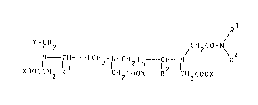Note: Descriptions are shown in the official language in which they were submitted.
,; ` `. "' '. fi S
-- 1 --
US~ OF AMIDE COMPLEX COMPO~DS
Cross-Reference to Related Ap~lications
This application is a continuation-in-part of Serial
No. 07/495,803, filed March 14, 1990, which is a
5 continuation of Serial No. 07/100,6~, filed September
24, 1987 (abandoned), both of which are entirely
incorporated by reference herein.
In addition, this application is related to U.S.
Application Serial Nos. 07/078,~07 (filed July 28, 1987),
07/063,355 (filed June 18, 1987), 07/020,301 (filed March
2, 1987), 07/020,300 (filed ~arch 2, 1987), 07/020,993
(filed March 2, 19~7), 07/020,992 (filed March 2, 1987),
06/936,055 (filed November 28, 1986), 06/876,497 (filed
June 20, 1986), and 06~627,143 (filed July 2, 1984), each
of which is a divisional, continuation or C-I-P of
- 06/573,184 (filed January 23, 1984), now U.S. Patent No.
4,647,447, which is a C-I-P of 06/401,594 (filed July 26,
1982), and this application is a C-I-P of all of said
applications directly or indirectly and all of which
applications are entirely incorporated by reference
herein.
Summary of the Invention
This invention relates to physiologically compatible
amide complex compounds and the use there~f in the
production of agents for organ-specific N~R diagnostics
and for cases involving patients with ren~l
insufficiency, as well as NMR msthods utilizing these
compounds.
European Patent Application Publication Number
263,059 claims compounds of Formula I R3
Y--CH2 CH2CO--N~ 4
N - -- CH--(CH2~1 -CH2) n CIH I (I)
XOOCCH2 R CHzCOOX R CH2COOX
wherein
nis o, 1 or 2,
10 Rl and R2 independently are hydrogen atoms, lower alkyl
groups, phenyl groups, benzyl groups or, if n
is O, jointly can also form a trimethylene or a
tetramethylene group,
R3 is a saturated, unsaturated, straight-chain or
branched-chain or cyclic aliphatic hydrocarbon
residue of up to 16 carbon atoms and, if R4 is a
hydrogen atom, at least one R3 :i.s a cycloalkyl
group, or an aryl or aralkyl group optionally
substituted by one or several di C1-C6-
alkylamino groups or by one or several C1-C6-
alkoxy groups,
R4 is a hydrogen atom, or a saturated,
unsaturated, straight-chain or branched-chain
or cyclic hydrocarbon residue of up to 16
carbon atoms, or
R3 and R4 jointly form a satuxated or uns~turated 5- or
6-membered ring which is option~lly substituted
by one or several of C1-C6-alkyl, C1-C5-hydroxy-
alkyl, optionally hydroxylated ~r C1-C6-alkoxy-
lated Cz-C6-acyl, hydroxy, carbamoyl, carbamoyl-
substituted C1-C6 alkyl residue, carbamoyl
substituted on the carhamoyl nitrogen by one or
two C1-C6-alkyl residue(s) -- which can also
form a ring optionally containing an oxy-gen
atom -- or C1-C6-acylamino or C1-C6-alkyl-amino;
this 5- or 6-membered ring opti~nally
containing a furkher nitrogen, ~xygen or sulfur
atom, or carbonyl group,
-- 3 --
X means a hydrogen atom and/or a metal ion
equivalent, R 3
Y is a COOX or ~ON\ group,
as well as their salts with organic and/or inorganic
bases.
Compounds havirlg the anion of one oF
these complex-forming amides and one or several
central ions of an element of atomic numbers 21-29,
31, 32, 38, 39, 42-44, 49, 57-83 and optionally one
or several cations of an inorganic and/or organic
base or amino acid are suited for the production of
NMR, X-ray and radiology diagnostic media.
It has now been found that surprisingly an
unexpected pharmacokinetic behavior is displayed by
compounds of this general Formula I if these compounds
contain at least one element of atomic numbers 21-29,
42, 44 or 58-70, i.e.,at least two of the substituents
X must stand for a metal ion equivalent of these ele-
ments, but in particular compounds of general
Formula I wherein
n is the number 1,
1 2
R a n d R are hydrogen atoms,
R3 is a saturated, unsaturated, straight-chain
or branched-chain or cyclic aliphatic
hydrocarbon residue of u~ to 16 carbon atoms
and, if R is a hydrogen atom, at least one R3 is a cycloalkyl
group, or an aryl or aralkyl group optionally
substituted by one or several di-C1-C6-
alkylamino groups or by one or several
C1-C6-alkoxy groups,
R4 is a hydrogen atom or a saturated, unsaturated,
straight-chain, branched-chain or cyclic
hydrocarbon residue of up to 16 carbon atoms,
X is a hydrogen atom and/or a metal ion
equivalent of at least one element of
atomic numbers 21-29, 42, 44 or 58-70,
R
/
Y is a COOX or CON~ group,
R
with the proviso that at least two of the substituents
X stand for a metal ion equivalent,
as well as their salts with organic and/or inorganic
bases.
Thus, for example, MAGNEVIST ~, thus far the
only NMR contrast medium permitted worldwide, is
distributed upon intravenous injection in an extra-
cellular fashion and is excreted via the kidneysby glomerular secretion. Passage of intact cell
membranes and extrarenal excretion are practically
not at all observed.
MAGNEVIST ~ is especially well suited for
the diagnosis of pathological regions (for example,
inflammations, tumors, infarctions, etc.).
Contrast media exhibiting an at least
partial extrarenal excretion would be desirable,
especially for patients with limited kidney function
(renal insufficiency) where MAGNEVIST G~is excreted
only very slowly and, in part, can be removed from the
organism only with the aid of a dialysis device.
r~ J ~ J
Consequently, there is a need ~or NMR contrast
media e~hibiting a di~ferent pharmacokinetic behavior and
thus higher organ specificity than MAGNEVIST~.
Accordingly, it is an object o~ this invention
to provide such compounds and media and methods of using
same.
Upon further study of the specification and
appended claims, further objects and advantages of this
invention will become apparent to those skilled in the
art.
It has been found that the above-mentioned
compounds surprisingly show the desired property:
renal elimination as well as excretion with the feces.
Surprisingly, elimination via the gallbladder,
however, is not the only extrarenal path of elimination:
in NMR studies on rats, upon intravenous administration
of the compounds of this invention, a contrast enhance-
ment of the gastrointestinal tract has also been
unexpectedly observed. The kidneys, as well as
implanted tumors, are likewise visualized with
improved contrast.
Elimination ~secretion) by way of the stomach
~` has the advantage that contras-ting of abdominal struc-
tures (e.g. pancreas) from the gastrointestinal tract
is made possible, with a simultaneous contrast enhance-
ment of pathological processes (tumors, inflammations).
Imaging of the renal system, of the liver and gallbladder,
and the bile ducts can moreover likewise be achieved.
Besides the improved visualization of ulcers and
stomach carcinomas, it is also possible to perform
studies on gastric acid secretion with the aid of
imaging procedures.
Accordingly, by making the compounds of this
invention available, help can be e~tended to patients
with renal insufficiency as well as patients suffering
from gastrointestinal disorders (at least 10% of the
population in the Western industrial countries).
-- 6
patients suspected of harboring such disease, must
submit to diagnos-tic tests. At present, two methods
suitable fo~ this purpose are utilized above all:
endoscopy and X-ray diagnos-tics with the aid of barium
contrast media.
These tests exhibit various drawbacks: they
carry the risk of radiation stress, cause trauma, are
connected with inconveniences, occasionally even with
risks for the patient, and thus can evoke psychological
stress. In most cases, these tests must be repeated;
their performance is relatively complicated, require the
patient's active cooperation (e.g. assumption of a
specific bodily attitude) and frequently cannot be
employed in case of frail and high-risk patients.
The object of providing novel diagnostic
methods for the identification and localization of
gastrointestinal diseases, which methods do not exhibit
these drawbacks, has thus likewise been attained by the
complex compounds and agents as mentioned above.
Their pharmacokinetics permit, even wi-thout
specific measures, an improvement in the diagnosis of
numerous diseases. The complexes for the most part
are excreted again in unchanged form and rapidly
so that, especially also in case of using relatively
toxic metallic ions, no damaging effects are observed
even at high dosage.
The practical use of the novel ccmplexes is
also facilitated by their favorable chemical stability.
~-" f ~ r ," ~
Compounds of formula I, wherein X is hydrogen,
are called "complexing agents", and those wherein at
least two of the substituents X are a met~l ion
equivalent are called "metal complexes~'.
For use in NMR diagnostics, the central ion of
the complex salt will be paramagnetic. These are, in
particular, the divalent and trivalent ions of the
elements of atomic numbers 21-29, 42, ~4 and 58-70.
Suitable ions include, for example, the chromium(III),
manganese(II), iron(II), cobalt(II), nickel(II),
copper(II), praseodymium(III), neodymium(III),
samarium(III) and ytterbium(III) ions. On account of
their very strong magnetic moment, the gadolinium(III~,
terbium(III), dysprosium(III), holmium(III); erbium(III)
and iron(III) ions are espscially preferrad.
Suitable alkyl substituents R1 and R2 are
hydrocarbons of 1-8, preferably 1-4 carbon atoms, such
as, for example, methyl, ethyl, propyl, isopropyl, n-,
sec- or tert.-butyl, isobutyl, and all is~mers of pentyl,
hexyl, heptyl and octyl.
Suitable aliphatic substituents R3 and R4 are
saturated (e.g., alkyl), unsaturated (e.g., alkenyl),
straight-chain or branched-chain or cycli- hydrocarbons
of up to 16 carbon atoms, pre~erably 1-10 C atoms, most
preferably saturated hydrocarbons of 1-10 carbon atoms,
especially saturated hydrocarbons of 1-5 -arbon atoms.
Examples include methyl, ethyl, propyl, i~opropyl, butyl,
isobutyl, pentyl, cyclopentyl, cyclohexyl, propenyl, etc.
Other suitable groups are the other alkyl
groups mentioned above for R1 and R2 and isomers
containing 9-16 C atoms as well as their ~lkenyl
counterparts.
When R4 is a hydrogen atom, at :east one R3 is
preferably C6-C10-aryl or C6-C10-Ar-C1-C6-alcyl group, e.g.,
phenyl or benzyl group, optionally substituted by one or
several ~e.g., up to three) di-C1- to C6-alkylamino groups
,
or by one or several (e.g., up to three) C1- to C6-alkoxy
groups.
In addition, when ~' i6 a hydrogen atom, R3 can
also preferably be a cycloalkyl group, as mentioned
above. The cycloalkyl group generally contains 3-16
carbon atoms, preferably ~-7 carbon atoms.
The heterocyclic 5- or 6-membered ring formed
by R3 and R4 with inclusion of the amide nitrogen can be
saturated, unsaturated andjor substituted and can
optionally contain a nitrogen, oxygen or sulfur atom or
carbonyl group.
The heterocycle can be substituted by hydroxy,
C1-C6-alkyl, e.g., methyl, ethyl, propyl, isopropyl,
butyl, C1-C5-hydroxyalkyl, e.g., hydroxymethyl,
hydroxyethyl, or by C2-C6-acyl (e.g., alkanoyl), for
example acetyl, propionyl, which can, if desired, be
substituted by hydroxy or C1-C6-alkoxy, e.g., methoxy,
ethoxy, etc.
A further substituent that can be mentioned is
carbamoyl, linked to the heterocycle dire-tly or
separaked by a C1-C6-alkylene group, for example
methylene, ethylene, propylene, and whicn can also be
substituted at the nitrogen, if desired, by one or two C1-
C6-alkyl residue(s), e.g., methyl, ethyl, propyl,
isopropyl, ~tc. The alkyl groups can, optionally, form a
ring, such as, for example, a pyrrolidine or piperidine
ring. The carbamoyl nitrogen can also be part of a
morpholine ring, i.e., the latter ring can have an 0
atom.
Another possible substituent on the heterocycle
that can be mentioned is an optionally C1-C6-alkylated or
C1-C6-acylated (e.g., alkanoylated~ primary or secondary
amino group, such as, for example, the methyl-, ethyl-,
acetyl-, propionyl-, amino-, etc., group.
If the heterocycle is substituted, the total
number of substituents is l to 3.
t ~
Suitable heterocycles are, for example: the
pyrrolidinyl, piperidyl, pyrazolidinyl; pyrrolinyl,
pyrazolinyl, piperazinyl, morpholinyl, imidazolidinyl,
oxazolidinyl, thiazolidinyl rings.
If not all of the acidic hydrogen atoms are
substituted by the central ion, then one, several, or all
remaining hydrogen atom(s) can be replace~ by cations of
inorganic and/or organic bases or amino acids. Suitable
inorganic cations include, for example, the lithium ion,
the potassium ion, the calcium ion and, in particular,
the sodium ion. Suitable cations of organic bases
include, inter alia, those of primary, secondary or
tertiary amines, e.g., ethanolamine, diethanolamine,
morpholine, glucamine, N,N-dimethylglucamine and
especially N-methylglucamine. Suitable c~tions of amino
acids include, for example, those of lysine, of arginine,
and of ornithine.
Introduction of amide groups for the production
of the complexing agents, i.e., of compounds of general
Formula I wherein X means hydrogen, takes place by
conventional partial conversion of activated carboxyl
groups into amide groups of the respectively suited
- tetra-, penta- and hexacarboxylic acids -- in
correspondence with the desired final pro~uct. All of
the synthesis pathways known to a person skilled in the
art are suitable for this procedure.
-- 10 --
One example is the reaction
of -the anhydrides or esters of gener~l E~ormulae II,
IV, V and VI:
VOC-C~ ~CH2-COV
N-Cj H- ( CH2- 1 -CH2 ) n-CI H-rl \ ( II )
ZOC-CH2 Rl CH2COOH R2 C~z-COZ
HOOC-C~ ~CH2-CO~l
/ N-CH- ( CH2-N-CH2 ) -CH-N \ ( IV )
R500C-CH2 Rl CH2COOH R CH -COZ
HOOC-CH2 ~ CH2COOH
~ N-CH-(CH2-N-CH2)n-~H-N ~ (V)
R500C-CH2 Rl CH2COOH ~2 CH2COOH
HQQC- CH `
.j / \
N -CH - t CH2-N -CH2 ) n-CH -N ( V I ) .
R OOC-CH2 ¦1 CH2COOH R ~~
wherein
Rl, R2 and n have the above-mentioned meanings,
V and Z jointly mean an oxygen atom, or V is a
hydroxy group and Z is the group.ing oR5~ wherein
R is a C1~C6~a1ky1 residue,
hs ~.3 .'; C.J ~ J
with amines oE general Formula III
HN ~ I I I ),
wherein ~3 and R4 have the meanings given above.
Examples of suitable amines include: dimethyl-
amine, diethylamine, di-n-propylamine, diisopropylamine,
di-n-butylamine, diisobutylamine, di-sec-butylamine,
N-methyl-n-propylamine, dioctylamine, dicyclohexyl-
amine, N-ethylcyclohexylamine, diisopropenylamine,
benzylamine, aniline, 4-methoxyaniline, 4-dimethyl-
aminoaniline, 3,5-dimethoxyaniline, 4-methoxybenzyl-
amine, morpholine, pyrrolidine, piperidine, N-methyl-
piperazine, N-ethylpiperazine, N-(2-hydroxyethyl~-
piperazine, N-(hydroxymethyl)piperazine, piperazino-
acetic acid isopropylamide, N-(piperazinomethyl-
carbonyl)morpholine, N-(piperazinomethylcarbonyl)pyr-
rolidine, 2-(2-hydroxymethyl)piperidine, 4-(2-hydroxy-
ethyl)piperidine 7 2-hydroxymethylpiperidine,
4-hydroxymethylpiperidine, 2-hydroxymethylpyrrolidine,
3-hydroxypiperidine r 4-hydroxypiperidine, 3-hydroxy-
pyrrolidine, 4-piperidone, 3-pyrroline, piperidine-3-
carboxylic acid amide, piperidine-4-carboxylic acid
amide, piperidine-3-carboxylic acid diethylamide,
piperidine-4-carboxylic acid dimethylamide, 2,6-
dimethylpiperidine, 2,6-dimethylmorpholine, N-acetyl-
piperazine, N-(2-hydroxypropionyl)piperazine,
N-(3-hydroxypropionyl)piperazine, N-(methoxyacetyl)-
piperazine, 4-(N-acetyl-N-methylamino)piperidine,
-
;
~Z~ u ~-1 ,J ~.., ,~
-- 12 --
piperidine-4-carboxylic acid (3-oxapentamethylene)amide,
piperidine-3-carboxylic acid 13-oxapentamethylene)amide,
N-~N',N'-dimethylcarbamoyl3piperazine, pyrazoline,
pyrazolidine, imidazoline, oxazolidine, thiazolidine, etc.
The saponification of any ester groups that
may still be present takes place according to methods
known to one skilled in ~he art, for example,by alkaline
hydrolysis.
The acid anhydrides of general Formula II
can be prepared conventionally, for example in accord-
ance with the mode of operation disclosed in U.S.
Patent 3,660,388 or DOS 1,695,050,
with acetic anhydride in pyridine. However, in certain
instances,it is especially advantageous to conduct the
lS step of splitting off water in a gentle fashion with
carbodiimides in a suitable solvent, such as, for ex-
ample dimethylformamide or dimethylacetamide.
The preparation of the monoanhydrides of gen-
eral Formula VI will be described by using as the
example the monoanhydride of diethylenetriaminepenta-
acetic acid ethyl ester starting with the monoethyl
ester of DTPA (J. Pharm. 5ci. 68 : 194, 1979):
N3-(2,6-Dioxomorpholinoethyl)-N6-(ethoxycarbonyl-
methyl)-3,6-diazaoctanedioic Acid
_________________________________________________
A suspension of 21.l g (50 millimoles) of
N3,N6-bis(carboxymethyl)-N9~(ethoxycarbonylmethyl)-
3,6,9-triazaundecanedioic acid in 250 ml of acetic
anhydride is agitated for 3 days at room temperature
after adding 42.2 ml of pyridine. Then the precip-
itate is suctioned off, washed three times with re-
spectively 50 ml of acetic anhydride and subsequently
~,J i~ ~.,J ~ ~, 3 ~1
- 13 ~
stirred for several hours with absolute diethyl ether.
Af-ter suctioning off the product, washing same with
absolute diethyl e~her and drying under vacuum at
40 C, 18.0 g (= 89% of theory) of a white powder is
obtained, mp 195-196 C.
Analysis (based on anhydrous substance):
Calculated: C 47.64 ll 6.25 N 10.42
Found: C 47.54 El 6.30 N 10.22
The reaction of the acid anhydrides to the
amides can be performed in the liquid phase. Suitable
reaction media include, for example, water, dipolar aprotic
solvents, such as acetonitrile, N-methylpyrrolidone,
dimethylformamide, dimethylacetamide, and the like, or
mixtures thereof. The reaction temperatures range
between about 0 C and 100 C, temperatures of
~0-80 C being preferred. The reaction periods xange
between 0.5 hour and 2 days, preferably between 1 hour
and 36 hours.
The esters of general Formula V are produced
conventionally, for example according to the processes
described in R.A. Guilmette et al., J. Pharm~ Sci.
68 : 194 (1979).
Aminolysis of the esters takes place in the
liquid phase, for example in a suitable higher-boiling
solvent, such as dimethylformamide, dimethylacetamide
or dimethyl sulfoxide. The reaction temperatures are
around 20 C to 200 C~ temperatures of 100-180 C
being preferred. The reaction times range between
2 hours and 2 days, reaction periods of between 4 houxs
and 36 hours being the preferred ones.
~ J~3~
1 'I -
Moreover, all methods known to a person skilled
in the art for converting carboxyl groups into amide
groups can be employed for the synthesis of the complex-
ing agents of Formula I according to this
invention, For example, the method by Krejcarek
and Tuc~er, Biochm. Biophys. Res. Comrnun. 77 : 581
(1977) via mixed anhydrides.
The resultant compounds of Formula I
wherein X is a hydrogen atom represent complex-forming
media. They can be isolated and purified, or they can
be converted without isolation into metal complexes of
general Formula I wherein at least two of the substi-
tuent X mean a metal ion equivalent.
The metal complexes are prepared conven~ionally by the methods
disclosed in Patents EP 71564, EP 130934 and DOS
3,401,052,by dissolving or suspending the metal oxide
or a metal salt (e.g.,the nitrate, acetate, carbonate,
chloride or sulfate) of the element of atomic numbers
21-Z9, 42, 44 or 58-70 in water and/or a lower alcohol
(such as methanol, ethanol or isopropanol) and reacting
with a solution or suspension of the equivalent amount
of the complex-forming acid of Formula I wherein
X means a hydrogen atom and subsequently, if desired,
substituting any acidic hydrogen atoms of acid groups
present by cations of inorganic and/or organic bases
or amino acids.
Neutralization is herein effected with the
air of inorganic bases (for example, hydroxides,
carbonates or bicarbonates) of, for example, sodium
potassium, lithium and/or organic bases such as, inter
alia, primary, secondary and tertiary amines, such as,
for example, ethanolamine, morpholine, glucamine,
N-methyl- and N,N-dimethylglucami~e, as well as basic
amino acids, such as, for example, lysine, arginine and
ornithine.
f~J ~ 2 s~
- 15 -
In order to prepare the neutral complex com-
pounds, it is possible, for example, to add to the
acidic complex salts in an aqueous solution or suspen-
sion such an amount of the desired bases that the
neutral point is reached. The resultant solution can
subsequently be evaporated to dryness under vacuum.
It is frequently advantageous to precipitate the thus-
formed neutral salts by adding water-miscible solvents,
such as, for example, lower alcohols (methanol, e-thanol,
isopropanol, etc.), lower ketones (acetone etc.),
polar ethers ~tetrahydrofuran, dioxane, 1,2-dimethoxy-
ethane, etc.), and to obtain in this way crystallized
products which can be readily isolated and easily
purified. It has proven to be especially advantageous t~
add the desired base to the reaction mixture as early
as during the complexing reaction, thereby saving a
process step.
If the acidic complex compounds contain
several free acidic groups, then it is frequently ex-
pedient to prepare neutral mixed salts containinginorganic as well as organic cations as the counterions.
This can be done, for example, by reacting
the complexing acid in an aqueous suspension or solu-
tion with the oxide or salt of the element yieldin~
the central ion and with half the amount of an organic
base needed for neutralization, isolating the thus-
formed complex salt, purifying same if desired, and
then combining same for complete neutralization with
the required amount of inorganic base. The sequence
of adding the bases can also be reversed.
The diagnostic media are likewise produced
in a manner known per se by suspending or dissolving
the complex compounds of this invention -- optionally
after adding the additives customary in galenic
pharmacy -- in an aqueous medium and subsequently
, . ' " ,~`. !,; ` C " ' ~
- 16 -
sterilizing the suspension or solution, if desired.
Suitable additives are, for example, physiologically
acceptable buffers (such as, e.g., trome-thamine), small
additions of complexing agents (such as, e.g., diethylene-
triaminepentaacetic acidj or, if necessary, electrolytessuch as, for example, sodium chloride or, if needed
antioxidants, such as ascorbic acid, for example.
If, for enteral administration or other
purposes, suspensions or solutions of the media of
this invention in water or a physiological saline solu-
tion are desirable, they can be mixed with one or several
auxiliary agents customary in galenic pharmacy (for
example methylcellulose, lactose, mannitol) and/or
tensides (e.g., lecithins, TWEENS(~), MYRJ(~).and/or
lS flavoring substances for taste improvement (e.g.
ethereal oils).
In principle, it is also possible to prepare
the diagnostic media of this invention even without
isolation of the complex salts. In any event, special
care must be directed toward effecting the chelate
formation so that the salts and salt solutions accord-
ing to this invention are practically devoid of
toxically active metal ions that are not complexed.
This can be ensured, for example, with the
aid of color indicators, such as xylenol orange, by
control titrations during the manufacturing process.
Consequently, the invention also relates to processes
for preparing the complex compounds and their salts.
The final safety feature resides in purification of
the isolated complex salt.
For nuclear spin tomography diagnostics in accord-
ance with this invention, the diagnostic media are ad-
ministered in a dosage of 1 ~mol/kg to 5 mmol/kg, prefer-
ably 10 ~mol to 0.5 mmol/kg of the complex sa3t according
to the invention. rn case of intravenous injection, aque-
ous formulations are ussd with a concentr~tion of 50
~mol/l to 2 mol/l, preferably 100 mmol/l to 1 mol/l. Rec-
tal as well as oral administration is preferably per-
formed with solutions of a concentration ~f 0.1 mmol to
100 mmol/l. The volumes administered ran~e from about
5 ml to 2 1, in dependence on the diagnostic problem.
Thus, the diagnostic media are intended for enteral and
parenteral administration to mammals, including humans.
The diagnostic media fulfill thQ variegated
requirements for suitability as contrast media for
nuclear spin tomography. Thus, they are ~xcellently
suited, upon oral or parenteral administration, for
improving the information content of the image obtained
with the aid of the nuclear spin tomograph, by increasing
the signal intensity. They show furthermore the high
efficacy necessary for burdening the body with minimal
amounts of foreign substances, and the go3d compatibility
required for maintaining the noninvasive -haracter of the
tests.
The high water solubility of the diagnostic
media permits production of highly concentrated solutions
so that the volume load on the circulation is maintained
within tolerable limits and dilution by b~dy fluids is
compensated, i.e., NMR diagnostic agents must show 100 to
1000 times the water solubility of that f~r in vitro NM~
spectroscopy. Furthermore, the agents of this invention
display not only high stability so that r~lease or ex-
change of the -- toxic per se -- ions not covalently
bound to the complexes takes place only e~tremely gra-
dually within the time wherein the contra~t media are
again entirely eliminated.
The agents of this invention can also be
utilized for radiation therapy. Thus, complexes of
gadolinium are excellently suited for neutron capture
therapy due to the large capture cross section. If the
medium of this invention is intended for use in the ver-
sion of radiation therapy proposed by R.L. Mills et al.
[Nature 336 : 787 (1988)~, then the centr~l ion must be
derived from a Mossbauer isotope, such as, for example,
s7Fe or ls1Eu
In their administration, the ag3nts of this
invention can also be given together with a suitable
vehicle, such as, for example, serum or physiological
saline solution and/or together with a protein, such as,
for example, human serum albumin. The dosage herein is
dependent on the type of cellular disorder and on the
properties of the metal complex utilized.
Consequently, the objective has been achieved
over-all of opening up novel possibilities in diagnostic
medicine by means of the recited complex -ompounds.
Without further elaboration, it is believed
that one skilled in the art can, using th~ preceding
description, utilize the present invention to its fullest
extent. The following preferred specific embodiments
are, therefore, to be construed as merely illustrative,
and not limitative of the remainder of the disclosure in
any way whatsoever.
In the foregoing and in the following examples, all
temperatures are set forth uncorrected in degrees Celsius
and unless otherwise indicated, all parts and percentages
are by weight.
The entire disclosures of all applic~tions, patents
and publications, cited above and below, ~nd of
corresponding application Federal Republi- of Germany
P 39 27 444.6, filed August 16, 1989, are hereby
incorporated by reference.
(~
- 19 -
Example 1
(a) 6-Carboxymethyl-3-ethoxycarbonylme-thyl-9-phenyl-
aminocarbonylmethyl-3,6,9-triazaundecanedioic Acid
__________________________________________________
At 0 C, 2.42 g (6 mmol) of N3-(2,6-dioxo-
morpholinoethyl)-N6-(ethoxycarbonylmethyl)-3,6-diaza-
octanedioic acid is combined in DMF with 4.16 ml
(3.04 g, 30 mrnol~ of triethylamine and 559 mg (6 mmol)
of aniline and stirred overnight at room temperature.
Then the clear solution is concentrated under vacuum
and the residue chromatoyraphed on silica gel with
dichloromethane/methanol/acetic acid/water (5:3:1:1)
as the mobile phase. The combined fractions are passed
over approximately 10 ml of "Amberlite" IR 120
(H+ form) and the acidic eluate is concentrated.
Yield: 2.35 g (79~)
Calculated: C 53.21 H 6.50 N 11.29
Found: C 53.01 H 6.55 N 11.26
(b) 3,6,9-Tris(carboxymethyl)-3,6,9-triazaundecane-
dioic Acid Phenyl Monoamide
_____._________________________________________
1.5 g (3 mmol) of the ethyl ester described
in Examplel(a) is dissolved in 2N NaOH and stirred for
2 hours at room temperature. A pH of 7 is set by addin~
"Amberlite" IR 120 (H form), the mixture is filtered
off, and the neutral solution is passed over about
16 ml of "Amberlite" IR 120 (H ). The acidic eluate
is concentrated and additionally dried under vacuum
at 50 C.
Yield: 1.3 y (92.5%)
Calculated: C 51.27 H 6.03 N 11.96
Found: C 51.19 H 5.99 N 11.91
iJ IJ ~1 ?.~
20 -
(c) Gadolinium Complex of 3,6,9-Tris(carboxymethyl)-
3,6,9-triazaundecanedioic Acid Phenyl Monoamide
________________________________________________
936 mg (2 mmol) of the complexing acid
obtained according to Example llb) is dissolved in
5 about 40 mi of water and combined at 80 C with 362 mg
(1 mmol) of Gd2O3. After 30 minutes, the almost clear
solution is filtered off and the filtrate is freeze-
dried.
Yield: 1.23 g (98.8%), based on anhydrous substance.
Calculated: C 38.S7 H 4.05 N 9.00 Gd 25.25
Found: C 38.33 H 4.10 N 9.03 Gd 24.99
Example 2
Preparation of a Solution of the N-Methylglucamine Salt
of the Gadolinium Complex of 3,6,9-Tris(carboxymethyl)-
3,6,9-triazaundecanedioic Acid Phenyl Monoamide
_______________________________________________________
1.87 g (3 mmol) of the gadolinium complex of
3,6,9-tris(carboxymethyl)-3,6,9-triazaundecanedioic
acid phenyl monoamide (Example 1) is suspended in 5 ml
of water pro injectione and combined with 0.586 g
(3 mmol) of N-methylglucamine, thus dissolving the
complex. The mixture is filled up with water to 10 ml,
the solution is introduced into a vial and subjected
to heat sterilization.
Tl relaxation (ltmmol sec) is:
25 in water: 3.79 + 0.16
in plasma: 5.45 + 0.68
- 21 -
~xample 3
Preparation of a So]ution of the Sodium Salt of -the
Gadolinium(III) Complex of 3,6,9-Tris(carboxymethyl)-
3,6,9-triazaundecanedioic Acid Phenyl Monoamide
_____________________________________________________
62.27 g (0~1 mol) of the gadolinium complex
obtained according to Example l(c) is suspended in
800 ml of water pro injectione (p.i.) and dissolved
at pH 7.2 by dropwise addition of normal sodium
hydroxide solution. After adding 0.2 g of tromethamine,
the mixture is filled up with water p.i. to 1000 ml, the
solution is dispensed into bottles and heat-sterilized.
Example 4
(a) 3,6,9-Tris(carboxymethyl)-3,6,9-triazaundecanedioic
Acid (N,N'-Diphenyl)diamide
___________________________________________________
42.88 g (120 mmol) of DTPA bis-anhydride
is suspended in 330 ml of dimethylormamde and cooled
in an ice bath under agitation to about 5 C. Within
50 minutes, a solution of 32.9 ml (360 mmol) of aniline
in 30 ml of dimethylformamide is added dropwise. The
mixture is stirred for another hour in an ice bath,
then overnight at room temperature. ~ter this time, a
slightly turbid solution has formed. The solvent is
removed under vacuum, and the smeary residue is
triturated with diethyl ether to remove traces of sol-
vent. The residue is combined with 500 ml of water anddissolved by adding 20 ml of llN sodium hydroxide
solution. The solution is combined with 3.5 g of
active carbon, filtered, and freeze-dried, thus
obtaining 73.9 g of the sodium salt of the title
compound as a powder.
~ J~3~) ~
- 22 -
(b) Gadolinium Comple~ of 3,6,9-Tris~carboxymethyl)-
3,6,g-triaZaUndeCanediOiC ACid IN,N~-DiPhenY1)-
diamide
__________________._____________________________
10.3 g of gadolinium oxide (30 mmol) is
heated under reflux with 10. 6 ml of glacial acetic
acid and 150 ml of water for 20 minu-tes. The solution
is filtered through a 0.1 ~m membrane filter, combined
with 35 . 3 g (60 mmol) of the ligand obtained accord-
ing to 4(a), and heated for 90 minutes to 80 C. The
solution is stirred with 2.1 g of active carbon for
30 minutes, filtered., and then passed in succession
over an anion exchange column (200 ml IRA-410) and
- 100 ml of cation exchanger (IRC-50). The eluates from
the columns a filtered through a 0.1 ~m membrane
filter and freeze-dried, thus obtaining 17.6 g of the
title compound as a white powder..
Analysis:
Calculated: C 44.75 H 4.33 Gd 22.54 N 10.04
Found: C 44.60 H 4.44 Gd 22.42 N 9.89
Example 5
(a) 3,6,9-Tris(carboxymethyl)-3,6,9-triazaundecanedioic
Acid (N,N'-Dibenzyl)diamide
___________________ _________________.. ____________
42.88 g (120 mmol~ of DTPA bis-anhydride is
suspended in 330 ml of dimethylformamide and cooled
in an ice bath under agitation to 5 C. Within one
hour, a solution of 39.3 ml (360 mmol) of benzylamine
in 30 ml of dimethylformamide is added dropwise. The
mixture is stirred for another hour in the ice bath,
then overnight at room temperature. After removal of
the solvent under vacuum, the residue i5 triturated with
- 23 -
diethyl ether, co~bined with 500 ml of water, and dis-
solved by adding 20 ml of llN sodium hydroxide solution.
A~ter freeze-drying, 71 g of -the sodium salt of the
title compound is obtained as a light-yellow powder.
(b) Gadolinium Complex of 3,6,9-Tris~carboxymethyl)-
3,6,9-triazaundecanedioic Acid (N,N'-Dibenzyl)-
diamide
________________________________________________
10.9 g of gadolinium oxide (30 mmol) is
heated under reflux with 10.6 ml of glacial acetic
acid and 150 ml of water for 20 minutes. The solution
is filtered o~er a 0.1 ~m membrane filter, combined
with 37 g (60 mmol) of the ligand obtained according
to 5(a), and heated for 90 minutes to 80~ C. The
solution is stirred.with 3 g of active carbon for
30 minutes, filtered, and then passed in succession
over an anion exchange column (200 ml IRA-410) and 100 ml
cation exchange column (100 ml IRC-50). The eluates
are filtered through a 0.1 ~m membrane filter and
freeze-dried, thus obtaining 18 g of the title com-
pound as a white powder.
Analysis:
Calculated: C 46.33 H 4.72 Gd 21.66 N 9.65
Found: C 46.46 H 4.49 Gd 21.50 N 9.81
f; '! f ~ ~I
~ w ~J
- 2~ -
Example 6
(a) 6-Carboxymethyl-3-ethoxycarbonylmethyl-9-benzyl-
aminocarbonylmethyl-3,6,9-triazaundecanedioic Acid
__________________________________________________
5.04 g (12.5 mmol) of N3-(2,6-dioxomorpholino-
ethyl)-N~-(ethoxycarbonylmethyl)-3,6~diazaoctanedioic
acid is suspended în 65 ml o~ dimeth~lformamide, stirred
in an ice bath and combined, in succession, with
8.7 ml (62.5 mmol) of triethylamine and 1.34 g
(12.5 mmol) of benzylamine. The mixture is then
stirred for another 2 hours in the ice bath, then
overni~ht at room temperature. The solvent is removed
by vacuum distillation, the residue is agitated with
diisopropyl ether, suctioned off, and dried. For
further purification, the residue is dissolved in such
a quantity of llN sodium hydroxide solution that a
pH of 7 is just attained. To this mixture is added
5 g of silica gel, the suspension is dried under vacuum,
and the residue is introduced into a column of 350 g
of silica gel charged with a mixture of chloroform/meth-
anol/glacial acetic acid/water (1750/1050/350/350).
The product is eluted with the same solvent and, after
evaporation of the solvent, 4.98 g of a colorless,
smeary substance is obtained which is dissolved in
35 ml of water and passed over a column with 35 ml of
the cation e~changer"IR 120". The column is washed
with 70 ml of water, the combined eluates are evap-
orated under vacuum, and the residue is triturated
with diethyl ether, thus obtaining 2.65 g of the title
compound as a white powder.
Analysis:
Calculated: C 54.11 El 6.71 N 10.97
Found: C 53.95 H 6.88 N 11.23
~ 47 ~
- 25 -
(b) 3,6,9-Tris(carboxymethyl)-3,6,9-triazaundecane-
dioic Acid Benzyl Monoamide
_____________._________________________________
A solution of 2.26 g of the compound
prepared according to Example 6(a) in 46 ml of lN
sodium hydroxide solution is allowed to stand for
2.5 hours at room temperature, and the solution is
then passed over a column of 110 ml of cation exchanger
"IR 120". Elution is first carried out with 200 ml of
water, and this fraction is discarded. Then, 600 ml
of 0.5N ammonia is used for elution; this eluate is
adjusted to pH 2.3 by adding "IR 120", and the solu-
tion is subjected to freeze-drying, thus obtaining
1.50 g of the title compound as a white powder.
Analysis:
Calculated: C 52.28 H 6.27 N 11.61
Found: C 52.44 H 6.32 N 11.80
(c) Gadolinium Complex of 3,6,9-1'ris(carboxymethyl)-
3,6,9-triazaundecanedioic Acid Benzyl Monoamide
______________ _________________________________
965 mg (2 mmol) of the compound produced in
accordance with Example 6(b) is heated with 40 ml of
water and 362 mg (1 mmol) of gadolinium oxide for one
hour to 80 C. The mixture is cooled to room tempera-
ture, the solution is filtered through a 0.1 ~m membrane
filter, and the compound is isolated by freeze-drying,
yielding 1.15 g of the title compound as a white powder.
Analysis:
Calculated: C 39.61 H 4.27 Gd 24.70 N 8.80
Found: C 39.50 H 4.48 Gd 24.61 N 8.97
~'J ~J '~
- 26 -
Example 7
Dysprosium(III) Complex of 3,6,9-Tris(carboxymethyl~-
3,6,9-tria~.aundecanedioic Acid senzyl Monoamide
_____________________________________________________
965 mg (2 mmol) of 3,6,9-tris(carboxyme-thyl~-
3,6,9-triazaundecanedioic acid benzyl monoamide
(Example 6b) is heated with 40 ml of water and 373 mg
(1 mmol) of dysprosium(III) oxide for one hour to 80 C.
The mixture is cooled to room temperature, the solution
is filtered through a 0.1 ~m membrane filter, and the
compound is isolated by freeze-drying, thus obtaining
1.22 g of the title compound as a white powder.
Analysis:
Calculated: C 39.29 H 4.24 Dy 25.31 N 8.73
Eound: C 39.41 H 4.51 Dy 25.19 N 8.70
Example 8
(a) 6-Carboxymethyl-3-ethoxycarbonylmethyl-9-tert-
butylaminocarbonylmethyl-3,6,9-triazaundecane-
dioic Acid
______________________________________________
At 0 C, 2.42 g (6 mmol) of N -(2r6-dioxo-
morpholinoethyl)-N6-(ethoxycarbonylmethyl)-3,6-diaza-
octanedioic acid is combined in DMF with 4.16 ml
(3.04 g, 30 mmol) of triethylamine and 0.64 g (6 mmol)
of tert-butylamine and stirred overnight at room tem-
perature. The clear solution is then concentrated
under vacuum and the residue chromatographed on silica
gel with dichloromethane/methanol/acetic acid/water
(5~3:1:1) as eluent. The combined fractions are
passed over about 10 ml of "Amberlite" IR 120
(H form), and the acidic eluate is concentrated.
Yield: 2.14 g (75~)
t~ 'J
-- 27 --
Calculated: C 50.41 H 7.62 N 1l.76
Found: C 50.26 ~l 7.66 N 11.80
(b) 3,6,9-Tris(Carboxymethyl)-3,6,9--triazaundecane-
dioic Acid tert-sutyl Monoamide
__.____________________________________________
1.43 g (3 mmol~ of the ethyl ester described
in Example 8(a) is dissolved in 2N NaOH and stirred
Eor 2 hours at room temperature. By addition of
"Amberlite" 120 (H form), the mixture is adjusted to
pH 7, filtered off, and the neutral solution passed
over abou-t 16 ml of "Amberlite" IR 120 (H ). The
acidic eluate is concentrated and further dried at 50 C
under vacuum. Yield: 1.20 g (89%).
Calculated: C 48.21 H 7.19 N 12.49
Found: C 48.13 H 7.24 N 12.41
(c) Gadolinium Complex of 3,6,9-Tris(carboxymethyl)-
3,6,9-triazaundecanedioic Acid tert-Butyl Monoamide
_________ ____ ______________ ________ ___ _ ______
897 mg (2 mmol) of the complex-forming aeid
obtained according to Example 8(b) is dissolved in
about 40 ml of water and combined at 80 C with 362 mg
(1 mmol) of Gd2O3. After 30 minutes, the almost elear
solution is filtered and the filtrate freeze-dried.
Yield: 1.19 g (99%), based on anhydrous substanee.
Calculated: C 35.87 H 4.85 N 9.30 Gd 26.09
Found: C 35.97 H 4.79 N 9.28 Gd 25.83
f~
- 2~ -
Example 9
(a) 6-Carboxymethyl-3-ethoxycarbonylmethyl-9-
(4-methoxyben~ylcarbamoylmethyl)-3,6,9-
triazaundecanedioic Acid
______________________________
14.10 g (35 mmol) of N -(2,6-dioxomorpholino-
ethyl)-N6~(ethoxycarbonylmethyl)-3,6-diazaoctanedloic
acid is combined in 175 ml of dimethylformamide at
0 C with 24.4 ml (175 mmol) of triethylamine and 4.67 ml
(35 mmol) of 4-methoxybenzylamine and stirred overnight
at room temperature. The solvent is extensively removed
by vacuum distillation and the residue is heated to
boiling with 600 ml of diisopropyl ether. After cool-
ing to room temperature, the mixture is decanted off
from the solvent. The residue is dissolved in 185 ml
of water and passed-over a column with 140 ml of cation
exchanger "IR 120" (H+ form), and the column is washed
with 200 ml of water. The combined eluates are con-
centrated to one-third under vacuum and then freeze-
dried, yielding 16.33 g of the title compound as a white
powder which still contains 1.2~ water and 1~ dimethyl-
formamide.
Analysis tafter correction of solvent proportions):
Calculated: C 53.33 H 6.71 N 10.36
Found: C 53.51 H 6.78 N 10.18
.
- - ~
- 29 -
(b) 3,6-Bis(carboxymethyl~-9-(4-methoxybenzylcarbamoyl-
methyl)-3,6,9-triazaundecanedioic Acid
___________~_______________~_______________ _______
12.34 g (22 mmol) of the ethyl ester
described in Example 9(a3 is dissolved in 200 ml of
water and 20 ml of llN sodium hydroxide solution and
stirred for 2 hours at room temperature. A pH of 2.3
is reached by addition of 140 ml of "Amberlite" IR 120
(H form). The mixture is filtered and the solution
subjected to freeze-drying.
10 Yield: 9.61 g (85% of theory?, water content 2.18~.
Analysis (after correction of water content):
Calculated: C 51.56 H 6.29 N 10.93
Found: C 51.37 H 6.44 N 10.89
(c) Gadolinium Complex of 3,6-Bis(carboxymethyl)-9-
(4-methoxybenzylcarbamoylmethyl)-3,6,9-
triazaundecanedioic Acid
_______________________________________________
5.24 g (10 mmol) of the complexing acid
obtained according to Example 9(b) is stirred in 200 ml
of water with 1.81 g ~5 mmol? of gadolinium oxide for
one hour at ~0 C, producing an almost clear solution.
The latter is filtered and the filtrate subjected to
freeze-dryin~.
Yield: 6.31 g, water content 3.7%.
Analysis (after correction of water content):
25 Calculated: C 39.63 H 4.38 N 8.40 Gd 23.99
Found: C 39.88 H 4.53 N 8.51 Gd 23.70
s ~
- 30 -
(d) N-Methylglucamine Salt of the Gadolinium Complex
of 3,6-Bis(carboxymethyl~-9-(4-methoxybenzyl-
carbamoylmethyl~-3,6,9-triazaundecanedioic Acid
________________________________________________
2 g of the gadolinium complex obtained
according to Example 9(c) is dissolved in 30 ml of
waterl combined with 1 equivalent of N-methylglucamine,
and the solution is concentrated by evaporation under
vacuum.
Yield: 2.30 g, water content 4%.
Analysis (after correction of water content):
Calculated: C 40.41 H 5.38 N 8.12 Gd 18.24
Found: C 40.52 H 5.11 N 8.27 Gd 18.39
(e) Sodium Sal-t of the Gadolinium Complex of
3,6-Bis(carboxymethyl)-~-(4-methoxybenzyl-
carbamoylmethyl)-3,6,9-triazaundecanedioic Acid
____________________________________________ __
0.5 g of the gadolinium complex obtained in
accordance with Example 9(c) is dissolved in lO ml o~
water, combined with l equivalent of sodium~hydroxide
dissolved in 5 ml of water, and the solution of the
title compound is subjected to freeze-drying, producing
0.55 g of the title compound as a white powder with a
water content of 4.5~.
Analysis (after correction of water content):
Calculated: C 38.37 H 4.10 Gd 22.83 N 8.13 Na 3.34
Found: C 38.40 H 4.45 Gd 22.43 N 8.10 Na 3.57
- . ~
~ ^ I ~ ' . ' ! '
- 31 -
Example 10
(a) 6-Carboxymethyl-3-ethoxycarbonylmethyl-9-
(N-undecylcarbamoylmethyl)-3,6,9-triaza-
undecanedioic Acid
_________________________________________
Under a nitrogen atmospherel 12.10 g
(30 mmol) of N3-(2,6-dioxomorpholinoethyl)-N6-
(ethoxycarbonylmethyl)-3,6-diazaoctanedioic acid in
1 liter of absolute dimethylformamide is combined at
0 C with 12.6 ml (91 mmol) of triethylamine and
5.14 g (30 mmol) of l-undecylamine and stirred for
16 hours at 20-25 C. After the reaction is completed,
the solvent is evaporated under vacuum and the remain-
ing oily residue is stirred with 1 liter of diethyl
ether. The thus-separated white powder is suctioned
off, rinsed with 1 liter of diethyl ether in portions,
and the product is dried at 40 C under vacuum.
Yield: 14.31 g (83%~, white powder.
Water content: 1.41
Dimethylformamide content: 0.8~
Analysis (after correction of solvent proportions):
Calculated: C 56.43 H 8.77 N 9.75
Found: C 56.25 H 8.89 N 9.48
(b) 3,6-Bis(carboxyme-thyl)-9-(N-undecylcarbamoyl-
methyl)-3,6,9-triazaundecanedioic Acid
__________________________________________ __
10 g (17.4 mmol) of the ethyl ester
described in Example lO~a) is dissolved in 200 ml of
2N sodium hydroxide solution and stirred for 2 hours
at room temperature. After the reaction is finished,
the mixture is cooled to 5 C and concentrated hydro-
chloric acid is added until a pH value of 2.15 has been
~J ~ C~
- 32 -
attained. The thus-separated white powder i5 suctioned
off and washed five times with 50 ml of ice water,
five times with diethyl ether/ethanol (8:2), five times
with S0 ml of diethyl ether, and five times with 50 ml
of n-pentane.
Yield: 8.25 g t86.7~, white powder.
Water content: 2.12%
Dimethylformamide content: < 0.05%
Analysis (after correction of water content):
10 Calculated: C 54.93 H 8.48 N 10.25
Found: C 54.78 H 8.67 N 9.98
(c) Gadolinium Complex of 3,6-Bis(carboxymethyl)-9-
(N-undecylcarbamoylmethyl)-3,6,9-tria~aundecane-
dioic Acid
__________________ _____________________________
At 80 C, 5.47 g (10 mmol) of the complex-
forming acid obtained according to Example lO(b) is
stirred in 500 ml of water with 1.81 g (5 mmol) of
gadolinium oxide for 4 hours, thus producing an almost
clear solution. The latter is filtered and the filtrate
subjected to free~e-drying.
Yield: 6.35 g (90.6%), white powder.
Water content: 4.2%
Analysis (after correction of water content):
Calculated: C 42.84 ~1 6.18 N 7.99 Gd 22.44
25 Found: C 42.91 H 6.25 N 7.87 Gd 22.40
- 33 -
(d) Mono-N-methylglucamine Sal-t of the Gadolinium
Complex of 3,6-Bis(carboxymethyl)-9-(N-undecyl-
carbamoylmethyl)-3,6,9-triazaundecanedioic Acid
2 g of the gadolinium complex obtained in
accordance with Example lO(c) is dissolved in 35 ml
of water, combined with one equivalent of N-methyl-
glucamine, filtered, and the solution is evaporated
under vacuum.
Yield: 2.25 g (88%), white powder.
Water content: 3.75%
Analysis (after correction of water content): ~
Calculated: C 42.89 H 6.75 N 7.82 Gd 17.55
Found: C 42.73 H 6.89 N 7.69 Gd 17.48
Example 11
5 (a) 6-Carboxymethyl-3-ethoxycarbonylmethyl-9-(1-hexa-
decylcarbamoylmethyl)-3,6,9-triazaundecanedioic
~cid
28.20 g (70 mmol) of N3-(2,6-dioxomorpholino-
ethyl)-N6-(ethoxycarbonylmethyl)-3,6-diazaoctanedioic
acid is combined in 320 ml of dimethylformamide at
0 C wi-th 28.8 ml (350 mmol) of triethylamine and
16.90 g (70 mmol) of 1-hexadecylamine and then stirred
for 24 hours at 20-25 C. The mixture is therea~ter
concentrated under vacuum and the residue stirred under
boiling heat with 1 liter of methyl tert-butyl ether.
After cooling to +10 C, the mixture is suctioned off,
the residue dried at 45 C under vacuum, taken up in
400 ml of water, and the solution is passed over a
column with 300 ml of ion e~changer "IR 120" (H Porm),
the column is washed with 0.5 liter of water, and the
combined eluates are concentrated under vacuum to about
- 3~ -
300 ml, and the title compound is isolated by freeze-
dryingl thus obtaining 36.5 g as a white powder.
Water content: 1.70%
Dimethylformamide conten-t: 0.7%
Analysis (after correction of solvent proportions):
Calculated: C 59.60 ll 9.38 N 8.69
Found: C 59.42 H 9.49 N 8.85
(b) 3,6-Bis(carboxymethyl)-9-(1-hexadecylcarbamoyl-
methyl)-3,6,9-triazaundecanedioic Acid
_______________________________________________
6.45 g (10 mmol) of the ethyl ester disclosed
in Example ll(a) is dissolved in 100 ml of water and
9.1 ml of llN sodium hydroxide solution and left for
2 hours at room temperature. Under agitation, the mix-
ture is combined with 65 ml of ion exchanger "Amberlite"
IR 120 (H form), thus setting a pH of 2.5. The mix-
ture is filtered and the solution is subjected to
freeze-drying, thus obtaining 5.30 g of the title
compound as a white powder.
Water content: 3.2%
Dimethylformamide content: ~ 0.05~
Analysis (after correction of water content):
Calculated: C 58.42 El 9.15 N 9.08
Found: C 58.59 H 9.44 N 8.95
- 35 -
~c) Monomeglumine Salt of -the Gadolinium Complex of
3,6-sis(carboxymethyl~-9-(1-hexadecylcarbamoyl-
methyl)-~,6,9-triazaundecanedioic Ac.id
_______________________________________________
A-t 80-85 C, 4.93 g (8 mmol) of the complex-
Eorming acid obtained according to Example ll(b~
is stirred in 170 ml of water for 2 hours with 1.45 g
(4 mmol) of gadolinium oxide and 1.56 g (8 mmol) of
N-methylglucamine. The almost clear solution is
filtered and Ereeze-dried.
~ield: 7.49 g of a white powder.
Water content: 2.8~
Analysis (after correction of water content):
Calculated: C 45.99 H 7.30 N 7.25 Gd 16.27
Found: C 46.21 H 7.55 N 7.08 Gd 16.18
By the same route as described in Example 11,
the monomeglumine salt of the europium complex of 3,6-
bis(carboxymethyl)-9-(1-hexadecylcarbamoylmethyl3-
3,6,9-triazaundecanedioic acid can be prepared as well.
Example 12
Preparation of a Solution of the Meglumine Salt of the
Gadolinium Complex of 3,6,9-Tris(carboxymethyl)-3,6,9-
tri.azaundecanedioic Acid Undecyl Monoamide
_____________________________________________________._
448.57 g (0.5 mol) of the compound described
in Example 10 ~d) is dissolved under heating in 600 ml o~
water pro injectione (p.i.). After addition of 4.92 g
(10 mmol) of the monohydrate of the calciumtrisodium
salt of DTPA, CaNa3DTPA, tlle solution is filled up with
water p.i. to 1000 ml. The solution is subjected to
ultrafiltration, dispensed into bo-tt].es, and heat-
sterilized, and is ready ~or use :Eor parenteral admini-
stration.
C 1 ' " ! ' '
- 36 -
Example 13
Production of a Powder Form of Administration
89.61 g (0.1 mol) o~ the meglumine salt disclosed in
Example lO(d) is finely ground up with 25 g of sucrose
and 5 g of "Pluronic" F 68 and lo mg of raspberry
flavoring. The powder is filled into bags and is ready
for oral administration.
The preceding examples can be repeated with similar
success by substituting the generically or specifically
described reactants and/or operating conditions of this
invention for those used in the preceding examples.
From the foregoing description, one skilled in the
art can easily ascertain the essential ch~racteristics of
this invention, and without departing from the spirit and
scope thereof, can make various changes and modifications
of the invention to adapt it to various usages and
conditions.