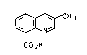Note: Descriptions are shown in the official language in which they were submitted.
- ~ t~ ~ ~ ':
O.Z. 0050/41088
Preparation of 3-methylquinoline-8-carboxylic acid
The present invention relates to a novel process
for the preparation of 3-methylquinoline-8-carboxylic
acid I
CH3
. ~ N. I
COyH
or a derivative thereof by reacting o-toluidine TI or a
derivative thereof
NHZ II
CH3
with methacrolein TII
HZ~CH3 III
pp~~~T H
in 70-90% strength by weight sulfuric acid in the pres-
ence of iodine or. of an iodine compound, and oxidizing
the resulting 3,8-dimethylquinoline IV or a derivative
thereof
,. ~ CH 3
~ ~ ~ Iv
CH3
with nitric acid in a solution containing sulfuric acid,
in the presence of vanadium ions to give 3-methylquinol-
ine-8-carboxylic acid.
EP-A 294 685 describes the synthesis of 3,8
dimethylquinoline from o-toluidine and methacrolein in
the presence of iodine in a solution containing sulfuric
acid.
EP-A 282 778 discloses the oxidation of 3,8-
dimethylquinoline and derivatives thereof to 3-methyl-
quinoline-8-carboxylic acid and derivatives thereof by
20~~~~~
- 2 - O.Z. 0050/41088
means of nitric acid in the presence of vanadium ions in
a solution containing sulfuric acid. However, it is
expressly pointed out that the purity of the educts is
critical for obtaining an advantageous result in this
reaction.
However, the prior art synthesis of 3-methyl-
quinoline-8-carboxylic acid I and its derivatives is
technically unsatisfactory since it requires the expen-
sive purification of the intermediate IV. If this
purification is omitted and the reaction mixture from the
synthesis of IV is used directly in the oxidation stage,
the yield of I decreases. In addition, the oxidation in
this case is accompanied by vigorous foaming, which makes
the reaction considerably more difficult in terms of
process technology. ..
The present invention is based on the observation
that the byproducts formed in the first reaction stage in
the synthesis of IV are responsible both for the lower
yields of I and for the foam formation in the oxidation
of IV to I.
We have found that these byproducts axe mainly
polymethacrylates, which are formed under the reaction
conditions of the fusion of II with III. When 1.4 moles
of III are used per mole of II, the amount of these
byproducts is about 5-10~ by weight, based on the 3, 8-
dimethylquinoline IV.
. It is an object of the present invention to
provide a one-stage process for the preparation of 3-
methylquinoline-8-carboxylic acid.
We have found that this object is achieved by a
process for the preparation of 3-methylquinoline-8-
carboxylic acid I
~ CH3 I
CO ZH
or a derivative thereof by reacting o-toluidine II or a
A ~ J
J ~s: t"J
- 3 - O.Z. 0050/41088
derivative thereof
NH Z I I
CH;
with methacrolein III
HZC III
~CH;
0
S or a derivative thereof in 70-90% strength by weight
sulfuric acid in the presence of iodine or of an iodine
compound, and oxidizing the resulting 3,8-dimethylquinol-
ine IV or a derivative thereof
w CH3
IV
CH;
with nitric acid in a solution containing sulfuric acid,
in the presence of vanadium ions, wherein, in the sul-
furic acid-containing reaction solution of the 3,8-
dimethylquinaline IV, the byproducts of the reaction are
first decomposed by oxidation, the resulting 3,8-
dimethylquinoline solution is concentrated by distilla-
tion and the 3,8-dimethylquinoline is then oxidized in
situ with nitric acid to give 3-methylquinolinecarboxylic
acid.
The core of the novel process is based on the
fact that the sulfuric acid-containing reaction solution
is freed from byproducts by oxidation, so that isolation
of the 3,8-dimethylquinoline is unnecessary. Consequent
ly, both fusion and oxidation can take place in succes
sion in one medium (in situ).
From 5 to 65% strength by weight aqueous nitric
acid is a particularly suitable oxidizing agent for the
oxidative decomposition of the byproducts of the fusion.
For the oxidative decomposition of the byproducts
I ~ 7~ i..) 'A mi
~~ '-~~~ ~) ~
- 4 - o.z. 0050/4loaa
of the reaction, nitric acid is added in amounts of from
1.2 to 3.0, preferably from 1.3 to 2, moles, based on
compound II.
This oxidation begins a~t about 50°C and takes
place at a sufficient rate at from about 70°C. If the
mixture is heated to above about 150°C, foam formation
begins. The optimum temperature range is from 70 to
130°C. If the reaction mixture is heated so slowly that
the byproducts are already partially decomposed before
the maximum temperature is reached, this oxidation can
also be carried out at higher temperatures.
After decomposition of the byproducts of the
reaction, the sulfuric acid-containing solution of 3,8-
dimethylquinoline is concentrated by distilling off
excess water and is oxidized in a known manner, without ,.
further pretreatment, by adding additional nitric acid.
The novel process is suitable for the preparation
of 3-methylquinoline-8-carboxylic acid and substituted 3
methylquinoline-8-carboxylic acids from the corresponding
o-toluidines, in particular those of the general formula
IIa
R1
RZ ( ~ IIa
R3 ~ NH1
CH3
where R1 to R3 are each hydrogen, halogen, organic carbon-
containing radicals and/or vitro groups; observations to
date have shown that the nature of the substituents has
only a slight effect on the process if they are inert
under the reaction conditions.
Preferred radicals Rl to R' in addition to hydro-
gen are the following groups:
halogen, such as fluorine, chlorine, bromine or iodine,
in particular chlorine, methyl and aryl, in particular
phenyl.
These radicals may in turn be interrupted by
~y ~5. ~ ~~y
- 5 - O.Z. 0050/41088
heteroatoms, such as nitrogen, oxygen and sulfur, or may
carry further inert radicals, such as halogen, nitro,
sulfonyl, arylsulfonyl and carboxyl.
The 3-methylquinoline-8-carboxylic acid I and
derivatives thereof, which are more readily obtainable by
the novel process, are used, for example, as crop protec
tion agents.
EXAMPLES
General
examples
of
the
process
for
the
preparation
of
3-
methylquinoline-8-carboxylic
acids
from
o-toluidines
IIa
and methacrolein III
Variant
1:
a) Fusion
1 mole of o-toluidine derivative was reacted with
1.4 moles of methacrolein according to Example 5 of EP-A .
294685.
b) Destruction of the byproducts
140 g of 65% strength by weight nitric acid and
the solution obtained from la) were added simultaneously
to a solution of 2 g ( 2 . 2 mol % ) of vanadium pentoxide
and 200 g of wate~e at 90C in the course of 2 hours.
After the end of the addition and a further 2
hours
at
90C,
the
reaction
mixture
was
heated
to
155C,
600 g of water distilling oft in the course of 6 hours.
c) Oxidation to quinolinecarboxylic acid
450 g of 65% strength by weight nitric acid were
then
added
to
this
concentrated
reaction
mixture
accord-
ing to Example 2 of EP-A 282778, at 155C, while passing
in 70 1/hour of air in the course of 10 hours.
Variant
2:
a) Fusion
300 ml of water were distilled of f at 150C and
150 mbar from a reaction mixture obtained similarly to
la) from 1 mole of o-toluidine.
b) Destruction of the polymethacrylates
140 g of 65% strength by weight nitric acid and
the solution obtained from 2a) were added simultaneously
~~~~~:c
- 6 - 0.2. 0050/41088
to a solution of 2 g ( 2 . 2 mol % ) of vanadium pentoxide
and 200 g of water at 90°C in the course of 2 hours. After
the end of the addition, the reaction mixture was left
for a further 2 hours at 90°C. The temperature was then
increased to 155°C and 300 ml of water was distilled off
in the course of 4 hours.
c) Oxidation to quinolinecarboxylic acid
The reaction mixture from 2b) was heated to 155°C
and was oxidized with 450 g of 65% strength by weight
nitric acid similarly to lc) while passing in 70 1/h of
air in the course of 10 hours.
Variant 3:
a) Fusion
1 mole of o-toluidine was reacted as described
under la). .
b) Destruction of the byproducts
140 g of 65% strength by weight nitric acid and
the solution obtained from 3a) were added simultaneously
to 200 g of water at 90°C in the course of 2 hours. After
a further 4 hours at 90°C, the temperature was increased
to 155°C, 600 g of water being distilled off in the course
of 6 hours.
c) Oxidation to quinolinecarboxylic acid
2 g (2.2 mol %) of vanadium pentoxide were added
to the concentrated solution of 3b). Oxidation was then
carried out with 450 g of 65% strength by weight nitric
acid as described under lc), at 155°C and while passing
in 70 1/hour of air.
Variant 4a
a) Fusion
1 mole of o-toluidine was reacted similarly to
la).
b) Destruction of the byproducts
The solution obtained from 4a) was treated as
described under lb).
c) Oxidation to quinolinecarboxylic acid
The reaction mixture treated in 4b) was oxidized
~~l~ifl_i)
- 7 - O.Z. 0050/41088
with 1,000 g of 65% strength by weight nitric acid in the
course of 20 hours and while passing in 70 1/h of air at
155°C, and was worked up in a conventional manner.
Variant 5:
a) Fusion
The fusion of 1 mole of o-toluidine was carried
out as described under la).
b) Destruction of the byproducts
300 g of an oxidation solution obtained as
described under, for example, lc) and 2 g (2.2 mol %) of
vanadium pentoxide were heated to 155°C. 140 g of 65%
strength by weight nitric acid and the reaction mixture
obtained from 5a) were added simultaneously to this
solution in the course of 4 hours, 600 g of water dis
tilling off. ..
c) Oxidation to quinolinecarboxylic acid
This solution from 5b), which had been simul-
taneously concentrated and freed from byproducts, Was
oxidized as described under lc).
The details of the experiments carried out are
summarized in Table 1.
- 8 - O.Z. 0050/41088
b
ml Q1 Q1 00 n N I~ N t~ N tf1 CO
V N ~ ~O t0 l0 10 ~D tf1 l0 t0 d~ M N
~rl dP
,1i a
/ \\
2
x
N
\ /
N n°1
Q
x
N
GG UUUUUxxxUUU
ro x xx
o xoo .
'
xxxxx~xxv~~
v U
.-i O
Cti/ O '"~,
\
m
W0.' N
xxxxxxUUxxx
H ~ ..
\
/
~
N
A1
a
z
t~
~
a~
ro
'"'~ N M 1f1 d' er .-1 ~ r-1 er ~-i
1-1
ro
Z m
"",iY. UUUUU~xxxUUU
.
ro
x
Z H
m m m N
~Nx xxxxx~
~~
xx
c
~
U
H ~ m m
w
ig xxxxxx~~xxx
/ .-
v
z
..
N
~'1
W H N M eh 1f1 t0 t'~ OD O1 O e-1
"!r'
- 9 - O.Z. 0050/41088
The effect of the reaction parameters on the des-
truction of the byproducts and on the yield of the subse-
quent oxidation to the quinolinecarboxylic acid was
investigated for the reaction of 6-chloro-o-toluidine IIb
with methacrolein II to give the quinoline IVb according
to the following example.
Variant 12:
a) Fusion
1.4 moles of rnethacrolein III were added to 1
mole (141.5 g) of 6-chlorotoluidine IIb according to
Example 5 of EF-A 294685.
b) Destruction of the byproducts
Y1 g of YZ $ strength by weight nitric acid (1.44
moles) and the solution obtained from lla) were added
simultaneously to a solution of X1 at TIC in the course
of tl hours.
After the end of the addition and a further tz
hours at T1°C, the reaction mixture was heated to 155°C,
XZ g of water being distilled off in the course of t3
hours.
c) Oxidation to7--chloro-3-methylquinolirie-8-carboxylic
acid Ib
The solution concentrated under llb) was oxidized
and worked up as described under lc).
The reaction parameters for the destruction of
the byproducts and the yields of the subsequent oxidation
of IVb to 7-chloro-3-methylquinoline-8-carboxylic acid Ib
are shown in Table 2.
n ° ~ r? <,'! ;' %-~
~.~ ~.d ~~' l7 ~ a wr
- 10 - O.Z. 0050/41088
. .n
,d H
H H
W G
O O
b b ~ 01 O1 CO L~ lC1(~ O Q1 O
ra N ~D ~D l0 W ~O ~O tD ~D lG
y u~
~ m
G
O
.a
O N
W Sa
n b m ~ l(?eh l0 O I~ I'rO I~ l0
U ~ N
9 i
.
H 'd
/
\\ W
2
s W
rv p p
O ~ ,fl
W 'G
\ O O
/ N
a .-~ O O O O O O O e4~O
~ ~ O O O O O O N O O
_ N pp t~ c'W0 t0 t0 vG N I~ W
U
d ~ " N
~
~
v7
O J-
3 b
O N .'
.'..~
1.!
d~ d~ l0 d~ d~ 00 ~ ~D d
~ ~
1J ~i rl
pe
s ~H
U O
N
1~
/
\\ 0 0 0 0 0 0 0 ~ o
~ d~ d~ d~ ~9'N o d'
E ..
y
/
v
a
v 30
~ t!76n Lf7If)1f11,A~l'~O l61
.O 'xiN 10 lO ~O t0 l0 lG M t~
W
?! O
N
~-1 ~ O ~ In O O o
G1 01 01 Ilktf1t~ O1 81 .-1
O
FJ H r'I ~-I
M
H
H '~O ~ r9 N M tP1N N N N N
s p y~.~ r1 ri ri rl r1
H ~
W 9
4-I
N O
s
a
..
v ~ on
~
N N O N N N N N N
as
U ,-1
A.
N M d~ tn 1D
W 'Ze r1 N th t!1ri rl ri ml n-I