Note: Descriptions are shown in the official language in which they were submitted.
2~2~ 3
-- 1
55-915.523
Condensed D.iazepinones
The invention relates to new condensed
diazepinones, processes for preparing them and
pharmaceutical compositions containing these compounds.
Condensed diazepinones with antiulcerative
properties and inhibitory effects on gastric acid
secretion are already known from EP-A-39519, EP-A-57428
US - A-3660380, US-A-3691159, US - A-4213984, US-A-4213985,
US-A - 4210648, US - A - 4410527, US-A-4424225, US-A-4424222
and US-A-4424226.
EP-~-156191 (US Patent 4550107) says, of condensed
diazepinones, that by introducing new aminoacyl groups,
compared with the compounds in the above mentioned
publications it is possible to produce compounds having
totally different and useful pharmacological properties.
Compared with the diazepinones disclosed in these
various patent publications as having antiulcerative or
antibradycardiac effects, certain novel condensed
diazepinones, despite being structurally similar
surprisingly have been found to possess another
different pharmacological activity. In particular the
new compounds are suitable for the treatment of
cholinergically induced spasms and motility disorders in
the gastrointestinal tract and in the region of the
outward-leading bile ducts, for the symptomatic
treatment of cystitis and spasms in urelithiasis by
lowering the pathologically raised tone of the hollow
organs, for the treatment of relative incontinence based
on a disordered correlation between sphincter and
detrusor tone, for the symptomatic therapy of bronchial
asthma and bronchitis by suppressing the muscariniclv
mediated part of the bronchoconstriction, and for the
treatment of ischaemic heart diseases by lowering heart
2 ~02~
rate whilst simultaneously suppressing
parasympathetically induced coronary spasm and lowering
the basal coronary tone. The new condensed diazepinones
show the effects described with a high selectivity and
are free from tachycardiac side effects, particularly in
the therapeutically useful dosage range.
Viewed fron~ one aspect therefore the present
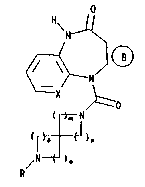
invention provides compounds of formula I
o
H\
--N
X ~ '.
~=0 ( I )
~N
~ ~n
~N ~o
R
(wherein ] ~ represents one of the groups
( S ) ( ~
X represents a =CH- group or a nitrogen atom;
R represents a straight chained or branched C14 alkyl
group optionally substituted by a phenyl group itself
optionally mono or disubstituted by chlorine, bromine,
fluorine, methyl or methoxy;
~2~0~
- 3 -
R4 and Rs, which may be the same or different, eachrepresents a hydrogen, fluorine, chlorine or bromine
atom or a C14 alkyl group;
R6 represents a hydrogen or chlorine atom or a methyl
~roup;
R7 and R8, which may be the same or different, each
represents a hydrogen atom or C14 alkyl group, and R8 may
also represent a halogen atom,
m, n, ~ and p each independently represents the number l
or 2 with the proviso that the sum of m+n+o+p must be no
greater than 6)
and the isomers and acid addition salts thereof.
Preferred compounds according to the invention
include compounds of formula I wherein:
either X represents a nitrogen atom and ] ~ represents
a group (S) or
X represents a =C~- group and ] ~ represents the group
(V);
R represents a methyl group,
R4 and Rs, which may be the same or different, each
represents a hydrogen, fluorine or chlorine atom or a
methyl or ethyl group; and
m and p each represents the number l and n and o each
represents the number l or 2,
and the isomers and salts thereof.
~ 9 2 .~
-- 4
Particularly preferred compounds according to the
invention include
5,11-dihydro-11-[[7-methyl-2,7-diazaspiro[4,4]non-2-
yl]carbonyl]-6~-pyrido[2,3-b][1,4]benzodiazepin-6-one;
5,11-dihydro-8-methyl-11-[[7-methyl-2,7-diazaspiro-
[4,4]non-2-yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzo-
diazepin-6-one;
5,11-dihydro-11-[[6-methyl-2,6-diazaspiro[3,4]oct-2-yl]-
carbonyl]-6~-pyrido[2,3-b][1,4]benzodiazepin-6-one;
5,11-dihydro-8-ethyl-11-[[6-methyl-2,6-diazaspiro[3,4]-
oct-2-yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-
one; and
5,11-dihydro-8-methyl-11-[[2-methyl-2,6-diazaspiro[3,4]-
oct-6-yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-
one;
and the isomers and salts thereof.
The compounds of formula I may occur in the form of
their acid addition salts. While physiologically
acceptable salts are of course preferred, other salts
may be useful as intermediates in the preparation of the
free bases or of physiologically acceptable salts.
Organic and inorganic acids which have proved suitable
for the preparation of physiologically acceptable salts
include, for example, hydrochloric, hydrobromic,
sulphuric, methylsulphuric, phosphoric, tartaric,
fumaric, citric, maleic, succinic, yluconic, malic, p-
toluenesulfonic, methanesulphonic and amidosulphonic
acids.
To illustrate the invention further the following
compounds are mentioned by way of example:
5 -
(+)-5,11-dihydro-11-[[7-methyl-2,7-diazaspiro[4,4]non-2-
yl]-carbonyl]-6H-pyrido[2~3-b][l~4]benzodiazepin-6-one
R-5,11-dihydro-11-[[7-methyl-2,7-diazaspiro[4,4]non-2-
yl]-carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one
S-5,11-dihydro-11-[C7-methyl-2,7-diazaspiro[4,4]non-2-
yl]-carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one
(+)-9-chloro-5,11-dihydro-11-[[7-methyl-2,7-
diazaspiro[4,4]non-2-yl]carbonyl-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
8-Chloro-5,11-dihydro-11-[[7-methyl-2,7-
diazaspiro[4,4]non-2-yl]-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
8-bromo-5,11-dihydro-11-[[7-methyl-2,7-
diazaspiro[4,4]non-2-yl]carbonyl]-6H-pyrido[2,3-
b][1,4~benzodiazepin-6-one
5,11-dihydro-8-ethyl-11-[[7-methyl-2,7-
diazaspiro[4,4]non-2-yl]carbonyl]-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
-
5,11-dihydro-9-methyl-11-[[7-methyl-2,7-
diazaspiro[4,4]non-2-yl]carbonyl]-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
5,11-dihydro-ll-[[7-ethyl-2,7-diazaspiro[4,4]non-2-
yl]carbonyl]-6H-pyrido[2,3-b][1,4~benzodiazepin-6-one
5,11-dihydro-8-methyl-11-[[7-methyl-2,7-
diazaspiro[4,4]non-2-yl]carbonyl]-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
-- 6
5,11-dihydro-11-[[7-ethyl-2,7-diazaspiro[4,4]non-2-
yl]carbonyl]-8-methyl-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
5,11-dihydro-11-[[7-ethyl]-2,7-diazaspiro[4,4]non-2-
yl]carbonyl]-9-methyl-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
9-chloro-5,11-dihydro-11-[[7-ethyl-2,7-
diazaspiro[4,4]non-2-yl]carbonyl]-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
5,11-dihydro-11-[[6-methyl-2,6-diazaspiro[3,4]oct-2-
yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one
5,11-dihydro-8-ethyl-11-[[6-methyl-2,6-
diazaspiro[3,4]oct-2-yl]carbonyl]-6H pyrido[2,3-
b][1,4]benzodiazepin-6-one
5-11-dihydro-8-methyl-11-[[6-methyl-2,6-
diazaspiro[3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
5,11-dihydro-9-methyl-11-[[6-methyl-2,6-
diazaspiro[3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
9-chloro-5,11-dihydro-11-[[6-methyl-2,6-
diazaspiro[3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one
5,11-dihydro-8,9-dimethyl~ll-[[6-methyl-2,6-
diazaspiro[3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one
5~11-dihydro-11-[[2-methyl-2~6-diazaspiro[3~4]oct-6-
yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one
~2~
-- 7 --
5,11-dihydro-11-[[6-(phenylmethyl)-2,6-
diazaspiro[3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
5,11-dihydro-8-methyl-11-[[2-methyl-2,6-
diazaspiro[3,4]oct-6-yl]carbonyl]-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one
5,11-dihydro-8-ethyl-11-[[2-methyl-2~6-
diazaspiro[3,4]oct-6-yl]carbonyl]-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one
5,11-dihydro-9-methyl~ [2-methyl-2,6-
diazaspiro[3,4]oct-6-yl]carbonyl]-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one
9-chloro-11-[[2-methyl-2,6-diazaspiro[3,4]oct-6-
yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one
8-chloro-11-[[2-methyl-2,6-diazaspiro[3,4]oct-6-
yl]carbonyl~-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one
5,11-dihydro-11-[[6-ethyl-2,6-diazaspiro[3,4]oct-2-
yl]carbonyl]-8-methyl-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one
5,11-dihydro-11-[[6-ethyl-2,6-diazaspiro[3,4~oct-2-
yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one
5,11-dihydro-11-[[6-ethyl-2,6-diazaspiro[3,4]oct-2-
yl]carbonyl]-9-methyl-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one
9-chloro-5,11-dihydro-11-[[6-ethyl-2,6-
diazaspiro[3~4]oct-2-yl]carbonyl]-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one
8 2~2~
5-11-dihydro-8-ethyl~ [[6-ethyl-2,6-
diazaspiro[3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one
5,11-dihydro-11-[[6-propyl-2,6-diazaspiro[3,4]oct-2-yl]-
carbonyl]-6H-pyrido[2,3-b][l,~]benzodiazepin-6-one
5,11-dihydro-8-methyl-11-[[6-propyl-2,6-
diazaspiro[3,4~oct-2-yl]carbonyl]-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one
~3-chloro-5,11-dihydro-11-[[6-propyl-2,6-
diazaspiro[3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
5,11-dihydro-9-methyl-11-[[6-propyl-2,6-
diazaspiro[3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one
5,11-dihydro-8-ethyl-11-~[6-propyl-2,6-
diazaspiro[3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one
5,11-dihydro-11-[[2-ethyl-2,6-diazaspiro[3,4]oct-6-
yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one
5,11-dihydro-11-[[2-ethyl-2,6-diazaspiro[3,4]oct-6-
yl]carbonyl]-8-methyl-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
5,11-dihydro-11-[[2-ethyl-2,6-diazaspiro[3,4]oct-6-
yl]carbonyl]-9-methyl-6H-pyrido[2,3-
b]tl,4]benzodiazepin-6-one
9-chloro-5,11-dihydro-11-[~2-ethyl-2,6-
diazaspiro[3,4]oct-6-yl]carbonyl]-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
- 9 -
5,11-dihydro-11-[[6-(2-methylpropyl)-2,6-
diazaspiro~3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
5,11-dihydro-8-methyl-11-[[6-(2-methylpropyl)-2,6-diaza-
spiro[3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzo-
diazepin-6-one
5,11-dihydro-9-methyl-11-[[6-~2-methylpropyl)-2,6-diaza-
spiro[3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzo-
diazepin-6-one
9-chloro~5,11-dihydro~ll-[[6-(2-methylpropyl)-2,6-diaza-
spiro[3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one
5,11-dihydro-11-[[6~methyl-2,6-diazaspiro[3,3]hept-2-
yl]-carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one
5,11-dihydro-8-methyl-11-[[6-methyl-2,6-
diazaspiro[3,3]hept-2-yl]carbonyl]-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one
5,11-dihydro-9-methyl-11-[[6-methyl-2,6-
diazaspiro[3,3]hept-2-yl]carbonyl]-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
5,11-dihydro-11-[[7-methyl 2,7-diazaspiro[3,5]non-2-
yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one
5,11-dihydro-8-methyl-11-[[7-methyl-2,7~
diazaspiro[3,5]non-2-yl]carbonyl]-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
9-chloro-5,11-dihydro-11-[[7-methyl-2,7-
diazaspiro[3,5]non-2-yl]carbonyl-6H-pyrido[2,3-
b][l,4]benzodiazepin-6 one
-- 10 --
5,11-dihydro-11-[[2-methyl-2,7-diazaspiro[3,5]non-7-
yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one
5,11-dihydro-9-methyl-11-[[2-methyl-2,7-
diazaspiro[3,5]non-7-yl]carbonyl]-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
8-chloro-5,11-dihydro-11-[[2-methyl-2,7-
diazaspiro[3,5]non-7-yl]carbonyl-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
8-bromo-5,11-dihydro-ll-[[2-methyl-2,7-
diazaspiro[3,5]non-7-yl]carbonyl]-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
(+)-6,11-dihydro-11~[[7-methyl-2,7-diazaspiro[4,4]non-2-
yl]carbonyl-5H-pyrido[2,3-b][1,5]benzodiazepin-5-one
R-6,11-dihydro-11-[[7-methyl-2,7-diazaspiro[4,4]non-2-
yl]carbonyl~-5H-pyrido[2,3-b][1,5]benzodiazepin-5-one
S-6,11-dihydro-11-[[7-methyl-2,7-diazaspiro[4,4]non-2-
yl]carbonyl]-5H-pyrido[2,3-b][1,5]benzodiazepin-5-one
(+)-6,11-dihydro-11-[[7-ethyl-2,7-diazaspiro[4,4]non-2-
yl]carbonyl]-5H-pyrido[2,3-b][1,5]benzodiazepin-5-one
6,11-dihydro-11-[[2-methyl-2,6-diazaspiro[3,4]oct-6-yl]-
carbonyl]-5H-pyrido[2,3-b][1,5]benzodiazepin-5-one
6,11-dihydro-11-[[6-ethyl-2,6-diazaspiro[3,4]oct-2-yl]-
carbonyl]-5H-pyrido[2l3-b][ll5]benzodiazepin-5-one
6,11-dihydro-11-[[6-propyl-2,6-diazaspiro[3,4]oct-2-yl]-
carbonyl]-5H-pyrido[2,3-b][1,5]benzodiazepin-5-one
6,11-dihydro-11-[[2-ethyl-2,6-diazaspiro[3,4]oct-6-yl]-
carbonyl]-5H-pyrido[2,3-b][1,5]benzodiazepin-5-one
6,11-dihydro-11-[[6-(2-methylpropyl)-2,6-
diazaspiro[3,4]-oct-2-yl]-carbonyl]-SH-pyrido[2,3-
b][1,5]benzodiazepin-5-one
6,11-dihydro-lI-[[6-methyl-2,6-diazaspiro[3,3]hept-2-
yl]-carbonyl]-5H-pyrido[2,3-b][1,5]benzodiazepin-5-one
6,11-dihydro-11-[[7-methyl-2,7-diazaspiro[3,5]non-2-yl]-
carbonyl]-5H-pyrido[2,3-b][1,5]benzodiazepin-5-one
6,11-dihydro-11-[[2-methyl-2,7-diazaspiro[3,5]non-7-yl]-
carbonyl]-5H-pyrido[2,3-b][1,5]benzodiazepin-5-one
(+)-5,10-dihydro-5-L[7-methyl-2,7-diazaspiro[4,4]non-2-
yl]carbonyl]-llH-dibenzo[b,e][1,4]diazepin-11-one
5,10-dihydro-5-[[6-methyl-2,6-diazaspiro[3,4]oct-2-yl]-
carbonyl]-llH-dibenzo[b,e][1,4]diazepin-11-one
(+)-4,9-dihydro-3-methyl-4-~[7-methyl-2,7-
diazaspiro[4,4]non-2-yl]carbonyl]-lOH-thieno[3,4-
b][1,5]benzodiazepin-10-one
(~)-4,9-dihydro-4-[[7-ethyl-2,7-diazaspiro[4,4]non-2-
yl]-carbonyl]-3-methyl-lOH-thieno[3,4-
b][1,5]benzodiazepin-10-one
4,9-dihydro-3-methyl-4-[[6-methyl-2,6-diazaspiro[3,4]-
oct-6-yl]carbonyl]-lOH-thieno[3,4~b][1,5]benzodiazepin-
10-one
4,9-dihydro-3-methyl-4-[[2-methyl-2,6-diazaspiro[3,4]-
oct-6-yl]carbonyl]-lOH-thieno[3,4-b][1,5]benzodiazepin-
10-one
:
4,9-dihydro-4-[[6-ethyl-2,6-diazaspiro[3,4]oct-2-
yl]carbonyl]-3-methyl-lOH-thieno[3,4-
b][1,5]benzo~iazepin-10-one
:.
- 12 -
4,9-dihydro-3-methyl-4-[[6-propyl-2,6-diazaspiro[3,4]-
oct-2-yl]carbonyl]-lOH-thieno[3,4-b]tl,5]benzodiazepin-
10-one
4,9-dihydro-4-[[2-ethyl-2,6-diazaspiro[3,4]oct-6-
yl]carbonyl]-3-methyl-lOH-thieno[3,4-
b][l,5]benzodiazepin-10-one
4,9-dihydro-3-methyl-4-[[6-(2-methylpropyl)-2,6-
diazaspiro[3,4]oct-2-yl]carbonyl]-lOH-thieno[3,4-
b][1,5]benzodiazepin 10-one
4,9-dihydro-3-methyl-4-[[6-methyl-2,6-diazaspiro[3,3]-
hept-2-yl]carbonyl]-lOH-thieno[3,4-b][1,5]benzodiazepin-
10-one
4,9-dihydro-4-[[6-methyl-2,6-diazaspiro[3,3]hept-2-
yl]carbonyl]-lOH-thieno[3,4-b][1,5]benzodiazepin-10-one
4,9-dihydro-3-methyl-4-[[7-methyl-2,7-diazaspiro[3,5]-
non-2-yl]carbonyl]-lOH-thieno[3,4-b][1,5]benzodiazepin-
10-one
4,9-dihydro-3-methyl-4-[[2-methyl-2,7-diazaspiro[3,5]-
non-7-yl]carbonyl]-loH-thieno[3~4-b][l~5~benzodiazepin
10-one
(+)-3-chloro-1-methyl-4-[[7-methyl-2,7-
diazaspiro[4,4]non-2-yl]carbonyl]-1,4,9,10-
tetrahydropyrrolo[3,2-b][1,5]benzodiazepin-10-one
(+3-1-methyl~4-~[7-methyl-2,7-diazaspiro~4,4]non-2-
yl]carbonyl]-1,4,9,10-tetrahydropyrrolo[3,2-
b][1,5]benzo-diazepin-10-one
3-chloro-1-methyl-4-[[6-propyl-2,~-diazaspiro[3,4~oct-2-
yl]carbonyl]-1,4,9,10-tetrahydropyrrolo~3,2-
b]~1,5]benzodiazepin-10-one
- 13 -
3-chloro-1-methyl-4-[[6-(2-methylpropyl)-2,6-
diazaspiro[3~4~oct-2-yl]carbonyl]-l/4~9~lo-
tetrahydropyrrolo[3,2-b][1,5]benzodiazepin-10-one
3-chloro-1-methyl-4-~[6-methyl-2,6-diazaspiro[3,4]oct-2-
yl]carbonyl]-1,4,9,10-tetrahydropyrrolo[3,2-
b][l,5]benzodiazepin-10-one and
3-chloro-1-methyl-4-[t6-methyl-2,6-diazaspiro[3,3]hept-
2-yl]carbonyl]-1,4,9,10-tetrahydropyrrolo[3,2-
b][1,5]benzodiazepin-10-one.
Viewed from another aspect the invention also
provides a process for the preparation of the compounds
according to the invention, said process comprising at
least one of the following steps:
a) (to prepare base-substituted condensed diazepinones
of formula Ia
\N~
~N)~ .
X
:; ( l a
n
R
- 14 - ~ ~2~
(wherein X, R, m, n, o and p are as hereinbefore defined
and ] ~ represents one of the groups (S), (U) or (V)
: as hereinbefore defined or a group (T')
~CH3
~/> ( T
R6
wherein R4, R5, R7 and R8 are as hereinbefore defined and
R6 represents a chlorine atom or a methyl group))
reacting a carbonic acid derivative of formula II
Y~
o
(wherein ] ~ and X are as hereinbefore defined and Y
represents a halogen atom, preferably bromine or
~ chlorine, or a group ORI1 where R11 represents an
: optionally halogen substituted C15 alkyl group, a phenyl
:~ group optionally substituted by halogen atoms or nitro
~ 2 ~
groups or a C715 aralkyl group) with a compound of
formula III
/
( t~--N
(1~ ~ )n (I ~ I)
/Nt ) o
R
(wherein R, m, n, o, and p are as hereinbefore defined)
or a metal compound of formula IIIa
/
( -~1
(~)n (Illa)
/N
:~ R
(wherein R,m,n,o and p are as hereinbefore defined and M
represents an alkali metal atom or 1 equivalerlt of an
alkaline earth metal atom);
b) (to prepare base substituted condensed diazepinones
of formula Ia) reacting a tricyclic compound of formula
IV
'
16 2 ~ 2 ~
, H
~ ~0
H
(wherein X and ] ~ are as hereinbefore defined) with a
:: chlorocarbonic acid derivative of formula V
i O
~CI
: ( ~N
(V)
N
(wherein R, m, n, o and p are as hereinbefore defined);
c) ~to prepare pyrrolo-condensed diazepinones of
formula Ib
H~ U C H
N ~N
~N
X ~ ( I b~
~N
tJ 3"
~N )~
:~- R
- 17 -
(wherein X, R, m, n, o and p are as hereinbefore
defined)~ hydrogenolysing a compound of formula Ia
wherein R6 represents a chlorine atom;
d) separating a compound of formula I thus obtained
into its isomers; and
e) converting a compound of formula I into an acid
addition salt thereof or an acid addition salt of a
compound of formula I into the free base.
The reaction of step (a) is carried out without or
preferably in the presence of a solvent such as water or
toluene or an alcohol such as methanol, ethanol or
isopropanol, but particularly preferably in the presence
of an aprotic polar solvent, e.g. tetrahydrofuran, 1,4-
dioxane, acetonitrile, N,N-dimethylformamide,
dimethylsulfoxide, hexamethylphosphoric acid triamide,
or mixtures thereof and at temperatures between -1~C
and the boiling point of the reaction mixture,
preferably between 40 and lOO~C. It has proved
convenient to use additional organic or inorganic bases,
e.g. alkali metal or alkaline earth metal hydroxides,
alkoxides or carbonates (e.g. sodium hydroxide, sodium
methoxide, potassium tert butoxide, sodium carbonate and
potassium carbonate), tertiary amines (e.g.
triethylamine, ethyldiisopropylamine and N,N-
dimethylaniline), pyridine or 4-(dimethylamino~pyridine,
and to carry out the reaction in the presence of an
excess of a compound of formula III.
If the spirodiamines of formula III and the carbonic
acid derivatives of formula II are used in equimolar
quantities, the hydrohalic acid salts of the desired
compounds of formula Ia are obtained directly (provided
that Y represents a halogen atom).
Metal compounds of formula IIIa can readily be
pr~pared in situ from compounds of formula III by
reacting with alkali or alkaline earth metals, e.g. with
- 18 -
sodium, potassium or barium, or with alkali metal or
alkaline earth metal hydrides, e.y. with sodium,
potassium or calcium hydride, or by reaction with alkali
metal or alkaline earth metal organometallic compounds,
e.g. with n-butyllithium or phenyllithium.
The reaction of step (b) is preferably carried out
in an inert organic solvent, e.g. in an aromatic
hydrocarbon such as toluene or xylene, in an ether such
as diisopropyl ether, tetrahydrofuran or dioxane, in a
ketone such as 3-pentanone, in a chlorinated aliphatic
hydrocarbon, such as 1,2-dichloroethane or in another
solvent, such as acetonitrile or dimethylformamide, or
in mixtures thereof, optionally in the presence of a
tertiary organic base such as pyridine, and at
temperatures up to the boiling point of the reaction
mixture, preferably at temperatures between +30 and
+100 C .
The hydrogenolysis of step (c) is conveniently
carried out in the presence of a catalyst based on a
metal of the VIIIth subgroup of the Periodic Table of
the elements, for example palladium on animal charcoal,
palladium on barium sulfate, Raney nickel or Raney
cobalt and at hydrogen pressures of 1 to 300 bar and
temperatures of 0C to 130C, in the presence of
solvents, e.g. alcohols (such as methanol and ethanol),
ethers (such as dioxane and tetrahydrofuran), carboxylic
acids (e.g. acetic acid) or tertiary amines (e.g.
triethylamine). If the work is done in the absence of
additional hydrogen chloride acceptors, e.g. sodium
carbonate, potassium hydrogen carbonate, triethylamine
or sodium acetate, the hydrochlorides of the desired
compounds are produced directly and may be isolated
after removal of the catalyst by evaporation of the
reaction solution. If, in place of hydrogen,formic acid
is used in the hydrogenolysis reaction described above,
the reaction will theoretically succeed even under
pressureless conditions. In this variant, it has proved
particularly useful to carry out the reaction with
- 19 -
formic acid in the presence of dimethylformamide as
solvent and palladium on charcoal as catalyst at
temperatures between 70 and 110C, and to carry out the
reduction with triethylammoniumformate in the presence
of excess triethylamine and palladium on animal charcoal
or palladium acetate and triarylphosphines, such as
triphenylphosphines, tris(o-tolyl) phosphines and tris-
(2,5-diisopropylphenyl)-phosphines, at temperatures
between 40 and 110C.
Bases of formula I thus obtained can subsequently be
converted into the acid addition salts thereof or, if
acid addition salts are obtained, they may be converted
into the free bases or other pharmacologically
acceptable acid addition salts.
If in the aminocarbonylated condensed diazepinones
of formula I according to the invention m and o each
represent 1, and n and p each represent 2, these
compounds are chiral because they have an asymmetric
carbon atom in the side chain. These compounds can
therefore occur as enantomeric (+) and (-) forms. The
invention includes the individual isomers as well as the
racemates.
Any racemates of the compounds of formula I may be
resolved by known methods, for example using an
optically active acid such as (+l or (-)tartaric acid or -
a derivative thereof such as (+~ or (-)diacetyltartaric
acid, (+) or (-~monomethyltartrate or (+)camphorsulfonic
acid.
According to a conventional method of isomer
separation the racemate of a compound of formula I may
be reacted with one of the above mentioned optically
active acids in equimolar quantities in a solvent and
the crystalline diasteromeric salts obtained are
separated on the basis of their different solubilities.
This reaction may be carried out in any type of solvent
provided that it has a sufficiently different solubility
for the salts. Methanol, ethanol or mixtures thereof
are preferred, e.~. in a ratio by volume of 50:50. Then
- 20 - 2~ 3
each of the diastereomeric salts is dissolved in water,
neutralised with a base such as sodium hydroxide or
potassium hydroxide and in this way the corresponding
free compound is obtained in the (+) or (-) form.
only one enantiomer is obtained if the methods of
synthesis described above are carried out with only one
enantiomer of formula III, IIIa or V.
The preparation of the carbonic acid derivatives of
formula II used as starting products is described in
detail in DE-A-3726908.
Compounds of formula III, which are generally new
and have not yet been described, can be obtained for
example by the following methods:
i) 2-Substituted 2,7-diazaspiro[4,4]nonanes of formula
III may be obtained following the procedures described
by Warner-Lambert Comp., in AU-A-83/18698 (see Derwent
107 300).
ii) 6-Substituted 2,6-diazaspiro[3,4]octanes of formula
III may be prepared for example starting from 1,1,2-
ethanetricarboxylic acid esters which, when reacted with
suitable 1,3,5-trisubstituted hexahydro-1,3,5-triazines
in the presence of catalytic quantities of
trifluoroacetic acid, yield 1-substituted 2-
pyrrolidinone-4,4-dicarboxylic acid esters in a high
yield. Reduction with lithium aluminium hydride leads
to 1-substituted 3,3-bis-hydroxymethyl-pyrrolidines
which can be reacted with concentrated aqueous
hydrombromic acid in a bomb-type tube to obtain the
corresponding bis-bromomethyl-pyrrolidine-hydrobromides
without difficulty. These react with p-toluenesulfonic
acid amides in the presence of concentrated potassium
hydroxide solution and using dioxane or dimethylform-
amide as solvents to obtain in a high yield 6
substituted 2-(4-methylbenzenesulfonyl~-2,~-
diazaspiro[3,4]octanes, which can }:e detosylated, for
example, with sodium-bis-t2-methoxyethoxy~-
2~2~
- 21 -
aluminiumhydride (RED-Al~ ), preferably in toluene
solution, to obtain the desired 6-substituted 2,6-
diazaspiro[3,4]octanes.
Alternatively, two cyanosuccinic acid esters may be
used for the raaction with the 1,3,5-trisubstituted
hexahydro-s-triazines instead of the 1,1,2-
ethanetricarboxylic esters. This results in 1-
substituted 4-cyano-4-carbalkoxy-2-pyrrolidinones, which
yield 1-substituted 3 aminomethyl-3-hydroxymethyl-
pyrrolidines when reduced with lithium aluminium
hydride. Reaction with concentrated aqueous hydrobromic
acid produces a good yield of 1-substituted 3-
(aminomethyl)-3-(bromomethyl)-pyrrolidines, which can
readily be cyclised, when treated with concentrated
sodium or potassium hydroxide solution in the presence
of water-miscible solvents, e.g. dioxane, ethanol,
methanol or dimethylformamide, to obtain the desired 6-
substituted 2,6-diazaspiro[3,4]octanes.
iii) 2-Substituted 2,6-diazaspiro[3,4~octanes of
formula III may, according to one of the variants given
in (ii) above, be obtained from readily accessible 6-
(phenylmethyl)-2,6-diazaspiro[3,4]octane by alkylating
in the 2-position in a conventional manner for example
by reacting with a suitable acylating agent followed by
reduction with lithium aluminium hydride, with the
benzyl group subsequently being removed by
hydrogenolysis.
iv) 2-Substituted 2,6-diazaspiro[3,3]heptanes of
formula III can be prepared in a conventional ~anner
from unsubstituted 2,6-diazaspiro[3,3]heptane which is a
known compound (see A. Litherland and F.G. Mann, J.
Chem. Soc. [London] 1938, 15~8; F. Govaert and M.
Beyaert. Bull. Soc. Chim. Belg. 55: 106, 112 [1946];
F.G. Mann and A. Litherland, Nature 141: 789-7gO
[1938]), for example by monoacylation and subsequent
\
- 22 -
careful re~uction with lithium aluminium hydride in
diethylether.
v) 7~Substituted 2,7-diazaspiro[3,5]nonanes of formula
III may be prepared from the 1-substituted 4-cyano-4-
piperidinecarboxylic acid esters which are obtainable
analagously to the procedures described by L. Ciszewski,
Pol. J. Chem. 62: 451-455 [1988]. For example, the 1-
substituted 4-cyano-4-piperidinecarboxylic acid esters
are catalytically hydrogenated in glacial acetic acid
and in the presence of platinum~IV)oxide and
concentrated sulphuric acid to obtain the corresponding
~-alanine esters. These may then be cyclised with
Grignard reagents, for example with ethyl magnesium
bromide, in ether and at O to 5C to obtain 7-
substituted 2,7-diazaspiro[3,5]nonan-1-ones which when
reduced with lithium aluminium hydride in ether are
converted into the desired 7-substituted 2,7-
diazaspiro[3,5]nonanes. Alternatively, the 7-
substituted 2,7-diazaspiro[3,5]non-1-ones may also be
obtained by saponifying the above mentioned ~-alanine
esters to obtain the corresponding ~-alanines and then
closing the azetidinone ring in a theoretically known
manner, e.g. by the action of triphenylphosphine and
tetrachloromethane or N-bromosuccinimide,
ethyldichlorophosphate, phenyldichlorophosphate or
phenylphosphonic acid dichloride, in the presence of
triethylamine and using acetonitrile as solvent.
vi) 2-Substituted 2,7-diazaspiro[3,5]-nonanes of
formula III may also be prepared from 7-(phenylmethyl)-
2,7-diazaspiro[3,5]nonane synthesised according to (v~
above, without any significant problems using the
reaction sequence specified in (iii) above.
The tricyclic compounds of formula IV are known from
patent literature or may be synthesised following
published methods from available starting materials.
- 23 ~
Chlorocarbonic acid derivatives of formula V may be
prepared in accordance with current methods from
spirodiamines of formula II and phosgene.
The condensed diazepinones of formula I and the acid
addition salts thereof have valuable properties; as
already mentioned, they exhibit selective spasmolytic
properties on peripheral organs, particularly the ileum
and bladder, and in view of the absence of any effect of
increasing heart rate, inhibiting gastric acid
secretion, inhibiting saliva or affecting the
accommodating powers of the eye in the therapeutic
dosage range they are suitable for use in human and
veterinary medicine for the treatment of cholinergically
induced spasms and motility disorders in the
gastrointestinal tract and in the region of the outward-
leading bile ducts, for the symptomatic treatment of
cystitis and spasm in urelithiasis by lowering the
pathologically raised tone of the hollow organs, for the
treatment of relative incontinence based on an
incongruity of sphincter and detrusor tone, for the
symptomatic treatment of bronchial asthma and bronchitis
by suppressing the muscarincally mediated part of the
bronchoconstriction, and for the treatment of ischaemic
heart disease by lowering heart rate and at the same
time suppressing parasympathetically induced coronary
spasm and lowering the basal coronary tone.
Thus viewed from a further aspect the present
invention provides a method of treatment of the human or
non-human ~preferably mammalian) body to combat
cholinergically induced spasm and motility disorders in
the gastrointestinal tract and in the region of the
outward leading bile ducts, to combat cystitis and spasm
in urelithIasis, to combat relative incontinence, to
combat bronchial asthma and bronchitis and to combat
ischaemic heart disease, said method comprising
administering a compound of formula I or a
physiologically acceptable acid addition salt thereof to
said body.
- 24 ~
Viewed from a further aspect the invention provides
the use of a compound of formula I or a physiologically
acceptable acid addition salt thereof for the
manufacture of a therapeutic agent for use in a method
of treatment to combat cholinergically induced spasm and
motility disorders in the gastrointestinal tract and in
the region of the outward leading bile ducts, to combat
cystitis and spasm in urelithiasis, to combat relative
incontinence, to combat bronchial asthma and bronchitis
and to combat ischaemic heart disease,
Viewed from a still further aspect the invention
provides a pharmaceutical composition comprising a
compound of formula I or a physiologically acceptable
acid addition salt thereof together with at least one
pharmaceutical carrier or excipient.
For this purpose the compounds of formula I or salts
thereof may be incorporated, in a conventional manner
into conventional pharmaceutical forms, e.g. solutions,
suppositories, plain or coated tablets, capsules or
infusions. For oral administration the daily dose is
generally between 0.01 and 10 mg/kg, preferably from
0.02 to 5 mg/kg, more particularly 0.05 to 2.5 mgJkg of
body weight, optionally administered in the form of
several, preferably one to three doses, to achieve the
desired results.
A favourable correlation between spasmolitic effects
on the one hand and the undesirable effects on heart
rate, pupil size, and the secretion of tears, saliva and
gastric acid, on the other hand, which occurs with
therapeutic agents having an anticholinergic activity
component is of particular importance to the therapeutic
use of the substances. The following experiments show
that the compounds according to the invention show
particularly favourable correlations in this respect.
A. Investi~ation of functional selectivity of the
antimuscarinic effect
- 25
Substances with antimuscarinic properties inhibit
the effects of exogenously supplied agonists or of
acetylcholine released from cholinergic nerve endings.
The following is a description of methods which are
suitable for determining spasmolitically effective
antimuscarinics.
"In vitro" OrgLan Preparations
Dissociation constants (KB-values) were determined in
vitro on the ileum and spontaneQusly beating atrium of
the guinea pig. The ileum was removed and incubated in
Krebs-~enseleit solution in an organ bath. Contractions
were induced by increasing concentrations of
methacholine (M) so that full concentration/activity
curves could be recorded. Then the M was washed out,
the test substance was added and left in contact for 30
minutes and another concentration/activity curve was
recorded with M.
The dosage ratio (DR), i.e. the extent of
displacement of the concentration/activity curve, made
it possible to calculate the dissociation constant
according to ~runlakshana and Schild ~Brit. J.
Pharmacol~ 14, 48, 1959).
In an isolated, spontaneously beating right atrium,
M reduced the heart rate~ as a function of the
concentration. By adding an antimuscarinic agent this
effect was cancelled again. Dissociation constants for
the muscarinic receptors of th~ atrium were obtained in
the manner described above. ~ comparison of the
dissociation constants obtained in two tissues made it
possible to identify substances ~ith a selective
spasmolitic effect. The results are shown in Table III.
"In vivo" Methods
The methods used had the purpose of confirming the
selectivity of the antimuscarinic effect. Those
~ - 26 - 2~2~
substances which had been selected on the basis of in
vitro tests were investigated for
1. Selectivity of the bronchospasmolitic activity in
guinea pigs.
2. The saliva-secretion inhibiting effect in the rat
and
3. In situ spasmolitic activity in guinea pigs.
1. Effect on M-receptors of the bronchii, heart and
bladder of anaethetised auinea pias
Method
, .
Male and female guinea pigs (body weight 550-600 g)
were anaethetised with urethane (1.4 g/kg, i.p.). A
cannula was inserted into the jugular vein for the
purpose of injecting the active substance. 220 I.U./kg
of heparin were injected intravenously. A cannula was
inserted in the trachea; the animals were artificially
respirated with oxygen-rich air using a positive
pressure pump (Braun-Melsungen~ at a rate of 80 beats
per minute. One branch of the tracheal cannula was
connected to a water manometer 10 cm high. The
respiration volume was adjusted so that the maximum
intratracheal pressure during respiration just reached
the pressure of a 10 cm water column.
~ part from a few modifications, the effect of the
active substances on bronchial tone was measured by the
method described by Konzett and Rossler (1940~. The
volume of respiration gas mixture produced by broncho-
constriction (overflow) which flowed through the water
manometer was measured by means of a tube-type pneumatic
tachometer (Fleisch, model 1000), connected to an SP
2040D differential pressure transducer tHSE). The
results were recorded with an IFD recorder. Before the
test the trachea was clamped for a short time to produce
2~2~
- 27 -
the maximum possible degree of bronchoconstriction for
calibration purposes. A cannula was inserted in the
left carotid artery; the arterial blood pressure was
measured using a pressure transducer (Bell and Howell,
4-327 I) in conjunction with an IFD recorder. The heart
rate was measured with a heart rate meter triggered by
arterial pulse waves.
A small median abdominal cut was made and the
bladder was connected to a power transducer under a
resting tension of l gram.
The active substances to be tested were injected
through the jugular vein and 5 minutes later the
increase in the tension of the bladder (in grams) the
bronchial resistance (in %) and the decrease in heart
rate (beats per minute) after the administration of
acetylcholine (50 ~g/kg i.v. and i.a.) were measured.
Dosage-dependent curves were plotted by giving the
percentage inhibition of bronchoconstriction,
bradycardia and the increase in the tension of the
bladder against the logarithm of the dose (mol/kg) of
the active substances to be tested. The results are
given as averages (for 4 to 6 animals). For the results
see Table I.
':
2. Salivation-inhibiting effect in the rat
Male THOM rats anaethetised with 1.2 g/kg urethane
were given increasing doses of the substance i.v. in
accordance with Lavy and Mulder (~rch. int. Pharmacodyn.
178, 437-445, (1969)). The salivation was initiated by
the subcutaneous administration o~ 2 mg/kg of
pilocarpine. The saliva was mopped up with blotting
paper and the area it occupied was determined by
planimetry every 5 minutes. The dosage o~ substance
which reduced the volume of saliva by 50% was determined
graphically. For the results see Table II.
3. In-situ spasmolytic effect on quinea pias
- 28 - 2~2~
Male guinea pigs (500 to 600 g body weight) were
anaethetised with urethane t1.2 g/kg, i.p.); cannulas
were inserted in the trachea, jugular vein and left
carotid artery. The animals were artificially
respirated with oxygen-rich air using a positive
pressure pump at a beat rate of 80 per minute. An
abdominal incision 3 to 4 cm long was made and about 15
cm of a movable loop of the small intestine (ileum) was
tied off at the distal end whilst the circulation of the
blood was maintained~ The proximal part was filled with
a Krebs-Ringer solution and a pressure meter with a
Millar micro-tip catheter (PC-450, 5F) was inserted into
the intestine. A glass tube was placed vertically in
the abdomen and fixed to the surrounding abdominal wall
so that when the glass tube was filled with Krebs-Ringer
solution the animal acted as his own organ bath.
The glass tube was filled with Krebs-Ringer solution
until the entire lower abdomen was immersed. The active
substances being tested were injected through the
jugular vein; 5 minutes later, contractions were
produced using methacholine (20 ~g/~g i.a.). By
recording the percentage suppression of the
methacholine-induced contractions against the logarithm
of the dosage (mol/kg) of the test substance,
dosage/activity curves were obtained.
The results were given as averages (for 4 to 8
animals~ (see Table II).
The following compounds, by way of example, were
tested using the above methods:
A = 5,11-dihydro-11-[[7-methyl-2,7-diazaspiro[4,4]non-2-
yl]carbonyl]-6H-pyrido[2,3-b]~1,4]benzodiazepin-6-one
B = 5,11-dihydro-8-methyl-11-[[7-methyl-2,7-
diazaspiro[4,4]non-2-yl]carbonyl]-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
~2~
- 29 -
C = 5,11-dihydro-11-[[6-methyl-2,6-diazaspiro~3,4]oct-2-
yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one
D = 5,11-dihydro-8-ethyl-11-[[6-methyl~2,6-
diazaspiro[3,4]oct-2-yl]carbonyl]-6~-pyrido[2,3-
b][1,4]benzodiazepin-6-one
E = 5,11--dihydro-8-methyl-11-[[2-methyl-2,6-
diazaspiro[3,4]oct-6-yl]carbonyl]-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one
and as comparison substances
X = 11-[[2-[(diethylamino)methyl]-1-piperidinyl]acetyl]-
5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one
(c.f. US Patent 4 550 107)
;
Y = 5,11-dihydro-11-[(4-methyl-1-piperazinyl)acetyl]-6H-
pyrido[2,3-b][1,4]benzodiazepin-6-one
(Pirenzepine, see US Patent No. 3 660 380)
and
; Z = Atropine.
- 30 - 2~2~
:~ Table I
Selectivity of the bronchospasmolytic activity in the
guinea pigo
Acetylcholine - Antagonism
_ _ = .. _
. ~ ¦ Tost Bronchii Bladdor Hoart Ratio o~
¦ Substanco - log ED,jo - log ED50 - log ED50 influ~nco o~
,~ l (mol kg'1) (mol kg~1) (mol kg 1) br~dycardia to
l i.v. i.v. i.v. bronchoconstriction
I
,
¦ A 6.44 5.48 5.10 22
'~' .__ . .. _.___
¦ B 6.65 6.29 5.45 16
C 7.30 6.79 5.88 26
~~ .. __.
D 6.97 6.82 6.01 9
., _ __
E 7.27 6.98 5.76 32
` -=.- -... _ .. __
X 5.58 4.93 5.84 0.5
Y 6.57 5.84 5.90 5
Z 8.09 7.28 7.57 3
. _ ~ .. ~ ..
-
.
- 31 -
Table II
Selectivity of the in situ spasmolytic activity in
relation to the salivation inhibiting effect.
Tost In silu spasmolysis S li~vatlon inhibition Ratio ot salivation .
Subst~tnco guinoa plg ilcum rat il~hibition to Ih~
- log ED60 -109 ED spasmolylic activity
. ~ . _... (mol kg~1) (mol kg'1)
¦ . _ 6.05 _ ~ 5.46 4
697 _~---~-- 637 Z
¦ - .... _ _ 5.4B . 5 . _ __ 3
Y 6.08 6.42 0.5
I _
l _ 7.2B 7.60 0.5 .
~2~
;: - 32 -
Table III
Dissociation constants (KR values) on the ileum and
spontaneously beating attrium of the guinea pig.
. _
T~st H~art llourn Soloc~ivity
Substanc~ KB Imol/l~ KB [mol/ll KB Hoart to
_ ____ _
A 1.23 X 10-6 2.69 X 10-7 4.~
: ~ B 2.51 X 10 7 5.89 X 10 B ._
. _ 3.39 X 10 7 7.24 X 10-8 4 7 __
D 1 1 X 10-7 2.24 X 10 8 4 9
. ~ E 4.68 X 10-7 6.46 X ~0-8 7.2
. ___ .
¦ X 1 05 10-7 6.17X 10 7 0.17
¦ Y 1 23 X 10-7 1.94 X 10 7 0.63
Z 1.41 X 10 9 8.13 X 10-~ 1.7
~ ..
Discussion of the results
The compounds of the invention, in low doses, inhibit
the effects of exogenously supplied acetylcholine or
methacholine on the smooth muscle of bronchii, bladder
or small intestine, without this agonistic effect
altering the heart rate (Tables I and II). For example,
substances C and E show a very marked selectivity for
the smooth muscle; 26- and 32- times lower doses are
needed in order to inhibit the acetylcholine induced
broncho-constriction, compared with the acetylcholine-
induced bradycardia (Table I). The compounds of the
invention not only show selectivity for the smooth
muscle compared with effects which are initiated by
cardiac muscarine receptors, but also higher doses are
- 33 - ~2
needed in order to inhibit the pilocarpine~induced
salivation (Table II).
The in vivo selectivity of these compounds for the
smooth muscle observed agrees with the in vitro tests.
The substances have a higher affinity for muscarine
receptors in the ileum than for cardiac muscarine
receptors (Table III).
The data show that the compounds of the invention
inhibit the effects of muscarine agonists on the smooth
muscle e.g. bronchii, bladder and ileum, at doses which
have no effect on heart rate or salivation. The
comparison substances Y (pirenzepine) and Z (atropine~
show no selectivity and influence all the above
mentioned effects in the same dosage range. Comparison
substance X shows a higher level of effect on cardiac
muscarine receptors.
All the compounds of the invention are characterised
by excellent stability to hydrolysis. It is therefore
possible to prepare solutions for parenteral
administration which will have a long shelf life.
The following non-limiting examples are intended to
illustrate the invention:
Mp denotes melting point, D denotes decomposition.
There are satisfactory elementary analyses, IR-, UV-, 1H-
NMR-spectra and frequently mass spectra for all the
compounds. Unless otherwise expressly mentioned, the
percentages, parts and ratios given are always by
weight.
Example 1
5,11-dihydro-11-[[7-methyl-2,7-diazaspiro[4,4]non-2-
Yllcarbonyl]-6H-pvrido r 2,3-bl[1,41benzodiaze~in-6-one
A mixture of 9.0 g (0.033 mol) of 11-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-
b] r 1,4]benzodiazepin-6-one, 4,3 g (0.041 mol) of
'3~
-- 34 --
anhydrous sodium carbonate, 5.6 g (0.04 mol) of 2-
methyl-2,6-diazaspiro[4,4]nonane and 100 ml of
acetonitrile were stirred for 30 minutes at a reaction
temperature of 50C. The solvent was then distilled off
in vacuo, the remaining highly viscous residue was taken
up in 30 ml of water, made alkaline with sodium
hydroxide and exhaustively extracted with
dichloromethane. The combined dichloromethane phases
were dried over sodium sulphate and evaporated down and
the residue was purified by chromatography on silica gel
(35-70 mesh) using dichloromethane/ethylacetate/
cyclohexane/methanol/conc. ammonia 50/11/9/9/1,
v/v/v/v/v, as eluent. The residue remaining after
evaporation of the suitable eluates was recrystallised
from watertmethanol 1/9 (v/v). 5.9 g (48 96 theory) oî
colourless crystal were obtained, Mp. 271-274C.
C2lH23Ns2 ~377-45)-
Calculated: C 66.83 H 6.14 N 18.55
Found: 67.00 6.35 18.85
Example 2
9-Chloro-5,11-dihydro-11-[[7-methyl-2,7-
diazaspiro[4,4]non-2-yI]carbonyl]-6H-pyrido[2,3-
b~[l,4lbenzodiazepin-6-one
Prepared analagously to Example l from 9-chloro-11-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one and 2-methyl-2,7-
diazaspiro[4,4]nonane in a yield of 41 g6 of theory.
Colourless crystals Mp. 257-259C (dimethylacetamide).
C21H22N52 (411-90)-
Calculated: C 61.24 H 5.38 C1 8.61 N 17.00
Found: 61.50 5.67 8.59 17.10
Example 3
- 35 - 2 ~ 2 ~
5,11-Dihydro-8-methyl-11-[[7-methyl-2,7-
diazaspiro[4,4]non-2-yl]carbonyl]-6H-pyrido[2,3-
b][1,4]benzodiaepin-6-one
. . _
Prepared analagously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-8-methyl-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one and 2-methyl-2,7-
diazaspiro[4,4]nonane in a yield of 71 % of theory.
Colourless crystals Mp. 212-214C (acetonitrile using
activated charcoal).
CzzHzsNsOz (391.48).
Calculated: C 67.50 H 6.44 N 17.89
Found: 67.89 6.20 18.00
Exam~le 4
8-Chloro-5,11-dihydro-11-[[7-methyl-2,7-
diazaspiro[4,4]non-2-yl]carbonyl]-6H-pyrido[2,3-
b][l~4]benzodiazepin-6-one
Prepared analagously to Example 1 from 8-chloro-11-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one and 2-methyl-2,7-
diazaspiro[4,4~nonane in a yield of 47 % of theory.
Colourless crystals Mp. 214-215C (acetonitrile).
Cz1HzzClN50z (411.90).
Calculated: C 61.24 H 5.38 Cl 8.61 N 17.00
Found: 61.00 5.38 8.72 17.10
Example 5
5,11-Dihydro-8-ethyl-ll-[[7-methyl-2,7-
diazaspiro[4,4]non-2-yl]carbonyl]-6H-pyrido[2,3-
b][1~4]benzodiazepin-6-one
2~ 3
- 36 -
Prepared analagously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-8-ethyl-6H-pyrido[2,3-
b][1,4]benzodiazepin 6-one and 2-methyl-2,7-
diazaspiro~4,4]nonane in a yield of 57 % of theory.
Colourless crystals Mp. 216-217C (from acetonitrile
using activated charcoal).
C23H27NsO2 (405-50)-
Calculated: C 68.13 H 6.71 N 17.27
Found: 68.30 6.81 17.30
Example 6
5,11-Dihydro-9-methyl-11-[[7-methyl-2,7-
diazaspiro[4,4]non-2-yl]carbonyl]-6H-pyrido[2,3-
b][l 4~benzodiazepin-6-one
Prepared analagously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-9-methyl-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one and 2-methyl-2,7-
diazaspiro[4,4]nonane in a yield of 9 % of theory.
Colourless crystals Mp. 253-255C (acetonitrile).
C2ZH2sNso2 (391-48)-
Calculated: C 67.50 H 6.44 N 17.89Found: 66.99 6Y4`7 17.62
Example 7
8-Bromo-5,11-Dihydro-11-[[7-methyl-2,7-
diazaspiro[4,4]non-2-yl]carbonyl]-6H-pyrido[2,3-
b~[l,4]benzodiazepin-6-one
Prepared analagously to Example 1 from 8-bromo-11-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one and 2-methyl-2,7-
diazaspiro[4,4~nonane in a yield of 64 % of theory.
- 37 -
Colourless crystals Mp. 227-229C (D.) (from
acetonitrile/ethanol 1/1 V/V).
C21H22BrN5O2 (456.36).
Calculated: C 55.27 H 4.86 Br 17.51 N 15.35
Found: 55.60 5.10 17.35 15.13
Example 8
5,11-Dihydro-11-[~7-ethyl-2,7-diazaspiro[4,4]non-2-
yllcarbonY11-6H-pyrido~2,3-b]~1,4~benzodiazepin-6-one
Prepared analagously to E~ample 1 from 11-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one and 2-ethyl-2,7-
diazaspiro[4,4]nonane in a yield of 85 % of theory.
Colourless crystals Mp. 214-215C (acetonitrile).
C22H25Nso2 (391-48)-
Calculated: C 67.50 H 6.44 N 17.89
Found: 67.44 6.70 18.10
Example 9
5,11-Dihydro-11-[[7-ethyl-2,7-diazaspiro[4,43non-2-
yl]carbonyl]-8 methyl-6H-pyrido[2,3-
b~ 41benzodiazepin-6-one
Prepared analagously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-8~methyl-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one and 2-ethyl-2,7-
diazaspiro[4,4]nonane in a yield of 74 % of theory.
Colourless crystals Mp. 178-180C (aGetonitrile~.
C23H27N502 (405 50~
Calculated: C 68.13 H 6.71 N 17.27
Found: 67.94 6.70 17.57
- 38 -
Exam~le 10
5,11-Dihydro-11-[[7-ethyl-2,7-diazaspiro[4,4]non-2-
yl]carbonyl]-9-methyl-6H-pyrido[2,3-
bl r 1.4lbenzodiazepin-6-one
Prepared analagously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-9-methyl-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one and 2-ethyl-2,7-
diazaspiro[4,4]nonane in a yield of 85 % of theory.
Colourless crystals Mp. 244-246C (acetonitrile).
C23Hz7NsO2 (405-50)-
Calculated: C 68.13 H 6.71 N 17.27Found: 67.83 6.92 17.17
Example ll
9-Chloro-5,11-Dihydro-11-[[7-ethyl-2,7-
diazaspiro[4,4]non-2-yl]carbonyl]-6H-pyrido[2,3-
b][1.4lbenzodiazepin-6-one
Prepared analagously to Example 1 from 9-chloro-11-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one and 2-ethyl-2,7-
diazaspiro[4r4]nonane in a yield o~ 75 % of theory.
Colourless crystals Mp. 224.0-225.5C (acetonitrile).
C22H24ClN5o2 (425.93),
Calculated: C 62.04 H 5.68 Cl 8.32 N 16.44
Found: 61.89 5.65 8.35 16.34
Example 12
5,11-Dihydro-11-[[6-methyl-2,6-diazaspiro[3,4]oct-2-
yl]carbon~l]-6H-pyrido[2,3-b] r 1 F 4~benzodiazepin-6-one
~ ~2 ~
- 39 -
a) 4,4-Bis-(ethox~carbonyl)-l-methyl-2-pvrrolidinone
A mixture of 221.6 g (0.90 mol) of triethyl 1,1,2-
ethanetricarboxylate, 116.3 g (0.90 mol) of 1,3,5-
trimethylhexahydro-1,3,5-tria~ine and 20.6 g (0.18 mol)
of trifluoroacetic acid was stirred for 20 hours at a
reaction temperature of 100C. ~fter cooling, the
mixture was diluted with l litre of toluene and then
extracted three times with 100 ml of 10% aqueous
hydrochloric acid. The aqueous hydrochloric acid
extracts were combined and extracted once with lOo ml of
ethylacetate. The organic phases obtained were
combined, shaken once with 200 ml of a saturated aqueous
sodium hydrogen carbonate solution and then washed three
times with 300 ml of water, dried over sodium sulphate
and evaporated down in vacuo. The colourless oil
obtained (yield 220 g, i.e. 91 ~ of theory) was used
without further purification in the following step.
b) 3,3 Bis-(hydroxymethyl~-1-methylpyrrolidine
To a suspension of 75.0 g (1.976 mol) of lithium
aluminium hydride in 1 litre of anhydrous
tetrahydrofuran, a solution of 160.0 g (0.658 mol) of
4,4-bis-(ethoxycarbonyl)-l-methyl-2 pyrrolidinone in 700
ml of dry tetrahydrofuran was added dropwise in such a
way that the reaction could be kept under control and
the tetrahydrofuran boiled gently. After the addition
had ended the mixture was refluxed for a further 4 hours
with stirring. It was then left to cool and 75 ml of
water, 75 ml of 15% sodium hydroxide solution and 215 ml
of water were added dropwise one after the other, with
stirring and external cooling with ice water. The
precipitate obtained was suction filtered, expended once
more with tetrahydrofuran and boiled, then suction
filtered again. The filtrates obtained were combined,
carefully dried over sodium sulphate and evaporated down
in vacuo. The colourless, viscous oil obtained in a
2~2~
- 40 -
yield of 71.4 g (75% of theory) was further processed in
tha next step without any more purification.
c) 3,3-Bis-lbromomethyl)-l-methylpyrrolidine
A mixture of 28.0 g (0.139 mol) of the above
mentioned compound and 250 ml of 63% aqueous hydrobromic
acid was heated in a bomb-type tube for 24 hours to
180C. A~ter cooling, the mixture was evaporated to
dryness in vacuo, the residue,-which dissolved with
relative difficulty in water, was taken up in 400 ml of
water and treated with excess potassium carbonate. ~he
suspension obtained was extracted exhaustively with
ethyl acetate, the extracts were combined and dried over
sodium sulphate. The residue remaining after
evaporation of the solvent (yield: 49.0 g, i.e. 94 % of
theory), a colourless oil, was used in the next step
without any further purification. RF . 9 (MaChereY_
Nagel, Polygram~R)SIL G/UV2s4, pre-coated plastic sheets
for TLC; ~luent: ethylacetate/methanol/conc. ammonia
100/30/3, v/v/v)O
d) 6-Methyl-2-[(4-methylphenyl)sulfonyl]-2,6-
diazaspiro- r 3,41Octane
To a solution of 51.3 g (0.3 mol) of p-
toluenesulfonamide and 33.6 g (0.6 mol) of potassium
hydroxide in 160 ml of water was added a solution of
80.0 g (0.295 mol? of 3,3-bis-(bromomethyl) 1-methyl-
pyrrolidine in 4.8 litres of dioxane and the resulting
mixture was refluxed. After 17 hours and then a further
24 hours, 10.0 g (0.0584 mol) of p-toluenesulfonamide
and 6.7 g (0.12 mol) of potassium hydroxide dissolved in
30 ml of water were again added and the mixture again
refluxed for 24 hours. The resulting reaction mixture
was evaporated down in vacuo, the residue remaining was
distributed between water and ethylacetate, the organic
layer was washed once with water, dried over sodium
.
- 41 -
sulphate and evaporated in vacuo using a rotary
evaporator. The residue remaining (64.0 g) was stirred
with diisopropylether and suction filtered, the suction
filter residue remaining was finally recrystallised from
hot cyclohexane using activated charcoal. 50.5 g (61 %
of theory) of colourless crystals were obtained, Mp. 83-
85C.
c14H20N22s (280-39)
Calculated: C 59.67 H 7.1~ N 9.99 S 11.43
Found: 56.66 7.16 10.30 11.51
e) 6-Methyl-2~6-diazaspiro r 3~4]octane
A mixture of 48.5 g (0.173 mol) of 6-methyl-2-[(4-
methyl-phenyl)-sulfonyl]-2,6-diazaspiro[3,4]octane, 199
ml (about 0.7 mol) of an approx. 3.5 molar solution of
sodium-bis-(2-methoxy-ethoxy)dihydroaluminate in toluene
and 300 ml of dry toluene were heated to 60C for 15
hours with stirring and to 80C for 6 hours. After the
reaction had ended, 20 % aqueous sodium hydroxide
solution was care~ully added dropwise, whilst external
cooling was carried out with ice water, until the
development of hydrogen had ceased, the toluene phase
was separated off, dried over sodium sulphate and
evaporated carefully using a Vigreux column at a
pressure of 50 mmHg. The desired compound had a boiling
point 22 ~Hg 8~-85C and proved to be a colourless,
readily mobile liquid smelling like an amine. Yield:
7.5 g (34 % of theory).
f) 5,11-Dihydro-11-[[6-methyl-2,6-diazaspiro[3,4]oct-2-
yl]-carbonyl-6H-pyrido[2 3-b]~1 4~benzodiaze~in-6-one
Prepared analagously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one and 6-methyl-2,6-
,
f~ ~ ~
- 42 -
diazaspiro[3,4]octane in a yield of 26 % of theory
Colourless crystals Mp. 225.5-227.0C (acetonitrile).
C20H21Ns2 (363-42)-
Calculated: C 66.10 H 5.82 N 19.27
Found: 66.185.82 19.17
Example 13
5,11-Dihydro-8-ethyl~ [[6-methyl-2,6-
diazaspiro[3,4]oct-2-yl]carbonyl-6H-pyrido[2,3-
bl~l,4]be_zodiazepin-6-one
Prepared analagously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-8-ethyl-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one and 6-methyl-2,6-
diazaspiro[3,4]octane in a yield of 47 % of theory.
Colourless crystals Mp. 215-217C (acetonitrile).
C22H25N502 (391-48)-
Calculated: C 67.50 ~ 6.44 N 17.89
Found: 67.27 6.41 17.88
Example 14
5,11-Dihydro-8-methyl-11-[[6-methyl-2,6-
diazaspiro[3~4]oct-2-yl]carbonyl-6H-pyrido[2 r 3~
b]~1 4~benzodiazepin-6-one
Prepared analagously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-8-methyl-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one and 6-methyl-2,6-
diazaspiro[3,4]octane in a yield of 34 % of theory
Colourless crystals Mp. 220-223C (acetonitrile).
C21H23Nso2 t377 45)
Calculated: C 66.83 H 6.14 N 18.55
Found: 66.59 6.12 18.41
2 ~
- 43 -
Example 15
5,11-Dihydro-9-methyl-11-[[6-methyl-2,6-
diazaspiro[3,4]oct-2-yl]carbonyl-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one
Prepared analagously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-9-methyl-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one and 6-methyl-2,6-
diazaspiro[3,4]octane in a yield of 41 % of theory
Colourless crystals Mp. 242-245C ~after re-
crystallisation twice from acetonitrile).
C21H23N50z (377-45)-
.
Calculated~ C 66.83 H 6.14 N 18.55
Found: 66.57 6.23 18.41
.Example 16
9-Chloro-5,11-dihydro-11-[[6-methyl-2,6-
diazaspiro[3,4]oct-2-yl]carbonyl-6H-pyrido[2,3-
b][l,4]benzodiazepin-6-one
Prepared analagously to Example 1 from 9-chloro-11-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one and 6-methyl-2,6-
diazaspiro[3,4]octane in a yield of 37 ~ of theory
Colourless crystals Mp. 229-230C (acetonitrile).
C20H20ClNsO2 (397,87).
.~ .
Calculated: C 60.38 H 5.07 8.91 N 17.60
Found: 60.21 5.01 8.90 17.~4
xample 17
5,11-Dihydro-11-[[2-methyl-2,6-diazaspiro[3,4]oct-6-yl]-
carbonyl-6H-pyrido r 2,3-b][1,4]benzodiazepin-6-one
~- %~2~
- 44 -
a) 4,4-Bis-(ethoxycarbonyl)-1-(phenylmethyl)-2-
pyrrolidinone
Prepared analagously to Example 12a from triethyl
1,1,2-ethanetricarboxylate and 1,3,5-tris
(phenylmethyl)-hexahydro-1,3,5-triazine in the presence
of trifluoroacetic acid in a yield of 90 % of theory.
Colourless, viscous oil which was used in the next stage
without any further purification.
b) 3 3-Bis-(hydroxymethyl)-l-(phenylmethYl)-pyrrolidine
Prepared analagously to Example 12b from 4,4-bis-
(ethoxycarbonyl)-l-(phenylmethyl)-2-pyrrolidinone and
lithium aluminium hydride in a yield of 78% of theory.
Colourless, highly viscous oil which after being left to
stand for one week at ambient temperature crystallised
out and was used without further processing in the next
step.
c) 3,3-Bis-(bromomethyl)-l-(phenylmethyl)-pyrrolidine-
hydrobromide
A mixture of 22.0 g (0.1 mol) of 3,3-bis-
(hydroxymethyl)-l-(phenylmethyl)-pyrrolidine and 130 ml
of 63 % aqueous hydrobromic acid was heated to 180C in
a bomb-type tube for 24 hours. After cooling, the
mixture was evaporated to dryness in vacuo, the
crystalline residue was stirred with 100 ml of cold
water and then suction filtered. It was re-crystallised
from boiling water and 35.0 g (82 % of theory) of
colourless crystals were obtained, Mp. 222-225CC.
C13Hl7Br2N x HBr (428.0)
Calculated: C 36.48 H 4024 Br 56.01 N 3.27
Found: 36.56 4.10 55.73 3.02
~ J
- 45 -
d) 6-(Phenylmethyl)-2-[(4-methylphenyl)sulfonyl]-2,6-
di-azaspiro[3,4]octane
Prepared analagously to Example 12d from 3,3-bis-
(bromomethyl)-l-(phenylmethyl)-pyrrolidine-hydrobromide,
potassium hydroxide and p-toluenesulfonamide in a yield
of 60 % of theory. The crystalline substance obtained
was further processed directly without re-
crystallisation or other purification.
e) 6-(Phenylmethyl)-2 6-diazaspiro r 3 4]octane
Prepared analagously to Example 12e from 6-
(phenylmethyl)-2-[(4-methylphenyl)sulfonyl~-2,6-
diazaspiro[3,4]octane and sodium-bis-(2-methoxyethoxy)-
dihydroaluminate in a yield of 41 % of theory.
Colourless liquid, Bp 18 mmHg 161-17~C and RF - 25
(Macherey-Nagel, Polygram ~R) SIL G/UV254, pre-coated
plastic sheets for TLC; eluent
dichloromethane/methanol/conc.aqueous ammonia
68/15/15/2, v/v/v/v).
f) 2-Methyl-6-(phenylmethyl)-2 6-diazaspiro[3 4]octane
10.8 g (0.0534 mol) of 6-(phenylmethyl)-2,6-
diazaspiro[3,4]octane were dissolved in 300 ml of
ethanol, mixed with 5 ml (about 0.062 mol) of a 37 %
aqueous formalin solution and refluxed for 50 minutes.
The mixture was allowed to cool, 5.0 g of Raney nickel
were added and the mixture was hydrogenated for 5 hours
at ambient temperature under 4 bar of hydrogen pressure.
The catalyst was filtered off, the filtrate was
evaporated down in vacuo, the residue remaining was
purified on silica gel by HPLC and using
dichloromethane/methanol/cyclohexane/conc.aqueous
ammonia 68/15/15/2 as eluent. Evaporation of suitable
fractions yielded the desired compound in the form of a
colourless viscous oil. Yield 6.1 g t53 ~ of theory).
- 46 ~
RF 0-54 (Macherey-Nagel, Polygram(R) SIL G/UV254, pre-
coated plastic sheets for TLC; eluent
dichloromethane/methanol/cyclohexane/conc.aqueous
ammonia 68/15/15/2, V/V/V/V).
q) 2-Methyl-2,6-diazaspiro[3,4~octane
4.0 g of 10 % palladium/animal charcoal catalyst
were added to a solution of 6.1 g (0. 0282 mol) of 2-
methyl-6-(phenylmethyl)-2, 6-diazaspiro[3,4]octane in 60
ml of ethanol and the mixture was then hydrogenated for
5 hours a~ ambient temperature under a hydrogen pressure
of 5 bar. It was filtered, the filtrate was evaporated
under reduced pressure (100 mmHg) and a colourless oil,
RF 0.1 was obtained as residue (Macherey-Nagel,
Polygram(R) SIL G/UV254, pre-coated plastic sheets for TLC;
eluent dichloromethane/methanol/cyclohexane/conc.aqueous
ammonia 68/20/10/5, V/V/V/V).
Yield: 3. 2 g (90 ~ of theory).
h) 5,11-Dihydro-11-[[2-methyl-2,6-diazaspiro[3,4]oct-2-
yl~carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one
Prepared analagously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-
b][l,4]benzodiaæepin-6-one and 2-methyl-2,6-
diazaspiro[3,4]octane in a yield of 46 ~ of theory.
Colourless crystals Mp. 273-275C (acetonitrile).
C20H2~N502 (363.42).
Calculated: C 66.10 H 5.82 N 19.27
Found: 66.25 5. 89 18.90
Example 18
5,11-Dihydro-11-[[6-(phenylmethyl)-2,6-
diazaspiro[3,4~oct-2-yl]carbonyl]-SH-pyridoi2,3-
b]rl,4]benzodiazepin-6-one
- 47 -
Prepared analagously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one and 6-(phenylmethyl)-2,6-
diazaspiro[3,4]octane in a yield of 20 % of theory.
Colourless crystals Mp. 193-195C (diisopropylether).
C26H2sN52 (439-52)-
Calculated: C 71.05 ~ 5.73 N 15.93
Found: 70.75 5.91 15.76
Example 19
5~11-Dihydro-8-methyl-11-[[2-methyl-2~6-
diazaspiro[3,4]oct-2-yl~carbonyl]-6H-pyrido[2,3-
b][l ~]benzodiazepin-6-one
Prepaxed analagously to Example 1 rom 11-
(chlorocarbonyl)-5,11-dihydro-8-methyl-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one and 2-methyl-2,6-
diazaspiro[3,4]octane in a yield of 43 % of theory.
Colourless crystals Mp. 184.0-184.5C (acetonitrile).
C21Hz3NsO2 (377 45)
Calculated: C 66.83 H 6.14 N 18.55
Found: 66.79 6.43 18.65
Example 20
5,11-Dihydro-8-ethyl-11-[[2-m~thyl-2,6-
diazaspiro[3,4]oct-6-yl]carbonyl]-6H-pyrido[2,3-
b][l 4lbenzodiazepin-6-one
Prepared analagously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-8-ethyl-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one and 2-methyl-2,6-
diazaspiro[3,4]octane in a yield of 34 % of theory.
Colourless crystals Mp. 245-~46C (acetonitrile)
C22H25Nso2 (391-48)-
` 2 ~ J~
- 48 -
Calculated: C 67.50 H 6.44 N 17.89
Found: 67.30 6.33 17.88
Example 21
5,11-Dihydro-9-methyl-11-[[2-methyl-2,6-
diazaspiro[3,4]oct-6-yl]carbonyl]-6H-pyrido[2,3-
blCl~ J~enzodiazepin-6-one
Prepared analagously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-9-methyl-6H-pyrido[2,3-
b][1,4]benzodiazepin-6-one and 2-methyl-2,6-
diazaspiro[3,4]octane in a yield of 41 % of theory.
Colourless crystals Mp. 284-285C (acetonitrile).
C2~H23N5O2 (377-45)-
Calculated: C 66.83 H 6.14 N 18.55Found: 67.08 6.12 18.85
Example 22
9-Chloro-5,11-dihydro-11-[[2-methyl-2,6-
diazaspiro[3,4]oct-6-yl]carbonyl]-6H-pyrido[2,3-
bl[1,4]benzodiazepin-6-one
Prepared analagously to Example 1 from 9-chloro-11-
(chlorocarbonyl)-5,11-dihydro-SH-pyrido[2,3-
b][1,4]benzodiazepin-6-one and 2-methyl-2,6-
diazaspiro[3,4]octane in a yield of 18 % of theory.
Colourless crystals Mp. 276-277C (after re-
crystallisation twice from acetonitrile).
C20H20ClNsO2 (397,87)-
Calculated: C 60.38 H 5.07 Cl 8.91 N 17.50
Found: 60.74 4.92 ~.91 17.77
Example 23
2 ~
- 49 -
8-Chloro-5,11-dihydro-ll-[[2-methyl-2r6-
diazaspiro[3,4~oct-6-yl~carbonyl]-6H-pyrido[2,3-
bl~l,41benzodiazepin-6-one
Prepared analagously to Example 1 from 8-chloro-11-
tchlorocarbonyl)-5,11-dihydro-6H-pyrido~2,3-
b][l,~]benzodiazepin-6-one and 2-methyl-2,6-
diazaspiro[3,4]octane in a yield of 50 % of theory.
Colourless crystals Mp. 231-232C (acetonitrile).
C20H20ClNsO2 (397.87).
Calculated: C 60.3~ H 5.07 Cl 8.91 N 17.60
Found: 60.62 5.20 8.61 17.54
Example 24
5,11-Dihydro-11-[[6-ethyl-2,6-diazaspiro[3,4]oct-2-yl]-
carbonyl]-8-methyl-6H-pyrido[2,3-b][1,4]benzodiazepin-6-
one
a) 4-Cvano-4-(ethoxycarbonyl)-1-ethyl-2-pyrrolidinone
Prepared analogously to Example 12a) from diethyl 2-
cyanosuccinate and 1,3,5-triethyl-hexahydro-1,3,5-
triazine in the presence of trifluoroacetic acid in a
yield of 89% of theory. Colourless oil, which was
further processed as a crude product without
purification.
b) 3-(Aminomethyl)-3-(hydroxymethyl)-1-ethylpyrrolidone
Prepared analogously to Example 12b) from ~-cyano-4-
(ethoxycarbonyl)-l-ethyl-2-pyrrolidinone and lithium
aluminium hydride in tetrahydrofuran in a yield of 74%
of theory. Colourless oil, RF . 25 (Macherey-Nagel,
Polygram~ SIL G/UV2s4, pre-coated pIastic sheets for TLC;
- 50 -
eluant: ethyl acetate/methanol/cyclohexane/conc. aqueous
ammonia 68/15/15/2, v/v/v/v).
c) 3-(Aminomethyl)-3-~bromomethyl)-1-ethylpyrrolidine-
dihydrobromide __ _
Prepared analogously to Example 12c) from 3-
(aminomethyl)-3-(hydroxymethyl)-1-ethylpyrrolidine and
63% aqueous hydrobromic acid in a yield o~ 94% of
theory. The crude, brownish salt was subjected to the
following reaction of cyclisation without any further
purification.
d) 6-Ethyl-2,6-diazaspiro~3 4]octane
The solution of 149.4 g (0.39 mol) of 3-
(aminomethyl)-3-(bromomethyl)-1-ethylpyrrolidine in 2
litres of dioxane was carefully mixed with a mixture of
130 g (3.25 mol) of sodium hydroxide and 120 ml of water
and then heated to boiling for 8 hours with stirring and
refluxing. After cooling, it was filtered, the aqueous
phase was removed from the filtrate and discarded, the
organic phase was freed from sol~ent under slightly
reduced pressure (100 mmHg) and using a Vigreux column.
The residue remaining yielded 15.8 g (29% of theory~ of
a colourless oil, bP.20 ~Hg 83-87C, which was identified
as the desired compound by MS, IR and 1H-NMR spectra,
after distillation in a water jet vacuum.
e) 5,11-Dihydro-11-[[6-ethyl-2,6-diazaspiro[3,4]oct-2-
yl]carbonyl]-8-methyl-6H-pyrido[2,3-b][1,4]-
benzodiazepin-6-one _ ,
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-8-methyl-6H-pyrido-
[2,3-b][1,4]benzodiazepin-6-one and 6-ethyl-2,6-
diazaspiro[3,4]octane in a yield of 27~ of theory.
Colourless crystals m.p. 216-217nC (acetonitrile).
3 ~
- 51 -
C22H2sNs2 (391- 48)-
Calc.: C 67. 50 H 6.44N 17.89
Found: 67.39 6.2017.60
Example 25
5,11-Dihydro-11-[[6 ethyl-2,6-diazaspiro[3,4]oc~-2-
yl]carbonyl]-6H-pyrido~2 3-b][1 4]benzodiazepin-6-one
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-b][1,4]-
benzodiazepin-6-one and 6-ethyl-2,6-
diazaspiro[3,4]octane in a yield of 15% of theory.
Colourless crystals m.p. 221-223C (acetonitrile).
C21H23Nso2 (377 45)
Calc.: C 66.83 H 6.14 N 18.55
Found: 66.77 6.32 18.32
Example 26
5,11-Dihydro-11-[[6-ethyl-2,6-diazaspiro[3,4]oct-2-yl]-
carbonyl]-9-methyl-6H-pyrido[2,3-b][1,4]benzodiazepin-6-
one
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-5,11~dihydro-9-methyl-6H-
pyrido[2,3-b][1,4]benzodiazepin-6-one and 6-ethyl-2,6-
diazaspiro[3,4]octane in a yield of 26~ of theory.
Colourless crystals m.p. 1~9-171C (acetonitrile).
C22H25N52 ( 391.48).
Calc.: C 67.50 H 6.44 N 17.89
Found: 67.46 6.09 17.49
Example 27
9-Chloro-5,11-dihydro-11-[[6-ethyl-2,6-diazaspiro[3,4]-
oct-2-yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-
,
2~2,~
- 52 -
one
Prepared analogously to Example 1 from 9-chloro-11-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-b][1,4]-
ben~odiazepin-6-one and 6-ethyl-2,6-
diazaspiro[3,4]octane in a yield of 19% of theory.
Colourless crystals, m.p. 158-160~C (acetonitrile) and RF
0.63 (~acherey-Nagel, Polygram~ SIL G/UVz54~ pre-coated
plastic sheets for TLC; eluant: dichloromethane/ethyl
acetate/methanol/cyclo-hexane/conc. aqueous ammonia
62/16.7/10/10/1.3, v/v/v/v/v).
C21H22ClN5~2 (411.90).
Calc.: C 61.24 H 5.38 Cl ~.61 N 17.00
Found: 61.25 5.36 8.63 17.08
5,11-Dihydro-11-[[6-propyl-2,6-diazaspiro[3,4]oct-2-
yllcarbonyl]-6H-pyrido r 2 3-b][1 41benzodiazepin-6-one
a) 4-Cyano-4-(ethoxycarbonyl)-1-propyl-2-pyrrolidinone
Prepared analogously to Example 12a) from diethyl
cyanosuccinate and 1,3,5-tripropyl-hexahydro-1,3,5-
triazine in the presence of trifluoroacetic acid in a
yield of 6~% of theory. Colourless liquid, RF . 72
(Macherey-Nagel, Polygram9 SIL G/~V2~4, pre-coated
plastic sheets for TLC; eluant: ethyl acetate/petroleum
ether 1/1, v/v).
b) 3-(Aminomethyl)-3-(hydroxymethyl)-1-
propylpyrrolidine
Prepared analogously to Example 12b~ from 4-cyano-4-
(ethoxycarbonyl)-l-propyl-2-pyrrolidinone and lithium
aluminium hydride in a yield of 28% of theory.
- 53 -
Colourless viscous oil, used in the next step without
further purification.
c) 3-(Aminomethyl)-3-(bromomethyl)-1-propylpyrrolidine-
dihydrobromide
Prepared analogously to Example 12c) from 3-
(aminomethyl)-3-(hydroxymethyl)-1-propylpyrrolidine and
63% aqueous hydrobromic acid in a yield of 91% of
theory. The brownish coloured salt was reacted without
further purification in the next step.
d) 6-Propyl-2,6-diazaspiro[3.4]octane
3-(Aminomethyl)-3-(bromomethyl)-1-propylpyrrolidine
was reacted with caustic soda, water and dioxan as
described in E~ample 24d). The crude product obtained
after processing, RF . 5 (Macherey-Nagel, Polygram3 SIL
G/UV254, pre-coated plastic sheets for TLC; eluant:
dichloromethane/methanol/cyclohexane/conc. a~ueous
ammonia 68/20/10/5, v/v/v/v) was purified by column
chromatography on silica gel using the eluant described
above. The title compound was obtained in a yield of
47% of theory - based on 3-(aminomethyl)-3-
(hydroxymethyl)-l-propylpyrrolidine - and identified as
the desired compound by MS and 1H-NMR spectrum.
e) 5,11-Dihydro-11-[[6-propyl-2,6-diazaspiro[3,4]oct-2-
yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-
one
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-b][1,4]-
benzodiazepin-6 one and 6-propyl-2,6-
diazaspiro[3,4]octane in a yield of 74~ of theory.
Colourless crys~als m.p. 208-209C (acetonitrile).
CzH2sN502 (391-48)
Calc.: C 67.50 H 6.44 N 17.89
- ` -
- 54 - ~JJq7
Found: 67.~Lo 6.39 17.60
Example 29
5,11-Dihydro-8-methyl-11-[[6-propyl-2,6-diazaspiro[3,4]-
oct-2-yl]carbonyl]-6H-pyrido[2,3-b]C1,4]benzodiazepin-6-
one
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-8-methyl-6H-pyrido[2,3-b]-
[1,4]benzodiazepin-6-one and 6-propyl-2,6-
diazaspiro[3,4]octane in a yield of 60% of theory.
Colourless crystals m.p. 219-221C (acetonitrile).
C23H27N502 (405.50)
Calc.~ C 68.13 H 6.71 N 17.27
Found: 68.02 6.74 17.00
Example 30
8-Chloro-5,11-dihydro-11-[[6-propyl-2,6-diazaspiro[3,4]-
oct-2-yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-
one
Prepared analogously to Example 1 from 8-chloro-11-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-b][1,4]-
benzodiazepin-6-one and 6-propyl-2,6-
diazaspiro[3,43Octane in a yield of 44% of theory.
Colourless crystals m.p. 210-212C (from
diisopropylether and acetonitrile).
C22H24ClN502 (425-93)
Calc.: C 62.04 H 5.68 Cl 8.32 N 16.44
Found: 61.85 5.68 8.39 16.38
Example 31
~ ~ 2 ~
5,11-Dihydro-8-ethyl-11-[[6-ethyl-2,6-diazaspiro[3,4]-
oct-2-yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-
one
Prepared analogously to Example 1 from 11-
(chlorocarbonyl~-5,11-dihydro-8-ethyl-6H-
pyrido[2,3-b][1,4]benzodiazepin-6-one and 6-ethyl-2,6-
diazaspiro[3,4]octane in a yield of 12% of theory.
Colourless crystals m.p. 190-191C (acetonitrile) and RF
0.65 (Macherey-Nagel, Polygram~ SIL G/UV254, pre-coated
plastic sheets for TLC; eluant: dichloromethane/ethyl
acetate/methanol/cyclohexane/conc. aqueous ammonia
62/16.7/10/10/3, v/v/v/v/v).
C23H27NsO2 (405-50)
Calc.: C 68.13 H 6.71 N 17.27
Found: 67.92 6.64 17.44
Example 32
5,11-Dihydro-9-methyl-11-[[6-propyl-2,6-diazaspiro[3,4]-
oct-2-yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-
one - -
Prepared analogously to ExampIe 1 from 11-
(chlorocarbonyl)-5,11-dihydro~9-methyl-6H-pyrido[2,3-b]-
~1,4]benzodiazepin-6-one and 6-propyl-2,6-
diazaspiro[3,4]octane in a yield of 39~ of theory.
Colourless crystals m.p. 183-184~C (acetonitrile) and RF
0.51 (Macherey-Nagel, Polygram~ SIL G/UV254, pre-coated
plastic sheets for TLC; eluant: dichloromethane/ethyl
acetate/methanol/cyclohexane/conc. aqueous ammonia
63.5/13/11/11/1.5, v/v/v/v/v).
C23H27N502 (405-50)
Calc.: C 68.13 H 6.71 N 17.27
Found: 68.00 6.52 17.10
Example 33
2~2~
- 56 -
5,11-Dihydro-8-ethyl-11-[[6-propyl-2,6-diazaspiro[3,4]-
oct-2-yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-
one
~` Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-8-ethyl-6H-pyrido[2,3-b]-
[1,4]benzodiazepin-6-one and 6-propyl-2,6-
diazaspiro[3,4]octane in a yield of 55% of theory.
Colourless crystals m.p. 170-172C (acetonitrile) and RF
0.52 (Macherey-Nagel, Polygram~ SIL G/ W2s4, pre-coated
plastic sheets for TLC; eluant: dichloromethane/ethyl
acetate/methanol/cyclohexane/conc. aqueous ammonia
63.5/13/11/11/1.5, v/v/v/v/v).
C24H29N5~2 (419.53)
Calc.: C 68.71 H 6.9t N 16.69
Found: 68.90 7.18 16.72
Example 34
5,11-Dihydro-11-[[2-ethyl-2,6-diazaspiro[3,4]oct-2-
yl~carbonyll-6H-pyrido r 2 3-bl r 1,4]benzodiazepin-6-one
a) 2-Acetyl-6-(PhenylmethYl)-2.6-diazasPiro~3.4loctane
37.7 ml (0.4 mol) of acetic anhydride are added
dropwise to a solution of 70.8 g (0.35 mol) of 6-
(phenylmethyl)-2,6-diazaspiro[3,4]octane in 300 ml of
ethanol with vigorous stirring and the resulting mixture
is then refluxed for 3 hours. It is concentrated by
evaporation in vacuo, made alkaline with 20% aqueous
sodium hydroxide solution and extracted exhaustively
with diethylether. The combined ether extracts are
dried over caustic potash, freed from solvent and the
residue remaining is finally distilled in a high vacuum.
The desired compound is obtained as a colourless oil,
bP.O 035 ~H9 155-168C in a yield of 69.3 g (81% of
theory).
~2~3 ~
- 57 -
b) 2-Ethyl-6-~phenylmethyl)-2 6-diazasPiro r 3 41octane
Prepared analogously to Example 12b) from 2-acetyl-
6-(phenylmethyl)-2,6-diazaspiro[3,4]octane and lithium
aluminium hydride in anhydrous tetrahydrofuran. The
desired compound is obtained as a colourless oil
bp.o2 ~Hg 91-93C amd RF 0.6 (Macherey-Nagel, Polygram
SIL G/UVzs4, precoated plastic sheets for TLC; eluant:
dichloromethane/methanol/cyclohexane/conc. aqueous
ammonia 68/15/15/2, v/v/v/v) in a yield of 73% of
theory.
c) 2-Ethyl-2, 6-diazaspiro r 3 41octane
Prepared analogously to Example 17g) from 2-ethyl-6-
(phenylmethyl)-2,6-diazaspiro[3,4]octane by catalytic
hydrogenation in the presence of 10% palladium/animal
charcoal. The desired compound is obtained as a
colourless oil, RF . 45 (Macherey-Nagel, Polygram~ SIL
G/UV2s4, precoated plastic sheets for TLC; eluant:
dichloromethane/methanol/cyclohexane/ethyl acetate/conc.
aqueous ammonia 62/10/10/16.7/1.3, v/v/v/v/v) in a yield
of 96% of theory.
d) 5,11-Dihydro-11-[[2-ethyl-2,6-diazaspiro[3,4]oct-6-
yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-
one
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-b][1,4]-
benzodiazepin-6-one and 2-ethyl-2,6-
diazaspiro[3,4]octane in a yield of 60% of theory.
Colourless crystals m.p. 251-254C (dioxan) and RF 0 3
(Macherey-Nagel, Polygram~ SIL G/UV254, pre-coated
plastic sheets for TLC; eluant: dichloromethane/ethyl
acetate/methanol/cyclohexane/conc. aqueous ammonia
63.5/13/11/11/1.~, v/v/v/v/v).
C21H23N5Oz (377-45)
-- 58 --
Calc.: C 66.83 H 6.14 N 18.55
Found: 66.61 6.20 18.40
Example 35
5,ll-Dihydro-11-[[2-ethyl-2,6-diazaspiro[3,4~oct-6-
yl]carbonyl]-8-methyl-6H-pyrido[2,3-b][1,4]benzo-
diazepin-6-one
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-8-methyl-6H-
pyrido[2,3-b][1,4]benzodiazepin-6-one and 2-ethyl-2,6-
diazaspiro[3,4]octane in a yield o~ 61% of theory.
Colourless crystals m.p. 213-215C (acetonitrile).
C22H2sNsOz (391-48)
Calc.: C 67.50 H 6.44 N 17.89
Found: 1 67.19 6.32 18.22
Example 36
5,11-Dihydro-11-[[2-ethyl-2,6-diazaspiro[3,4]oct-2-yl]-
carbonyl]-9-methyl-6H-pyrido[2,3-b][1,4]benzodiazepin 6-
one
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-9-methyl-6H-pyrido[2,3-b]-
[1,4]benzodiazepin-6-one and 2-ethyl-2,6-
diazaspiro[3,4]octane in a yield of 56% of theory.
Colourless crystals m.p. 251-253C (acetonitrile~.
C22H2sNs2 (391-48)
Calc.: C 67.50 H 6.44 N 17.89
Found: 67.32 6.49 18.31
Example 37
9-Chloro-5,11-dihydro-11-[[2-ethyl-2,6-diazaspiro[3,4~-
oct-2-yl]carbonyl]-6H-pyrido[2,3-b]~1,4]benzodiazepin-6-
one ~
2 ~ 3
- 59 -
Prepared analogously to Example 1 from 9-chloro~
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-b][1,4]-
benzodiazepin-6-one and 2-ethyl-2,6-
diazaspiro[3,4]octane in a yield of 45% of theory.
Colourless crystals m.p. 248-250C (acetonitrile).
CzlH22ClNsO2 (411-9~)
Calc.: C 61.24 H 5.38 Cl 8.61 N 17.00
Found: 61.31 5.22 8.90 16.98
Example 38
5,11-Dihydro-11-[[6-(2-methylpropyl)-2,6-
diazaspiro[3,4]-oct-2-yl]carbonyl]-6H-
pyr do[2 3-b]~1 4]benzodiazepin-6-one
a) 4-Cyano-4-(ethoxycarbonyl)-1-(2-methylpropyl)-2-
pyrrolidinone
Prepared analogously to Example 12a) in a yield of
74% of theory from cyanosuccinic acid and hexahydro-
1,3,5-tris-(2-m~thylpropyl)triazine using
trifluoroacetic acid. Colourless oil, RF . 8 (Macherey-
Nagel, Polygram~ SIL G/UV2s4, pre-coated plastic sheets
for TLC; eluant: ethyl acetate/petroleum ether 1:1,
v/v) .
b) 3-(Aminomethyl)-3-(hydroxymethyl)-1-(2-
methylpropyl)-pyrrolidine
Prepared analogously to Example 12b) from 4-cyano-4-
(ethoxycarbonyl)-1-(2-methylpropyl) 2-pyrrolidinone by
reduction with lithium aluminium hydride in a yield of
35% of theory. Colourless oil, RF . 2~ (Macherey-Nagel,
Polygram~ SIL G/UV254, pre-coated plastic sheets for TLC;
eluant: dichloromethane/methanol/cyclohexane/conc.
aqueous ammonia 68/15/15/2, v/v/v/v).
2~2~
- 60 -
c) 3-(Aminomethyl)-3-(bromomethyl)-1-(2-methylpropyl)-
pyrrolidine-dihydrobromide
Prepared analogously to E~ample 12c) from 3-
(aminomethyl)-3-hydroxymethyl-1-(2-methylpropyl)-
pyrrolidine and 63% aqueous hydrobromic acid. The
brownish salt is used in the following step without
further purification.
d) 6-(2-Methylpro~yl)-2,6 diazaspiro~3l4]octane
Prepared analogously to Example 28d) from 3-
(aminomethyl)-3-(bromomethyl)-1-(2-methylpropyl)-
pyrrolidine-dihydrobromide and caustic soda in the
presence of water and dioxan in a yield of 62% of
theory. Colourless oil, RF . 45 (Macherey-Nagel,
Polygram~ SIL G/UV254, pre-coated plastic sheets for TLC;
eluant: dichloromethane/methanol/cyclohexane/conc.
aqueous ammonia 68/20/10/2, v/v/v/v).
e) 5, 11-Dihydro-11-[[6-(2-methylpropyl)-2,6-diazaspiro-
[3,4]oct 2-yl]carbonyl3-6H-pyrido[2,3-b][1,4]benzo-
diazepin-6-one
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-b] [1,43-
benzodiazepin-6-one and 6-(2-methyl-propyl)-2,6-
diazaspiro[3,4]octane in a yield of 49% of theory.
Colourless crystals m.p. 199-~01C (acetonitrile) and RF
0.48 (Macherey-Nagel, Polygram~ SIL G/UVz54~ pre-coated
plastic sheets for TLC; eluant: dichloromethane/ethyl
acetate/methanol/cyclohexane/conc. aqueous ammonia
63.5/13/11/11/1.5, v/v/vjv/v).
C23H27NsO2 (405-50)
Calc.: C 68.13 H 6.71 N 17.27
Found: 67.96 6.84 17.47
Example 39
- ~2~3~
- 61 -
5,11-Dihydro-8-methyl-11-[[6-(2-methylpropyl)-~,6-
diazaspiro[3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-b]-
r 1 4]benzodiazepin-6-one
Prepared analogously to Example 1 ~rom 11-
(chlorocarbonyl)-5,11-dihydro-8-methyl-6H-
pyrido[2,3-b][1,4]benzodiazepin-6-one and 6-(2-
methylpropyl)-2,6-diazaspiro[3,4]octane in a yield of
52% o~ theory. Colourless crystals m.p. 1~6-188C
(acetonitrile).
C24H29N502 (419.53)
Calc.: C 68.71H 6.97N 16.69
Found: 68.67 6.99 16.47
Example 40
5,11-Dihydro-9-methyl-11-[[6-(2-methylpropyl)-2,6-
diazaspiro[3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-b]-
r 1 4]benzodiazepin-6-one
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-9-methyl-6H-pyrido[2,3-b]-
[1,4]benzodiazepin-6-one and 6-(2-methylpropyl)-2,6-
diazaspiro[3,4]octane in a yield of 24~ of theory.
Colourless crystals, m.p. 163-165C (acetonitrile).
C24H29N502 (419.53)
Calc.: C 68.71 H 6.97 N 16.69
Found: 68.62 6.72 16.67
Example 41
9-Chloro-5,11-dihydro-11-[[6-(2-methylpropyl)-2,6-
diazaspiro[3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-b]-
r 1~4]benzodiaze~in-6-one
Prepared analogously to Example 1 from 9-chloro-11-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-b][1,4]-
benzodiazepin-6-one and 6-(2-methylpropyl)-2,6-
' ~ t'J
- 62 -
diazaspiro[3,4]octane in a yield of 45% of theory.
Colourless crystals, m.p. 177-179C tdiisopropylether).
C23H26ClNsO2 (439.94)
Calc.: C 62.79 H 5.96 Cl 8.06 N 15.92
Found: 62.98 6.29 8.07 16.08
Exam~le 42
6,11-Dihydro-11-[[7-methyl-2,7-diazaspiro[4,4]non-2-yl]-
carbonyll-5H-pyrido[2,3-b][1,5]benzodiazepin-5-one
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-6,11-dihydro-5H-pyrido[2,3-b][1,5]-
benzodiazepin-5-one and 2-methyl-2,7-
diazaspiro[4,4]nonane in a yield of 84% of theory.
Colourless crystals, m.p. 212-214C (acetonitrile).
C21H23N502 (377-45)
Calc.: C 66.83 H6.14 N 18.55
Found: 66.86 6.33 18.73
~'
Example 43
:`;
6,11-Dihydro-11-[[7-ethyl-2,7-diazaspiro[4,4]non-2-
yl]carbonvl]-5H-pyrido[2.3-b][1,5]benzodiazepin-5-one
Prepared analogously to Example l from 11-
(chlorocarbonyl)-6,11-dihydro-5~-pyrido[2,3-b][1,5]-
benzodiazepin-5-one and 2-ethyl~2,7-diazaspiro[4,4]-
nonane in a yield of 68% of theory. Colourless
crystals, m.p. 151.0-152.5C (acetonitrile1.
C22H2sN502 (391.48)
Calc.: C 67.50 H 6.44 N 17.89
Found: 67.65 6.55 18.04
Example 44
- 63 -
6,11-Dihydro~ [[6-methyl-2,6-diazaspiro[3,4]oct-2-yl]-
carbonyl]-5H-pyrido~2 3-b][1,51benzodiazepin-5-one
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-6,11-dihydro-5H-pyrido[2,3-b][1,5]-
benzodiazepin-5-one and 6-methyl-2,6-
diazaspiro[3,4]octane in a yield of 38% of theory.
Colourless crystals, m.p. 239-241C (acetonitrile).
C20H21N5O2 (363.42)
Calc.: C 66.10 H 5.82 N 19.27
Found: 65.99 5.77 19.17
Example 45
6,11-Dihydro-11-[[2-methyl-2,6-diazaspiro[3,4]oct-6-
y~llcarbonyl]-5H-pyrido[2,3-b]~1,51benzodiazepin-5-one
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-6,11-dihydro-5H-pyrido[2,3-b][1,5]-
benzodiazepin-5-one and 2-methyl-2,6-diazaspiro[3,4]-
octane in a yield of 50% of theory. Colourless
crystals, m.p. 220-221~C (acetonitrile).
CzoH2lNsO2 (363-4~)
Calc.: C 66.10 H 5.82 N 19.27
Found: 65.88 6.08 19.00
Example 46
6,11-Dihydro-11-[[6-ethyl-2,6-diazaspiro[3,4]oct-2-yl]-
carbonyll-5H-pyrido~2,3-bl r 1,51benzodiazePin-5-one
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-6,11-dihydro-5H-pyrido[2,3-b][1,5]-
benzodiazepin-5-one and 6-ethyl-2,6-
diazaspiro[3,4]octane in a yield of 10% of theory.
Colourless crystals, m.p. 196-200~C (acetonitrile~ and RF
0.62 (~acherey-Nagel, Polygram~ SIL G/UV254, pre-coated
- 64 -
plastic sheets for TLC; eluant: dichloromethane/ethyl
acetate/methanol/cyclo-hexane/conc. aqueous ammonia
62/16.~/10/10/1.3, v/v/v/v/v).
C2~H23NsO2 (377-45)
Calc.: C 66.83 H 6.14 N 18.55
Found: 66.26 6.11 18.14
~m~
6,11-Dihydro-11-[[6-propyl-2,6-diazaspiro[3,4]oct-2-
yl]carbonyl]-5H-pyrido[2,3-bl~l.51benzodiazepin-5-one
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-6,11-dihydro-5H-pyrido[2,3-b][1,5]-
benzodiazepin-5-one and 6-propyl-2,6-
diazaspiro[3,4]octane in a yield of 51% of theory.
Colourless crystals, m.p. 195-197C (acetonitrile) and RF
~` 0.58 (conditions as in Example 46).
C22H25NsO2 (391.48)
Calc.: C 67.50 H 6.44 N 17.89
Found: 67.46 6.36 17.98
Example 48
6,11-Dihydro-11-[[2-ethyl-2,6-diazaspiro[3,4]oct-6-yl]-
carbonyl~-5H-pyrido r 2,3-b U1 51benzodiazepin-5-one
:`
Prepared analogously to Example 1 but using
tetrahydrofuran instead of acetonitrile as solvent, from
ll-(chlorocarbonyl)-6,11-dihydro-5H-pyrido[~,3-b]-
[1,5]benzodiazepin-5-one and 2-ethyl-2,6-
diazaspiro[3,4]-octane in a yield of 61% of theory.
Colourless crystals/ m.p. 186-189C (acetonitrile) and RF
0.32 (Macherey-Nagel, Polygram~ SIL G/UV254, pre-coated
plastic sheets for TLC; eluant: dichloromethane/ethyl
- 65 -
acetate/methanol/cyclo-hexane/conc. aqueous ammonia
63.5/13/11/11/1.5, v/v/v/v/v).
C21H23N5O2 (377 45)
Calc.: C 66.83 H 6.14N 18.55
Found: 67.00 6.17 18.73
Exa~nple 49
6,11-Dihydro-11-[[6-(2-methylpropyl)-2,6-
diazaspiro[3,4]-oct-2-yl]carbonyl]-5H-
~ Pyridor2,3-b] r1~5lbenzodiazepin-5-one
.
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-6,11-dihydro-5H-pyrido[2,3-b][1,5]-
benzodiazepin-5-one and 6-(2-methylpropyl)-2,6-
diazaspiro[3,4]octane in a yield of 60~ of theory.
Colourless crystals, m.p. 187-189C (acetonitrile).
C23H27NsO2 (~05 50)
Calc.: C 68.13 H 6.71 N 17.27
Found: 67.95 6.77 17O47
Example 50
5,10-Dihydro-5-[[7-methyl-2,7-diazaspiro[4,4]non-2-yl]-
carbonyl]-llH-dibenzo[b,e][1.4]diazepin-11-one
Prepared analogously to Example 1 from 5-
(chlorocarbonyl)-5,10~dihydro-llH-dibenzo[b,e][1,4]-
diazepin-ll-one and 2-methyl-2,7-diazaspiro[4,4]nonane
in a yield of 75% of theory. Colourless crystals, m.p.
190C (acetonitrile).
C22H24N4O2 (376.46)
Calc.: C 70.19 H 6.43 N14.88
Found: 70.14 6.4314.86
Example 51
~2~
-- 66 --
4,9-Dihydro-3-methyl-4-[[7-methyl-2,7-diazaspiro[4,4]-
non- 2-yllcarbonyll-1OH-thienor3 4-b1 rl 51benzodiazepin-
10-one
Prepared analogously to Example 1, but using
dichloromethane instead of acetonitrile, from 4-
(chlorocarbonyl)-4,9-dihydro-3-methyl-lOH-thieno[3,4-b]-
[1,5]benzodiazepin-10-one and 2-methyl-2,7-diazaspiro-
[4,4]nonane in a yield of 44% of theory. Colourless
crystals, m.p. 213-214C (acetonitrile).
C21H24N42S (396051)
Calc.: C 63.61 H 6.10 N 14.13 S 8.09
Found: 63.30 6.19 14.21 7.94
Example 52
4,9-Dihydro-4-[[7-ethyl-2,7-diazaspiro[4,4]non-2-yl]-
carbonyl]-3-methyl-lOH-thieno[3,4-b][1,5]ben20diazepin-
10-one
Prepared analogously to Example 51 from 4-
(chlorocarbonyl)-4,9-dihydro-3-methyl-lOH-thieno[3,4-b]-
[1,5]benzodiazepin-10-one and 2-ethyl-2,7-
diazaspiro[4,4]nonane in a yield of 65% of theory.
Colourless crystals, m.p. 215-216C (acetonitrile).
C22H26N42S (410.54)
Calc.: C 64.36 H 6.38 N 13.65 S 7.81
Found: 64.28 6.33 13.87 7.91
Exam~le 53
4,9-Dihydro-3-methyl-4-[[6-methyl-2,6-diazaspiro[3,4]-
oct-2-yl]carbonyl]-lOH-thieno[3,4-b][1,5]benzodiazepin-
10-one
Prepared analogously to Example 1 from 4-
(chlorocarbonyl)-4,9-dih~dro-3-methyl-lOH-thieno~3,4-~]-
67
[1,5]benzodiazepin-lo-one and 6-methyl-2,6-
diazaspiro[3,4]octane in a yield of 52% of theory.
Colourless crystals, m.p. 200-203C (acetonitrile).
2S (382.49)
Calc.: C 62.80 H 5.80 N 14.65 S 8.38
j Found: 62.51 5.68 14.67 8.40
ExamPle 54
4,9-Dihydro-3-methyl-4-[[2-methyl-2,6-diazaspiro[3,4]-
oct-6-yl]carbonyl]-lOH-thieno[3,4-b][1,5]benzodiazepin-
10-one
Prepared analogously to Example 1 from 4-
(chlorocarbonyl)-4,9-dihydro-3-methyl-lOH-thieno-
[3,4-b][1,5]benzodiazepin-10-one and 2-methyl-2,6-
diazaspiro[3,4]octane in a yield of 28% of theory.
Colourless crystals, m.p. 135-137C [acetonitrile).
C20H22N42S (382.49)
Calc.: C 62.80 H 5.80 N 14.65 S 8.38
Found: 62.62 5.81 14.70 8.54
Example 55
4,9-Dihydro-4-[[6-ethyl-2,6-diazaspiro[3,~]oct-2-
yl]carbonyl]-3-methyl-lOH-thieno[3,4-b][1,5]benzo-
diazepin-10-one
Prepared analo~ously to Example 1 from 4-
(chlorocarbonyl)-4,9-dihydro-3-methyl-lOH-thieno-
[3,4-b][1,5]benzodiazepin-10-one and 6-ethyl-2,6-
diazaspiro[3,4]octane in a yield of 30% of theory.
Colourless crystals, m.p. 208-210C (acetonitrile).
.~
- 68 -
CzlH24N42S (396.51)
Calc.: C 63.61 H 6.10 N 14.13 S 8.09
Found: 63.35 6.25 14.32 8.19
Example 56
4,9-Dihydro-3-methyl-4-[[6-propyl-2,6-diazaspiro[3,4]-
oct-2-yl]carbonyl]-lOH-thieno[3,4-b][1,5]benzodiazepin-
10-one
Prepared analogously to Example 1 from 4-
(chlorocarbonyl)-4,9-dihydro-3-methyl-lOH-thieno[3,4-b]-
[1,5]benzodiazepin-10-one and 6-propyl-2,6-
diazaspiro[3,4]octane in a yield of 44% of theory.
Colourless crystals, m.p. 180-181C (acetonitrile) and RF
0.54 (Macherey-Nagel, Polygram~ SIL G/UV254, pre-coated
plastic sheets for TLC; eluant: dichloromethane/ethyl
acetate/methanol/cyclohexane/conc. a~ueous ammonia
63.5/13/11/11/1.5, v/v/v/v/v).
CzH26N402S (410.54)
Calc.: C 64.36 H 6.38N 13.65 S 7.81
Found: 64.26 6.35 13.64 7.71
Example 57
4,9-Dihydro-4-[[2-ethyl-2,6-diazaspiro[3,4]oct-6-ylJ-
carbonyl]-3-methyl-lOH-thieno[3,4-b][1,5Jbenzodiazepin-
10-one
Prepared analogously to Example 1 from 4-
(chlorocarbonyl)-4,9-dihydro-3-methyl-lOH-thieno[3,4-b]-
[1,5]benzodiazepin-10-one and 2-ethyl-2,6-
diazaspiro[3,4]octane in a yield of 61% of theory.
,J ,~ J~3
-- 69 --
Colourless crystals, m~p. 216-217C (acetonitrile) and RF
0.47 (conditions as in Example 56).
C21H24N42S (396.51)
Calc.: C 63.61 H 6.10 N 14.13 S 8.09
Found: 63.51 6.10 14.27 8.08
xample 58
4,9-Dihydro-3-methyl-4-[[6-(2-methylpropyl)-2,6-
diazaspiro[3,4]oct-2-yl]carbonyl]-lOH-thieno[3,4-b]-
r 1~5]benzodiaæepin-lo-one
Prepared analogously to Example 1 from 4-
(chlorocarbonyl)-4,9-dihydro-3-methyl-lOH-
thieno[3,4-b][1,5]benzodiazepin-10-one and 6-(2-
methylpropyl)-2,6-diazaspiro[3,4]octane in a yield of
71% of theory. Colourless crystals, m.p. 186-188C
(acetonitrile).
C23H28N42S (424.56)
Calc.: C 65.07 H 6.65N 13.20 S 7.55
Found: 65.07 6.6113.30 7.42
Example 59
3-Chloro-1-methyl-4-[[7-methyl-2,7-diazaspiro[4,4]non-2-
yl]carbonyl]-1,4,9,10-tetrahydropyrrolo[3,2-b][1,5]-
benzodiazepin-lo-one
Prepared analogously to Example 1 from 3-chloro-4-
(chlorocarbonyl)-l-methyl-1,4,9,10-tetrahydropyrrolo-
[3,2-b][1,5]benzodiazepin-10-one and 2-methyl-2,7-
diazaspiro[4,4]nonane in a yield of 80% of theory.
Colourless crystals, m.p. 205-206C (acetonitrile).
C21H24ClN502 (413.92)
Calc.: C 60.94 H 5.84 Cl 8.57 N 16.92
Found: 61.13 5.56 8.78 17.20
Example 60
- 70 -
1-Methyl-4-[[7-methyl-2,7-diazaspiro[4,4]non-2-
yl]carbonyl]-1,4,9,10-tetrahydropyrrolo[3,2-b][1,5]-
enzodiazepin-10-one
3.5 g (~.46 mmol) of 3-chloro-1-methyl-4-[[7-methyl-
2,7-diazaspiro[4,4]non-2-yl]carbonyl]-1,4,9,10-
tetrahydropyrrolo[3,2~b][1,5]benzodiazepin-10-one were
dissolved in 350 ml of hot ethanol and after the
addition of 3 g of palladium on animal charcoal (20~)
the mixture was hydrogenated for 2 hours under a
hydrogen pressure of 50 bar and at a temperature of
40C. The catalyst was filtered off, the filtrate was
evaporated down ln vacuo, the crystalline hydrochloride
was taken up in 20 ml of water, the resulting solution
was made alkaline with sodium hydroxide solution and
extracted exhaustively with dichloromethane. The
combined extracts were dried over sodium sulphate and
concentrated by evaporation and the residue remaining
was recrystallised from n-propanol. 1.1 g (34% of
theory) of colourless crystals are obtained, m.p.
223-225OC.
C2l~25N5o2 (379.47)
Calc.: C 66.47 H6.64 N 18.46
Found: 66.43 6.84 18.44
Example 61
3-Chloro-1-methyl-4-[[6-propyl-2,6-diazaspiro[3,4~oct-2-
yl]carbonyl]-1,4,9,10-tetrahydropyrrolo[3,2-b][1,5]-
benzodiazepin-10-one
Prepared analogously to Example 1 from 3-chloro-4-
(chlorocarbonyl)-1-methyl-1,4,9,10 tetrahydropyrrolo-
[3,2-b][1,5]benzodiazepin-lo-one and 6-propyl-2,6-
diazaspiro[3,4]octane in a yield of 51% of theory.
Colourless crystals, m.p. 164-165C (acetonitrile).
C22H26ClNsO2 (427.94)
Calc.: C 61.75 H 6.12 Cl 8.28 N 16.37
~ ~ 2 ~
- 71 -
Found: 61.48 5.99 8.42 16.37
Example 62
3-Chloro-l-methyl-4-[[6-(2-methylpropyl)-2,6-diazaspiro-
[3,4]oct-2~yl]carbonyl]-1,4,9,10-tetrahydropyrrolo-
r 3,2-b][1 ~]benzodiazepin-lo-one __
Prepared analogously to Example 1 from 3-chloro-4-
(chlorocarbonyl)-l-methyl-1,4,9,10-tetrahydropyrrolo-
[3,2-b][1,5]benzodiazepin-10-one and 6-(2-methylpropyl)-
2,6-diazaspiro[3,4]octane in a yield o~ 23% of theory.
Colourless crystals, m.p. 163-165C (acetonitrile).
C23H2sClN5Oz (441-96)
Calc.: C 62.51 H 6.39 Cl 8.02 N 15.85
Found: 62.47 6.39 8.18 15.89
Example 63
3-Chloro-l-methyl-4-[[6-methyl-2,6-diazaspiro[3,4]oct-2-
yl]carbonyl]-1,4,9,10-tetrahydropyrrolo[3,2-b]~1,5]-
benzodiazepin-10-one
Prepared analogously to Example 1 from 3-chloro-4-
(chlorocarbonyl)-l-methyl-1,4,9,10-tetrahydropyrrolo-
[3,2-b][1,5]benzodiazepin-10-one and 6-methyl-2,6-
diazaspiro[3,4]octane in a yield of 61% of theory.
Colourless crystals, m.p. 215-217C (acetonitrile) and
RF 0-7 (Macherey-Nagel, Polygram~ SIL G/UV2s4, pre-coated
plastic sheets for TLC; eluant:
dichloromethane/methanol/ c~clohexane/conc. aqueous
ammonia 68/15/15/2, v/v/v/v).
CzoH22ClNs2 (399-88)
Calc.: C 60 . 07 H 5 . 55 C1 8. 87 N 17 . 51
Found: 60.13 5.39 9.02 17.64
Example 64
~2~
- 72 -
5,11-Dihydro-11-[[6-methyl-2,6-diazaspiro[3,4]oct-2-
yl]carbon~l]-6H-pyrido r 2 3-bl[1 4]benzodiazePin-6-one
5.36 g (0.0425 mol) of 6-methyl-2,6-diazaspiro-
[3,4]octane were added dropwise to a mixture consisting
of 22.5 ml of a 20% solution of phosgene in toluene,
100 ml of acetonitrile and 4.75 g (0.045 mol) of
anhydrous sodium carbonate, whilst cooling externally
with ice. The mixture was stirred for a further 60
minutes at ambient temperature, then 9.0 g (0.0428 mol)
of 5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one
were added to the reaction mixture and it was then
refluxed for 4 hours. The boiling hot mixture was
filtered, the precipitate was washed thoroughly three
times with 10 ml of hot acetonitrile and the combined
filtrates were evaporated down to a total volume of
50 ml in vacuo. They were left to cool and kept
occasionally stirred with a glass rod for 2 hours at 0
to 5C, the crystal slurry formed was suction filtered,
recrystallised from acetonitrile and colourless crystals
were obtained, m.p. 225.5-227.0C which were found to be
identical, according to the mixed melting point, IR and
1H-NMR spectrum, to a preparation made according to
Example 12.
Yield: 5.4 g (35~ of theory).
The following were obtained in the same way:
~ 5,11-dihydro-11~[[7-methyl-2,7-diazaspiro[4,4]non-2-
yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one,
m.p. 272-274C (acetonitrile);
5,11-dihydro-8-methyl-11-[[7-methyl-2,7-diazaspiro[4,4]-
non-2-yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin 6-
one, m.p. 212-214C (acetonitrile);
J
- 73 -
5,11-dihydro-8-ethyl-11-[[6-methyl-2,6-diazaspiro[3,4]-
oct-2-yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-
one, m.p. 215-217C (acetonitrile);
5,11-dihydro-8-methyl-11-[[2~methyl-2,6-diazaspiro[3,4]-
oct-6-yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-
one, m.p. 184.0-184.5C (acetonitrile).
Example 65
5,11-Dihydro-11-[[6-methyl-2,6-diazaspiro[3,4]oct-2-yl]-
carbonyl]-6H-pyrido r 2.3-b][1,4]benzodiazepin-6-one
To a suspension of 2.6 g (9.5 mmol) of 11-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-b][1,4]-
benzodiazepin-6-one in 30 ml of dimethylformamide, a
solution of 1.2 g (9.51 mmol) of 6-methyl-2,6-
diazaspiro-[3,4]octane in 10 ml of dimethylformamide was
added dropwise at ambient temperature and with stirring.
The initially clear solution turns cloudy within a few
minutes. After stirring for half an hour at ambient
temperature the colourless solid was suction filtered
and washed thoroughly with three times 3 ml of ice cold
ethanol. The colourless monohydrochloride of the
desired compound obtained was dissolved in 10 ml of
water, mixed with a saturated aqueous potassium
carbonate solution until a clearly alkaline reaction
occurred and then filtered. The resulting solid was
washed thoroughly with water, then dried in a vacuum
drying chamber at 50C and over diphosphorus pentoxide;
then recrystallised from hot acetonitrile and dried ln
vacuo once more. 2.35 g (68~ of theory) of colourless
crystals were obtained, m.p. 225.5-227.0C, which was
found to be identical to a preparation made according to
Example 12, judging by the mixed melting point, IR and
H-NMR spectra.
Example 66
.
- 7~ -
1-Methyl-4-[[7-methyl-2,7-diazaspiro[4,4]non-2-
yl]carbQnyl]-1,4,9,10-tetrahydropyrrolo[3,2-b][1,5]-
benzodiazepin-10-one
4.02 g (9.71 mmol) of 3-chloro-1-methyl-4-[[7-
methyl-2,7-diazaspiro[4,4]non-2-yl]carbonyl]-1,4,9,10-
tetrahydropyrrolo[3,2-b][1,5]benzodiazepin-10-one were
dissolved in a mixture of 5 ml of 85% formic acid and
25 ml of dimethylformamide and refluxed for 3 hours
after the addition of 0.5 g of 10% palladium/activated
charcoal. 7.0 ml of formic acid were added, the mixture
was refluxed for a further 6 hours and, after the
addition of a further 4.0 ml of formic acid and 0.8 g of
10% palladium/activated charcoal, the mixture was
refluxed for a further 8 hours. It was then filtered
while hot, ths filtrate was evaporated down ln vacuo and
the residue was purified by column chromatography
(silica gel: dichloromethane/ethyl
acetate/methanol/conc. ammonia 3.5:1.5:0.46:0.0~,
v/v/v/v). 1.14 g (31% of theory) of colourless crystals
were obtained, m.p. 223-225C (n-propanol), which was
found according to thin layer chromatography, IR, UV and
H-NMR spectra, to be identical to a preparation obtained
according to Example 60.
Example 67
l-Methyl-4-[[7-methyl-2,7-diazaspiro[4,4]non-2-yl]-
carbonyl]-1,4,9,10-tetrahydropyrrolo[3,2-b][1,5]-
benzodiazepin-1_-one
A mixture of 4.14 g (0.01 mol) of 3-chloro-4-[[7-
methyl-2,7-diazaspiro[4,4]non-2-yl]carbonyl]-1,4,9,10-
tetrahydropyrrolo[3,2-b][1,5~benzodiazepin-10-one,
83.3 mg (0.001 mol) of 2:1 tris(o tolyl)phosphine-
palladium acetate catalyst, 2.025 g (0.044 mol) of
formic acid and 5.77 g (0.057 mol) of trie~hylamine in
200 ml of tetrahydro~uran were heated to 100C in an
2 ~ 2 ~
- 75 -
autoclave for 40 hours under a nitrogen atmosphere. The
mixture was filtered and evaporated down in vacuo, the
residue was made alkaline with sodium hydroxide solution
and extracted exhaustively with dichloromethane. The
dried and concentrated organic phases were purified by
column chromatography as in Bxample 66. 1.44 g (38~ of
theory) of colourless crystals were obtained, m.p.
223-225C (n-propanol), found to be identical to a
preparation obtained in Example 60, according to the
thin layer chromatography, mixed melting point and IR
spectrum.
Example 68
5,11-Dihydro-8,9-dimethyl-11-[~6-methyl-2,6-diazaspiro-
[3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-b][1,4]-
benzodiazepin-6-one
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-8,9-dimethyl-6H-
pyrido[2,3-b][1,4]benzodiazepin-6-one and 6-methyl-2,6-
diazaspiro[3,4]octane in a yield of 59% of theory.
Colourless crystals, m.p. 247-249C.
C22H2sNso2 (391-48)-
Calc.: C 67.50 H 6.44N17.89
Found: 67.25 6.52 18.10
Example 69
5,11-Dihydro-8,9-dimethyl~ [[2-ethyl-2,6-diazaspiro-
[3,4]oct-6-yl]carbonyl]-6H-pyrido[2,3-b][1,4~benzo-
diazepin-6-one hydrochloride
3.01 g (0.00998 mol) of 11-(chlorocarbonyl)~-5,11-
dihydro-8,9-dimethyl-6H-pyrido[2,3-b][1,4]benzodiazepin-
6-one, 1.4 g (0.00998 mol) of 2-ethyl-2,6-
diazaspiro[3,4]octane and 100 ml of anhydrous
acetonitrile were heated to 60 D C for 2 hours with
2~
- 76 -
stirring. The colourless crystalline deposit
precipitated after cooling was suction filtered and
recrystallised from dry boiling ethanol. 2.3 g (52% of
theory) of colourless crystals were obtained, m.p.
268-270C.
C23H27NsO2xHCl (441.97).
Calc.: C 62.51 H 6.39 Cl 8.02 N 15.85
Found: 62.38 6.34 8.1615.61
Example 70
5,11-Dihydro-8,9-dimethyl-11~[[2-methyl-2,6-diazaspiro-
[3,4]oct-6-yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzo-
diazepin-6-one
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-8,9-dimethyl-6H-pyrido-
[2,3-b][1,4]benzodiazepin-6-one and 2-methyl-2,6-
diazaspiro~3,4]octane in a yield of 30% of theory.
Colourless crystals, m.p. 292-294C.
C22H2sNs2 (391-48)-
Calc.: C 67.50 H 6.44 N 17.89
Found: 66.91 6.39 17.71
Example 71
5,11-Dihydro-11-[[6-methyl-2,6-diazaspiro[3,3]hept-2-
yl]-carbonyl]-6H-pyrido[2,3-b]~1,4~benzodiazepin-6-one
a) 2,2-Bis(bromomethyl)-1 3-propanediamine-
dihydrobromide
40.65 g (0.1 mol~ of 2,6-bis-[(4-methylphenyl)-
sulphonyl]-2,6-diazaspiro[3,3]heptane and 300 ml of
concentrated aqueous hydrobromic acid were heated to
180C in a glass autoclave for 12 hours with shaking.
After cooling, the reaction mixture was diluted to three
times the volume and then filtered. The filtrate was
- 77 -
evaporated down in a water jet vacuum, the light brown
residue remaining was carefully triturated with 2x
lO0 ml of absolute ethanol and suction filtered, the
crystals obtained were finally recrystallised from a
boiling mixture of 3 parts ethanol and one part water
(by volume). Colourless crystals, m.p. 283-285C.
Yield: 35.7 ~ (85% of theory).
C5Hl2Br2N2X2HBr (421-80)-
Calc.: C 14.24 H 3.35 Br 75.78 N 6.64
Found: 14.39 3.36 75.32 6.36
b) 5 11-Dihydro~ [~6-methyl-2.6-diazaspiro~3 3]hept-
2~yl]carbonyll-6~-pyrido[2,3-bl[I 4]benzodiazePin-6-
one
A suspension of 16.87 g (0.04 mol) of 2,2-bis-
(bromomethyl)-1,3-propanediamine-dihydrobromide in
500 ml of acetonitrile was combined, with stirring and
at ambient temperature, with a solution of 6.4 g
(0.16 mol) of sodium hydroxide in 25 ml of water, added
dropwise. After stirring for 4 hours at ambient
temperature the mixture was dried with anhydrous sodium
carbonate and filtered. 9.0 g (0.033 mol) of ~1-
(chlorocarbonyl)-5,11-dihydro-6H-
pyrido[2,3-b][1,4]benzodiazepin-6-one and 4.3 g
(0.041 mol) of anhydrous sodium carbonate were added to
the filtrate and stirred for 30 minutes at a reaction
temperature of 50C. The solvent was then distilled off
ln vacuo, the highly viscous residue remaining was
dissolved in 300 ml of ethanol, mixed with 4 ml (about
0.05 mol) of a 37% aqueous formalin solution and
refluxed for 50 minutes. It was left to cool, 5.0 g of
Raney nickel were added and the mixture was hydrogenated
for 5 hours at ambient temperature under 4 bar of
hydrogen pressure. The catalyst was filtered off, the
filtrate was evaporated down ln vacuo and the residue
remaining was purified by chromatography on silica gel
(35-70 mesh) using dichloromethane/ethyl
- 78 -
acetate/cyclohexane/methanol/conc. ammonia 50/11/9/9/1
v/v/v/v/v as eluant. The residue remaining after
evaporation of the suitable eluates was recrystallised
from hot acetonitrile. 3.3 g t29~ of theory) of
colourless crystals were obtained, m.p. 226-228~C.
C19H~9N5O2 (349-39)-
Calc.: C 65.32 H 5.48 N 20.04
Found: 65.08 5.29 20.31
.
The following compound was obtained analogously:
5,11-dihydro-8,9-dimethyl 11-[[6-methyl-2,6-diazaspiro-
[3,3]hept-2-yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzo-
diazepin-6-one, m.p. 296-298C.
s2 (377-45)-
Calc.: C 66.83 H 6.14 N 18.55
Found: 67.05 6.25 18.75
Example 72
5,11-Dihydro-11-[[7-methyl~2,7-diazaspiro[3,5]non-2-yl]-
carbonyl]-6H-pyridor2~3-b][l 4]benzodiazepin-6-one
:
a) Ethyl-4-(aminomethyl)-1-methyl-4-piperidine
- carboxylate
52.0 g (0.265 mol) of ethyl 4-cyano-1-methyl 4-
piperidine carboxylate were dissolved in 400 ml of
glacial acetic acid and, after the addition of ~0 ml of
conc ntrated sulphuric acid, hydrogenated in the
presence of 3 g of platinum(IV~oxide at an initial
hydrogen pressure of 40 bar up to the end of the
hydrogen uptake at ambient temperature. The catalyst
was separated off, the filtrate was freed from solvent
in vacuo, the residue was mixed with 100 g of ice and,
with stirring and good external cooling using a mixture
of ice and common salt, concentrated sodium hydroxide
solution was added dropwise until the mixture had a pH
- 79 -
of 8. Whilst external cooling was continued, 250 g of
anhydrous potassium carbonate was added in batches and
the stiff slurry thus formed was exhaustively extracted
with ether. The combined ether solutions were dried
over potassium carbonate and concentrated by evaporation
and the residue remaining was reacted in the following
step without further purification. Yield: 40.0 g (75%
of theory) of a colourless oil.
b) 7-Methyl-2L7-diazaspiro[3,5]nonane
37.5 g (0.187 mol~ of ethyl 4-(aminomethyl)-1-
methyl-4-piperidine carboxylate in 100 ml of anhydrous
diethylether were added slowly dropwise, with stirring,
to a Grignard solution, cooled to 0 to 5C, consisting
of 13.6 g (0.559 g-atom) of magnesium, 61.1 g
(0.561 mol) of bromoethane and 350 ml of diethylether.
The mixture was stirred for a further 2 hours at 0C and
for 4 hours at ambient temperature. The mixture, cooled
to 0 to 5C again, was then very slowly mixed, with
stirring, with 50 ml of 10% aqueous ammonium chloride
solution and made clearly ammoniacal with 50 ml of conc.
ammonia. The ether layer was separated off, the aqueous
phase was exhaustively extracted with ether, the
combined ether extracts were washed twice with 50 ml of
water, dried over sodium sulphate and evaporated ln
vacuo. The oily residue was taken up in 80 ml of
anhydrous diethylether, the resulting solution was added
dropwise to a suspension of 5.9 g (0~16 mol) of lithium
aluminium hydride in 200 ml of dry diethylether so that
the ether boiled only gently. After all had been added,
the mixture was stirred for a further 4 hours at ambient
temperature and refluxed for 1 hour. It was left to
cool and, with stirring and external cooling with ice
water, 6 ml of water, 6 ml of 15% sodium hydroxide
solution and 18 ml of water were added dropwise. The
precipitate obtained was suction filtered, suspended
once more with diethylether and boiled, and then suct~on
::--`` 2~
- 80 -
filtered again. The combined filtrates were dried over
sodium sulphate and concentrated by evaporation. The
colourless oil obtained in a yield of 19.0 g (72% of
theory) was further processed in the next step without
any further purification~
c) 5,11-Dihydro-11-~[7- eth~l-2.7-diazaspiro[3,5]non-2-
yl]carbo_yll-6H-Pyrido r 2,3-bl r 1,4lbenzodiazepin-6-
one
.
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-b][1,4]-
benzodiazepin-6-one and 7-methyl-2,7-diazaspiro[3,5]-
nonane in a yield of 61~ of theory. Colourless
crystals, m~p. 201-203C (acetonitrile~.
C21H23NsO2 (377-45)-
Calc.: C 66.83 H 6.14 N 18.55
Found: 66.95 6.27 18.80
r~
- 81 -
Example 73
5,11-Dihydro-8,9-dimethyl-11-[[7-methyl-2,7-diazaspiro-
[3,5]non-2-yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzo-
diaze~in-6-one _ __ _
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-8,9-dimethyl-6H-
pyrido[2,3-b][1,4]benzodiazepin-6-one and 7-methyl-2,7-
diazaspiro[3,5]nonane in a yield of 46% of theory.
Colourless crystals, m.p. 222-224C (acetonitrile).
C23H27N502 (405 50)
Calc.: C 68.13 H 6.71 N 17.27
Found: 67.95 6.80 17.49
Example 74
5,11-Dihydro-11-[[2-methyl-2,7-diazaspiro[3,5]non-7-
yl]carbonyl]-6H-pyrido[2 3-b][1 4]benzodiazepin-6-one
a) 2-Methyl-7-tphenylmethyl)-2~7-diazaspiror3,5]nonane
11.55 g (0.0534 mol) of 7-(phenylmethyl)-2;7-
diazaspiro[3,5]nonane, obtained analogously to Example
72a) and 72b), were dissolved in 300 ml of ethanol,
mixed with 5 ml (about 0.062 mol) of a 37% aqueous
formalin solution and refluxed for 50 minutes. The
mixture was left to cool, 5.0 g of Raney nickel were
added and the mixture was hydrogenated for 5 hours at
ambient temperature under 4 bar of hydrogen pressure.
The catalyst was filtered off, the filtrate concentrated
hy evaporation in vacuo, the residue remaining was
purified on silica gel by HPLC using
dichloromethane/meth-anol/cyclohexane/conc. aqueous
ammonia 68/15/15/2 as eluant. Evaporation of suitable
fractions yielded the desired compound in the form of a
viscous colourless oil. Yield: 6.7 g (54% of theory).
RF 0.58 (Macherey-Nagel, Polygram~ SIL G/UV254, pre-coated
- 82 -
plastic sheets for TLC; eluant:
dichloromethane/methanol/cyclohexane/conc. aqueous
ammonia 68/15/15/2 v/v/v/v).
b) 2-Methyl-2,7-diazaspiro~3,5]nonane
To a solution of 6.5 g (0.0282 mol) of 2-methyl-6-
(phenylmethyl)-2,6-diazaspiro[3,4]octane in 60 ml of
ethanol, 4.0 g of 10% palladium/animal charcoal catalyst
were added and the mixture was then hydrogenated for 5
hours at ambient temperature under a hydrogen pressure
of 5 bar. It was filtered, the filtrate was
concentrated by evaporation under reduced pressure
(100 mmHg) and a colourless oil was obtained as residue,
RF . 13 (Macherey-Nagel, Polygram~ SIL G/UV2s4, pre-coated
plastic sheets for TLC; eluan~:
dichloromethane/methanol/cyclo-hexane/conc. aqueous
ammonia 68/20/10/5, v/v/v/v).
Yield: 3.2 g (81% of theory).
c) 5 11-Dihydro-11-[[2-methyl-2,7-diazaspiro[3,5]non-7-
yl]carbonyl]-6H-pyrido r 2,3-b~[1 4]benzodlaze~in-6-
one
Prepared analogously to Example 1 from I1-
(chlorocarbonyl)-5,11-dihydro-6H-pyrido[2,3-b][1,4]-
benzodiazepin-6-one and 2-methyl-2,7-diazaspiro[3,5]-
nonane in a yield of 59% of theory. Colourless
crystals, m.p. 186-188C (diisopropylether).
C21H23NsO2 (377 45)
Calc.: C 66.83 H 6.14 N 18.55
Found: 67.05 6.15 18.79
2 0 2 ~ 3
. .
- 83 -
.
Example 75
5,11-Dihydro-8,9-dimethyl-11-[[2-methyl-2,7-diazaspiro-
` [3,5]non-7-yl]carbonyl]-6H-pyrido[2,3-b][1,4]benzo-
diazepin-6-one
.,.,~
Prepared analogously to Example 1 from 11-
(chlorocarbonyl)-5,11-dihydro-8,9-dimethyl-6H-
pyrido[2,3-b][1,4]benzodiazepin-6-one and 2-methyl-2,7-
diazaspiro[3,5]nonane in a yield of 43% of theory.
: Colourless crystals, m.p. 2~5-247C (acetonitrile).
~: C23H27NsO2 (405.50).
Calc.: C 68.13 H 6.71 N 17.27
Found: 68.05 6.86 17.41
2J ~J ~
-~:
- 84 -
The preparation of some pharmaceutical
administration forms will now be described by means of
some Examples:
Example I
Tablets containing 5 mg of 5,11-dihydro-11-[[6-methyl-
2,6-diazaspiro[3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-b]-
r 1,4~benzodiazepin-6-one
Composition:
1 tablet contains-
Active substance 5.0 mg
Lactose 148.0 mg
Potato starch 65.0 mg
Magnesium stearate 2.0 mg
220.0 mg
A 10% mucilage is prepared from potato starch by
heating. The active substance, lactose and remaining
potato starch are mixed together and granulated with the
above mucilage through a screen with a mesh size of
1.5 mm. The granules are dried at 45C, rubbed through
the same screen again, mixed with magnesium stearate and
compressed to form tablets.
Weight of tablet: 220 mg
Punch: 9 mm
Example II
Coated tablets containing 5 mg of 5,11-dihydro-11-[[6-
methyl-2,6-diazaspiro[3,4]oct-2-yl]carbonyl]-6~-
~yrido[2,3-b] r 1.4]benzodiazepin-6-one
The tablets prepared in Example I are coated in a
conventional manner with a coating consisting
essentially of sugar and talc. The finished coated
tablets are polished with beeswax.
- ` -
- 85 -
; Weight of coated tablet: 300 mg
:
Example III
Ampoules containin~ 10 mg of 5,11-dihydro-11-[[6-methyl-
2,6-diazaspiro[3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-b]-
[1 4]benzodiazepin-6-one_
Composition:
1 ampoule contains:
Active substance 10.0 mg
Sodium chloride 8.0 mg
Distilled water ad 1 ml
The active substance and sodium chloride are
dissolved in distilled water and then made up to the
volume specified. The solution is filtered sterile and
transferred into 1 ml ampoules.
Sterilisation: 20 minutes at 120C.
Example IV
Suppositories containing 20 mg of 5,11-dihydro-11-[[6-
methyl-2,6-diazaspiro[3,4]oct-2-yl]carbonyl]-6H-
pyrido[2,3-b][1,4]benzodiazepin-6-one
Composition:
1 suppository contains:
Active substance 20.0 mg
Suppository mass (e.g. Witepsol W 45~ 1 680.0 mq
1,700.0 mg
The finely powdered active substance is suspended in
the molten suppository mass which has been cooled to
40C. At 37C the mass is poured into slightly chilled
suppository moulds.
Weight of suppository 1.7 g
-- ~ ` 2 ~ 2 ~
- 86 -
Example V
~.
Drops containing 5,11-dihydro-11-[[6-methyl-2,6-
diazaspiro[3,4]oct-2-yl]carbonyl]-6H-pyrido[2,3-b]-
r 1.4~benzodiazepin-6-one
Composition:
100 ml o~ drops solution contain:
Methyl p-hydroxybenzoate 0.035 g
Propyl p-hydroxybenzoate 0.015 g
Aniseed oil 0.05 g
Menthol 0.06 g
Pure ethanol 10.0 g
Acti~e substance 0.5 g
Sodium cyclamate 1.0 g
Glycerol 15.0 g
Distilled water ad100.0 ml
The active substance and sodium cyclamate are
dissolved in about 70 ml of water and glycerol is added.
The p-hydroxybenzoates, aniseed oil and menthol are
dissolved in ethanol and this solution is added to the
aqueous solution with stirring. It is then made up to
100 ml with water and filtered to remove any suspended
particles.