Note: Descriptions are shown in the official language in which they were submitted.
RV3 011090 1 LUDR3.~
FIELD OF THE INVENTION
Absorbents for Removal of Toxins from Whole Blood.
BACKGROUND OF THE INVENTION
It is well accepted in the art that it is preferable to undertake the
cleansing of
whole blood rather than blood plasma in extracorporeal circuits, since such a
procedure requires substantially less equipment and also reduces the
requirement of
supervision by additional professionally trained personnel, who are not always
available in every hospital. In the cleansing of whole blood, it is not
necessary to
expand substantial efforts in the prior removal of cells ( i.e., leuco-,
erthyro- and
thrombocytes), for example in the filter, which also requires constant
supervision.
Heretofore, the cleaning of whole blood ex-vivo, utilized absorbents of
activated
charcoal or activated charcoal bearing certain coatings, such as those
provided by
solutions of polyacrylic acid or polyacrylic acid and polyethylene imine (see
USSR SU
732,207).
Such activated charcoal absorbents, because of their grounding in activated
charcoal, however suffer from the disadvantage of reduced mechanical stability
in
particular at high pressures, as well as a low level of selectivity, with
respect to the
biomacromolecules to be removed.
As a consequence of the foregoing, considerable experimentation was
undertaken to replace absorbents based on charcoal (as well as, for other
reasons,
those based on other inorganic materials) by, for example, modified natural or
synthetic polymers which had a higher mechanical stability and a higher level
of
selectivity for the elimination of certain body generated, in particular
pathogenic
biomacromolecules, in body fluids such as blood, plasma, or serum.
,2026717
RV3 011090 2 LUDR3.0-041
By use of the process of suspension polymerization, certain porous
homopolymers as well as co- or terpolymers of vinyl containing monomers, for
example, acrylic acid received particular attention, as the carrier materials.
Such acrylic acid polymers are presently available in commerce, for example,
"TSK-Gel Toyopearl~, manufactured by Toyo Soda Kogyo Co., Ltd, Japan and Toso
Haas, Philadelphia, PA, USA) and Fractogel~ TSK (manufactured by Merck GmbH,
Darmstadt, Federal Republic of Germany). Such substances are designated as
hard
gels which, in a chemical sense, are substantially identical, and, due to the
presence
of OH groups, are also hydrophilic.
These materials not only in their original form, but especially after
modification
(activation) by reaction first with an oxirane containing compound, for
example,
epichlorhydrin and the subsequent reaction with ammonia, an amino, or carboxyl
containing compound, or with cyanuric chloride, may be used as carrier
materials
(see for example J. Chromatogr. 239, 747-754 (1982)) and Toya Soda
Kenkyuhokoku, 25 (2), 81-88 (1981)). As further examples of such modified
products
which could be used as absorbents or carrier materials in absorbents, there
may be
mentioned those which are activated with glutaraldehyde and then reduced, for
example, with sodium borohydride (see Shin-jikkenkagaku-koza, ed. S. Ishii,
Maruzen,
Tokyo, 141 (1978)).
Such activated carriers offer the possibility of having a specific operating
mode.
By the use organic bridging members of different chemical structure and length
(generally known as spacers) one may introduce specific covalent organic
ligands.
Thus, one can produce so-called specifically tailored absorbents of higher
selectivity
with respect to the biomacromolecules which one wishes to remove from the
system.
Thus, in EP-A 83 112 042, certain absorbents are suggested as particularly
suitable for the selective removal of VLDL (lipoprotein of very low density)
and/or LDL
(lipoprotein of low density) from body fluids such as blood or plasma in an
extracorporeal circuit. Such materials utilize as carrier material Toyopearl~
TSK of the
type HW 75, 65, 60 as well as 55 (having a grain size of approximately 50 to
100 ~,m
. 20 2677
RV3 011090 3 LUDR3.0-041
(see in comparison Examples 1 and 2 to 4 of the EP application), however
having
different exclusion limits, to which, after reaction with epichlorhydrin a
ligand, such as
heparin or chondroitin-polysulfate is covalently bound.
In DE-OS 36 17 672, there are named a substantial number of porous
adsorbents including those which are suitable for the elimination of
pathogenic
biopolymers from aqueous fluids such as body fluids, for example, blood,
plasma or
serum, which comprise an organic solid phase as the carrier material which,
via a
covalently bound bridge member (spacer), which may be a mercapto-, amino-,
and/or carboxyl group containing mono-, oligo-, or polymer and is covalently
bonded
to a ligand, suitably a polycarboxy acid or a derivative thereof which can be
converted
into the free acid form. The carrier material is pretreated with a coupling
agent
suitably an epoxy compound such as a diglycidal compound which can
subsequently
be reacted (derivatized) with an amino compound such as ammonia to form the
bridge member. The thus modified carrier material is then further reacted, for
example with a polycarboxy acid or a derivative thereof which is further
activated with
a carbamino acid ester, for example, N-ethoxy-carbonyl-2-ethoxy-1,2-dihydro-
quinoline, to form the ligand.
Many other materials may also be used as ligands. However, especially
preferred are polymerizates or copolymerizates of acrylic acid and as the
carrier
material, the commercially available Fractogel~ may be mentioned.
It is disclosed in GIT Fachz. Lab. 27, 380-389 (1983) that all types of
Fractogel~ TSK, that is to say, types HW 40, 50, 55 and 65 comprise spherical
and
entirely porous particles having a grain size for the subtype S in the range
of 25-
40~m and for the subtype F, in the range of 32-63~.m. There is an exception in
type
HW 40 in the form of subtype C, which comprises a grain~size range of 50-
100~,m.
From Figure 4 of the above-identified publication, it is clear that type HW 40
exists in a size range of only 102 to 104 daltons. Thus, as a result of this
relatively
small pore diameter, it is unusable for the separation of larger
biomacromolecules,
for example, LDL which has a molecular weight greater than 106.
2026717
RV3 011090 4 LUDR3.0-041
The term "exclusion limit" is understood to be the minimum molecular weight
of a molecule which, in gel permeation chromatography cannot (anymore) enter
into
the pores of the absorbent.
It is further to be deduced from the foregoing publication, see in particular
page 385, first column, last paragraph, that utilizing absorbents of a smaller
particle
size, leads to a substantial improvement in the efficiency of separation
between the
biopolymers to be eliminated (at constant selectivity). For this reason, in
such
chromatography where high demands are made on the absorptive properties, in
particular the separation efficiency, the finest possible, that is to say
"super fine"
material, with a grain size in the range of 20 to 50~m is used.
A spacer and a ligand, for example, a polycarboxy acid, suitably a polyacrylic
acid, or a Polymyxin, for example Polymyxin B, may be used in conjunction with
known carrier materials formed from homo-, co-, or terpolymers of acrylic acid
or
methacrylic acid, such as for example, the above identified commercially
available
materials to provide substrates which can be utilized for the removal of
biomacromolecules, for example, LDL and endotoxins from blood plasma. This is
particularly so when the carrier material is chosen from the point of view of
porosity,
that is to say, the exclusion limit wherein, with respect to efficiency of
separation, the
smallest particle size is sought.
When utilized in an extracorporeal circuit with whole blood on the other hand,
particles of a grain size of at least 50um should be utilized. This
requirement is based
on the fact that the largest blood cell particles present in whole blood have
a diameter
of approximately 20~m, so that the sieve which holds back the absorbed
material
must have a pore width of at least 40~.m, in order to let the blood cells
through. It
has been found that utilizing packed columns of absorbent particles of a size
of
50~cm, there is sufficient room between these particles to permit the blood
cells to
pass through.
2026717
RV3 011090 5 LUDR3.0-041
Unexpectedly however, it has been found that the foregoing carrier materials
having a covalently bound spacer and a ligand covalently bound thereto, for
example,
a polycar-bonic acid such as polyacrylic acid or Polymyxin B, having a
particle size
greater than 50~.m ostensibly utilizable with whole blood, when utilized for
example,
for the separation of LDL and endotoxins which require an exclusion limit of
at least
5 x 105 daltons (measured with lypoproteins), are not suitable for the removal
of such
biomacro-molecules because an entirely undesired thrombocyte aggregation then
occurs.
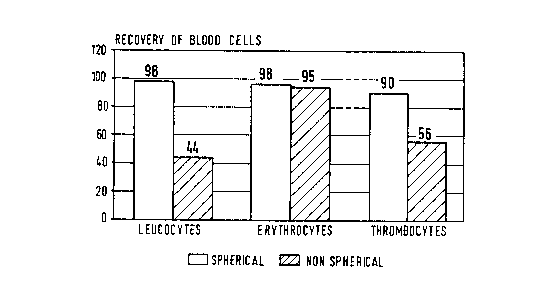
This phenomenon of blood cell incompatibility is shown in Figure 1 (dashed
surfaces) when what one would consider as suitable absorbent for the removal
of
LDL and endotoxin from full blood (that is to say, one with a particle size
greater than
um and a cut-off barrier of approximately 5x105 daltons measured with
lipoproteins) was tested and found unsuitable. Here, a carrier material, i.e.,
the
15 commercially available Toyopearl~ (HW 75 SC) was used which has a spacer
and a
ligand in accordance with Example 1 hereof (this material is, as stated above,
is made
of copolymer of glycidylmethacrylate, erythrodimethacyrlate and ethylene
glycol.
However, while this material has the appropriate particle size and exclusion
limit, it is
not spherical but rather exists in the form of irregularly formed aggregates.
SUMMARY OF THE INVENTION
The purpose of the present invention is to provide a absorbent material which
may be used in an extracorporeal circuit with whole blood which has, suitably,
a high
selectivity with respect to removal of biomacro molecules in particular, LDL
and
endotoxin while having an acceptable compatibility with blood cells.
This task is achieved in accordance with the absorbents comprising a porous
carrier material of a homo-, co-, or terpolymerizate of acrylic acid and/or
methacrylic
acid with a particle range of between 50 and 250 mmol and an exclusion level
of at
least 5 x 105 daltons, as well as organic ligands which are covalently bound
to the
carrier material via a spacer, wherein the carrier material has a spherical
shape.
.202677
RV3 011090 6 LUDR3.0-041
A further problem to be solved by this invention is the preparation of an
absorbent, in particular for the removal of endotoxins from whole blood which
gives
rise to the lowest possible level of retention for thrombocytes. This is
solved by an
absorbent wherein the spacer contains no double bonds.
It has been found that in order to formulate absorbents, which are in large
measure suitable for the removal of endotoxins from whole blood that it is
desirable
to provide the carrier material with a relatively long covalently bound spacer
having,
for example, 13 atoms. It has also been shown that the presence of double
bonds,
which occur in the spacer as a result of a requirement of production, gives
rise to an
undesired retention level of thrombocytes (see Fgure 2). An example of a
spacer
containing two double bonds is the 13 atom spacer which is introduced in the
reaction of OH containing carrier materials such as Fractogel~ and Toyopearl~
with
ethylenediamine and glutaraldehyde. Surprisingly, it has been found that the
hydration of double bonds, for example with sodium borohydride in the
conventional
manner, gives rise to an absorbent which retains much lower amounts of
thrombocytes (see Figure 2, right).
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a block plot showing recovery of blood cells of different types
for
spherical and non-spherical carriers.
Figure 2 is a block plot showing recovery of blood platelets using different
types of spacers.
Figure 3 is a comparative pressure/flow diagram for adsorption in whole blood
and saline.
Figure 4 (a) and (b) are electron micrographs of non spherical and spherical
carrier material.
2p2s717
RV3 011090 7 LUDR3.0-041
Figure 5 is a block diagram showing the influence of spacers of different
length
on the LDL adsorption capacity.
Figure 6 a block diagram showing the recovery of blood cells of the three
different types.
Figure 7 shows the structure of a 13 atom spacer beofre and after hydration.
The invention may be further illustrated by the following examples.
2026717
RV3 011090 8 LUDR3.0-041
EXAMPLE 1
In this example, there will be described the formation of an absorbent
suitable
for the removal of LDL from whole blood wherein, as well as in Example 2,
there is
utilized as the starting carrier material Toyopearl~, under the internal
characterization,
HW 70 SC (56H), manufactured by Toyo Haas Company, Philadelphia, PA, U. S. A.,
under the this material was spherical (see Fgure 4b), has a size range of
between 70
and 150um. This product is a copolymer of ethylene glycol,
glycidylmethacrylate and
erythritol-dimethacrylate.
The absorbent utilized for the following comparison tests for blood cell
compatibility comprises, as the carrier material, the known commercial product
Toyopearl~ 76 SC, which may be distinguished from the foregoing described
product
in that it is not spherical. Furthermore, it demonstrated (as a result of
particle
aggregation) an irregular condition (see Figure 4a) at the same particle size.
The foregoing carrier material is amino derivatized in the usual manner,
namely
as follows:
a) epichlorhydrin followed by ammonia, whereby a 5 atom spacer is
introduced; b) with epichlorhydrin and ethylenediamine, whereby an 8 atom
spacer is introduced;
c) with 1,4-butandiol-diglycidoxyether and then ammonia, whereby a 13 atom
spacer is introduced.
The preparation of the amino derivatized carrier (b) has been carried out as
follows:
The Toyopearl~ gel was washed on a glass filter (G-1 ) with distilled water
and
dried in vacuo at 60°C. Fifty grams of the dried gel, 300 ml of 1 N-
NaOH, and 11 ml
of epichlorhydrin were placed in a 500 ml-separable flask and stirred for 3
hours at
30°, filtered by a glass filter (G4) and washed with cold distilled
water. This gel was
placed in a 500 ml-separable flask with 63 ml of ethylenediamine, 35 mol
distilled
water and stirred for 1.5 hours at 80°C. After the reaction, the
mixture was filed by
2026717
RV3 011090 9 LUDR3.0-041
a glass filter (G4), the gel was washed with acetone several times to remove
unreacted ethylenediamine and dried in vacuo at 60°C overnight.
For the introduction of polyacrylic acid as a ligand 200 mg of polyacrylic
acid
were dissolved in 12 ml at 0.15 M aqueous sodium chloride to which were added
200
~mg of N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline as activating agent.
After 30
minutes, the above described amino derivatized carrier material was added to
the
polyacrylic acid solution. The mixture was permitted to react at room
temperature for
12 hours. The composite absorbent on the spherical carrier material was
subjected
to a battery of tests:
A1 Whole Blood Measurament/S~ecific Binding Caaacitv
These were carried out with an absorbent in accordance with Example 1 for
the determination of the LDL binding capacity obtained from fresh blood drawn
from
healthy volunteers.
2 ml. of the absorbent of Example 1 were charged to a 10 m. diameter column.
ml. of fresh blood treated with ACDA (citrate anticoagulant) (1:1 ) and pumped
through the absorber at the rate of 1 ml. per minute. The blood cells were
measured
20 before and after this treatment.
The absorbent produced in accordance with Example 2 however was utilized
for the measurements of the endotoxin binding capacity in physiological
aqueous
sodium chloride (standard: E.coli 055:85). For an absorbent produced in
accordance
with Example 1 (a), an LDL binding capacity of 8 mg/ml. of absorbent was
given. In
vitro tests with human blood which were carried out to determine the effect of
spacer
lengths in the absorbents according to Examples 1 a, 1 b and 1 c, with respect
to LDL
capacity, are shown in Figure 5.
Blood Cell Compatibility
An absorbent was produced in accordance with Example 1, however, utilizing
the non-spherical absorbent (Toyopearl~ (HW 75 SC). Tests were carried out
with
20 ml. of blood/2 ml. of absorbent. The results are illustrated in Figure 1.
They show
.2026717
RV3 011090 10 LUDR3.0-041
a substantial superiority of the absorbent in the spherical form over the non-
spherical
form, with respect to compatibility with leuco- and thrombocytes.
Toxicological Experiments
The following toxicological experiments were carried out with respect to the
LDL absorbent:
1. Physicochemical tests (USP XXI)
2. Systematic Acceptability in the Mouse (USP XXI)
3. Intracutaneous Test with Rabbits (USP XXI)
4. Sensitization Test with Guinea Pigs (DECD Guideline No. 406)
5. Hemolysis Test with Rabbits
6. Agar Overlay Test (Compare Evaluation of Hemodialysis Membranes, US
Depart. Health and Welfare, 1977, Publ. No. NIH-77/1234).
7. Ames Test, DECD Guideline No. 471 ).
None of these tests showed the presence of any toxic effect. The absorptive
material is therefore biologically compatible.
Pressure Stability
400 ml. of absorber produced in accordance with Example 1 was packed into
an absorption housing of 48 mm. diameter.
Whole blood was pumped over 15 minutes through the absorber at a plurality
of rates. The pressure was measured at the filter input. The pressure was
found to
be constant over 15 minutes. The pressure flow diagram is shown in Figure 3.
The
stability of pressure, as well as the linearity of the pressure flow diagram,
shows that
the absorber is stable at these pressures.
Utilizing the same housing and similarly utilizing 400 m. of an absorbent
produced in accordance with Example 1, a particle count was carried out in
accordance with the provisions of DIN Schedule 58 362 (determination of
particulate
impurity levels).
20267t7.
RV3 011090 11 LUDR3.0-041
Size of Particles (gym) 25-50 51-100 Over 100
after ~assaae of 10 liters
Number of Particles 31 2 0
All of these experiments were carried out with sterilized absorbent which had
been previously autoclaved at 121 °C for 30 minutes.
E~-
The spherical carrier material described in Example 1 was treated, in a manner
well known in the art, with epichlorhydrin and subsequently with
ethylenediamine or
with 1,4-butandiol-diglycidoxyether, followed by ethylenediamine to produce an
activated carrier material.
In this procedure, 40 grams of carrier material were dissolved in 125 ml. of a
1 molar aqueous sodium hydroxide solution with 8.25 ml. of epichlorhydrin or
with
1,4-butandiol-diglycidoxyether, followed by 47 ml. of ethylenediamine.
The thus activated carrier material was incubated for 12 hours with a 5%
aqueous solution of glutardialdehyde. These procedures introduced into the
carrier
material - a 13 atom spacer and a 19 atom spacer respectively, each containing
two
double bonds. The structure of the 13 atom spacer (before and after hydration)
is
shown in Figure 7, wherein the term "POL." shows a polymyxin ligand bound
thereto.
Thereafter, Polymyxin B was covalently bound to the ligand in the conventional
manner in which the coupling reaction was carried out in 250 ml. of a 10 mM
solution
of magnesium chloride containing 0.6 g. of Polymyxin B sulfate to yield
absorbent (I).
A portion of the absorbent with the 13 atom spacer was hydrated in the usual
manner with 5% aqueous sodium borohydride to hydrate the double bonds of the
spacer to yield absorbent (ll).
.202677
RV3 011090 12 LUDR3.0-041
The retention of thrombocytes was examined, utilizing 20 ml. of blood/2 ml.
of absorbent with (I) (with unreduced spacer), as well as (II) (with reduced
spacer)
for the 13 atom spacer as well as the 19 atom spacer. The surprising result,
as
5 shown in Figure 2, was that (II) showed the retention of a substantially
smaller
number of thrombocytes.
.202677
RV3 011090 13 LUDR3.0-041
EXAMPLE 3
In accordance with the detailed description set forth immediately below,
spherical carrier material based upon methacrylic acid terpolymer was amino
derivatized in accordance with Example 1, an 8 atom spacer introduced and
bonded
to the polyacrylic acid in a covalent manner as described in Example 1,
whereby an
absorbent was obtained which proved itself to be useful in the separation of
LDL from
whole blood.
The starting carrier material was a terpolymer of methacrylamide, N-methylene-
bis-methylacrylamide and allylgylcidylether, having a size distribution of
spherical
particles in the region of 50 to 200 ~.m and an exclusion limit of > 5 x 105
daltons.
These particles have a macroporous structure comprising channels, openings and
cavitations. The pore volume (determined by the mercury method) was 1.74
cm3/g.
and the mean pore diameter was 35 nm. The specific BET-surface (isothermal
nitrogen absorption) was 183 m2/g.
Toxicological determinations (USP XXI) showed that the carrier material and
the absorbent produced therefrom are toxicologically harmless. It was also
shown
further to be biologically compatible.
The method utilized in Example 1 showed the LDL binding capacity of the
absorbent from whole blood to be 3.6 mg./ml.
This value is less than that obtained for the measurements of the material
produced in accordance with Example 1 (a) which can be explained by the fact
that
the absorbent of the present Example 3 has a smaller exclusion limit than the
absorbent of Example 1.
The blood cell compatibility of the absorbent is carried out in accordance
with
procedures of Example 1. The results are shown in Figure 6. The absorbent thus
illustrates an unexpectedly low level of retention for leucocytes,
erythrocytes and
thrombocytes.