 7.89(t,1H), 8.19(d,1~); mass spectrum (-ve FAB,
DMSO/GLY): 420 (M-~) ; microanalysis, found: C,65.8; H,5.4; N,14.0;
H20, 3.0~; C26H23N50.HC1Ø75H20Ø33 EtOAc requlres C,65.6; H,5.6;
N,14.0; H20, 2-7Z;
~Lxample 22): 2-Lthyl-7-methyl-4-[(2'-(1~-tetrazol-5-yl)b~phenyl-
4-yl)methoxy]quinoline hydrochloride, m.p. 213-215C (dec.); NMR
(d6-DMSO): 1.43(t,3H), 2.59(s,3H), 3.19(q,2H), 5.65(s,2~), 7.22(d,2H),
7.54-7.75(m,8H), 8.09(s,1H), 8.19(d,1H); mass spectrum (-ve FAB,
DMSO/GLY): 420 (M-L) ; microanalysis, found: C,68.5; ~,5.3; N,15.3~;
C~6H23N50.~Cl requires C,68.2; H,5.3; N,15.3X;
(~xample 23): 2,6-Dimethyl-4-[(2' (1~-tetrazol-5-yl)biphenyl-4-yl)-
methoxylquinoline hydrochloride, m.p. 200-202C (dec.); NMR (d6-DMSO):
2.56(s,3~), 2.89(s,3H), 5.62(s,2H), 7.22(d,2H), 7.54-7.72(m,7H),
7.91(dd,1H), 8.06(s,1H), 8.18(d,1L); mass spectrum (-ve FAB,
DMSO/GLY): 406 (M-H) ; microanalysis, found: C,67.1; ~,4.8; N,15.4X;
C25L21N50.HClØ25H20 requires C,67.0; ~,5.0; N,15.6X;
(Bxample 24): 2,8-Dimethyl-4-[(2'~ tet~azol-5-yl)biphenyl-4-yl)-
methoxy]quinoline hydrochloride, m.p. 193-195C (dec.); NMR (d6-DMSO):
2.82(s,3H), 2.99(s,3H), 5.63(s,2H), 7.21(d,2~), 7.53-7.72(m,8H),
7.89(d,1H), 8.16(d,1H); mass spectrum (-ve FAB, DMSO/GLY): 406 (M-H) ;
microanalysis, found: C,67.5; H,5.0; N,15.6%; C25H21N50.~Cl requires
C,67.6; H,5.0; N,15.8%;
(Example 25): 2-~thyl-4-[(2'-(lL-tetra~ol-5-yl)~iphenyl-4-yl)methoxy]-
quinoline hydrochloride, m.p. 178-181C (dec.); NMR (d6-DMSO):
1.48(t,3H), 3.22(q,2H), 5.68(s,2H), 7.23(d,2~), 7.5-7.8(m,7H),
7.83(t,1H), 8.08(t,1~), 8.32(t,2H); 13C NMR: (benzylic CH2) 71.9;
mass spectrum (-ve FAB, DMSO/GLY): 406 (M-H) ; microanalysis, found:
C,68.0; ~,5.1; N,15.~%; C25H21N50.HCl requires C,67.6; ~,5.0; N,15.8%.
(Example 26): 6,8-Dimethyl-2-ethyl-4-l(2'-(1~-tetrazol-S-yl)biphenyl-
4-yl)methoxylquinoline hydrochloride, m.p. 195-197C (dec.); NMR
~d6-DMSO): 1.4(t,3H), 2.S(s,3H), 2.81(s,3H), 3.34(q,2H), 5.65(s,2H),
7.22(d,2H), 7.54-7.72(m,8~), 7.89(s,1H); mass spectrum (-ve FAB,
DMSO/GLY): 434 (M-~) ; microanalysis, found: C,68.7; H,5.6; N,14.8%;
,, ,, ~ r~
- 24 -
C27H25N50.HCl requires C,68.7; H,5.5; N,14.9Z;
(Example 27): 6-chloro-~-~ethyl-4-[(2~ -t~razol-5-yl)biphen
4-yl)methoxy]quinoline hydrochloride m.p. 197-198C tdec.); NMR
(d6-DMSO): 2.9~s,3a), 5.61(s,2H), 7.22(d,2H), 7.53-7.77(m,7H),
8.08(dd,1H), 8.21-8.28(m,2H); 13C NMR: (benzylic C~2) 72; mass
spectrum (-ve FAB, DMSO/GLY): 426 (M-H)-; microanalysis, found:
C,62.2; H,4.1; N,15.1; C1,15.0~; C24H18N5ClO.HC1 requires C,62.1;
H,4.1; N,15.1; C1,15.3%;
(Example 28): 7-Chloro-2-ethyl-4-~(2'-(1~-tetrazol-5-yl)biphenyl-
4-yl)methoxy]qulnoline hydrochloride m.p. 170-172C (dec.); NMR
(d6-DMS0): 1.43(t,3H), 3.18(q,2H), 5.64(s,2H), 7.21(d,2H),
7.5-7.75(m,7H), 7.81(dd,1~), 8.29(d,1~), 8.40(d,1~); mass spectrum
(-ve FAB, DMSO/GLY): 440 (M-H) ; microanalysis, found: C,61.8; ~,4.3;
N,14.2; Cl,14.3; H20, 2.1%; C25H2oN5C10.HC1Ø5~20 requires C,61.6;
H,4.5; N,14.4; C1,14.6; H20, 1.8X; and
(Example 29): 8-Chloro-2-ethyl-4-t~2'-(lH-tetrazol-5-yl)biphenyl-
4-yl)methoxy]quinoline hydrochloride m.p. 146-148C (dec.); NMR
(d6-DMS0): 1.38(t,3H), 3.08(q,2H), 5.52(s,2H), 7.19(d,2H), 7.38(t,1H),
7.51-7.75(m,7~), 8.02(dllH), 8.18(dd,1H); mass spectrum (-ve FAB,
DMSOtGLY): 440(M-H) ; microanalysis, found: C,60.8; H,4.6; N,14.1X;
C25H20N5ClO.HCl.H20 requires C,60.5; H,4.6; N,14.1X.
___.___ ______
The necessary starting materials of formula III were
obtained in yields of 20-70X using an analogous procedure to that
described in Example 7 but starting from the appropriate quinolines of
formula IV. The compounds of formula III had the following
properties:
(9): 5-Cyano-2-ethyl-4-(~2'-(2-triphenylmethyl-2H-tetrazol-5-yl)-
biphenyl-4-yl]methoxy)quinoline, isolated as a foam; NMR (d6-DMSO):
1~34(t,3H), 2.96(q,2H)t 5.55(s,2H), 6.82-6.93(m,6H), 7.14(d,2H),
7.25-7.36(m,9H), 7.40-7.65(m,6H), 7.80(dd,1H), 7.93(t,1H), 8.16(d,1H),
8.30(d,lH);
(10): 2-Ethyl-6-trifluoromethyl-4-([2'-(2-triphenylmethyl-2H-
tetrazol-5-yl)biphenyl-4-yl]methoxy)quinoline, isolated as a foam;
NMR: 1.55(t,3H), 3.12(q,2H), 5.35(s,2H), 6.91(s,1H), 7.05-7.10(m,6H),
7.33-7.45(m,13H), 7.52-7.69(m,3H), 7.99(dd,1H), 8.10(dd,1H),
8.24(d,1H), 8.64(s,1H);
- 25 ~
(11): 2-Ethyl-8-trifluoromethyl-4-([2'-(2-triphenylmethyl-2H-
tetrazol-5-yl)biphenyl-4-yl]methoxy)quinoline, isola~ed as a foam;
NMR: 1.45(t,3H), 3.01(q,2H), 5.19(s,2H), 6.80(s,1H), 6.88-7.0(m,6H),
7.18-7.55(m,17H), 8.05(dd,2H), 8.32(d,1H); 13C NMR: (benzylic CH2)
69.96;
(12): 2-Ethyl-6-methoxy-4-([2'-(2-triphenylmethyl-2H-tetrazol-5-
yl)biphenyl-4-yl]methoxy)quinoline, isolated as a foam; NMR:
1.37(t,3H), 2.90(q,2H), 3.80(s,3H), 5.19(s,2~), 6.70(s,1H)9
6.91-6.97~m,68), 7.19-7.35(m,14H), 7.42-7.52(m,4H), 7.89-7.95(m,2~);
13C NMR: (benzylic C~2) 69.72;
(13): 2-Ethyl-8-methoxy-4-(l2'-(2-~riphenylmethyl-2~-tetrazol~5-
yl)biphenyl-4-yl]methoxy)quinoline, isolated as a foam; NMR (d6-DMS0):
1.33(t,3~), 2.88(q,2H); 3093(s,3~), 5.31(5,2~), 6.81-6.92(m,~H);
7.10-7.12(m,4H), 7.25-7.70(m,15~), 7.85(d,2H);
(14): 2-Ethyl-5,7-dimethoxy-4-([2'-(2-triphenylmethyl-2H-tetrazol-
5-yl)biphenyl-4-yl]methoxy)quinoline, isolated as a foam; NMR:
1.39(t,3~), 2.89(q,2H), 3.87(s,3H), 3.93(s,3~), 5.14(s,2H),
6.46(d,1H), 6.58(s,1H),6.86-6.95(m,6H), 7.01(d,1H), 7.i6-7.29(m,11~),
7.35-7.52(m,5~), 7.91-7.95(m,1~); 13C NMR: (benzylic CH2) 69.63;
(15): 2-Ethyl-6,7-dimethoxy-4-([2'-~2-triphenylmethyl-2~-tetrazol-
5-yl)biphenyl-4-yl]methoxy)quinoline, m.p. 211C (decomp.); NMR
(d6-DMS0): 1.39(t,3H), 2.90(q,2H), 3.87(s,3~), 4.03(s,3~), 5.20(q,2H),
6.65(s,1H), 6.94-7.00(m,6~), 7.19-7.33(m,13H), 7.39-7.55(m,5H),
7.94(dd,1~); 13C NMR: (benzylic CH2) 69.72;
(16): 2-Ethyl-5,8-dimethoxy-4-([2'-(2-triphenylmethyl-2H-tetrazol-
5-yl)biphenyl-4-yl~methoxy)quinoline, m.p. 94-97C; NMR (CDC13):
1.41(t,3H), 3.04(q,2H), 3.85(s,3H), 4.04(s,3H), 5.18(s,2~),
6.75(d,2~), 6.88-6.98(m,7H), 7.18-7.29(m,11H), 7.37-7.56(m,5~),
7.91-7.96(m,1H); 13C NMR: (benzylic CH2) 69.85;
(17): 2-Ethyl-5,6,7-trimethoxy-4-(12~-(2-triphenylmethyl-2H-
tetrazol-5-yl)biphenyl-4-yl]methoxy)quinoline, m.p. 90-95C; NMR:
1.39(t,3H), 2.90(q,2H), 3.78(s,3H), 3.96(s,3H), 4.00(s,3H),
5.19(s,2H), 6.63(s,1H), 6.92-6.98(m,6H), 7.18-7.56(m,17H),
7.91-7.95(m,1H); 13C NMR: (benzylic C~2) 70.26;
(18): 7-Cyano-2-ethyl-4-([2'-(2-triphenylmethyl-2H-tetrazol-5-
yl)biphenyl-4-yl]methoxy)quinoline , m.p. 172-175C; NMR (d6-DMSO):
1.33(t,3H), 2.94(q,2H), 5.36(s,2H), 6.81-6.90(m,6H), 7.18(d,2H),
~ 26 ~ J J~ 2 ~
7.25-7.36(m,10H), 7.43(d,2H), 7.47-7.70(m,4H), 7.85(dd,1~),
8.13(d,1H), 8.39(d,1H);
l Note: Prepared by alkylation of a 70:30 mixture of 7-cyano-
2-ethyl-4-quinolone and 5-cyano-2-ethyl 4-quinolone and pur~fied by
flash chromatography using ethyl acetate/dichloromethane (2:98 v/v) as
eluant.]
(19): 2-Ethyl-7-methoxy-4-([2'-(2-triphenylmethyl-2H-tetrazol-5-
yl)biphenyl-4-yllmethoxy)quinoline , m.p. 159-161C; NMR: 1.39(t,3H),
2.92(q,28), 3.93(s,3H), 5.12(s,2H), 6.62(s,1~), 6.90-6.95(m,7H),
7.21-7.55(m,17H), 7.95-7.80(m,2H~; 13C NMR: (benzylic CH2) 69.69;
[ Note: Prepared by alkylation of a 80:20 mixture of 2-ethyl-
7-methoxy-4-quinolone and 2-ethyl-5-methoxy~-4-quinolone and purified
by flash chromatography using ethyl acetate~hexane (50:50 v/v) as
eluant.]
(20): 6-Çarbomethoxy-2-ethyl-4-([2~-(2-triphenylmethyl-2H-tetrazol-
5-yl)biphenyl-4-yl]methoxy)quinoline, m.p. 179-181C; NMR (d6-DMSO):
1.33(t,3H), 2.89(q,2H), 3:85(s,3H), 5.39(s,Z~), 6.81-6.95(m,6H),
7.12-7.23(m,3H), 7.24-7.38(m,9H), 7.43(d,2H), 7.50-7.69(m,3H),
7.82(dd,1H), 7.95~d,1H), 8.17(dd,1H), 8.76(d,1H);
(21): 2-Ethyl-5-methyl-4-~(2r-(2-triphenylmethyl-2H-tetrazol-5-yl)-
biphenyl-4-yl]methoxy)quinQline; m.p. 179-181C (dec.); NMR:
1.38(t,3~), 2.78(s,3H), 2.90(m,2H), 5.15(s,2~), 6.69(s,1H),
6.9-6.98(m,6H), 7.13-7.34(m,13H), 7.4-7.57(m,5H), 7.85(d,1~),
7.94(dd,1H); 13C NMR: (benzylic CH2) 70.27; microanalysis, found:
C,79.3; H,5.9; N,10.5%; C45H37N50.~20 requires C,79.3; H,5.7; N,10.3X;
(22): 2-Ethyl-7-methyl-4-(12'-(2-triphenylmethyl-2H-tetrazol-5-
yl)biphenyl-4-yl]methoxy)quinoline; m.p. 205-206C (dec.); NMR:
1.39(t,3~), 2.52(s,3H), 2.94(q,2H),'5.14(s,2~), 6.66(s,1H),
6.90-6.95(m,6H), 7.17-7.32(m,14H), 7.39-7.43(m,3H), 7.79(s,1H),
7.96-8.02(m,2H); 13C NMR: (benzylic CH2) 69.69; microanalysis, found
C,81.6; H,5.9; N,10.6%; C45H37N50 requires C,81.4; H,5.6; N,10.6%;
(23): 2,6-Dimethyl-h-(E2'-(2-triphenylmethyl-2H-tetrazol-5-yl)-
biphenyl-4-yl]methoxy)quinoline, isolated as a foam; NMR: 2.41(s,3H),
2.69(s,3H), 5.15(s,2H0, 6.67(s,1~), 6.91-6.95(m,6H0, 7.22-7.25(m,13H),
7.44-7.51(m,4H), 7.89-7.99(m,3~);
(24): 2,8-Dimethyl-4-(12'-(2-triphenylmethyl-2H-tetrazol-5-yl)-
biphenyl-4-yl]methoxy)quinoline; m.p. 175-177C (dec.); NMR:
- 27 ~
2.7(s,3H), 2.78(s,3H), 5.14~s,2H), 6.69(s,1H), 6.87-6.95(m,6H),
7.15-7.3(m,14H), 7.38-7.54(m,4H), 7.94-8.02(m,2N); microanalysis,
found: C,81.6; H,5.2; N,10.9%; C44H35N50 requires C,81.3; H,5-4;
N,10.8X;
(25): 2-Ethyl-4-(~2'-(2-triphenylmethyl-2H-tetrazol-5-yl)biphenyl-
4-yl]methoxy)quinoline; m.p. 173-174C (dec.); NMR: 1.4(t,3H),
2.96(q,2H), 5.16(s,2H), 6.73(s,1H), 6.9-6.94(m,6H), 7.18-7.32(m,13H),
7.33-7.55(m,4H), 7.67(dt,1H), 7.99(m,2H), 8.11(d,1H); microanalysis,
found: C,81.1; H,5.4; N,10.9X; C44H35N50 requires C,81.4; H,5.4;
N,10.8Z;
(26): 6,8-Dimethyl-2-ethyl-4-([2'-(2-triphenylmethyl-2H-tetrazol-5-
yl)biphenyl-4-yl~methoxy)quinoline; m.p. 183-185C (dec.); NMR:
1.4(t,3H), 2.37(s,3H), 2.76(s,3H), 2.95(q,2H), 5.13(s,2H), 6.68(s,1~),
6.90-6.95(m,6H), 7.17-7.35(m,13H), 7.43-7.50(m,4H), 7.76(s,1H),
7.95(m,lH);
(27): 6-Chloro-2-methyl-4-([2'(2-triphenylmethyl-2H-tetrazol-5-
yl]methoxy)quinoline; m.p. 182-184C (dec.); NMR: 2.68(s,3H),
5.15(s,2H), 6,75(s,1H), 6.B7-6.97(m,6H), 7.15-7.35(m,13H),
7.4-7.62(m,4H), 7.9(d,1H), 7.98(m,1H), 8.08(d,1H);
(28): 7-Chloro-2-ethyl-4-([2~-(2-triphenylmethyl-2H-tetrazol-5-yl)-
biphenyl-4-yl]methoxy)quinoline; m.p. 176-178C (dec.); NMR:
1.53(t,3H), 3.08(q,2H), 5.29(s,2~), 6.86(s,1H), 7.02-7.10(m,6H),
7.32-7.48(m,14H), 7.52-7.70(m,3H), 8.08-8.18(m,3H~; 13C NMR ~benzylic
C~2) 70.~1;
(29): 8-Chloro-2-ethyl-4-(l2'-(2-triphenylmethyl-2H-tetrazol-5-yl)-
biphenyl-4-yl]methoxy)quinoline; m.p. 156-158C ~dec.); NMR:
1.42(t,3H), 3.05(q,2H), 5.16(s,2H), 6.8(s,1~), 6.87-6.98(m,6H),
7.15-7.33(m,14H), 7.38-7.55(m,3H), i.78(dd,1H), 7.95-8.06(m,2H).
Using an analogous procedure to that described in ~ y~.,
1955, Coll. V . III, pages 374 and 593, the following quinolones of
formula IV were obtained in yields of 20-60%:-
2-Ethyl-6-trifluoromethyl-4-quinolone, m.p. 288-289 C;
2-Ethyl-8-trifluoromethyl-4-quinolone, m.p. 162-163 C;
5-Cyano-2-ethyl-4-quinolone, m.p.250 C (dec.);
7-Cyano-2-ethyl-4-quinolone, (isolated as a 70:30 mixture of 7-CN and
_ 28
5-CN isomers);
2-Ethyl-6-methoxy-4-quinolone, m.p. 210-212 C;
2-Ethyl-7-methoxy-4-quinolone, (isolated as an 80:20 mixture of 7-OMe
and 5-OMe isomers);
2-Ethyl-8-methoxy-4-quinolone, m.p. 196-198 C;
2-Ethyl-5,7-dimethoxy-4-quinolone, m.p. 242-Z44 C;
2-Ethyl-5,8-dimethoxy-4-quinolone, m.p. 196-198 C;
2-Ethyl-6,7-dimethoxy-4-quinolone, m.p. 284-287 C;
2-Ethyl-5,6 t 7-trimethoxy-4-quinolone, m.p. 226-228 C;
~ethyl 2-ethyl-4-hydroxyquinol-6-ylcarboxylate, 245 C (dec.);
2-Ethyl-5-methyl-4-quinolone, m.p. 264-266C ;
2-Ethyl-7-methyl-4-quinolone, m.p. 242-244C ;
2-Ethyl-~,8-dimethyl-4-quinolone, m.p. 264-266C; and
8-Chloro-2-ethyl-4-quinolone, m.p. 183-184C.
7-chloro-2-ethyl-4-quinolone (isolated as a 43.5:56.5) mixture of 7-Cl
and 5-Cl isomers).
[ Note: these compounds were separated by flash chromatography on
silica, eluting with methanol/dichloromethane (1:9 v/v) as eluant.].
2,6-Dimethyl-4-quinolone and 2,8-dimethyl-4-quinolone were obtained as
~0 described in Ann. Chem., 1982, 1656-1676.
6-Chloro-2-methyl-4-quinolone was obtained as described in Synthesis,
1'~87, 482-3.
Bxample 30
2M Aqueous sodium hydroxide solution (3.8 ml) was added to a
solution of 6-carbomethoxy-2-ethyl-4-l(2'-(2-triphenylmethyl-2H
tetrazol-5-yl)biphenyl-4-yl)methoxy]quinoline in methanol (10 ml) and
dioxane (15 ml). The solution was heated to reflux for 1 hour,
cooled, and the solvent removed by evaporation. Uater (40 ml) was
added and the solution extracted with ethyl acetate (40 ml). The
aqueous phase was acidified with concencrated hydrochloric acid (2 ml)
and the resulting suspension dissolved in dioxane (20 ml). The
solution was stirred for 1 hour then evaporated to a yellow gum.
Crystallisation from methanol gave 6-carboxy-2-ethyl-4-1(2'-~
tetrazol-5-yl)biphenyl-4-yl)methoxy3quinoline hydrochloride (0.24 g)
-- 29 ~ ? ~
as a white powder, m.p. 161-164~C; NMR (d6-DMS0): 1.45(t,3~),
3.24~q,2R), 5.68(s,2H), 7.24(d,2H), 7.53 7.76~m,7~), 8.47(d,2~),
8.77(d,1H); mass spectrum (-ve FAB, DMS0/GLY): 450 (M-8) ;
microanalysis, found: C,61.3; H,4.9; N,13.3; H20, 1.7X;
C26H21N503.HCl.MeQHØ5~20 requires C,61.3; H,5.1; N,13.2; E20, 1.7%.
~xample 31
Using an analogous procedure to that described in Example 7,
but starting from 2-ethyl-6-(~ert-butyldimethylsilyloxy)-4-([2'-
(2-triphenylmethyl-2H-te~razol-5-yl)biphenyl-4-yljmethoxy)quinoline,
itself isolated as a foam INMR: 0.25(s,6~)~ 1.02(s,9H), 1.38(t,3H),
2.94(q,2H), 5.20(s,2H), 6.68(s,1H), 6.88-6.98(m,6H), 7.17-7.34(m,14H),
7.40-7.60(m,4H), 7.89-8.00(m,2H); 13C NMR: (benzylic CH2) 69.52]
starting from 2-ethyl-6-(tert-butyldimethylsilyloxy)-4-quinolone,
there ~as obtained 2-ethyl-6-hydroxy-4-1(2'-~1~-tetrazol-5-
yl)b1phenyl-4-yl)methoxy]quinoline hydrochlorlde, m.p. 189-191C; NMR
(d6-DMS0): 1.42(t,3H), 3.14(q,2H), 5.61(s,2H), 7.22(d,2~
7.49-7.72(m,9H), 8.20(d,1H); mass spectrum (-ve FAB, DMS0/GLY): 422
(M-H) ; microanalysis, found: C,62.7; H,4.8; N,14.4; H20, 4.4%;
C25H21N502.HCl.H20 requires C,62.8; H,4.8; N,14.6; H20, 3.8%.
Examples 32-54
Using an analogous procedure to that described in Example 7,
but starting from the appropriate triphenylmethyl tetrazole (III), the
following compounds of formula I were obtained in yields of 70-90~:-
(Example 32): 2-~thyl-6-methylthio-4-[(2'-(lH-tetrazol-5-yl)biphe~yl-
4-yl)methoxy~quinoline hydrochloride, m.p. 156-158C; NMR (d6-DMS0):
1.40(t,3H), 2.62(s,3H), 3.17(q,2H), 5.66(s,2H), 7.20(d,2H),
7.51-7.70(m,7H), 7.90-7.95(m,2H), 8.25(d,1H); mass spectrum (-ve FAB,
DMS0/GLY): 452 (M-H) ; microanalysis, found: C,63.2; H,4.8; N,13.7X;
C26H23N50S.HClØ25.H20 requires C,63.2; H,4.8; N,14.2X;
(~xample 33): 2-Ethyl-7-hydroxymethyl-4-[~2'-(1~-tetrazol-5-
yl)biphenyl-4-yl)methoxy]quinoline hydrochloride, m.p. 228-230C; NMR
(d6-DMS0): 1.41(t,3H), 3.15(q,2H), 5.06(s,2H), 5.62(s,2H), 7.20(d,2H),
7.55(d,2H), 7.56-7.62(m,3H), 7.65-7.72(m,2H), 7.99(d,2H),
8.12-8.20(m,1H); mass spectrum (-ve FAB, DMS0/GLY): 436 (M-~) ;
- 30 ~ 6~
microanalysis, found: C,65.7; H,5.1; N,14.8X; C26~23N502.HCl requires
C,65.9; H,5.1; N,14.8X;
(Exam~le 34): ~-Ethyl-6-methylsulphonyl-4-[(2'~ -tetrazol-5-
yl~biphenyl-4-yl)methoxy~quinoline hydrochloride, m.p. 149-151C; N21R
S (d6-DMS0): 1.40(t,3H), 3.11(q,2H), 3.30(s,3H), 5.59(s,2H), 7.20~d,2H),
7.53-7.70(m,7H), 8.20(d,1H), 8.33(dd,1H), 8.67(d,1H); mass spectrum
(-ve FAB, DMS0/GLY): 484 (M-H) ; microanalysis, found: C,58.3; H,5.0;
N,12.2Z; C26H23N503S.HCl.H20 requires C,57.8; H,4.9; N,13.0X;
~Example 35): 2-Bthyl~6,7-methylenedioxy-4-[(2'~ -tetrazol-
5-yl)biphenyl-4-yl)methoxy]quinollne hydrochloride, m.p. 174-175C;
NMR (d6-DMSO): 1.40(t,3H), 3.11(q,2H), 5.62(s,2H), 6.35(s,2H),
7.19(d,2H), 7.45-7.76(m,9H); mass spectrum (-ve FAB, DMS0/GLY): 450
(M-~) ; microanalysis, found: C,61,6; H,4.8; N,13.2; H20, 4.3%;
C26H21N503.HCl~1.2H20 requires C,61.3; H,4.8; N,13.7; H20, 4.2Z;
(~xample 36): 2-Ethyl-6-(2-fluoroethoxy)-4-l(2'-(1~-tetrazol-
5-yl)biphenyl-4-yl)methoxy]q~inoline hydrochloride, m.p. 161-163C;
NMR (d6-DMS0): 1.40(t,3H), 3.12(q,2H), 4.32-4.55(m,2H),
4.66-4.98(m,2~), 5.64(s,2H), 7.20(d,2H), 7.50-7.81(m,9H), 8.24(d,1H);
mass spectrum (-ve FAB, DMS0/GLY): 468 (M-H) ; microanalysis, found:
C 63 1; H 4 9; N,12.9; H20, 1-7Z; C27H24N52F HCl 5H20 q
C,63.0; H,4.8; N,13.6; H20, 1.8%;
~Example 37): 7-Carboethoxy-2-ethyl-4-l(2'-(lH-tetrazol-5-yl)-
biphenyl-4-yl)methoxy]quinoline hydrochloride, m.p. 226-228C; NMR
(CD3CN/CF3C02H): 1.55(t,3H), 1.62(t,3H), 3.31(q,2H), 4.60(q,2H),
5.75(s,2H), 7.42(d,2H), 7.52~s,1H), 7.70(d,2H), 7.73-7.78(m,2H),
7.85-7.91(m,2H), 8.44(dd,1H), 8.60(d,1H), 8.78(d,1H); mass spectrum
(-ve FAB, DMS0/GLY): 479 (M-H) ; microanalysis, found: C,64.7; H,5.0;
2 ' %; C28H25N503.Hcl.o.lH2o requires C,64.9; H,5.1;
N,13.5; H20, 0-3%;
~ xample 38): 2-~thyl-6-(2,2,2-trifluoroethoxy)-4-[(Z'-(lH-tetrazol-
5-yl)biphenyl-4-yl)m~thoxy~quinoline hydrochloride, m.p. 140 141~C;
NMR (d6-DMS0): 1.41(t,3H), i.14(q,2H), 5.03(q,2H), 5.68(s,2H),
7.20(s,2H), 7.52-7.75(m,8H), 7.85(dd,1~), 8.28(d,1H), mass spectrum
(-ve FAB, DMS0/GLY): 504 (M-H)-; microanalysis, found: C~5800; H,4.7;
; H20, 2-6%; C27H22N502F3 HCl~H2o requireS C,57.9; H,4.3;
N,12.S; H20, 3-2%;
(Example 39): 6-Carboxamido-2-ethyl-4-l(2'-(lH-tetrazol-5-yl)-
_ 31 - ~ s ~ ;, 2~
biphenyl-4-yl)methoxy]quinoline hydrochloride, m.p. 178-180C; NMR
(d6-DMS0): 1.44(t,3~), 3.21(q,2H), 5.69(s,2H), 7.22(d~2H),
7.57-7.75(m,6H), 8.36(d,1~), 8.46(dd,2H), 8.76(d,1H); mass spectrum
(+ve FAB, DMS0/GLY): 451 (M+H)+; microanalysis, found: C,62.4; H,5.1;
N,16~ 2~ 1-7~; C26H22N602-HCl-0-25H20-0-4CH30H requires C~62-3;
H,5.0; N,16.5; H20, 1.8X;
(~xample 40): 2-Bthyl-6-trifluoromethoxy-4-[(2'-(1~-tetrazol-
5-yl)biphenyl-4-yl)m~thoxylq~inoline hydro~hlorlde, m.p. 180-182C;
NMR (d6-DMSO): 1.43(t,3H), 3.18(q,2H), 5.65(s,2~), 7.20(d,2H),
7.52-7.73(m,7H), 8.03-8.15(m,2H), 8.41(d,1H); mass spec~rum (-ve FAB~
DMSOtGLY): 490 (M-H?-; microanalysis, found: C,59.0; ~,3.6; N,13.2~;
C26H20N502F3.~Cl requires C,59.1; H,4.0; N,13.3Z;
~xample 41): 6-Cyano-2-ethyl-4-[(2'-(1~-tetrazol-5-yl~biphenyl-
4-yl)methoxy]quinollne hydrochloride, m.p. 153-155~C; NMR (d6-DMS0):
1.41(t,3H), 3.15~q,2H), 5.62(s,2H), 7.20(d,2H), 7.53-7.75(m,7H),
8.22-8.39(m,2H), 8.79(s,1H); mass spectrum (+ve FAB, DMS0/GLY): 433
(M+H)+; microanalysis, found: C,66.9; H,4;3; N,17.6~; C26H2oN60.~Cl
requires C,66.6; H,4.5; N,17.9Z;
(Bxample 42): 2-Ethyl-6-for~yl-4~1(2'~ -tetrazol-5-yl)biphenyl-4-
-
yl)me~hoxy]quinoline hydrochloride, m.p. 142-144C; NMR (d6-DMS0):
1.45(t,3~), 3.20(q,2H), 5.70(s,2H), 7.22(d,2H), 7.52-7.75(m,7H),
8.34-8.49(m,2~), 8.85(s,1H), 10.22(s,1H); mass spectrum (+ve FAB~
DMS0/NBA): 436 (M+H)+; microanalysis, found: C,65.0; H,4.6; N,14.4X;
C26H21N502.HClØ5H20 requires C,64.9; H,4.8; N,14.6Z;
(Example 43): 6-Dimethylamino~2-ethyl-4-1(2'-(1 -~etrazol-5-
yl)biphenyl-4-yl)methoxy]quinoline dihydrochloride, m.p. 206-209C;
NMR (d6-DMSO): 1.40(t,3H), 3.07(s,6H), 3.12(q,2~), 5.63(s,2H~,
7.05(d,1H), 7.21(d,2~), 7.45(s,lH), 7.51(s,1H), 7.55-7.65(m,3H),
7.66-7.75(m,3H), 8.23(d,1H); mass spectrum (+ve FAB, DMS0/NBA): 451
(M+H)+; microanalysis, found: C,61.8; H,5.4; N,16.0; C1,13.2~;
C27H26N60.2HCl requires C,61.9; H,5.4; N,16.1; Cl,13.6Z;
(Example 44): 2-Ethyl-6-nitro-4-[(2'-(lH-tetrazol-5-yl)biphenyl-
4-yl)me~hoxylquinoline hydrochloride, m.p. 149-152C; NMR (d6-DMSO):
1.45(t,3H), 3.18(q,2~), 5.66(s,2H), 7.22(d,2H), 7-54-7-75tm,7~).
8.45(d,1H), 8.68(dd,1H), 8.95(d,1H); mass spectrum (+ve FAB,
DMSO/NBA)o 453 tM~H)+; microanalysis, found: C,59.3; H,4.5; N,16.8;
~2~ 3 9X; C25H20N603.HCl.H20 requires C,59.2; H,4.5; N,16-6; H20,
- 32 -
~t~3~
3.6Z;
(Example 45): 6-Cyano-2-methyl-4-~(2'~ -tetrazol-5-yl)biphe~yl~
4-yl)methoxy]quinoline hydrochloride, m.p. 280-282C; N~R (d6-DMS0):
2.90(s,3~), 5.63(s,2H), 7.21(d,2H), 7.57-7.79(m,8H), 8.25-8.41(m,2H),
8.79(s,1H); mass spectrum (-ve FAB, DMS0/GLY): 417 (M-H) ;
microanalysis, found: C,66.0; H,4.5; N,17.8X; C25H18N60.~Cl requ~res
C,66.0; H,4.2; N,18.5~;
(Example 46): 2-Ethyl-6-fluoro-4-[(2'-(1~-tetrazol-5-yl)biphenyl-
4-yl)methoxy]quinoline hydrochloride, m.p. 185-186C; NMR (d6-D~S0):
1.44(t,3H), 3.20(q,2H~, 5.67(s,2H), 7.20(d,2H), 7.50-7.78(m,7~),
7.93-8.08(m,2H), 8.36-8.49(m,1H); mass spectrum (-ve FAB, DMSO~GLY):
424 (M-H) ; microanalysis, found: C,64.7; ~,4.4; N,14.8~;
C25H20N5FO.HCl requires: C,65.0; H,4.6; N~15.2%;
t~xam~le 47): 2-~thyl-6-isopropoxy-4-[(2~ -tetrazol-5-yl)-
biphenyl-4-yl~methoxy]qu~noline hydrochloride, m.p. 177-180C (dec);
NMR (d6-DMSO): 1.36(d,6~), 1.4(t,3H), 3.16(q,2H), 4.83(m,1H),
5.66(s,2H), 7.20(d,2H), 7.48-7.65(m,6H), 7.65-7.75(m,3H), 8.28(d,1~);
mass spectrum (-ve FAB, DMS0/GLY): 464 (M-H) ; microanalysis, found:
, ; H2o,o-2~; C2g~27Ns2 ~cl o l(c~3)2cHoH o-25H
requires C,66.3; H,5.7; N,13.7; ~2' 9%;
(Example 48): 5-Chloro-2-ethyl-4-t(2'-(1~-tetrazol-5-yl)biphenyl-
4-yl~methoxy]quinoline hydrochloride, m.p. 189-190C (dec); NMR
(d6-DMS0): 1.41(t,3H), 3.14(q,2~), 5.63(s,2~), 7.19(d,2~),
7.50-7.63(m,5B), 7.63-7.74(m,2H), 7.84(d,1H), 7.94(t,1~), 8.28(d,1H);
mass spectrum (-ve FAB, DMS0/GLY): 440 (M-~) ; microanalysis, found:
C,62.8; H,4.2; N,14.7%; C25H20N5ClO.HCl requires C,62.8; H,4.4;
N,14.6X;
(Example 49): 2-Trifluoromethyl-4-[(2'-(1~-te~razol-5-yl)biphenyl-4-
yl)methoxy]quinoline, m.p. 187-190C (dec); NMR (d6-DMSO): 5.53(s,2H),
7.20(d,2H), 7.50-7.80(m,8H), 7.90(dt,1H), 8.12(d,1H), 8.28(dd,1H),
13.0(brs,1H); mass spectrum (-ve FAB, DMS0/GLY): 446 (M-H) ;
microanalysis, found: C,~4.3; H,3.3; N,15.5%; C24H16N5F30 requires
C,64.4; H,3.6; N,15.7~;
(Example 50) 2-Hethoxymethyl-4-[(2'-(1~-tetrazol-5-yl)biphenyl-
4-yl)methoxy]quinoline hydrochloride, m.p. 169-171C; NMR (d6-DMSO):
3.50(s,3H), 4.98(s,2H), 5.67(s,2H), 7.22(d,2H), 7.54-7.74(m,7~),
7.84(t,1~), 8.08(t,1~), 8.32-8.38(m,2H); mass spectrum (-ve FAB,
-- 33 -- ~ ~ C~
DMS0/GLY~: 422 (M-~ ; microanalysis, found; C,65.2; ~,4.8; N,15.2%;
C~5~21N502.HCl requires C,65.3; H,4.8; N,15.2%;
(~xample 51~: 2-~thoxymethyl-4-l(Z~ -tetrazol-5-yl)biphenyl-
4-yl)methoxy]quinoline hydrochloride, m.p. 164-166C; NMR (d6-DMSO):
1.25(t,3H), 3.68(q,2H), 4.99(s,2H), 5.68(s,2~), 7.21(d,2~),
7.54-7.72(m,7H), 7.84(t,1H), a.08(t,1~), 8.34(d,2H); mass spectrum
(-ve FAB, DMS0/GLY): 436(M-a) ; microanalysis, found; C765.6; ~,5.2;
N,14-4X; C26~23N502.HCl requires C,65.9; H,5.1; N,14.8%;
(Example 52~: 2,3-Dimethyl-4-[(2'-(1~-tetrazol-5-yl)biphenyl-
4-yl)methoxy]quinoline hydrochloride, m.p. 155-156C (dec); NMR
(d6-DMS0): 2.35(s93~), 2.95(s,3H), 5.38(s,2~), 7.16(d,2~), 7.47(d,2H),
7.53-7.76(m,4~), 7.85(t,1H), 8.05(dt,1~), 8.19(d,1H~, 8.38(d,1~); mass
spectrum (+ve FAB, DMS0/NBA): 408 (M~H)~; microanalysis, found:
C,66.8; H,4.9; N,15.3X; C25H21N50.HC1Ø25C~30H requires C,67.1;
~,5.1; N,lS.5%;
~Example 53): 2-~3,3,3-Trifluoropropyl)-4-[(2'-(1~-tetrazol-5-
-yl)biphenyl-4-yl~methoxg]quinoline hydrochloride, m.p. 204-206C
(dec); NMR (d6-DMS0): 2.92-3.14(m,2H), 3.40-3.51(m,2~), 5.64(s,2~),
7.21(d,2H), 7.50-7.64(m,4H), 7.65-7.87(m,4H), 8.08(t,1~),
8.25-8.35(m,2H); mass spectrum (~ve FAB, DMS0/NBA): 476 (M+~)+;
microanalysis, found: C,60.8; ~,3.9; N,13.7%; C26~20N5F30.HCl requires
C,61.0; H,4.1; N,13.7X;
(~xample 54): 2-~ydroxymethyl-4-1(2' (1~-tetrazol-5-yl)biphenyl-
4-yl)methoxy3quinoline hydrochloride, m.p. 199-201C (dec); NMR
(d6-DMS0): 5.07(s,2H), 5.66(s,2H), 7.22(d,2~)~ 7.53-7.63(m,4H),
7.65-7.75(m,3H), 7.83(t,1H), 8.09(t,1~)~ 8.32(d,1H), 8.39(d,1H); mass
spectrum (-ve FAB, DMS0/GLY): 408 (M-H) ; microanalysis, found:
C,64.2; H,4.7; N,15.5%; C24~19N502.~Cl requires C,64.6; ~,4.5;
N,15.7X;
The necessary s~arting materials of formula III used in
Examples 32-53 uere obtained in yields of 20-7~% using an analogous
procçdure to ~hat described in Example 7 but starting from the
appropriate quinolones of formula IV. The compounds of formula III
had the following properties:-
(32): 2-Ethyl-6-methylthio-4-([2'-(2-triphenylmethyl-2~-tetrazol-
_ 34 ~ .3 ~ ~ ~
-5-yl)biphenyl-4-y3methoxy)quinoline, m.p. 164-166C; NMR: 1.37(t,3~),
2.50(s,3H), 2.92(q,2H), 5.19(s,2H), 6.70(s,1H), 6.91-6.95(m,6H),
7.19-7.34(m,14H), 7.44-7.60(m,4H), 7.89-7.99(m,2H); 13C NMR: (benzylic
CH2) 69-82;
(33): 2-Ethyl-7-hydroxymethyl-4 ([2~-(2-triphenylmethyl-2H-tetrazol-
5-yl)biphenyl-4-yl]methoxy)quinoline, m.p. 99-102C; NMR(d6-DMSO):
1.29(t,3H), 2.82(q,2H), 5.10(s,2H), 5.31(s,2H), 6.84-6.93~m,6H),
7.02(s,1H), 7.15(d,2H), 7.27-7.38(m,9H), 7.44(d,2H), 7.48-7.83(m,7H);
(34): 2-Ethyl-6-methylsulphonyl-4-(~2'-(2-triphenylmethyl-
2H-tetrazol-5-yl)biphenyl-4-yl]methoxy)quinoline, lsolated as a foam;
NMR: l.l9(t,3H), 2.76(q,2H), 2.89(s,3H), 5.03(s,ZH), 6.61(s,1H),
6.72-6.77(m,6H), 7.00-7.12(m,13H), 7.22-7.35(m,3H), 7.74-7.78(m,1H),
7.85-7.98(m,2H), 8.67(s,1H); 13C NMR- (benzylic CH2) 70.52;
(35): 2-Ethyl-6,7-methylenedioxy-4-([2'-(2-triphenylmethyl-
2H-tetrazol-5-yl)biphenyl-4-yl]methoxy)quinoline, isola~ed as a foam;
NMR 1.38(t,3H), 2.90(q,2H), 5.15(s,2H), 6.09(s,2H), 6.65(s,1H),
6.88-6.98(m,6H), 7.16-7.55(m,19H), 7.96-8.03(m,1H); 13C NMR (benzylic
CH2) 69-75;
(36): 2-Ethyl-6-(2-fluoroethoxy)-4-([2'-(2-triphenylmethyl-2H-
tetrazol-5-yl)biphenyl-4-yl]methoxy)quinoline, m.p. 173-175C; N~R:
1.30(t,3H), 2.84(q,2H), 4.03-4.19(m,2H), 4.51-4.74(m,2H), 5.11(s,2H),
6.64(s,1H), 6.85-6.90(m,6H), 7.12-7.46(m,18H), 7.85-7.89(m,2H); 13C
NMR: (benzylic C~2) 69.93;
(37): 7-Carboethoxy-2-ethyl-4-([2'-(2-triphenylmethyl-2H-tetrazol-
5-yl)biphenyl-4-yllmethoxy)quinoline, m.p. 160-163C; NMR (d6-DMSO):
1.33(t,3H), 1.38(t,3H), 2.92(q,2H), 4.38(q,2H), 5.35(s,2H),
6.84-6.89(m,6H), 7.16(d,2H), 7.21(s,1~), 7.29-7.31(m,9H), 7.42(d,2H),
7.52-7.65(m,3H), 7.83-7.89(m,2H), 8.10(d,1H), 8.46(d,1H);
(38): 2-Ethyl-6-(2,2,2-trifluoroethoxy~-4-([2'-(2-triphenylmethyl-
2H-tetrazol-5-yl)biphenyl-4-yl]methoxy)quinoline, m.p. 147-149C; NMR:
1.40(t,3H), 2.95(q,2H), 4.38(q,2H), 5.21(s,2H), 6.73(s,1~),
6.92-7.00(m,6H), 7.21 7.55(m,18H), 7.93-8.00(m,2H); 13 NMR: (benzylic
CH2) 70.00;
(39): 6-Carboxamido-2-ethyl-4-([2'-(2-triphenylmethyl-2H-tetrazol-
5-yl)biphenyl-4-yl]methoxy)quinoline, isolated as a foam; NMR:
1.31(t,3H), 2.87(q,2H), 5.38(s,2H), 6.82--6.89(m,6H), 7.13(d,2H),
7.31-7.34(m,9H), 7.45(d,2H), 7.54-7.63(m,3H), 7.80-7.94(m,3H),
- 35 - ~J
8.14(dd,1H), 8.70(d,1H);
(40): 2-Ethyl-6-trifluorom~thoxy-4-([2t~(2-triphenylmethyl-
2~-tetrazol-5-yl)biphenyl_4_yl]methoxy)quinoline, m.p. 144-146C; NMR:
1.38~t,3H), 2.95(q,2H), 5.18(s,2H), 6.75(s,1H), 6.90-6.97(m,6~),
7.20-7.32(m,13H), 7.39-7.55(m,4H), 7.95-8.05(m,3H); 13C NMR: (benzylic
CH2) 70.22;
(41): 6-Cyano-2-ethyl-4-([2'-(2-triphenylmethyl-2H-tetrazol-
5-yl)biphenyl-4-yl]methoxy)quinoline, m.p. 178-179C; NMR (d6-DMSO):
1.33(t,3H), 2.92(q,2H), 5.36(s,2H), 6.80-6.90(m,6H), 7.16-8.02(m,20H),
8.42(s,1H);
(42): 2-Ethyl-6-formyl-4-([2' (2-triphenylmethyl-2H-tetrazol-
5-yl)biphenyl-4-yl~methoxy)quinoline, m.p. 164-166C; NMR (d6-DMSO):
1.34(t,3H), 2.92(q,2H), 5.40(s,2H), 6.85-6.91(m,6H), 7.17-7.33(m,13H),
7.46-7.66(m,4H), 7.82(dd,1H), 8.05(dq,2H), 8.59(d,1H), 9.91(s,1H);
(43): 6-Dimethylamino-2-ethyl-4-(l2'-2-triphenylmethyl-2~-tetrazol-
5-yl)biphenyl-4-yl]methoxy)quinoline, isolated as a foam; NMR
(d6-DMS0): 1.28(t,3H), 2.83~q,2H), 2.94(s,6H), 5.35(s,2H),
6.82-6.94(m96H), 6.98(s,1H), 7.08(d,1~), 7.15(d,2H), 7.27-7.40(m,9H),
7.41-7.70(m,6a), 7.72-7.84(m,2H);
(44): 2-Ethyl-6-nitro-4-([2'-(2-triphenylmethyl-2H-tetrazol-
5-yl)biphenyl-4-yl~methoxy)quinoline, isolated as a foam; NMR
(d6-DMS0): 1.34(t,3H), 2.94(q,2H), 5.42(s,2H), 6.84-6.89(m,6H),
7.18(d,2~), 7.30-7.36(m,9H), 7.42-7.65(m,6H), 7.84(d,1~), 8.07(d,1H),
8.41(dd,1H), 8.88(d,1H);
(45): 6-Cyano-2-methyl-4-([2'-(2-triphenylmethyl 2H-tetrazol-
5-yl)biphenyl-4-yl]methoxy)quinoline, m.p. 188C (dec.); NMR
(d6-DMS0): 2.65(s,3H), 5.35(s,2H), 6.82-6.89(m,6H), 7.16-7.34(m913H),
7.43-7.67(m,4H), 7.86(dd,1H), 7.98(s,2H), 8.42(s,1H);
(46): 2-Ethyl-6-fluoro-4-([2'-(2-triphenylmethyl-2H-tetrazol-
5-yl)biphenyl-4-yl]methoxy)quinoline; m.p. 183-184C; NMR: 1.40(t,3H),
2.95(q,2H), 5.18(s,2H), 6.75(s,1H), 6.87-6.98(m,6H), 7.17-7.35(m,13H),
7.36-7.58(m,4H), 7.70(dd,1H), 7.93-8.05(m,2~); microanalysis, found:
C,78.8; H,5.1; N,10.5X; C44H34N5F0 requires C,79.1; H,5.1; N,10.5%;
(47): 2-Ethyl-6-isopropoxy-4-([2'-(2-triphenylmethyl-2H-tetrazol-
5-yl)biphenyl-4-yl]methoxy)quinoline, isolated as a foam; NMR:
1.35(d,6H), 1.38(t,3H), 2.90(q,2H), 4.64(m,1H), 5.19(s,2H),
6.69(s,1H), 6.90-6.98(m,6H), 7.15-7.35(m,14H), 7.38-7.55(m,4H),
_ 36 - ~f~
7.92(d,1H), 7.95(dd,1H);
(48): 5-Chloro-2-ethyl-4-([2'-(triphenylmethyl-2H-tetrazol-
5-yl)biphenyl-4-yl]me~hoxy)quinoline; m.p. 180-181C (dec.); NMR
(d6-DMS0): 1.3(t,3H), 2.85(q,2H), 5.35(s,2H), 6.80-6.92(m,6H),
7.13(t,3H), 7.23-7.37(m,9H), 7.40-7.67(m,7H), 7.74-7.88(m,2H);
(49): 2-Trifluoromethyl-4-~[2'-(2-triphenylmethyl-2H-tetrazol-
5-yl)biphenyl-4-yl]methoxy)quinoline, isolated as an amorphous solid;
NMR (ds-DMSO): 5.48(s,2H), 6.8-6.94(m,6H), 7.19(d,2H),
7.24-7.38(m,9H), 7.4-7.69(m,7H), 7.8-7.94(m,2~), 8.07-8.17(m,2H); 13C
NMR: (benzylic CH2) 70.45;
(50): 2-Methoxymethyl-4-([2'-(2-triphenylmethyl-2H-tetrazol-
5-yl)biphenyl-4-yl]methoxy)quinoline; m.p. 174-176C; NMR: 3.52(s,3H),
4.73(s,2H), 5.19(s,2H), 6.89-6.94(m,6H), 7.06(s,1H), 7.18-7.30(m,13H),
7.30-7.53(m,4H), 7.69(dt,1H), 7.98(m,2H), 8.14(dd,1H); microanalysis,
found: C,78.7; ~,5.2; N,10.4~; C44H35N502Ø25H20 requires C,78.9;
H,5.3; N,10.5X;
(51): 2-Ethoxymethyl-4-([2' (2-triphenylmethyl-2H~tetrazol-
5-yl)biphenyl-4-yl]methoxy)quinoline; m.p. 156-158C; N~R: 1.29(t,3H),
3.66(q,2H), 4.77(s,2H), 5~20(s,2H), 6.90-6.94(m 6H), 7.09(s,1H),
7.18-7.29(m,13H), 7.30-7.53(m,4H), 7.68(dt,1H), 7.96-8.03(m,2H),
8.14(dd,1H); microanalysis, found: C,79~0; H,5.5; N,10.3~; C45H37N502
requires C,79.5; H,5.5; N,10.3X;
(52): 2,3-Dimethyl-4-([2'-(2-triphenylmethyl-2H-tetrazol-
5-yl)biphenyl-4-yl]methoxy)quinoline; m.p. 170-172C; NMR: 2.37(s,3H),
2.73(s,3H), 4.95(s,2~), 6.90-6.99(m,6H), 7.18-7.35(m,1~H),
7.37-7.58(m,4H), 7.64(dt,1H), 7.95-8.07(m,3H);
(53): 2-(3,3,3-Trifluoropropyl)-4-([2'-(2-~riphenylmethyl-~H-
tetrazol-5-yl)biphenyl-4-yl]methoxy)quinoline, isolated as an
amorphous solid, m.p. 182-184C; NMR (d6-DMS0): 2.7-3.0(m,2~),
3.05-3.20(m,2H), 5.32(s,2H), 6.80-6.90(m,6H), 7.12-7.65(m,18H),
7.70(dt,1H), 7.87(dt,2H), 8.03(d,1H);
The starting material used in Example 54 was obtained as follows:-
(i) Using an analogous procedure to that described in Example 7
but starting from the appropriate quinolone of formula IV, there was
thus obtained 2-ethoxycarbonyl-4-([2'-(2-triphenylmethyl-2H-tetrazol-
_ 37 - ~t~
5-yl)biphenyl-4-yl]methoxy)quinoline; ~.p. 146-147C (dec.); NMR:
1.51(t,3H), 4.57(q,2H), 5.23(s,2H), 6.89-6.94(m,6H), 7.18-7.30(m,13H),
7.40-7.53(m,4H~, 7.68(s,1H), 7.75(dt,1H), 8.0(m,1H), 8.17(d,1~),
8.26(d,1H).
(ii) Lithium borohydride (11 mg) was added to a solution of
2-ethoxycarbonyl-4-([2'-(2-triphenylmethyl-2H-tetrazol-5-yl)biphenyl-
4-yl]methoxy)quinoline (346 mg) in tetrahydrofuran (4 ml) and the
mixture stirred for 18 hours. ~ater (20 ml) was added to the mixture
and a white solid precipitated. The solld was collected by
filtration, dissolved in ethyl acetate and the solution dried (MgS04).
The solvent was removed by evaporation and the residue crystallised
from ethyl acetate/hexane to give 2-hydroxymethy-4-~2'-(2-
triphenylmethyl-2H-tetrazol-5-yl)biphenyl-4-yl]methoxy)quinoline
(213 mg), as a white crystalline solid, m.p. 183-185C (dec.); NHR:
4.85(s,2H), 5.18(s,2H), 6.70(s,1H), 6.85-6.98(m,6H), 7.15-7.35(m,13H),
7.35-7.58(m,4H), 7.70(dt,1H), 7.95-8.08(m,2H), 8.15~d,1H);
microanalysis, found: C,78.7; ~,4.7; N,10.7X; C43H33N502 requires
C,79.2; H,5.1; N,10.7X.
Bxample 55
Using an analogous procedure to that described in Example 7,
but starting from 6-(tert-butyloxycarbonyl)aminomethyl-2-ethyl-4-([2'
(2-triphenylmethyl-2H-tetrazol-5-yl)biphenyl-4-yl]methoxy)qu1noline,
itself isolated as a foam [NMR: 1.40(t,3H), 1.45(s,9H), 2.96(q,2H),
4.37(d,2H), 4.83(brs,1H), 5.18(s,2H), 6.89-6.99(m,6~),
7.18-7.35(m,14H), 7.40-7.55(m,3H), 7.62(dd,1H), 7.92-8.02(m,3H); 13C
NMR. (benzylic CH2) 70.02] starting from 6-(tert-butyloxycarbonyl)-
aminomethyl-2-ethyl-4-quinolone, there was thus obtained
6-aminomethyl-2-ethyl-4-[(2'-(lH-tetra~ol-5-yl)biphenyl-4-yl)methoxy]-
quinoline dihydrochloride, m.p. 150-153C; NMR (d6-DMSO): 1.45(t,3H),
3.20(q,2H), 4.22-4.33(m,2H), 5.69(s,2H), 7.23(d,2H), 7.52-7.75(m,7H),
8.17(dd,1H), 8.34-8.46(m,2H), 8.62(brs,2H); mass spectrum (~ve FAB,
DMS0/N8A): 437 (M+H)+; microanalysis, found: C,57.9; H,5.7; N,14.4;
Cl,14.5; H20, 3.7Z; C26H2~N60.2.25HCl.1.25H20Ø25(C2~5)20 requires
C,57.9; H,5.5; N,15.0; Cl,14.3; H20, 4-0%-
Using an analogous procedure to that described in ~ y~.,
1955, Coll. Vol. III, pages 374 and 593, the following quinolones used
in Examples 31, 32, 35-47 and 49-54 may be obtained in yields of
20-60%:-
2-Ethvl-6-(tert-butyldimethylsilyloxy)-4-quinolone, m.p. 197-198C;
2-Ethyl-6-methylthio-4-quinolone, m.p. 196-199C;
2-~thyl-6,7-methylenedioxy-4-quinolone, m.p. 250C (dec.);
2-Ethyl-6-(2-fluoroethoxy)-4-quinolone, m.p. 267-269C;
7-Carboethoxy-2-ethyl-4-quinolone, m.p. 220-223C;
2-Ethyl-6-(2,2,2-trifluoroethoxy)-4-qu$nolone, m.p. 260C (dec.);
6-Carboxamido-2-ethyl-4-quinolone, m.p. ~300C;
2-Ethyl-6-trifluoromethoxy-4-quinolone, m.p. 258-260C;
6-Cyano-2-ethyl-4-quinolone, m.p. 290C (dec.);
2-Ethyl-6-formyl-4-quinolone, m.p. 285C (dec.);
6-Dimethylamino-2-ethyl-4-quinolone, m.p. 237-239C;
2-Ethyl-6-nitro-4-quinolone, m.p. >280~C;
6-Cyano-2-methyl-4-quinolone, m.p. 297-8C;
2-Ethyl-6-fluoro-4-quinolone, m.p. 243-245C;
2-Ethyl-6-isopropoxy-4-quinolone, m.p. 174-177C;
2-Trifluoromethyl-4-quinolone, m.p. 210-212C;
2-Methoxymethyl-4-quinolone, m.p. 186-188C;
2-Ethoxymethyl-4-quinolone, m.p. 146C;
2,3-Dimethyl-4-quinolone, m.p. >300C;
2-Ethoxycarbonyl-4-quinolone, m.p. 214-215C;
2-(3,3,3-Trifluoropropyl)-4-quinolone, m.p. 240-242C.
2-Ethyl-7-hydroxymethyl-4-quinolone used in Example 33 was
obtained as follows:-
Lithium aluminium hydride (0.6 g) was added to a solution of
7-carboethoxy~2-ethyl-4-quinolone (2.65 g) in T~F (200 ml) and the
resulting suspension stirred for 2 hours at ambient temperature.
Water (100 ml) was slowly added, followed by ethyl acetate ~100 ml).
The suspension was filtered and washed with ethyl acetate (100 ml).
The phases of the filtrate were separated and the aqueous layer
re-extracted with ethyl acetate (100 ml). The combined organic phases
were dried (MgS04) and evaporated to give a solid which on
recrystallisation from ethyl acetate gave 2-ethyl-7-hydroxymethyl-
4-quinolone as a white powder, m.p. 254-257C.
cç;~
2-Ethyl-6-methylsulphonyl-4-quinolone used in Example 34 was
obtained as follows:-
Oxone (920 mg) was added to a solution of 2-ethyl-
6-methylthio-4-quinolone (220 mg) in methanol (10 ml) and water (1
ml). The suspension uas stirred at ambient temperature for 1 hour
then evaporated to give a yellow solid. Saturated sodium bicarbonate
solution (10 ml) was added and the resulting precipitate collected by
filtration and dried to give 2-ethyl-6-methylsulphonyl-4 quinolone
(150 mg) as a light yellow powder, m.p. 283C.
5-Chloro-2-ethyl-4-quinolone used in Example 48 was obtained
as follows:-
A mixture of 4-benzyloxy-5-chloro-2-ethyl quinoline (A) (1.8
g) and a solution of hydrogen bromide in acetic acid ~45% w/v; 30 ml)
was heated at 100C for 4 hours. The mixture was diluted with
ice/water and basified to pH 9 with 5M aqueous sodium hydroxide
solution, when a solid precipitated. The mixture containing the solid
precipitate was extracted with ethyl acetate (50 ml), and the solid
was then collected by filtration. The solid was purified by flash
chromatography, eluting with methanol/dichloromethane (4:96 v/v) to
give 5-chloro-2-ethyl-4-quinolone as a white solid, m.p. 236-239C;
NMR (d6-DMSO): 1.25(t,3~), 2.58~q,2H), 5.89(s,1~), 7.2(dd,1~),
7.42-7.53(m,2H), 11.4(brs,1H); microanalys1s, found: C,63.2; H,4.8;
N~6-6X; C11H1oNClO requires C,63.6; ~,4.8; N,6.8Z.
The starting material (A) was obtained as follows:-
Sodium hydride (60X dispersion in mineral oil; 1.95 g) was
added to a stirred solution of a mixture of 7-chloro-2-ethyl-
-4-quinolone and 5-chloro-2-ethyl-4-quinolone (43.5:56.5, 10 g) in DMF
(100 ml). The mixture was stirred until evolution of hydrogen had
ceased and benzylbromide ~8.25 g) was added. The mixture was stirred
for 18 hours. The solvent was removed by evaporation and the residue
partitioned between uater (80 ml) and dichloromethane (2 x 100 ml).
The organic layer was washed with saturated sodium chloride solution
(50 ml) and dried (MgS04). The solvent was removed by evaporation and
the resultant yellow oil was purified by flash chromatography, eluting
with increasing concentration of e~hyl acetate in dichloromethane.
_ 40 _ c~ ~ f~
The product was eluted with ethyl acetate/dichloromethane (10:-90 v/v)
to give 4-benzyloxy-5-chloro-2-ethylquinoline as a white solid (2.6
g), m.p. 78-80C; NMR: 1.39(t,3H), 2.94(q,2H), 5.38(s,2H), 6.72(s,1H),
7.30-7.55(m,6H), 8.0(d,1H), 8.13(d,1H).
S 6-(tert-butyloxycarbonyl)aminomethyl-2-ethyl-4-quinolone used in Example 55 was obtained as follows:-
Cobalt (II) chloride (2.87 g) was added to a suspension of
2-ethyl-6-cyano-4-quinolone (1.2 g) in methanol (100 ml~. Sodium
borohydride (2.24 g) was added in small portions to the resulting
purple suspension and the mixture sti~red for 2 hours during which
time a colloidal precipitate appeared. Excess borohydride was
destroy~d by careful acidification with 2M hydrochlor~c acid and the
mixture was then made basic with lM sodium hydroxide solution.
Di-tert-butyl dicarbonate (1.32 g) was added to the resulting slurry
and stirring was continued for 1 hour. The slurry was filtered
through diatomaceous çarth and washed with methanol (100 ml). The
filtrate was evaporated and the residue was extracted with
dichloromethane, dried (MgS04) and evaporated to give a cream foam.
Trituration with diethyl ether gave 6-(tert-butyloxycarbonyl)-
aminomethyl-2-ethyl-4-quinolone (1.2 g) as a cream powder, m.p. 228C
(dec.).
Ex~le 56
Using an analogous procedure to that described in Example 7,
but starting from 4-[(2'-(2-triphenylmethyl-2H-tetrazol-5-yl)-
biphenyl-4-yl)methoxylquinoline (A), there was obtained in 60X yield
4-l(2'-(1~-tetrazol-5-yl)biphenyl-4-yl)methoxy]quinoline
hydrochloride, as a white solid, m.p. 162-164C; NMR (d6-DMSO):
5.7(s,2H), 7.2(d,2H), 7.45-7.8(complex m,7H), 7.9(t,1H), 8.1(dt,1H),
8.3(dt,2H), 9.2(dd,1H); mass spectrum (-ve FAB, DMS0/GLY): 378 (M-H) ;
microanalysis, found: C,66.6; H,4.2; N,16.4%; Cr3~17N50.HCl requires:
C,66.4; H,4.3; N,16.8%.
The starting material (A) was obtained as follows:-
(i) Powdered potassium acetate (17.5 g) was added to a solution
of 5-[2-(4'-bromomethylbiphenylyl)]-2-triphenylmethyl-2H-tetrazole (50
~ s~
- 41 -
g) (obtained as described in European Patent Application, Publication
No. 0291969) and 1,4,7,10,13,16-hexaoxacyclooctadecane (100 mg) in
1,2-dimethoxyethane (DME) (600 ml), and the mixture was heated under
reflux for 20 hours. Insoluble material was removed by filtration,
and the residue triturated w1th a mixture of ethyl acetate and hexane
(1:4 v/v) to give 5-12-[4'-acetoxymethylbiphenylyl]-2-triphenylmethyl-
-2H-tetrazole (B) (41.8 g), as a cream powder, m.p. 119-121C; NMR
(CDC13): 2.1(s,3H), 5.0ts,2~), 6.8-6.95(complex m,8H),
7.2-7.55(complex m,14H), 7.9-8.0(m,1~).
(ii) A solution of compound (B) (41.8 g) in tetrahydrofuran (THF)
(200 ml) was added over a period of 40 minutes to a suspension of
lithium borohydride (4.1 g) in T~F (400 ml) stirred at 0C under an
atmosphere of argon. The mixture was stirred at ambient temperature
for 20 hours and then cooled to 0C. 20% Aqueous citric acid solution
(40 ml)-was added and the mixture uas diluted with saturated sodium
chloride solution ~600 ml). The mixture was extracted with ethyl
acetate (2 x 500 ml) and the extracts were washed with water (500.ml)
and saturated sodium chloride solution (500 ml). The extracts were
dried (MgS04) and volatile material removed by evaporation. The
residue ~as purified by flash chromatography, eluting with ethyl
acetate/hexane (2:3 v/v), to g~ve 5-[2-(4'-hydroxymethylbiphenylyl)]-
-2-triphenylmethyl-2~-tetrazole (C) (17.4 g), as a white solid, m.p.
168-169C (after recrystallisation from a mixture of ethyl acetate and
hexane (1:9 v/v)); NMR (CDC13): 4.6(s,2H), 6.85-7.0(m,6H),
7.2-7.5(complex m,16~), 7.9-8.0(m,1~).
(iii) Sodium hydride (60~ dispersion in mineral oil; 80 mg) was
added to a stirred solution of compound (C) (0.99 g) in DMF (10 ml).
The mixture was stirred until evolution of hydrogen ceased and then
4-chloroquinoline (0.33 g) was added. The mixture was stirred for 24
hours, poured into water (100 ml) and extracted with ethyl acetate (2
x 50 ml). The extracts were washed with water (40 ml) and saturated
sodium chloride s~lution (40 ml) and then dried (MgS04). Volatile
material was removed by evapora~ion and the residue purified by flash
chromatography, eluting with ethyl acetate/hexane (1:1 v/v), to give
4-[(2'-(2-triphenylmethyl-2~-tetrazol-5-yl)biphenyl-4-yl)methoxy]-
quinoline (A) (0.80 g), as a foam; NMR (CDC13): 5.2(s,2H), 6.8(d,1H),
6.85-6.95(m,6H), 7.15-7.55(complex m,17H), 7.7(t,1H), 7.95-8.25(m,3H),
- 42
8.7(d,1H).
Example 57 (Note: all parts by weight)
The compounds of the invention may be administered for
therapeutic or prophylactic use to warm-blooded animals such as man in
the form of conventional pharmaceutical compositions, typical examples
of which include the following:-
a) psule (for oral administration)
Active ingredient * 20
Lactose powder 578.5
o Magnesium stearate 1.5
b) Tablet (for oral administration)
Active ingredient * 50
Microcrystalline cellulose 400
Starch (pregelatinised) 47.5
lS Magnesium stearate 2.5
c) In~ectable Solution (for intravenous administration)
Active ingredient * 0.05 - 1.0
Propylene glycol 5.0
Polyethylene glycol (300) 3.0 - 5.0
Purified water to lOOX
d) Iniectable Suspension (for intramuscular administration)
Active ingredient * 0.05 - 1.0
Methylcellulose . 0.5
Tween 80 0-05
Benzyl alcohol 0.9
Benzalkonium chloride 0.1
Purified water to lOOX
Note: the active ingredient * may typically be an Example described
hereinbefore and will conveniently be present as a pharmaceutically
acceptable acid-addition salt, such as the hydrochloride salt.
Tablets and capsules formulations may be coated in conventional manner
? ,
- 43 -
in order to modify or sustain dissolution of the active ingredient.
Thus, for example, they may be coated with a conventional enterically
digestible coating.
,,3 ~ 7,
Scheme 1
~r ~~
~L~J C~.oR
~,~5
x~ Q
RS ~R~ s
~'1
Clt3~,~H
R5-
"~l
3r ~ ~ ; I r
Note: R = lo~er alkyl, benzyl, phenyl; Tr = triphenylmethyl (trityl)
~eagents: a) BuLi/T~F; ZnC12/Et O; Pd(Ph3P)4
b) Bu3Sn.N~/toluene; ~Cl/toluene
c) Tr.Cl/E~3N~C~2cl7
d) N-bromosuccinlmi~e/azoisobutyronitrile/CC14
~'~iJ ~9~2~,
ChPmical Pormulae
~5
R~Rs Ia
~1~
~L
Rs
_ ~6 - ,~d ~ a ~
Chemical Pormulae
(continued)
R~
R o
~lal X ~o.oR~
~F2~s
Hal ~,X Nr3N~L
R~
Rc~
R3~ R
YII ~