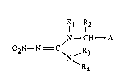Note: Descriptions are shown in the official language in which they were submitted.
2~25~7~
PS/5-17741/1~2/=
Guanidine derivatives
The present invention relates to novel derivatives of nitroguanidines, processes for their
preparation, pesticides containing these compounds, and their use in the control of pests.
The nitroguanidines according to the invention are those of the ~ormula I :
1 l2
~N--CH--A
02N--N = C~N ,R3 (I)
~R4
.
in which Rl is hydrogen, Cl-C4alkyl or C3-C6cycloalkyl, R2 is hydrogen or Cl-C4alkyl, R3
is hydrogen, Cl-C4alkyl or C3-C6cycloalkyl, R4 is hydrogen, Cl-C~aLcyl, C3-C6cycloalkyl
or a radical .CHR5-B, or R3 and R4 together aTe -(CH2)4- or -(CH2)s-~ Rs is hydTogen or
Cl-C4alkyl, A is an unsubstituted or mono- to tetrasubsti~lted aromatic or nonaromatic,
monocyclic or bicyclic heterocyclic radical, one to two substituents being selected from
the group comprising Cl-C3haloalkylj cyclopropyl, halocyclopropyl, C2-C3alkenyl,C2-C3aLcynyl, C2-C3haloalkenyl, C2-C3haloalkynyl, Cl-C3haloalkoxy, Cl-C3aL~cylthio,
Cl-C3haloalkylthio, allyloxy, propargyloxy, allylthio, propargylthio, haloallyloxy,
haloallylthio, cyano and nitro, and one to four substituents being selected from the group
comprising Cl-C3alkyl, Cl-C3alkoxy and halogen, and B is phenyl, cyanophenyl,
nitrophenyl, halophenyl having 1 to 3 halogen atoms, 3-pyridyl, 5-thiazolyl, 5-thiazolyl
which is monosubstituted to disubstituted by substituents from the group comprising
Cl-C3alkyl, Cl-C3haloalkyl, cyclopropyl, halocyclopropyl, C2-C3alkenyl, (~2-C3alkynyl,
Cl-C3alkoxy, C2-C3haloalkenyl, C:2-C3haloalkynyl, Cl-C3haloalkoxy, Cl-C3alkylthio,
Cl-C3haloalkylthio, allyloxy, propargyloxy, allylthio, propargylthio, haloallyloxy,
haloallylthio, halogen, cyano and nitro; or 3-pyridyl which is substituted by one or two
radicals from the group comprising Cl-C3haloalkyl, cyclopropyl, halocyclopropyl,
C2-C3alkenyl, C2-C3alkynyl, C2-C3haloalkenyl, C2-C3haloaLkynyl, Cl-C3haloalkoxy,Cl-C3alkylthio, Cl-C3haloalkylthio, allyloxy, propargyloxy, allylthio, propargylthio,
.
- 2025~72
haloallyloxy, haloallylthio, cyano and nitro, or by one to four radicals from the group
comprising Cl-C3alkyl, Cl-C3alkoxy or halogen; and their salts with inorganic acids; with
the exception of the compounds l-nitro-2-(pyrid-3-ylmethyl)guanidine,
1-nitro-2-(pyrid-2-ylmethyl)guanidine, l-nitro-2-(pyrid-4-ylmethyl)guanidine,
1-nitro-2-(1-oxopyrid-3-ylmethyl)guanidine and
1-(benzimidazol-2-ylmethyl)-2-nitroguanidine.
Heterocyclic compounds known from the literature which contain a nitroguanidine
structure are known from EP-A-192,060 as insecticides. However, the biological
properties of ~hese compounds are not entirely satisfactory in pest control. The individual
compounds excluded from the definition of the forrnula I are described in the literature as
intermediates for pharrnaceutically active compounds with antileukaernic and
antimicrobial actions. These compounds likewise have the biological properties of the
remaining substances of the formula I. When used in agrochemicals and as active
substance in agrochemicals, they are therefore likewise a subject of the present invention.
The compounds of the formula I according to the invention also include the salts with
agrochemically acceptable inorganic acids. ~xamples of such acids are hydrochloric acid,
hydrobromic acid, sulfuric acid, phosphoric acid and nitric acid, and acids which have the
same central atom and higher or lower oxidation levels, such as perchloric acid, nitrous
acid or phosphorus acid.
If at least one of the radicals R1, R3 or ~4 is hydrogen, the compounds of the forrnula I can
also exist in the tautomeric forrns Ia or Ib.
/N--CHR2 A
02N--NH--C~ (Ia)
NR3R4
~NRl- CHR2- A
02N- NH--C~ (Ib)
N--R3 (or R4)
Formula I according to the invention is to be understood as meaning here that the forms Ia
and Ib are included in the term formula I.
The ring systems included in the definition of the heterocyclic radical A contain at least
2Q12~72
one hetero atom as a ring member, i.e. at least one of the atoms which forms thering-shaped basic body on which the ring systems are based is other than carbon. In
principle, all atoms of the periodic system of the elements are capable of acting as ring
members if they can form at least two covalent bonds, in which case the heterocyclic
radical is preferably unsaturated and bonded via a carbon atom as a ring member to the
basic body of the nitroguanidine of the formula I. Unsaturated ring systems of the
definition A contain one or more double bonds, and such ring systems are preferably
polyunsaturated and generally have aromatic character. Ring systems which contain at
least one nitrogen atom as the hetero atom are preferred. Such rings af the definition A
customarily contain one to three hetero atoms from the series comprising oxygen, sulfur
and nitrogen, the maximum of oxygen or sulfur atoms in each case being one. Preferred
ring systems made the definition of A being those where the heterocyclic radical A
contains one to three hetero atoms from the series comprising oxygen, sulfur and nitrogen,
where one hetero atom is always nitrogen and the maximum of oxygen atoms or sulfur
atoms being one. In particular, examples of heterocycles of the definition A according to
the invention can be found in the group of basic bodies of the following structures:
o
~ 3 ~ ~3 ~s3 5~N
E ~ E
o~3 s~o3s~s~ o 3 o~o3
202~072
- 4 -
od~sJ sd~l o~N3 o~oJ s~3 S~N3
E E E
5~0 o~o3 o~ J 0~0~ o~53
~s~l o~s~ o~N3 O~NJI 0~
E E E
N~ 3~ ~N ~ ~N N~l` 3 ~N~N
s os o J s o-- s d~ s s s J
sD[~ 5 ~ s~N3 S ~NJIS ~N~ ~3
E E E
N~ ~;3 ~3 '
y
~,3 N~3 ,ND~3 ~N~3
Y Y
2~2~072
N~3 ~N~ ' INN~ N . ~ N .
Y
N ~ I . N - N
~N~ ~o~ N~ ~N ~ N~ ~N ~ N
O O O Y S
N~N N~D ~ ~ N
~ ~ S S ~ Y
~ N ~ N ~
:
~n~3
N~N N~N N~N N~N
o~N~l~ o~N~J~3 o~Nl;~3 s~N1~3
..- ~ '
:. .
, .
- 2~2~2
s~N1~3 and
In the above forrnulae, E is (: I-C3alkyl and Y is hydrogen, Cl-C3alkyl or cyclopropyl.
The heterocycles A which were listed as examples can be unsubstituted or, depending on
-the substitution possibilities of the ring system, carry up to four substituents such as are
indicated under formula I. These heterocycles are preferably bonded to the guanidine body
via a carbon atom in their unsubsti~uted form, or they carry one to three substituents from
the group comprising halogen, Cl-C3alkyl, Cl-C3haloalkyl, Cl-C3haloalkoxy or
Cl-C3alkoxy. Very particularly preferred heterocycles A are pyridyl radicals or thiazolyl
radicals, for example 3-pyridyl, 2-halopyrid-5-yl, 2j3-dihalopyrid-5-yl, 2-halothiazol-4-yl,
I-oxopyrid-3-yl, 1-oxo-2-halopyrid-5-yl and 1-oxo-2,3-dihalopyrid-5-yl.
In the definition of the formula I according to the invention, the individual generic terms
are to be understod as follows:
~ .
The halogen atoms suitable as substituents are fluorine and chlorine as well as brornine
and iodine, preferred substituents being fluorine, chlorine and bromine. Halogen here is to
be understood as a substituent in its own right or as part of a substituent such as in
haloalkyl, haloalkylthio, haloalkoxy, halocycloalkyl, haloalkenyl, haloalkynyl,
haloallyloxy or haloallylthio. The alkyl, alkylthio, alkenyl, aLI~ynyl and alkoxy radicals
suitable as substituents can be straight-chain or branched. Examples of such alkyls which
may be mentioned are methyl, ethyl, propyl, isopropyl, butyl, i-butyl, sec-butyl or
ten-butyl. Suitable aL~oxy radicals which may be mentioned, inter aIia, are: methoxy,
ethoxy, propoxy, isopropoxy or butoxy and their isomers. Alkylthio for example is
methylthio, ethylthio, isopropylthio, propylthio or the butylthio isomers. If the alkyl,
alkoxy, alkenyl, alkynyl or cycloalkyl groups suitable as substituents are substituted by
halogen, they can be partially halogenated or even perhalogenated7 in which case the
definitions given above hold for halogen, alkyl and alkoxy. Examples of the alkyl
elements of these groups are methyl which is monosubstituted to trisubstituted by fluorine,
chlorine and/or bromine, for exarnple CHFz or CF3; ethyl which is monosubstituted to
pentasubstituted by fluorine, chlorine and/or bromine, for example CH2CF3, CF2CF3,
CF2CCl3, CF2CHCl2, CF2CHF2, CF2CFC12, CF2CHBr2, CF2CHCIF, CF2CHBrF or
072
CCIFCHClF; propyl or isopropyl, each of which is monosubstituted to heptasubstituted by
fluorine, chlorine and/or bromine, for example CH2CHBrCH2Br. CF2CHFCF3,
CH2CF2CF3 or CH(CF3)2; butyl which is monosubstituted to nonasubstituted by fluorine,
chlorine and/or bromine, or one of its isomers, for example CF(CF3)CHFCF3 or
CH2(CF2)2CF3; 2-chlorocyclopropyl or 2,2-difluorocyclopropyl; 2,2-difluorovinyl,2,2-dichlorovinyl, 2-chloroalkyl, 2,3-dichlorovinyl or 2,3-dibromovinyl.
If the defined alkyl, alkoxy or cycloalkyl groups are substituted by other substituents, they
can be monosubstituted or polysubstituted by an identical or by different substituents from
among those mentioned. Preferably, one or two further substituents are present in the
substituted groups. Cylcoalkyl radicals ~vhich are suitable as substituents are, for example,
cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl. Alkenyl and alkynyl groups contain
one unsaturated carbon-carbon bond. Typical representatives are allyl, methallyl or
propargyl, but also vinyl and ethynyl. The double or triple bonds in allyloxy,
propargyloxy, allylthio or propargylthio are separated from the site where they are linked
to the hetero atom (O or S) preferably by a saturated carbon atom.
Sub-groups which must be emphasized from among the compounds of the formula I are
those in which either
a) Rl and R3 independently of one another are hydrogen, methyl, ethyl or cyclopropyl, or
b) R2 is hydrogen, or c) R4 is hydrogen or methyl.
In the event that R4 is the group -CHRs-B, those compounds in which Rs is hydrogen are
prefer~ed. The preferred ernbodiments of B are phenyl, pyridyl or thiazolyl, each of which
is unsubstituted or substituted by one to three radicals from the group comprising halogen,
Cl-C3alkyl, Cl-C3haloalkyl, Cl-C3haloalkoxy or Cl-C3alkoxy.
In a particularly preferred group of compounds of the formula I, Rl and R3 independently
of one another are hydrogen, methyl, ethyl or cyclopropyl, R2 is hydrogen, R4 is hydrogen
or methyl, and A is pyridyl, l-oxopyridyl, thiazolyl, or pyridyl, l-oxopyridyl or thiazolyl,
each of which is substituted by one to three substituents from the group comprising
halogen, Cl-C3alkyl, Cl-C3haloalkyl, Cl-C3haloalkoxy or C~-C3alkoxy.
The following are to be mentioned as preferred individual compounds of the formula I:
1 -(2-chloropyrid-5-ylmethyl)- 1 -methyl-2-nitroguanidine,
~2~ 2
- 8 -
1-(2-chloropyrid-5-ylmethyl)-2-nitroguanidine,
1-methyl-2-nitro-3-(pyrid-3-ylmethyl)guanidine,
1 -methyl-2-nitro- 1 -(pyrid-3-ylmethyl)guanidine,
1 ,2-dimethyl-3-nitro- 1 -(pyrid-3-ylmethyl)guanidine,
1 -(2-chloropyrid-5-ylmethyl)- 1 ,2-dimethyl-3-nitroguanidine,
1-(2-chlorothiazol-S-ylmethyl)- 1 -methyl-2-nitroguanidine,
1-(2-chloropyrid-5-ylmethyl)-2,2-dimethyl-3-nitroguanidine,
1 -(2-chloropyrid-5-ylmethyl)-2-methyl-3-nitroguanidine,
-1, 1 -dimethyl-2-nitro-3-(pyrid-3-ylmethyl)guanidine,
1 -ethyl-2-nitro-3-(pyrid-3-ylmethyl)guanidine,
1 -ethyl-2-(2-chloropyrid-5-ylmethyl)-3-nitroguanidine,
1 -ethyl-1-methyl-2-(2-chloropyrid-5-ylmethyl)-3-nitroguanidine,
1 -(2-chlorothiazol-5-ylmethyl)-2,2-dimethyl-3-nitroguanidine,
1-(2,3-dichloropyrid-5-ylmethyl)-2-methyl-3-nitroguanidine,
1 -ethyl-2-(2,3-dichloropyrid-5-ylmethyl)-3-nitroguanidine,
1 -(2,3-dichloropyrid-5-ylmethyl)- 1 -methyl-2-nitroguanidine,
1 -ethyl- 1- (2,3-dichloropyrid-5-ylmethyl) -2-nitroguanidine,
1- (2-chloro- 1 -oxopyrid-S -ylmethyl)- 1 -methyl-2-nitroguanidine,
1 -ethyl- 1 -(2-chloro- 1 -oxopyrid-S-ylmethyl)-2-nitroguanidine,
1-(2-chloro- 1-oxopyrid-5-ylmethyl)-2-methyl-3-nitroguanidine,
1 -ethyl- 1- (2-chloro- 1 -oxopyrid-5 -ylmethyl) -3-nitroguanidine,
1 -(2-chlorothiazol-5-ylmethyl)-2-methyl-3-nitroguanidine,
1-(~-chlorothiazol-5-ylmethyl)-2-nitroguanidine and
1 -ethyl-2-(2-chlorothiazol-5-ylmethyl)-3-nitroguanidine.
The compounds of the formula I according to the invention can be prepared in analogy to
known processes. For example, the compound of the formula I is obtained either when
a) an amine of the formula II
H-NRl-CHR2-A (II)
is reacted with a nitroisothiourea of the forrnula III
2~2C~Q72
,s--CH3
02N--N C~N~R3 (III),
in which Rl, R2, R3, R4 and A have the meanings given under formula I, or when
b) a compound of the formula IV
- Hal-ClEIR2-A (~)
is reacted with a nitroguanidine derivative of the formula V
02N--N--C~ (V),
NR3R4
in which Rl, R2, R3, R4 and A have the meanings given under formula I and Hal ishalogen, preferably chlonne or bromine, in the presence of a base.
The compounds of the formula I in which R3 and R4 are hydrogen can also be obtained by
reacting an amine of the formula Il
H-NRI-CHR2-A (II),
in which Rl, R2 and A have the meanings given under formula 1, either with
l-methyl-l-nitroso-2-nitroguanidine, of the formula VI,
2 ~ (Vl)
NH2
or with nitroguanidine, of the formula VII,
~NH2
02N--N = C (VII).
2025~72
- 10-
Yariant a) of the process (tl + II > I) according to the invention is advantageously
carried out in an inert solvent at temperatures between 0C and +150C, in particular
between +40C and +120C. Solvents which are particularly suitable are alcohols such as
methanol, ethanol or isopropanol. Variant b) (I~ + V ~ I) is advantageously car~ied
out in an inert polar aprotic solvent such as acetonitrile or dimethylformamide, at
temperatures between +50C and the boiling point of the reaction mixture. Suitable bases
are carbonates such as potassium carbonate. Hydrides such as sodium hydride can also be
-employed as bases. When sodium hydride is employed in acetonitrile or
dimethylformarnide, it is possible to lower the reaction temperature to +10C to +25C,
without this resulting in a considerably longer reaction time. II is advantageously reacted
with VI in aqueous, alcoholic solutions at temperatures between 0C and +25C. In this
reaction, N-nitrosomethylamine is formed as a by-product. II is customarily reacted with
VII in an aqueous medium at temperatures between +40C and +100C, preferably
between +60C and +80C.
Those guanidine derivatives of the formula I in which Rl, R3 and R4 have those meanings
which are not hydrogen, can be prepared from compounds of the sub-formula Ia
i2
NH-CH--A
02N--N = C~ (Ia)
NH2
in which R2 and A have the meanings given under formula I, by alkylation reaction in the
presence of a base, using the alkylation reagents
Rl-Hal,
R3-Hal or
R4-Hal
in which Rl, R3 and R4 have the meanings given under formula I which are not hydrogen,
and Hal is halogen, preferably chlorine or bromine.
Since it is known that aLI~ylation reactions of the above type hardly ever proceed
7 2
regiospecifically, this alkylation step is preferably suitable for preparing tertiary amino
groups, i.e. in particular for those cases in which all three remaining hydrogen atoms are to
be substituted simultaneously on the guanidine nitrogen atoms by radicals Rl, R3 and R~
of the same type.
On the other hand, with alkylations of the above type it is possible to obtain in a simple
manner those guanidine derivatives of the formula I in which all radicals Rl, R3 and R4
have a meaning other than hydrogen, if it is desired to introduce only one of these radicals
-in each case, in place of a hydrogen atom. If sterical hindering is sufficiently effective by
either the radicals present in the guanidine structure, such as -CHR2-A, or the radicals Rl,
R3 or R4 to be introduced, the alkylation reaction of this type can still be used for
preparing secondary guanidine amino groups. If the compounds of the formula Ia are
reacted only with one or two equivalents of all~ylation reagent, the results are mixtures of
isomers as well as alkylation products having different degrees of alkylation. However,
these mixtures can be separated into their individual components using customaryseparation methods, so that, inter alia, it is also possible to select the desired product.
The reaction conditions chosen for the alkylation reactions mentioned are a temperature
between 0C and +150~, preferably between +10C and +50C. Suitable bases are alkali
metal hydrides and alkaline earth metal hydrides, such as sodium hydride or calcium
hydride, or carbonates, such as sodium carbonate or potassium carbonate. The alkylation
reaction is preferably carried out in a polar, aprotic solvent, such as acetoni~ile or
dimethylformamide.
The intermediates of the forrnulae II, III, IV, V, VI and VII are known or can be prepared
in analogy to known processes.
It has now been found that the compounds of the formula I according to the invention are
valuable active substances in pest control and are well tolerated by warm-blooded species,
fish and plants. In particular, the use of the active substances according to the invention
relates to insects and arachnids which occur in crop plants and ornamental plants in
agriculture, in particular in cotton, vegetable and fruit plantations, in forests, in the
protection of stored products and materials, and in the hygiene sector, in particular on
domestic animals and productive livestock. They are active against all or individual stages
of development of normally sensitive, but also resistant species. In this context, their
action can become apparent by direct destruction of the pests or only after sorne time, for
2~7~
- 12-
example during ecdysis, or by reduced egg production and/or hatching rate. The above
mentioned pests include:
from the order of Lepidoptera, for example
Acleris spp., Adoxophyes spp., Aegeria spp., Agrotis spp., Alabama argillaceae, Amylois
spp., Anticarsia gemmatalis, Archips spp., Argyrotaenia spp., Autographa spp., Busseola
fusca, Cadra cautella, Carposina nipponensis, Chilo spp., Choristoneura spp., Clysia
ambiguella, Cnaphalocrocis spp., Cnephasia spp., Cochylis spp., Coleophora spp.,Crocidolomia binotalis, Cryptophlebia leucotreta, Cydia spp., Diatraea spp., Diparopsis
castanea, Earias spp., Ephestia spp., Eucosma spp., Eupoecilia ambiguella, Euproctis spp.,
Fuxoa spp., Grapholita spp., Hedya nubiferana, Heliothis spp., Hellula undalis, Hyphantria
cunea, Keiferia lycopersicella, Leucoptera scitella, Lithocollethis spp., Lobesia botrana,
Lymantria spp., Lyonetia spp., Malacosoma spp., Mamestra brassicae, Manduca sexta,
Operophtera spp., Ostrinia nubilalis, Pammene spp., Randemis spp., Panolis flammea,
Pectinophora gossypiella, Phthorimaea operculella, Pieris rapae, Pieris spp., Plutella
xylostella, Prays spp., Scirpophaga spp., Sesamia spp., Sparganothis spp., Spodoptera spp.,
Synanthedon spp., Thaumetopoea spp., Tortrix spp., Trichoplusia ni and Yponomeuta
spp.;
from the order of Coleoptera, for example
Agriotes spp., Anthonomus spp., AtomaIia linearis, Chaetocnema tibialis, Cosmopolites
spp., Curculio spp., Dermestes spp., Diabrotica spp., Epilachna spp., Eremnus spp.,
Leptinotarsa decemlineata, Lissorhoptrus spp., Melolontha spp., Orycaephilus spp.,
Otiorhynchus spp., Phlyctinus spp., Popillia spp., Psylliodes spp., Rhizopertha spp.,
Scarabeidae, Sitophilus spp., Sitotro~a spp., Tenebrio spp., Tribolium spp. and
Trogoderma spp.; from the order of Orthoptera, for example Blatta spp., Blattella spp.,
Gryllotalpa spp., Leucophaea maderae, Locusta spp., ~eriplaneta spp. and Schistocerca
spp.;
from the order of Isoptera, for example
Reticulitermes spp.; from the order of Psocoptera, for example Liposcelis spp.; from the
order of Anoplura, for example Haematopinus spp., Linognathus spp., Pediculus spp.,
Pemphigus spp. and Phylloxera spp.; from the order of Mallophaga, for example
Damalinea spp. and Trichodectes spp.;
from the order of Thysanoptera, for example
E~rankliniella spp., Hercinothrips spp., Taeniothrips spp., Thrips palmi, Thrips tabaci and
Scirtothrips aurantii;
from the order of Heteroptera, for example
Cimex spp., Distantiella theobroma, Dysdercus spp., Euchistus spp., Lurygaster spp.,
202~72
Leptocorisa spp., Nezara spp., Piesma spp., Rhodnius spp., Sahlbergella singularis,
Scotinophara spp. and Triatoma spp.;
from the order of :Homoptera, for example
Aleurothrixus floccosus, Aleyrodes brassicae, Aonidiella spp., Aphididae, Aphis spp.,
Aspidiotus spp., Bemisia tabaci, Ceroplaster spp., Chrysomphalus aonidium,
Chrysomphalus dictyospermi, Coccus hesperidum, Empoasca spp., Eriosoma larigerum,
Erythroneura spp., Gascardia spp., Laodelphax spp., Lecanium corni, Lepidosaphes spp.,
Macrosiphus spp., Myzus spp., Nephotettix spp., Nilaparvata spp., Paratoria spp.,
Pemphigus spp., Planococcus spp., Pseudaulacaspis spp., Pseudococcus spp., Psylla spp.,
Pulvinaria aethiopica, Quadraspidiotus spp., Rhopalosiphum spp., Saissetia spp.,Scaphoideus spp., Schizaphis spp., Sitobion spp., Trialeurodes vaporariorum, Trioza
erytreae and Unaspis citri;
from the order of Hymenoptera, for example
Acromyrmex, Atta spp., Cephus spp., Diprion spp., Diprionidae, Gilpinia polytoma,
Hoplocampa spp., Lasius spp., ~onomorium pharaonis, Neodiprion spp., Solenopsis spp.
and Vespa spp.;
from the order of Diptera, for example
Aedes spp., Antherigona soccata, Bibio hortulanus, Calliphora erythrocephala, Ceratitis
spp., ~hrysomyia spp., Culex spp., Cuterebra spp., Dacus spp., Drosophila melanogaster,
Fannia spp., Gastrophilus spp., Glossina spp., Hypoderrma spp., Hyppobosca spp.,Liriomyza spp., Lucilia spp., Melanagromyza spp., Musca spp., Oestrus spp., Orseolia
spp., Oscinella frit, Pegomyia hyoscyami, Phorbia spp., Rhagoletis pomonella, Sciara spp.,
Stomoxys spp., Tabanus spp., Tannia spp. and Tipula spp.;
from the order of Siphonaptera, for example
5~eratophyllus spp., Xenopsylla cheopis,
from the order of Acarina, for example
Acarus siro, Aceria sheldoni, Aculus schlechtendali, Amblyomma spp., Argas spp.~Boophilus spp., Brevipalpus spp., Bryobia praetiosa, Calipitrimerus spp., Chorioptes spp.,
Dermanyssus gallinae, Eotetranychus carpini, Eriophyes spp., Hyalomma spp., Ixodes
spp., Olygonychus pratensis, Ornithodoros spp., Panonychus spp., Phyllocoptruta oleivora,
Polyphagotarsonemus latus, Psoroptes spp., Rhipicephalus spp., Rhizoglyphus spp.,
Sarcoptes spp., Tarsonemus spp. and Tetranychus spp.; and
from the order of Thysanura, for example
Lepisma saccharina.
The good pesticidal action of the compounds of the formula I according to the invention
2~ 7~
- 14-
corresponds to a destruction rate (mortality) of at least 50-60 % of the pests mentioned.
The action of the compounds according to the invention and of the compositions
containing them can be broadened considerably by adding other insecticides and/or
acaricides, and it can be adapted to suit the given circumstances. Examples of suitable
additives are representatives of the following classes of active substances:
organophosphorus compounds, nitrophenols and derivatives, formamidines, ureas,
carbarnates, pyrethroids, chlorinated hydrocarbons and Bacillus thuringiensis preparations.
The compounds of the formula I are employed in unaltered form or, preferably, together
with the auxiliaries conventionally used in the art of formulation, and they can therefore
be processed in a known manner to give, for example, emulsifiable concentrates, directly
sprayable or dilutable solutions, dilute emulsions, wettable powders, soluble powders,
dusts, granules and also encapsulations in polymeric substances. The application methods
such as spraying, misting, dusting, scattering or pouring, as well as the compositions, are
selected to suit the intended aims and the prevailing conditions. The compounds of the
formula I are furthermore suitable for use in seed treatment, in which case the seed can be
treated or dressed with the active substance or with a formulation containing the active
substance before sewing or the ac~ve substance can be applied to the seed furrow during
sowing.
The formulation, i.e. the compositions, preparations or formulations containing the active
substance of the formula I or combinations of these active substances with otherinsecticides or acaricides, and, if desired, a solid or liquid additive, are prepared in a
known manner, for example by intimatcly mixing and/or grinding the active substances
with extenders, for example with solvents, solid carriers, and, if desired, surface-active
compounds (surfactants).
The following are suitable as solvents: aromatic hydrocarbons, preferably the fractions Cg
to C12 of alkylbenzenes such as xylene mixtures or alkylated naphthalenes, aliphatic or
cycloaliphatic hydrocarbons, such as cyclohexane, paraffins or tetrahydronaphthalene,
alcohols such as ethanol, propanol or butanol, and glycols as well as their ethers and esters
such as propylene glycol, dipropylene glycol ether, ethylene glycol, ethylene glycol
monomethyl ether or ethylene glycol monoethyl ether, ketones such as cyclohexanone,
isophorone or diacetanol alcohol, strongly polar solvents such as N-methyl-2-pyrrolidone,
dimethyl sulfoxide or dimethylformamide, or water, vegetable oils such as rapeseed oil,
21~2~072
- 15-
castor oil, coconut oil or soya oil; silicone oils may also be suitable.
Solid carriers which are generally used, for example for dusts and dispersible powders, are
ground natural minerals, such as calcite, talc, kaolin, montmorillonite or attapulgite. To
improve the physical properties, it is also possible to add highly-disperse silicas or
highly-disperse absorptive polymers. Suitable particulate, adsorptive carriers for granuies
are porous types, for example pumice, brick grit, sepiolite or bentonite, and also
non-sorptive caTrier materials, such as calcite or sand. Moreover, a large number of
granulated materials of inorganic or organic nature can be used, such as, in particular,
dolomite or comminuted plant residues.
Suitable surface-active compounds are non-ionic, cationic and~or anionic surfactants
having good emulsifying, dispersing and wetting properties, depending on the nature of
the active substance of the formula I to be formulated or on the combinations of these
active substances with other insecticides or acaricides. Surfactants are also to be
understood as meaning mixtures of surfactants.
Anionic surfactants which are suitable can be either so-called water-soluble soaps or
water-soluble synthetic surface-active compounds.
Suitable soaps are the alkali metal salts, alkaline earth metal salts or substituted or
unsubstituted ammonium salts of higher fatty acids (C10-C22), such as the sodium salts or
potassium salts of oleic or stearic acid, or of natural mixtures of fatty acids which can be
obtained, for example, from coconut or tallow oil. Mention must also be made of the fatty
acid methyltaurinates as surfactants.
HoweYer, so-called synthetic surfactants are used more frequently, in particular fatty
sulfonates, fatty sulfates, sulfonated benzimidazole derivatives or alkylarylsulfonates.
The fatty sulfonates or fatty sulfates are generally in the form of alkali metal salts, alkaline
earth metal salts or substituted or unsubstituted ammonium salts, and generally have an
alkyl radical having 8 to 22 C atoms, alkyl also including the alkyl moiety of acyl radicals,
for example the sodium or calcium salt of ligninsulfonic acid, of the dodecylsulfuric ester
or of a fatty alcohol sulfate mixture prepared from natural fatty acids. This group also
includes the salts of the sulfuric esters and sulfonic acids of fatty alcohol/ethylene oxide
adducts. The sulfonated benzimidazole derivatives preferably contain 2 sulfonyl groups
2~25~72
- 16-
and one fatty acid radical having about 8-22 C atoms. Examples of alkylarylsulfonates are
the sodium, calcium or triethanolamine salts of dodecylbenzenesulfonic acid, of
dibutylnaphthalenesulfonic acid or of a naphthalenesulfonic acidlformaldehyde
condensation product. Other suitable compounds are the corresponding phosphates, such
as the salts of the phosphoric ester of a p-nonylphenoV(4-14)-ethylene oxide adduct, or
phospholipids.
Suitable non-ionic surfactants are mainly polyglycol ether derivatives of aliphatic or
cycloaliphatic alcohols, saturated or unsaturated fatty acids and alkylphenols, which can
colltain 3 to 30 glycol ether groups and 8 to 20 carbon atoms in the (aliphatic)hydrocarbon radical and 6 to 18 carbon atoms in the alkyl radical of the alkylphenols.
Other non-ionic surfactants which are suitable are the water-soluble polyethylene oxide
adducts with polypropylene glycol, ethylenediaminopolypropylene glycol and
alkylpolypropylene glycol which have 1 to 10 carbon atoms in the alkyl chain and which
contain 20 to 250 ethylene glycol ether groups and 10 to 100 propylene glycol ether
groups. The abovementioned compounds customarily contain 1 to 5 ethylene glycol units
per propylene glycol unit.
Examples of non-ionic surfactants which may be mentioned are
nonylphenolpolyethoxyethanols, castor oil polyglycol ethers, polypropylene/polyethylene
oxide adducts, tributylphenoxypolyethoxyethanol, polyethylene glycol and
octylphenoxypolyethoxyethanol. Other suitable substances are fatty acid esters of
polyoxyethylenesorbitan, such as polyoxyethylenesorbitan trioleate.
The cationic surfactants are mainly quaternary ammonium salts which contain at least one
alkyl radical having 8 to 22 C atoms as N-substituents and which have lower halogenated
or free alkyl, benzyl or lower hydroxyalkyl radicals as further substituents. The salts are
preferably in the form of halides, methylsulfates or ethylsulfates, for example
stearyltrimethylammonium chloride or benzyldi~2-chloroethyl)ethylammonium bromide.
The surfactants customary in the art of formulation are described, for example, in the
following publications:
"Mc (~utcheon's Detergents and Emulsifiers Annual", Mc Publishing Corp., Glen Rock,
NJ, USA, 1988,
H. Stache, "Tensid-Taschenbuch [Surfactant Guide]",2nd edition, C. Hanser VerlagMunich, Yienna 1981,
M. and J. Ash. "Encyclopedia of Surfactants", Vol. I-III, Chemical Publishing Co., New
York,1980-1981.
As a rule, the pesticidal preparations contain 0.1 to 99 %, in particular 0.1 to 95 %, of the
active substance of the formula I or combinations of this active substance with other
insecticides or acaricides, l to 99.9 % of a solid or liquid additive and 0 to 25 %, in
particular 0.1 to 25 %, of a surfactant. While concentrated compositions are often
preferred as commercially available goods, the end user generally uses dilute preparations
containing considerably lower concentrations of active substance. Typical application
concentrations are between 0.1 and 1,000 ppm, preferably between 0.1 and 500 ppm. The
application rates per hectare are generally 1 to l,000 g of active substance per hectare,
preferably 25 to 500 g/ha.
In particular, preferred formulations have the following composition: (% = percent by
weight)
Emulsi~lable concentrates
~ctive ingredient:1 to 90 %,5 to 20 % being preferred
Surface-active agent:1 to 30 %, preferably 10 to 20 %
Liquid carrier:5 to 94 %, preferably 70 to 85 %
Dusts:
Active ingredient:0.1 to lO %, preferably 0.1 to 1 %
Solid carrier:99.9 to 90 %, preferably 99.9 to 99 %
SusPension concentrates:
Active ingredient:S to 75 %, preferably 10 to 50 %
Water: 94 to 24 %, preferably 88 to 30 %
Surface-active agent:1 to 40 %, preferably 2 to 30 %
Wettable powders:
Active ingredient:0.5 to 90 %, preferably 1 to 80 %
Surface-active agent:0.5 to 20 %, preferably 1 to 15 %
202~72
- 18-
Solid carrier material: 5 to 95 %, preferably 15 to 90 %
Granules:
Active ingredient:0.5 to 30 %, preferably 3 to 15 %
Solid carrier:99.5 to 70 %, preferably 97 to 85 %
The compositions can also contain further additions such as stabilizers, for example
epoxidized or unepoxidized vegetable oils (epoxidized coconut oil, rapeseed oil or soya
oil), defoamers, for example silicone oil, preservatives, viscosity regulators, binders,
tackifiers and also fertilizers and other active substances for achieving specific effects.
The examples which follow are intended to illustrate the invention. They do not restrict
the invention.
:
Preparation exarnples
Example H 1: 1- (2-chloropvrid-5 -~lmethyl) -1 -methvl-2-n;tro ~u anid;ne
,Q CH2 N--f = N ---N02
Cl CH3 NH2
A mixture of 3.2 g of N-methyl(2-chloropyrid-5-yl)methylam;ne, 2.7 g of S-methylN-nitroisothlourea, 0.2 g of potassium hydrogen sulfate and 75 ml of ethanol~is refluxed for
4,5 hours. The reaction mixture is filtered while hot. When the filtrate has cooled to 0C,
the crude E~roduct crystallizes. Recrystallization from ethanol results in pure
1-(2-chloropyrid-5-ylmethyl)-1-me~yl-2-nilroguanid;ne in the form of a colourless
crystallizate having a melting point of 163-165C.
Example H2: 1-~2-chloropyrid-5-vlmethvl)-2-nitro~uanidine
~ C~2 - NH--IC = N--N02
C NH2
A suspension of 4.0 g of nitroguanidine and 5.4 g of (2-chloropyrid-5-yl)methylamine in 80
ml of water is heated for 3 hours at +80C. When the reaction mixture has cooled to +20C,
2 ~ 7 ~
- 19-
the product is precipitated within 16 hours in the form of a colourless crystallizate. The
crystal powder is separated o-ff, washed with methanol and dried. This gives 2.0 g of
1-(2-chloropyrid-5-ylmethyl)-2-nitroguanidine having a melting point of 195-197C.
Example H3 1-(2-chloropvrid-5-Ylmethv1)-2-(4-chlorobenzvl)-1-methvl-3-nitro~uanidine
Cl~ CH2 N--C ~ CH2 ~3 Cl
N02
To a solution of 4.0 g of 1-(2-chlorpyrid-5-ylmethyl)-1-methyl-2-nitroguanidine ;n 50 ml of
dimethylformamide there is added at 10-15C 0.7 g of 55 % sodium hydride in small
portions. After 20 minutes, a solution of 2.65 g of 4-chlorobenzyl chloride in 5 ml of
dimethylformamide is added dropwise at 10-15C. The reaction mixture is stirred for 20
hours at room temperature and then poured into 200 ml of ice-water. The aqueous solution
is extracted using chloro~orm. The organic phase is separated off, dried and evaporated.
Purificatil)n by column chromatography on silica gel gives 1-(2-chloropyrid-5-ylmethyl)-2-
(4-chlorobenzyl)-1-methyl-3-nitroguanidine having a melting point of 130-132C.
The compounds of the formula I listed in the table below can be prepared in an analogous
manner.
2~2~72
- 20 -
Table 1:
Rl I Rl 2
N--CH--A
O2N - N = c~ , R3
R4
Compound A Rl R2 R3 R4 Physical data
No.
1.01~ ,J CH3 H H H m.p.163-165C
Cl N'
1.02~ J H H H H m.p.195-197C
1.03~ J H H CH3 H m.p.162-163C
N
1.04 ~NJ CH3 H H H m.p.l34-136C
1.05 ~N~ CH3 H CH3 H
Resin, lH-NMR (CDCl3):
2.96 (s,3~1),
3.04 (s,3H),
4.70 (s,2H),
8.10 (broad s,1H)
cl~NJ CH3 H CH3 H m.p.129-131C
cl1N~ H H C2Hs H m.p.125-127C
1.08 ~ H H C3H7-n H
Cl N
- 21 -
Compound A R1 R2 R3 R4 Physical data
No.
.09J~' H H ~ H
1.10- J~ H H C3H7-i H
1.11,¢~/ H H C4H9-n H m.p.88-90C
1.12~ H H C4Hg-s H
1.14~ H H CH3 CH3 m.p.158-160C
1.15~¢~ H H CH3 C2Hs m.p.ll5-119C
1.16~¢~ H H CH3 C4H9-n
1.17"~/ H H C2HS C2Hs Resin
1.18~¢~ H H C4Hg-n C4Hg-n
1.19 ~¢~ H H -CH2CH2-CH2CHz-
1.20 ~¢~ H H H ~3CH2--m.p.154-155C
CN
1.21 J~' H H H
2 ~ 2
Compound A R1 R2 R3R4 Physical data
No~
1.22 ~/ H H H ~ CH2--
1.23 ~¢~/ H H H Cl~ C~
Cl N
1.24 ~/ C2Hs H H H m.p.136-138C
1.25 ~ J~ ~ H H H
1.26 "¢~ C3H7-n H H H
1.27 J~ C2~ls H CH3 H m.p.107-109C
1.28 J~ ~ H CH3 H
1.29 ~¢~ CH3 H C2~s H
1.30 ~¢~1~ C2Hs H C2Hs H - . :
1.31 J~' ~ H C2Hs H
1.32 J~ CH3 H C3H7-n H
1.33 J~ C2I~s H C3H7-n H
2~2~7~
- 23 -
Compound A R1 R2 R3 R4 Physical data
No.
1.34 '¢~/ CH3 H ~ H
1.35 ~¢~/ C2~5
1.36 J~ CH3 H C3H7-i H
Cl N
1.37 J~ ~ H C3H7-; H
Cl N
1.38 ~¢~/ c~3 H CH3 CH3
1.39 ~¢~ C2Hs I H CH3 C~I3
1.40 J~ CH3 H C2Hs C2Hs
Cl N
1.41 ~¢~ H H CH3 ~ C~2 -
1.42 ~¢~/ CH3 H H ~ CH2--
1.43 J~ c~3 H H ~ CH2--
1.44 J~ CH3 H H Cl~ CH
1.45 ¢~/ C2Hs H H H
2102~07~
- 24 -
Compound A R1 R2 R3 R4 Physical data
No.
1.46 ~ ~ H H H
1.47 ¢~ C2~I~ H CH3 H
1.48 ~ H H CH3 H
1.49 ¢~ C2H5 H H H
o
1.50 ¢~ [~ H H H
o
CH3 H CH3 H
1.52 ~ CH3 CH3 H H
1.53 J~ c3H7-n H CH3 H
1.54 '~3f C2Hs CH3 H H
~2~72
Compound A R1 R2 R3 R4 Physical data
No.
.
1.55 ~¢~ CH3 H H H
1.56 . J~' CH3 H (: H3 H
1.57 ~¢~ H H CH3 CH3
1.58 ~¢~ ~ H H H
1.59 ~¢~ C2~Is H H H
1.60 ~ C2Hs H C~3 H
1.61 ~ CH3 H H H
1.62 J~ CH3 H CH3 H
1.63 ~¢~ c2~s H H H
1.64 '¢~/ C2H5 H CH3 H
1.65 ,¢~1' H H CH3 CH3
1.66 ,~ ~ H CH3 H
2~25072
Compound A R1 R2 R3 R4 Physical da~a
No.
~
CH3JbNJJ CH3 H H H
-1.68 ~ CH3 H CH3 H
CH3 N
H3J~Ni C2Hs H H H
H3~N~ C2Hs H CH3 H
~ ~ cH3J?~ H H CU3 CH3
;~ 1.72 . 1~ :~ ~ H CH3 H
1.73 1?~ CH3 H H H
: 1.74 ~/ CH3 H CH3 H
cP3 N
1.75 .1~ C2Hs H H H
CF3 N
CF3~NJI C2Hs H CH3 H
2~2~072
Compound A R1 R2 R3 R4 Physical data
No.
1.77 J~l' H H C~I3 CH3
c~3 N
.78 CF J~ H H H
CH3 H H H
` Cl N
C~
1.80 J' J CH3 H CH3 H
c~
1.81 J~ J C2H5 H H H
c~,~
1.82 J~ J c2~s H CH3 H
Cl N~
~ ~ ~ C~
Cl NJ H H CH3 CH3
1.84 ~ ~ H H H
1.85 ~ D~ H OEI3 H
cl J~3 CH3 H H H m.p.112-114C
Cll~ CH3 H CH3 H
202~7~
- 28 -
Compound A R1 R2 R3 R4 Physical data
No.
1.88 J~L C2H5 H H H
N
1.89 l~ C2Hs H CH3 H
1.90 J~l~ C2H5 H C2Hs H
N_
c1~s J~ H H CH3 CH3 m.p.159-160C
Cl~ H H CH3 Cl~;~ CH2--
1.93 1~ H H CH3 H m.p.168-170C
1.94 l~l ~ H H H
c~ ~ H CH3 H
1.96 ~3 CH3 H H H
1.97 ~ ,11 CH3 H CH3 H
1.98 ~3 ~ H H H
202~07~
- 29 -
Compound A R1 R2 R3 R4 Physical data
No.
.. ~ , .
1.99 ~ CH3 H H H m.p.203-205C
o
1.100 ~ H H CH3 CH3
o
1.101 ,~ H H CH3 CH3
Cl N
1.102 ~ CH3 H CH3 H
o
1.103 ~1~ t 2Hs H C~H3 H
o
1.104 ~ <1 H CH3 H
o
1.105 ~[~ CH3 H H H m.p.198-200C
2Q~5Q7~
- 30 -
Compound A R1 R2 R3 R4 Physical data
No.
-
~ 1.106 ~ H H H H m.p.201-202C
-1.107 ~ H H CH3 H m.p.148-150C
1.108 ~ H H CH3 CH3 m.p.145-146C
1.109 ~ H H C2H5 H m.p.113-116C
1.110 ~ H CH3 H H m.p.158-160C
1.111 ~cl CH3 H H H m.p.210-212C
.112 ~CI CH3 H H H m.p.202-203C
H CH3 CH3 H m.p.161-163C
1.114 c l~ H H H H m.p.158-160C
cl~ H ` H C2Hs H m.p.135-136C
1.116 ~ ~ CH3 H H
2~2~2
- 31 -
Compound A Rl 1~2 R3 R4 Physical data
No.
1.117 J~ CH3 CH3 CH3 H
1.118 ~¢~/ CH3 CH3 C2Hs H
I.llg ,¢;~1' CH3 CH3 ¦> H
1.120 ~¢~/ CH3 CH3 C3H7-n H
1.121 ,¢~ H CH3 CH3 H
1.122 ~¢~/ H OEl3 C2H5 H
1.123 J~ H CH3 ~ H
1.124 ~¢;~ H CH3 C3H7-n H
1.125 c~ H C2Hs CH3 CH3
1.126 ~¢~ H CH3 CH3 CH3
1.127 ~ H C2Hs CH3 H
1.128 ~¢~ H C2Hs C2Hs - H
-: ~02~07~
Compound A Rl R2 R3 R4 Physical data
No.
~.
1.129 ,ll J CH3 C2Hs H H
1-130 cl '~NJ CH2 C2Hs CH3 H
Cl~
1.131 J~ ~ H H CH3 H m.p.l73-175C
~: , : cl~
~; ~ 1-132 cl~llNJ H H C2Hs H m.p.l59-161C
cl~
1.133 ~ ~I H H ~ H
;~ ~ Cl~ ~
1.134 ~ J H H C3H7-n H
c~
-135 CI~NJ H H CH3 C2Hs
Cl~
cl ~NJ CH3 H C2Hs H
~: cl~
1.137 J` 'J CH3 H c3H7-n H
1.138 ~ ~I H H -(CH2)4-
1-139 cl ~NJ H H -(CH2)s-
1.140 ,l~ J H CH3 -CH2)4-
2 ~ Q 7~
- 33 -
Compound A Rl R2 R3 R4 Physical data
No.
1.14i ~¢~ H CH3 -(CH2)s-
1.142 ~¢~/ H H ~ H
1.143 ,1~ CH3 H ~3 H
1.144 ,¢~1' H H ~ H
1.145 ~JÇI~ CH3 H ~ H .
1.146 ~¢~/ C2Hs H {¦ H
1.147 ~¢~ C2Hs H ~ H
1.148 JÇI~ H CH3 H -cu2 ~
CN
1.149 J~ H CH3 H -
1.15û ~lÇl~ H CH3 H -CH2 ~ c
1.151 ~[~ C3H7-n H H H
~5~2
- 34 -
Compound A R1 R2 . ~3 R4 Physical data
No.
1.152,ll J C3H7-n ~ CH3
1.153~ll J C3H7-n H C2Hs H
1.154 ~ C3H7-n H H H
1.155J~L~ C3H7-n H CH3 H
1.156C,J~l C3H7-n H C2Hs H
N ~
1.157cl ~ H H H -CH2 ~
1.158 ~ H H H -CH2 ~CI
1.159c1~5~-- CH3 H H -CH2 ~3
1.160 ~ CH3 H H -CH2~CI
N
1.161c 1~3 C3H7-i H H H
Cl--~3 C3H7-i H CH3 H
N
1.163 ~ C3H7-i H C2H5 H
- 35 -
Compound A Rl R2 R3 E~4 Physical data
No.
.
1.164 ~¢~ C3H7-i H CH3 H
1.165 ~1~1~ C3H7-i H C2Hs H
1.166 ~/ CH3 H H H
1.167 ,¢~1' C2Hs H H H
1.168 J~l' H H CH3 H
1.169 ~¢~ H H C2Hs H
1.170 JÇI~ H H CH3 CH3
1.171 ~1~1~ H H CH3 C2Hs
1.172 c~¢ ~/ CH3 H CH3 H
1.173 ~ H H H H m.p.207-209C
1.174 c~¢~ CH3 H H -CH2~3--cl m-p 130-132C
1.175 ~ H H C"H9-n H m.p.l52-153C
7 2
- 36 -
Compound A Rl R2 R3 R4Physical data
No.
1.176 ,[~ H H CH3 H
cl ~ .
o
Formulation examples (% = percent by weight)
Example Fl: Emulsion concentrates a) b) c)
Active substance No. 1.05 25 %40 %50 %
Ca-dodecylbenzenesulfonate 5 %8 % 6 %
Castor oil polyethylene glycol ether
(36 mol of EO) 5 %
Tributylphenol polyethylene glycol
ether (30 mol of EO) - 12 % 4 %
Cyclohexanone - 15 % 20 %
Xylenemixture 65 %25 %20 %
Emulsions of any desired concentration can be prepared ~rom such concentrates bydiluting them with water.
Example F2: Solutions a) b)c) d)
Active substance No. 1.05 80 % 10 %5 %95 %
Ethylene glycol monomethyl
ether 20 % - - -
Polyethylene glycol MW 400 - 70 %
N-methyl-2-pyrrolidone - 20 %
Epoxidized coconut oil - - 1 % 5 %
Petroleum ether (boiling
range 160-190C) - ^94 %
2 ~ 7 ~
The solutions are suitable for use in the form of very small droplets.
Example F3: Granules a)b) c) d)
Active substanceNo. 1.05 5 %10 % 8 % 21 %
Kaolin 94 % - 79 % 54 %
Highly-disperse silica 1% - 13 % 7 %
Attapulgite - 90 % - 18 %
The active substance is dissolved in methylene chloride, the solution is sprayed onto the
carrier, and the solvent is then evaporated in vacuo.
Example F4: Dust a)b)
Active substance No. 1.05 2 %5 %
Highly-disperse silica 1%5 %
Talc 97 %
Kaolin - 90 %
Ready-to-use dusts are ob~ained by intimately mixing the carriers with the active
substance.
Example FS: ~ettable powder a)b) c)
Active substance No. 1.01
or 1.03 25 %50 % 75 %
Na ligninsulfonate 5 %5 %
Nalauryl sulfate 3 % - 5 %
Na diisobutylnaphthalene-
sulfonate - 6 % 10 %
Octylphenol polyethylene glycol
ether (7-8 mol of EO) - 2 %
Highly-disperse silica 5 %10 % 10 %
Kaolin 62 %27 %
The active substance or the active substance combination is mixed with the ad~itives, and
the rnixture is thoroughly ground in a suitable mill. This gives wettable powders which
2~2~72
- 38 -
can be diluted with water to give suspensions of any desired concentration.
Example F6: Emulsion concentrate
Active substance No. 1.01 or 1.03 10 %
Octylphenol polyethylene glycol ether
(4-S mol of EO~ 3 %
Ca dodecylbenzenesulfonate 3 %
Castor oil polyglycol ether
(36 mol of EO) 4 %
Cyclohexanone 30 %
Xylene mixture 50 %
Emulsions of any desired concen~ation can be prepared from this concentrate by diluting
it with water.
Example F7: Dust a) b)
Active substance No. 1.02 5 % 8 %
Talc 95 %
Kaolin - 92 %
Ready-to-use dusts are obtained by mixing the active substance with the carrier and
grinding the rnixture on a suitable mill.
Example F8: Extruder granules
Active substance No. 1.06 10 %
Na ligninsulfonate 2 %
Carboxymethylcellulose 1 %
Kaolin 87 %
The active substance or the active substance combination is mixed with the additives, and
the mixture is ground and moistened with water. This mixture is extruded, granulated and
then dried in a stream of air.
202~a7~
-39-
Example F9: Coated ~ranules
Active substance No. 1.04 3 %
Polyethylene glycol (MW 200) 3 %
Kaolin 94 %
In a mixer, the ~mely-ground active substance or the active substance combination is
applied uniformly to the kaolin moistened with polyethylene glycol. In this manner,
dust-free coated granules are obtained.
Example F10: Suspension concentrate
Active substanceNo. 1.01 40 %
Ethylene glycol 10 %
Nonylphenol polyethylene glycol ether
(15 mol of EO) 6 %
Na ligninsulfonate 10 %
Carboxymethylcellulose 1 %
Silicone oil in the form
of a 75 % aqueous emulsion 1 %
Water 32 %
The ~mely-ground active substance or the active substance combination is mixed
intimately with the additives. This gives a suspension concentrate from which suspensions
of any desired concentration can be prepared by diluting with water.
Biological examples
Example Bl: Action against Nilaparvata lu~ens
Rice plants are treated with a spray liquor containing 400 ppm of the active substance and
prepared from an aqueous emulsion. After the spray coating has dried on, the rice plants
are colonized with cicada larvae of stage 2 and 3. The evaluation is carried out after 21
days. The percentage reduction of the population (% action) is deterrnined by comparing
the number of surviving cicadas on the treated plants with those on the untreated plants.
In this test, the compounds of Table 1 have a good action against Nilaparvata lugens. An
`B ~12
- 40 -
action of more than 80 % is shown, in particular, by the compounds 1.01, 1.03, 1.04, 1.05,
1.07, 1.14, l.lS, 1.24, 1.86, 1.93, 1.107, 1.108, 1.113 and l.llS.
Example B2: Action against Nephotettix cinctice~s
Rice plants are treated with a spray liquor containing 400 ppm of the active substance and
prepared from an aqueous emulsion. After the spray coating has dried on, the rice plants
are colonized with cicada larvae of stage 2 and 3. The evaluation is carried out after 21
days. The percentage reduction of the population (% action) is determined by comparing
the number of surviving cicadas on the treated plants with those on the untreated plants.
In this test, the compounds of Table 1 have a good action against Nephotettix cincticeps.
An action of rnore than 80 % is shown, in particular, by the compounds 1.01, 1.06, 1.14,
l.lS and 1.91.
Example B3: Action a~ainst Diabrotica balteata larvae
Maize seedlings are sprayed with a spray liquor containing 400 ppm of the activesubstance and prepared from an aqueous emulsion. After the spray coating has dried on,
the maize seedlings are colonized with 10 Diabrotica balteata larvae of the second stage
and placed in a plastic container. The evaluation is carried out after 6 days. The
percentage reduction of the population (% action) is determined by comparing the number
of dead larvae on the treated plants with those on the untreated plants.
In this test, the compounds of Table 1 have a good action against Diabrotica balteata. An
action of more than 80 % is shown, in particular, by compound 1.01.
Example B4: Action a~ainst Heliothis virescens caterpillars
Young soya plants are sprayed with a spray liquor containing 400 ppm of the active
substance and prepared from an aqueous emulsion. After the spray coating has dried on,
the soya plants are colonized with 10 Heliothis virescens caterpillars of the first stage and
placed in a plastic container. The evaluation is carried out after 6 days. The percentage
reduction of the population, or the percentage reduction of feeding damage (% action), is
determined by comparing the number of dead caterpillars and the damage by feeding on
the treated plants with those on the untreated plants.
In this test, the compounds of Table 1 have a good action against Heliothis virescens. An
action of more than 80 % is shown, in particular, by the compounds 1.01, 1.07 and 1.110.
2~ 0~2
- 41 -
Example B5: Action a~ainst Spodoptera littoralls caterpillars
Young soya pl~nts are sprayed with a spray liquor containing 400 ppm of the active
substance and prepared from an aqueous emulsion. After the spray coating has dried on,
the soya plants are colonized with 10 Spodoptera littoralis caterpillars of the third stage
and placed in a plastic container. The evaluation is carried out after 3 days. The
percentage reduction of the population, or the percentage reduction of feeding damage (%
action~, is determined by comparing the number of dead caterpillars and the damage by
feeding on the treated plants with those on the untreated plants.
In this test, the compounds of Table 1 have a good action against Spodoptera littoralis. An
action of more than 80 % is shown, in particular, by the compounds 1.01, 1.03, 1.07, 1.14,
1.86,1.107 and 1.108.
Example B6: Action against Aphis craccivora
Pea seedlings are infected with Aphis craccivora and then sprayed with a spray liquor
containing 400 ppm of the active substance and incubated at 20C. The evaluaùon is
carried out after 3 and 6 days. The percentage reduction of the population (% action) is
determined by comparing the number of dead aphids on the treated plants with those on
the untreated plants.
In this test, the compounds of Table 1 have a good action against Aphis craccivora. An
action of more than 80 % is shown, in particular, by the compounds 1.01, 1.03, 1.04, 1.05,
1.06, 1.07, 1.14, 1.15, 1.24, 1.86, 1.91, 1.93, 1.99, 1.107, 1.114 and 1.115.
.
Example B7: Systemic action against Nilaparvata lugens
Pots with rice plants are placed in a solution~ of the aqueous emulsion, containing 400
ppm of the active substance. The rice plants are then colonized with larvae of stages 2 and
3. The evaluation is carried out after 6 days. The percentage reduction of the population
(% action) is determined by comparing the number of cicadas on the treated plants with
those on the untreated plants.
In this test, the compounds of Table 1 have a good action against Nilaparvata lugens. An
action of more than 80 % is shown, in particular, by the compounds 1.01, 1.03, 1.04, 1.06,
l.Q7, 1.14, 1.15, 1.86, i.93, 1.107, 1.114 and 1.115.
202~7~
- 42 -
Exam~le B8: Systemic action against Nephotettix cincticePs
Pots with rice plants are placed in a solution, of the aqueous emulsion, containing 400
ppm of the active substance. The rice plants are then colonized with larvae of stages 2 and
3. The evaluation is carried out after 6 days. The percentage reduction of the population
(% action) is deterrnined by comparing the number of cicadas on the treated plants with
those on the untreated plants.
In this test, the compounds of Table 1 have a good action against Nephotettix cincticeps.
-An action of more than 80 % is shown, in particular, by the compounds 1.01, 1.03, 1.04,
1.06,1.14,1.15,1.86,1.91, and 1.114.
Exarnple B9: Svstemic action a~ainst Mvzus persicae
Pea seedlings are infected with Myzus persicae, the roots are then placed in a spray liquor
containing 400 ppm of the active substance, and the plants are incubated at 20C. The
evalution is carried out after 3 and 6 days. The percentage reduction of the population (%
action) is determined by comparing the number of dead aphids on the treated plants with
those on the untreated plants.
In tnis test, the compounds of Table 1 have a good action against Myzus persicae. An
action of more than 80 % is shown, in particular, by the compounds 1.01, 1.06, 1.14, and
1.86.
Example Blû: Action a ainst Anthonomus ~randis adults
Young cotton plants are sprayed with a spray liquor containg 400 ppm of the active
substance and prepared from an aqueous emulsion. After the spray coating has ~ied on,
the cotton plants are colonized with 10 adult Anthonomus grandis and placed in a plàstic
container. The evaluation is carried out after 3 days. The percentage reduction of the
population, or the percentage reduction feeding damage (% action), is determined by
comparing the number oE dead beetles and the damage by feeding on the treated plants
with those on the untreated plants.
In this test, the compounds of Table 1 have a good action against Anthonomus grandis. An
action of more than 80 % is shown, in particular, by the compounds 1.01, 1.14, 1.15, 1.86,
1.91 and 1.93.
- 43 -
Example B11: Action against Bemisia tabaci
Dwarf bean plants are placed in gauze cages and colonized with Bemisia tabaci adults
(whitefly). Once eggs have been deposited, all the adults are removed, and, 10 days later,
the plants and the nymphs on the plants are treated with a spray liquor of the active
substances to be tested (concentration 400 ppm), prepared from an aqueous emulsion. 14
days after the active substance has been applied, the batches are evaluated for % hatching
by comparing them with the untreated control batches.
-In this test, the compounds of Table 1 have a good action against Bemisia tabaci. An
action of more than 80 % is shown, in particular, by the compounds 1.01, 1.06, 1.07, 1.14,
1.86,1.107 and 1.108.
Example B 12: Action against Lucilia cuprina ~reenbottle flies
Small portions (30-50 eggs) of freshly deposited eggs of the greenbottle fly species Lucilia
cuprina are placed in test tubes in which 4 ml of nutrient medium have previously been
mixed with 1 ml of test solution containing 16 ppm of the active substance to be tested.
After the culture medium has been inoculated, the test tubes are sealed with a cotton-wool
ball and incubated in an incubator for 4 days at 30C. Up to this point in time, larvae of
approximately 1 cm in length (stage 3) develop in the untreated medium. If the substance
is active, the larvae are either dead at this point in time or their growth is markedly
retarded. The evaluation takes place after 96 hours.
In this test, compounds of Table 1 have a good action against Lucilia cuprina.